Simple Summary
Mobile applications in clinical treatment are becoming increasingly popular among cancer patients and survivors. The COVID-19 pandemic demonstrated the importance of digital interventions in patient monitoring. We conducted a scoping review and classified Mobile Health (mHealth) trials into sub-groups based on intervention methodologies, lifestyle variables, and their effectiveness on cancer health outcomes. Our study identified the key elements of the mHealth approach for cancer care, including interactive support, personalized suggestions, active participation of users, wearable technology and rigorous theory-based solutions. We also established a taxonomy that can be employed by application developers and medical specialists in developing future mHealth cancer care solutions.
Abstract
Mobile Health (mHealth) has a great potential to enhance the self-management of cancer patients and survivors. Our study aimed to perform a scoping review to evaluate the impact and trends of mobile application-based interventions on adherence and their effects on health outcomes among the cancer population. In addition, we aimed to develop a taxonomy of mobile-app-based interventions to assist app developers and healthcare researchers in creating future mHealth cancer care solutions. Relevant articles were screened from the online databases PubMed, EMBASE, and Scopus, spanning the time period from 1 January 2016 to 31 December 2022. Of the 4135 articles initially identified, 55 were finally selected for the review. In the selected studies, breast cancer was the focus of 20 studies (36%), while mixed cancers were the subject of 23 studies (42%). The studies revealed that the usage rate of mHealth was over 80% in 41 of the 55 studies, with factors such as guided supervision, personalized suggestions, theoretical intervention foundations, and wearable technology enhancing adherence and efficacy. However, cancer progression, technical challenges, and unfamiliarity with devices were common factors that led to dropouts. We also proposed a taxonomy based on diverse theoretical foundations of mHealth interventions, delivery methods, psycho-educational programs, and social platforms. We suggest that future research should investigate, improve, and verify this taxonomy classification to enhance the design and efficacy of mHealth interventions.
Keywords:
cancer; m-health; mobile apps; health outcomes; scoping review; taxonomy; intervention; treatment 1. Introduction
Due to technological advancements and superior treatment interventions, individuals diagnosed with cancer are now living longer [1]. However, the COVID-19 pandemic has had a significant impact on cancer care, causing delays in diagnosis and treatment for many patients [2]. As the global population grows and ages, the burden of cancer continues to increase [3]. Recent data shows that approximately 10 million lives were lost due to cancer in 2020, excluding non-melanoma skin cancer. Despite the progress in cancer research and treatment, cancer remains a major health challenge and one of the leading causes of mortality worldwide [3]. Hence, there is an urgent need to strengthen long-term supportive care services as the cancer population grows [4]. Currently, cancer patients and survivors face a daunting amount of responsibilities and information related to managing their illnesses and recovery. To address this challenge, healthcare providers must equip patients with the skills and knowledge required to effectively self-manage their conditions. This includes problem-solving, decision-making, resource utilization, coordination with healthcare providers, and taking appropriate actions to promote their own health and wellbeing [5].
While traditional face-to-face interventions have been beneficial in cancer care [6,7], they are not always feasible due to a lack of services, financial coverage, distance, or incapacity [8]. Fortunately, digital interventions can help overcome this challenge, as the rapid rise of mobile technology has made psychological interventions accessible to a much wider population of survivors [9,10]. The use of short message service (SMS) and applications (apps) through mobile devices such as smartphones and tablets in the health domain is referred as mHealth [11]. Further, mHealth technology has already proved its importance in the management of cancer patients, particularly in the areas of supportive care and follow up [12,13].
Mobile apps offer several benefits, including the ability to gather self-reported measures, providing user-friendly experiences, tools for managing personal health, immediate access to vital data, and reducing potential research biases [14,15]. Moreover, real-time mHealth apps offer more personalized care by providing relevant healthcare information at a low cost, and they encourage patients to meet the goals established by healthcare professionals [16,17]. Combining an app with human supervision can significantly increase patient engagement, while also improving cancer health outcomes [18,19].
Due to its complex and multi-faceted nature [20], defining a proposed mHealth intervention and evaluating its effects requires a clear and precise taxonomy [21]. A taxonomy is a useful tool for creating classifications based on relationships and has the aim of enhancing conceptual understanding and predictions [21]. The development of a taxonomy is an ongoing process that necessitates continual feedback and modification from users [21]. Theoretical foundations have important value for categorizing the assessment of mHealth intervention outcomes [22,23,24]. The health and well-being of cancer survivors are significantly affected by various factors, such as different delivery mechanisms, social media influence, and psycho-educational programs [24]. Several types of interactive health communications, including social networking, have already been used as classification methods [21]. After integrating a scoping review with taxonomy, it could support and influence future research in this field by identifying and addressing gaps, inadequacies, and trends in the existing evidence [25].
The primary objectives of this review were: (1) to explore published studies that used mobile-based interventions among cancer patients, (2) to investigate research trends and provide recommendations for the adherence and usage of mobile-app-based interventions for cancer care management, and (3) to examine the effectiveness of mobile-app-based interventions on cancer health outcomes. Hence, this analysis classified mHealth studies into various subcategories depending on the type of intervention strategies, lifestyle factors, and their impact on health outcomes for diverse cancer types and its usage rate or adherence for cancer care management. Additionally, the secondary objective of this review was to develop a taxonomy of mobile-app-based interventions for app developers and healthcare researchers that could assist in the development of future mHealth cancer care solutions.
2. Materials and Methods
In this study, a scoping review was conducted to identify articles that implemented the following search strategy, study selection and data extraction method, and fulfilled the selection criteria outlined below. The studies were classified based on the methods and clinical outcomes.
2.1. Search Strategy
The search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Review (PRISMA-ScR) guidelines [26]. We searched for related studies in different online databases, including PubMed, Scopus, and EMBASE, published between 1 January 2016 and 31 December 2022. The search for the studies was conducted from 1 October 2022 to 31 December 2022. The authors performed a rigorous search based on medical subject headings (MeSH) terms and relevant publication text keywords that had been identified beforehand. These search terms included ‘Mobile application’ or ‘mHealth’ or ‘Mobile apps’; ‘Cancer’ or ‘Cancer survivors’ or ‘Neoplasms’; ‘Intervention’ or ‘Treatment’.
The detailed search strategy and results are shown in Figure 1.
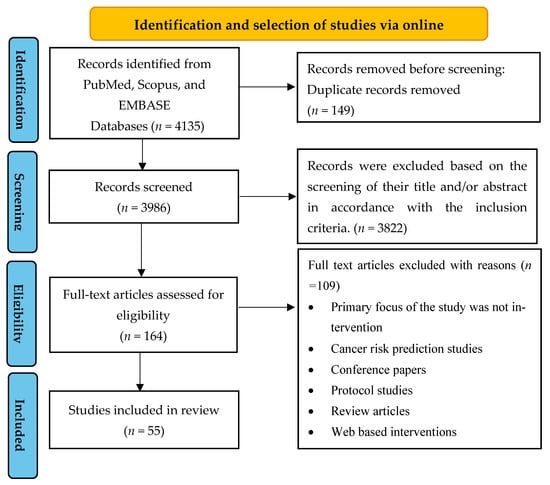
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Review (PRISMA-ScR) flow chart of study identification and selection of studies.
2.2. Eligibility Criteria
The following selection criteria were applied for the inclusion of articles: (1) a focused on cancer survivors and cancer patients undergoing treatment, (2) assessed lifestyle and psychological interventions using mobile apps, (3) utilized apps for assisting patients or survivors in self-managing their health on a routine basis, (4) contained one of the following design types: randomized controlled trial (RCT), pilot study, prospective clinical trial, quasi-experimental study, feasibility study, observational or pre-test post-test study, (5) included only original research, and (6) were written in the English language only.
Studies were excluded based on the following criteria: (1) aimed at preventing or detecting cancer, (2) used telecommunication technologies such as websites, telephones, or wearables alone, (3) did not assess lifestyle or psychosocial factors or engagement with mobile apps, (4) did not focus on intervention, (5) focused on the design, development, or usefulness of mobile health apps, (6) were review articles, trial protocols, trial registrations, conference papers, book chapters, notes, brief reports, letters, editorials, case studies, and (7) were written in non-English languages.
2.3. Study Selection and Data Extraction
Two authors of this study (ED and NS) conducted an independent review of the titles and abstracts of the entire search yield to identify eligible articles. If an article was considered potentially significant by either reviewer, the complete text of the publication was retrieved. In the event of a disagreement, a third reviewer (AC) decided the final article based on the inclusion and exclusion criteria. All duplicate articles were removed. An initial screening was conducted based on the titles and abstracts to identify articles that fulfilled the inclusion criteria. For those that could not be rejected with certainty, full-text articles were obtained. The authors then reviewed the full-text versions of each article to identify those that met the inclusion criteria. Subsequently, the following information was systematically extracted from each included study: study characteristics (country of origin, year of publication, and sample size), patient characteristics (mean age, gender, and type of cancer), intervention characteristics (duration, mobile app with or without interactive support and/or wearables), and intervention focus (physical and psychosocial/lifestyle variables). The selected studies were categorized based on their methods and outcomes, as shown in Figure 2.
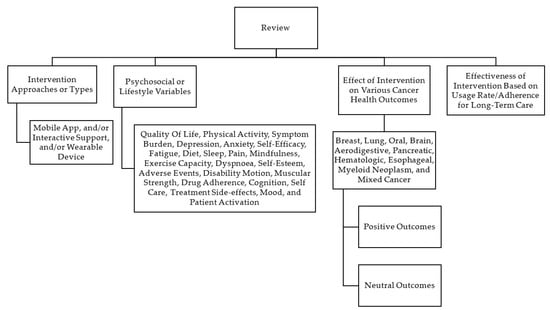
Figure 2.
Categorization of studies included in the scoping review based on methods and outcomes.
2.4. Taxonomy
Our taxonomy was classified on the basis of dimensions that could potentially represent the key characteristics of users’ interaction and engagement with the interventions delivered through mobile devices.
The four dimensions of this taxonomy are shown in Figure 3 and include the classification of mHealth interventions according to
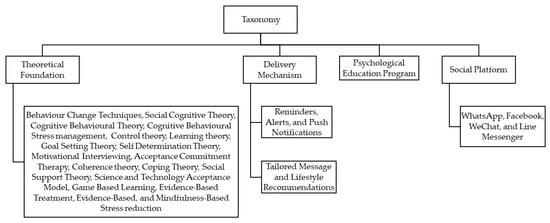
Figure 3.
Flow of classification of the studies via taxonomy.
- Theoretical foundation or behavioural techniques [22,23,24].
- Delivery mechanism (through reminders/alerts or tailored messages/lifestyle recommendations) [24].
- Psycho-educational program [24].
- Various social platforms [21,24].
3. Results
3.1. Scoping Review
3.1.1. Study Selection
The electronic databases’ search yielded a total of 4135 articles from PubMed (n = 564), EMBASE (n = 587), and Scopus (n = 2984). After removing duplicates, 3986 articles were assessed based on their titles and abstracts. Among these, 3822 were excluded because they did not meet the inclusion criteria exclusively. The remaining 164 articles underwent full-text screening, and 109 were subsequently excluded for different reasons, such as being non-intervention studies, cancer risk prediction studies, conference papers, protocol studies, review articles, and web-based interventions (see Figure 1). Ultimately, a total of 55 studies were included in this analysis.
3.1.2. General Characteristics of the Studies
Out of the fifty-five studies, nineteen were conducted in North America (eighteen in United States and one in Canada), eighteen in Asia (nine in South Korea, two each in Iran, Turkey, China, and Taiwan, and one in Japan), fourteen in Europe (three in Sweden, two each in Switzerland and Spain, and one each in United Kingdom, Ireland, Denmark, Norway, Netherlands, Slovenia, and Germany), two in Australia (Australia and New Zealand), and one in South America (Brazil). One study was conducted at multiple centres, with study participants recruited across five European nations, namely Austria, Greece, Ireland, Norway, and the UK.
In terms of study the designs of the selected studies, there were thirty-five randomized controlled trials, six feasibility studies, five pilot studies, three quasi-experimental studies, two pre-post studies, and one each of baseline/post study, prospective clinical trial and randomized open-label trial (see Table 1).
3.1.3. Characteristics of Research Participants
Most of these articles targeted breast cancer only (20 studies) or breast cancer and other types of cancer (denoted by the term mixed cancer, 23 studies). Two studies focused on each of the following: prostate cancer, pancreatic cancer and lung or/and non-small cell lung cancer. The remaining six studies focused on oral cancer, brain tumour, aerodigestive cancer, myeloid neoplasm, hematologic cancer and oesophageal cancer. The number of participants across the 55 studies varied between 10 and 829. The mean age of participants ranged from 14.2 years to 72 years. The duration of mobile-app-based interventions varied between 4 and 32 weeks. The current review included studies with both younger and older cohorts. Paediatric and adolescent cancer studies [27,28,29,30] accounted for four of the fifty-five cancer trials. The remaining 51 studies focused on adults and older patients.
3.1.4. Measurement Tools
Subjective self-reported questionnaires and electronic patient-reported outcome measures that had previously been validated or applied in cancer research were used in all of the included studies.
3.2. Categorisation of Studies
The studies included in our analysis were categorized based on their interventional approaches and clinical outcomes (see Figure 2). The categories were classified as below.
3.2.1. Interventional Approaches/Types
All studies in our analysis used various intervention methods to improve cancer health outcomes. Of these, twenty-six studies solely relied on mobile-based interventions, seventeen studies used mobile-based interventions with interactive support, six studies utilized mobile-based interventions with wearable devices, and six studies combined mobile-based interventions with wearable devices and interactive support (see Table 1).
The mobile-based interventions in the studies monitored cancer health outcomes while providing motivational texts, educational support, coping skills training, and game-based learning. When integrated with wearable devices, the mHealth apps collected real-time data and offered feedback. The interventions with interactive support were provided by healthcare professionals, qualified counsellors, or researchers, and involved personalized assistance, treatment, coaching, guidance, counselling, and motivation delivered via phone calls, mobile apps, or face-to-face interactions.
3.2.2. Psychosocial/Lifestyle Variables Assessed
There were forty-three studies that assessed quality of life, fifteen studies that focused on physical activity, ten studies that targeted anxiety, nine studies that addressed symptom burden and management, seven studies each for fatigue, loneliness/depression, nutrition/diet, and self-efficacy, six studies for sleep quality, and five studies each for pain and mindfulness. Additionally, three studies investigated exercise capacity, two studies examined smoking cessation/abstinence, and one study each focused on dyspnoea, adverse events, disability motion, muscular strength, drug adherence, cognition, self-care activities, patient activation (self-management of illness), treatment side effects, self-esteem, and utilization of supportive care services. These variables have been listed under the ‘focus of the study’ in Table 1.
3.2.3. Effects of Interventions on Various Cancer Health Outcomes
The term “positive outcomes” referred to any improvement in participants’ health as a result of using mobile apps. Positive outcomes were found in 19 of 20 breast cancer studies, 19 of 23 mixed cancer studies, two studies each for prostate and lung cancer, and one study each for oral, brain, pancreatic, and aerodigestive cancer. Neutral outcome implied no significant improvement in the health outcomes of the participants and was seen in nine studies [27,29,30,31,32,33,34,35,36]. Improvements in cancer health outcomes are summarized in Table 1.

Table 1.
Characteristics of included studies and mobile-based interventions.
Table 1.
Characteristics of included studies and mobile-based interventions.
| Author, Year, Country | Cancer Type | Sample Size | Study Design | Gender (%) Mean Age (Years) | Mobile App and/or Interactive Support and/or Wearables Device | Study Focus | Study Duration | Improvements in Health Outcomes |
|---|---|---|---|---|---|---|---|---|
| Egbring et al., 2016 [18] Switzerland | Breast Cancer | 139 | RCT | 100% females 53 (yrs) | Mobile app + interactive support | Daily functional activity and adverse events | 6 weeks | Daily functional activity and patient awareness of severity of symptoms |
| Lozano et al., 2019 [37] Spain | Breast Cancer | 80 | Prospective test-retest quasi-experimental study | 100% females 51.80 (yrs) | Mobile app only | QoL, physical activity, body composition and physical activity motivation | 8 weeks | QoL, physical activity and body weight |
| Allicock et al., 2021 [38] USA Ballcock | Breast Cancer | 22 | Feasibility study | 100% females 52.23 (yrs) | Mobile app + wearable device | Physical activity and diet | 8 weeks | Physical activity and diet habits |
| Yanez et al., 2020 [39] USA | Breast Cancer | 78 | RCT | 100% females 52.54 (yrs) | Mobile app + interactive support | QoL, symptom burden (breast cancer related) | 6 weeks | Breast cancer well-being (disease specific QoL) and symptom burden |
| Cinar et al., 2021 [40] Turkey | Breast Cancer | 64 | RCT | 100% females 45.7 (yrs) | Mobile app + interactive support | QoL, distress | 12 weeks | QoL and distress |
| Handa et al., 2020 [29] Japan | Breast Cancer | 95 | RCT | 100% females 49.9 (yrs) | Mobile app only | QoL (Anxiety and depression), health literacy | 12 weeks | No improvement |
| Uhm et al., 2017 [16] South Korea | Breast Cancer | 339 | Quasi-experimental study | 100% females 50.3 (yrs) | Mobile app + wearable device | QoL, physical measurements and self-reported physical activity | 12 weeks | QoL and physical activity |
| XU et al., 2021 [41] China | Breast Cancer | 126 | RCT | 100% females 47.93 (yrs) | Mobile app + interactive support | QoL (anxiety and depression), discomfort symptoms and self-efficacy | 16 weeks | QoL and self-efficacy |
| Ghanbari et al., 2021 [42] Iran | Breast Cancer | 82 | RCT | 100% females 46.45 (yrs) | Mobile app + interactive support | Anxiety and self-esteem | 4 weeks | Anxiety and self-esteem |
| Sheean et al., 2021 [43] USA | Breast Cancer | 35 | RCT | 100% females 55.11 (yrs) | Mobile app + interactive support | QoL, symptom burden, lifestyle behaviours (nutrition and physical activity) | 12 weeks | QoL and physical activity |
| Kuhar et al., 2020 [44] Slovenia | Breast Cancer | 91 | Non-randomized controlled prospective cohort Study | 100% females 51.7 (yrs) | Mobile app only | QoL | Throughout chemotherapy | QoL |
| Lozano-Lozano et al., 2020 [45] Spain | Breast Cancer | 78 | RCT | 100% females 52.5 (yrs) | Mobile app only | QoL, disability, motion, muscular strength | 8 weeks | QoL, disability, motion and muscular strength |
| Kim et al., 2018 [46] South Korea | Breast Cancer | 72 | RCT | 100% females 51 (yrs) | Mobile app only | QoL, drug adherence, side effects depression and anxiety | 3 weeks | QoL, compliance to medication and side effects |
| Rosen et al., 2018 [47] USA | Breast Cancer | 84 | RCT | 100% females 53 (yrs) | Mobile app only | QoL and mindfulness | 8 weeks | QoL and mindfulness |
| Lengacher et al., 2018 [48] USA | Breast Cancer | 13 | Pilot Study | 100% females 57 (yrs) | Mobile app only | QoL, fatigue, depression, pain, sleep quality, stress, FOR, anxiety, cognition and mindfulness | 6 weeks | QoL, fatigue, depression, pain, sleep quality, anxiety, stress, FOR, anxiety, cognition and mindfulness |
| Ferrante et al., 2020 [49] USA | Breast Cancer | 35 | RCT | 100% females 61.54 (yrs) | Mobile app + wearable device | QoL, weight management, diet and physical activity, cardiopulmonary fitness, social cognitive theory variables and anthropometric outcomes | 24 weeks | QoL, waist circumference, healthy eating and calorie reduction techniques |
| Hou et al., 2020 [50] Taiwan | Breast Cancer | 112 | RCT | 100% females 42 (yrs) | Mobile app only | QoL | 12 weeks | QoL |
| Fjell et al., 2020 [51] Sweden | Breast Cancer | 149 | RCT | 100% females 49 (yrs) | Mobile app + interactive support | QoL and symptom burden | 18 weeks | QoL and symptom burden |
| Öztürk et al., 2021 [52] Turkey | Breast Cancer | 57 | RCT | 100% females 51.44 (yrs) | Mobile app only | QoL and symptom burden | 8 weeks | QoL and symptom burden |
| Bandani-Susan et al., 2021 [53] Iran | Breast Cancer | 38 | RCT | 100% females 46.34 (yrs) | Mobile app only | Cancer-related fatigue | 7 weeks | Fatigue |
| Mendoza et al., 2017 [27] USA | Mixed Cancer | 59 | RCT | 59.3% females 16.6 (yrs) | Mobile app + wearable device + interactive support | QoL and physical activity | 10 weeks | No improvement |
| Blair et al.,2021 [54] USA | Mixed Cancer | 54 | RCT | 55% females 69.6 (yrs) | Mobile app + wearable device + Interactive support | QoL and physical activity | 13 weeks | Physical activity |
| Kubo et al., 2019 [55] USA | Mixed Cancer | 97 | RCT | 69% females 59 (yrs) | Mobile app only | QoL, fatigue, distress, sleep, mindfulness, pain, anxiety and depression, and posttraumatic growth | 8 weeks | QoL |
| Puszkiewicz et al., 2016 [56] UK | Mixed Cancer | 11 | Pre–post study | 82% females 45 (yrs) | Mobile app only | QoL, physical activity, well-being, fatigue, sleep, anxiety and depression | 6 weeks | Physical activity and sleep quality |
| Yang et al., 2019 [57] China | Mixed Cancer | 58 | RCT | 34% females 52.53 (yrs) | Mobile app only | QoL and pain | 4 weeks | QoL and pain |
| Mikolasek et al., 2021 [58] Switzerland | Mixed Cancer | 100 | Feasibility study | 74% females 53.2 (yrs) | Mobile app only | QoL, anxiety, fatigue, distress, sleep and mindfulness | 20 weeks | QoL, anxiety, fatigue, distress, sleep disruptions and mindfulness |
| Walsh et al., 2021 [59] Ireland | Mixed Cancer | 123 | RCT | 86% females 57.42 (yrs) | Mobile app + Wearable device | QoL, fatigue, self-efficacy, loneliness, exercise self-efficacy, social support for physical activity, functional exercise capacity, diet and physical activity | 12 weeks | BMI, waist circumference and physical activity |
| Poort et al., 2021 [30] USA | Mixed Cancer | 25 | Pilot study | 56% females 28 (yrs) | Mobile app only | QoL, self-efficacy for coping with cancer, self-efficacy for managing emotions and perceived emotional support | 12 weeks | No improvement |
| Robertson et al., 2020 [60] USA | Mixed Cancer | 78 | RCT | 91% females 55.1 (yrs) | Mobile app + wearable device | Physical activity | 16 weeks | Physical activity |
| Pappot et al., 2019 [12] Denmark | Mixed Cancer | 20 | Pre–post study | 70% females 25 (yrs) | Mobile app only | QoL | 6 weeks | QoL |
| Jibb et al., 2017 [28] Canada | Mixed Cancer | 38 | One-group baseline/poststudy | 43% females 14.2 (yrs) | Mobile app only | QoL, pain and self-efficacy | 4 weeks | QoL and pain |
| Livingston et al., 2020 [31] Australia | Mixed Cancer | 82 | RCT | 71% females 59.5 (yrs) | Mobile app only | Unmet psychological needs | 16 weeks | No improvement |
| Børøsund et al., 2020 [61] Norway | Mixed Cancer | 25 | Feasibility study | 84% females 48 (yrs) | Mobile app + interactive support | QoL, anxiety and depression, fatigue, stress | 8 weeks | QoL, anxiety, fatigue and stress |
| Ham et al., 2019 [62] South Korea | Mixed Cancer | 63 | RCT | 86% females 44.1 (yrs) | Mobile app only | QoL, depression and anxiety | 10 weeks | Depression and anxiety |
| Benze et al., 2019 [63] Germany | Mixed Cancer | 40 | Feasibility study | 70% females 57 (yrs) | Mobile app only | QoL, pain, distress and symptoms | 24 weeks | QoL and symptom burden |
| Greer et al., 2019 [64] USA | Mixed Cancer (high anxiety) | 145 | RCT | 74% females 56.45 (yrs) | Mobile app only | QoL, anxiety and depression | 12 weeks | QoL, anxiety and depression |
| Maguire et al., 2021 [65] Austria, Greece, Ireland, Norway and UK | Mixed Cancer | 829 | RCT | 82% females 52.4 (yrs) | Mobile app + interactive support | QoL, symptom burden, supportive care needs, work limitations, anxiety and self-efficacy | 18 weeks | QoL, symptom burden, anxiety and self-efficacy |
| Ormel et al., 2018 [66] Netherland | Mixed Cancer | 32 | RCT | 13% females 33.6 (yrs) | Mobile app only | Physical activity | 12 weeks | Physical activity |
| Krebs et al., 2019 [67] USA | Mixed Cancer | 38 | RCT | 71% females 57.11 (yrs) | Mobile app + interactive support | Smoking cessation | 4 weeks | Higher confidence to quit |
| Casillas et al., 2019 [68] USA | Mixed Cancer | 71 | RCT | 53% females 21 (yrs) | Mobile app + interactive support | Survivorship care knowledge and self-efficacy | 8 weeks | Survivorship care attitude and self-efficacy |
| Rico et al., 2020 [69] Brazil | Mixed Cancer | 87 | RCT | 56% females 45.2 (yrs) | Mobile app + interactive support | QoL side effects | 12 weeks | QoL (side effects) |
| Chung et al., 2022 [70] South Korea | Mixed Cancer | 41 | RCT | 80% females 41.78 (yrs) | Mobile app only | QoL and sleep quality | 10 weeks | Sleep quality |
| Merz et al., 2022 [33] USA | Mixed Cancer | 45 | RCT | 60% females | Mobile app only | QoL, utilization of supportive care services and patient activation (self-management of illnesses) | 12 weeks | No Improvement |
| Sundberg et al., 2017 [71] Sweden | Prostate cancer | 130 | Feasibility study | 100% males 69 (yrs) | Mobile app + interactive support | QoL and symptom burden | 5–8 weeks | QoL and symptom burden |
| Lee at al., 2019 [72] South Korea | Prostate cancer | 100 | Randomized open-label trial | 100% males 69.44 (yrs) | Mobile app + wearable device+ interactive support | Physical functions | 12 ± 1 weeks | Physical functions |
| Ji et al., 2019 [73] South Korea | Non-small cell lung cancer | 64 | Prospective clinical trial | 70% males 59.23 (yrs) | Mobile app + wearable Device | QoL, exercise capacity and dyspnea | 12 weeks | QoL, exercise capacity and dyspnea |
| Park et al., 2019 [74] South Korea | Lung cancer | 90 | Pilot study | 46% males 55.1 (yrs) | Mobile app + wearable + interactive support | QoL, exercise capacity and symptom management | 12 weeks | Exercise capacity and symptom management. |
| Wang et al., 2020 [75] Taiwan | Oral cancer | 100 | Quasi experimental | 92% males 57.01 (yrs) | Mobile app + interactive support | QoL (symptoms), cancer needs | 12 weeks | QoL and cancer care needs |
| De Tommasi et al., 2020 [76] New Zealand | Brain tumour | 10 | Feasibility study | 60% females 53.8 (yrs) | Mobile app only | QoL (illness-related), psychological distress and mindfulness capacity | 8 weeks | QoL and mindfulness |
| Rettig et al., 2018 [77] USA | Aerodigestive cancer | 29 | RCT | 62% males 55 (yrs) | Mobile app + interactive support | Smoking abstinence | 8 weeks | Smoking abstinence |
| Gustavell et al., 2019 [32] Sweden | Pancreatic and Periampullary cancer | 26 | RCT | 61% males 66.5 (yrs) | Mobile app only | QoL, self-care activity | 32 weeks | No Improvement |
| Keum et al., 2021 [78] South Korea | Pancreatic cancer | 33 | RCT | 63% males 61.5 (yrs) | Mobile app + interactive support | QoL and nutrition | 12 weeks | QoL and nutrition |
| Chow et al., 2021 [19] USA | Hematologic cancer | 41 | RCT | 48.8% females 45.1 (yrs) | Mobile app + wearable + interactive support | QoL, physical activity, self-efficacy and diet | 16 weeks | No improvement |
| Loh et al., 2022 [35] USA | Myeloid neoplasm | 22 | Pilot study | 68% males 72 (yrs) | Mobile app + wearable + interactive support | QoL, physical activity, fatigue and mood | 8–12 weeks (two cycles of chemotherapy) | No improvement |
| Yang et al., 2021 [36] South Korea | Oesophageal cancer | 30 | Pilot study | 100% males 59 (yrs) | Mobile app + interactive support | Physical activity and nutrition | 8 weeks | No improvement |
QoL, quality of life; RCT, randomized controlled trial; yrs, years; FOR, fear of recurrence.
3.2.4. Effectiveness of Mobile-App Based Interventions Based on the Usage Rate/Adherence for Cancer Care Management
To comprehend the general trends for app adherence or usage rate in cancer care management, we tracked the participants’ usage of the applications and positive/neutral outcomes across all cancer-type studies (see Table 2).

Table 2.
Cancer type, cancer health outcomes, duration and usage rate of mobile-app-based interventions for different cancer types.
There is no established standard measure or threshold for defining good or bad adherence rates in app-based interventions for cancer care [79]. Adherence or usage rate was evaluated using various criteria outlined in the selected articles. For the majority of the included studies, we assessed adherence/usage rate based on data collection completeness, which refers to the percentage of enrolled patients who completed the research. The remaining studies had their own specific criteria for evaluating adherence. The study’s usage rate was defined as the percentage of enrolled users who used the app as intended, based on data logging of the application or until the end of the intervention [38,73]. Nearly 70% of the total studies included in this scoping review reported an 80% usage rate. Therefore, we determined 80% as a suitable threshold for comparing the usage rates of all the included studies. Of the included studies, 41 indicated an adherence rate of 80% or greater, while 14 reported less than 80% adherence (see Table 3). One study had a notably high dropout rate of 60%, despite having a short intervention period of only four weeks. This was attributed to the participants’ advanced age and lack of experience with the game-based app [67].

Table 3.
Distribution of studies based on mobile app usage rate and duration.
Based on our analysis, the definition of dropouts varied across studies. Some studies considered users who logged in only once or used the app once or twice as dropouts [79,80], while another study set a minimum weekly usage of 90 min [39]. Therefore, for the present investigation, the dropout rate was defined as the percentage of users who stopped using the mHealth app [80]. The review presented usage rate and study duration results for each cancer type (see Table 2).
3.3. Taxonomy
Out of the fifty-five studies, forty-nine were classified based on the proposed taxonomy’s four different dimensions. However, six studies could not be categorized according to the taxonomy as they did not fit into any of its dimensions [12,18,19,33,51,77]. The classification of articles based on the four dimensions of the taxonomy outlined in Figure 3 is presented in Table 4.

Table 4.
Taxonomy of the included studies based on four dimensions.
3.3.1. Theoretical Foundations or Behavioural Techniques
A total of 22 studies utilized a theoretical foundation to encourage behavioural engagement in their mobile-based interventions. Among these studies, the most commonly used theory was social cognitive theory (SCT), which was applied in seven studies, followed by behaviour change techniques (BCTs) in five studies. Additionally, control theory (CT), learning theories (LTs), and goal setting theory (GST) were implemented in three studies each.
One study by Lozano et al. [37] incorporated a variety of BCTs, such as reinforcement, facilitation, self-monitoring, goal setting, performance feedback, and goal review. By incorporating cognitive behavioural therapy (CBT) strategies, some studies used stress management techniques, relaxation training, behavioural activation, cognitive restructuring, problem solving approaches, activity planning, and pacing, along with techniques for generating new thoughts, staying present, summarizing, and reviewing [61,62,64,70].
3.3.2. Delivery Mechanism (Reminders/Alerts or Tailored Messages/Lifestyle Recommendations)
The studies included in the current taxonomy have been classified based on their intervention methods, which include reminders/alerts/notifications and personalized lifestyle recommendations/tailored messages. Out of the total 20 mHealth studies, alerts, reminders, or push notifications were used as a means of intervention. These interventions led to improved quality of life (QoL), pain-related outcomes, activity levels, anxiety, fatigue, symptom burden, exercise ability, sleep, stress, disability motion, muscular strength, mindfulness, and cancer care needs. However, only two studies failed to show any significant improvements [31,34].
On the other hand, thirteen studies provided personalized recommendations or tailored messages as their means of intervention, which motivated cancer patients to change their lifestyle habits or achieve their goals. These interventions resulted in improvements in various aspects of health, such as QoL, physical activity, symptom burden, exercise capacity, dyspnoea, smoking, self-efficacy, diet, sleep, weight, and even survivorship care attitudes. However, four studies did not show any significant improvements [27,29,35,36].
3.3.3. Psychoeducational Program
Seven studies included psychoeducation as a component of their interventions for cancer patients. These apps provided information on cancer, exercises, balanced diet, and therapeutic interventions for managing fatigue, pain, sleep, anxiety, depression, self-esteem, and stress.
3.3.4. Various Social Platforms
Five studies utilized social media platforms to provide cancer care services. Platforms such as WeChat app, Facebook, WhatsApp, and LINE app enabled patients to join groups and interact with each other while also receiving support and guidance from a healthcare provider. These studies demonstrated the potential of social media in facilitating peer support and improving patients’ psychological and social well-being.
4. Discussion
4.1. Summary and Findings
The objective of this review was to examine the characteristics of intervention studies and evaluate the impact of mobile health technologies on cancer health outcomes, adherence, and usage rates among cancer patients. This review analysed 55 studies that utilized mobile technology to enhance psychosocial or lifestyle habits in cancer patients and survivors. The studies included various cancer populations, age groups, mHealth interventions, and cancer outcome measures. The secondary objective was to develop a taxonomy based on mobile applications and interventions. All the studies were classified into four distinct categories: theoretical foundation, delivery mechanism, psycho-educational programs, and various social platforms. Despite the diverse range of studies included in the review, the results demonstrated that mobile health interventions were effective and well-received.
Several interventional strategies were employed to enhance cancer health outcomes. Although cancer survivorship is characterized by persistent physical and psychological challenges that make lifestyle modifications and management more challenging, the majority of these interventions had a favourable impact on cancer health outcomes [59].
In 32 of 43 trials, mHealth treatments enhanced at least one component of cancer patients’ quality of life. This is consistent with another meta-analysis that also reported improvements in quality of life among cancer patients [82]. Of the seven psycho-educational interventions based on mHealth, three resulted in an improvement in quality of life. In contrast, a meta-analysis found that internet-based psycho-educational treatments reduced depression and fatigue but had no effect on distress or quality of life [75].
Out of a total of 15 studies focused on physical activity, 11 reported a positive impact on patients’ physical activity levels. Similarly, another systematic review found that mobile applications had a positive impact on physical activity among cancer patients, utilizing various theoretical frameworks. The only difference was that this particular review focused specifically on the effectiveness of gamification interventions for improving cancer health outcomes [83].
Our review included several studies, which indicated that symptom management was improved in 7 out of 9 studies, and anxiety levels were enhanced in 7 out of 10 studies. These results align with another review that emphasized the beneficial effects of digital interventions on anxiety and symptom management. However, the latter review encompassed a wider range of interventions, such as web-based platforms, mobile apps, tele monitoring, and telemedicine in cancer care, whereas our review focused only on mobile-based interventions for cancer care [84].
Several studies reported that testing the effectiveness of their intervention was challenging due to a small sample size [28,30,32]. One study identified a communication barrier between cancer patients and healthcare professionals, which negatively impacted the patients’ health outcomes. The report suggested that if healthcare providers had offered additional support, the patients would have used the app more frequently [30].
Previous studies have shown that high drop-out rates pose a significant challenge to the success of digital health initiatives in terms of improving adherence and providing support and follow-up [85]. Our review found that the duration of the research did not have a significant impact on the adherence rate of most mobile applications. While mobile devices may appear to be a practical solution in healthcare, users may initially use them but then fail to continue using them regularly over time [31]. For instance, two trials included in our review showed that elderly users lacked familiarity with the mobile devices, resulting in drop-out rates of 59% and 60% [32,68]. One study from our review attributed its 55.2% completion rate to one-way communication, which they believed reduced patient engagement [69]. This is consistent with a review that found that two-way text messaging improves medication adherence [86]. Our review also identified other factors that could affect the effectiveness of mobile-based interventions, such as small sample size, paper-based questionnaires, and the absence of a control group [30,31,59,61]. Lower usage rates were also associated with how data was obtained, with objective measures (such as number of log-ins) being more reliable than self-reported questionnaires [37]. The reasons for dropouts in our review included aggravated sickness, old age, technical challenges with devices/apps, fatigue, mortality, personal reasons, lack of intervention support, and unfamiliarity with the mobile device. In our review, three studies examined approaches to addressing the problem of attrition [31,62,67]. These studies suggested that a blended healthcare delivery model, which combines mobile interventions with face-to-face consultation or telephone support, could be effective. Additionally, time flexibility was found to be essential for cancer survivors to fully benefit from mHealth solutions. The studies also recommended providing a variety of interventions, such as survivorship features and different exercises, to address this issue.
After conducting the review, several significant themes emerged from analysing various mHealth solutions. As a result, we have put forward a taxonomy that categorizes all treatments based on their theoretical foundations, delivery methods, psycho-educational tools, and social platforms. Whilst mHealth treatments have huge amount of potential, it is important to develop them using a theory and evidence-based methodology [24,59]. BCTs help in designing and presenting difficult treatments in a systematic way [59]. From the analysis, we discovered that BCTs can potentially lead to favourable lifestyle modifications [38,46,56,60,67]. Some of these techniques used in the apps included goal setting (behaviour), action planning, performance feedback, self-monitoring, instructions on how to perform behaviour, graded tasks, prompts and cues, or social reward [37,56,59]. Besides this, patients in the included studies took an active role in managing their own health, which may have resulted in a rise in the usage of supportive care services. Self-management strategies such as psycho-education therapies, exercise programs, and (online) self-care interventions have shown to promote patient activation by giving them knowledge and training problem solving and coping skills [80]. In three of the included studies, gamified apps were also implemented to improve cancer health outcomes [47,60,68].
We discovered that nearly 84% of the articles included in our review showed a positive response, which is consistent with prior reviews that have demonstrated improvements in cancer health outcomes [87,88,89]. It is possible that this is because the trials in our investigation had access to self-monitoring tools as well as automated sensors, online social support, and, most importantly, real-time feedback systems.
Previously, a taxonomy was developed to distinguish various technology modalities in clinical applications [90]. Furthermore, the credible classification of treatments in terms of BCTs exists, as indicated in prior studies [22,90,91,92]. Based on existing related work [23,93], we propose a framework for mobile-based interventions in cancer care. In our taxonomy, classification is conducted based on four dimensions: theoretical foundations, delivery mechanisms, psychoeducational materials, and social support via social media.
The widespread use of social media platforms such as Twitter, Facebook, and YouTube, as well as online support, represents a significant opportunity for mHealth apps in cancer survivorship [24,94]. In our study, three out of four studies successfully implemented mHealth solutions with social networks, demonstrating improvements in quality of life and self-esteem, and decreased cancer care needs. Our findings suggest that online mobile-based solutions can effectively enhance psychosocial and quality of life outcomes, while also reducing anxiety levels in cancer patients [95]. These results are consistent with Attai et al. [96], who found that participating in a Twitter support group increased cancer knowledge and decreased anxiety levels among patients.
4.2. Study Strengths
Our research showed the usefulness and effectiveness of mobile-based treatments for cancer care management. The present review provided an in-depth analysis of mobile technology for lifestyle changes in the cancer population. The proposed taxonomy can be used as a starting point for the methodical characterization of mHealth solutions, which despite their wide range, are often described in similar words [97]. This taxonomy is important, as it defines and categorizes the key characteristics of the users’ interaction and engagement in mHealth interventions on cancer patients; therefore, it could be considered in the creation of mobile apps focused on cancer management in the future. In addition, most of the included studies showed good adherence to mHealth interventions, indicating the promising application of mHealth in cancer management.
4.3. Study Limitations
First, we were unable to conduct a meta-analysis due to the multiplicity of study designs and efficacy assessments. Second, the methodological issues (small sample size and lack of control groups) deterred the synthesis and assessment of overall evidence strength. Third, as the mean age of the participants from the selected studies varied from 14.2 years to 72 years, we were unable to discuss the implications of elderly people older than 75 years. Fourth, the search results for this study were based on MeSH terms and relevant keywords along with their combinations found in cancer care literature reviews. Fifth, the review was limited to English language articles only. Finally, it did not include any grey literature.
5. Conclusions
This research focused on identifying the factors that impact the effectiveness of mobile-based apps and provided a taxonomy classification that explains the design and efficacy of interventions for cancer care treatment and management. Various factors have been identified as facilitators of successful mHealth interventions, including guided supervision, personalized suggestions, a strong theoretical basis, and the use of wearable technology. These elements have been shown to improve both adherence to treatment regimens and the overall efficacy of mHealth interventions. However, despite these positive findings, there are still barriers to the widespread adoption of mHealth, such as technical difficulties with devices and apps, fatigue, and lack of intervention support. Additionally, various factors such as old age, mortality, personal reasons, and unfamiliarity with mobile devices can lead to dropouts from mHealth programs. Therefore, while the benefits of mHealth are clear, further research is needed to address the challenges that may hinder its effectiveness and ensure that mHealth interventions are accessible and useful to a diverse range of individuals. The results highlighted the need for well-designed trials and robust theory-based mHealth interventions in determining the efficacy and impact of mobile health interventions in cancer care. With the advancements and expansion of mHealth technologies, there are increasing opportunities for these mobile apps in personalized health care and behavioural change for cancer patients. For future studies, we highly recommend investigating, improving and verifying this taxonomy classification in order to enhance the efficacy of mHealth interventions for cancer care. These targets are aligned with the goals of participatory health informatics, where the work for defining the types of interventions that foster the participation of patients in their own healthcare is in progress. Furthermore, as per recommendations, future research should look into how to expand this taxonomy to address core aspects of cancer self-management with a holistic approach to the adherence to pharmacological treatments in cancer and addressing the challenges of disease, patient, and socioeconomic factors in the cancer care domain.
Author Contributions
Conceptualization, E.D., S.S.-A., I.A.C.G. and A.N.B.; methodology, E.D. and A.N.B.; writing—original draft preparation, E.D. and S.M.; writing—review and editing, M.U., S.Q. and U.U.; supervision, L.F.-L., F.J.N.-B. and S.S.-A.; funding acquisition, L.F.-L. and S.S.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Taipei Medical University and the Taipei Medical University Hospital under a joint research grant (109TMU-TMUH-11). This study was also partially funded by the European Union’s Horizon H2020 iHelp project under the research grant GA: 101017441 and MOST, Taiwan: 110-2923-E038-001-MY3 and the European Union’s Horizon H2020 LifeChamps under the research grant GA: 875329.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foster, C. The Need for Quality Self-Management Support in Cancer Care. BMJ Qual. Saf. 2021, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Weinberg, D.S.; Edelman, M.J.; Horwitz, E.M.; Uzzo, R.G.; Fisher, R.I. A war on two fronts: Cancer care in the time of COVID-19. Ann. Intern. Med. 2020, 172, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef]
- McCorkle, R.; Ercolano, E.; Lazenby, M.; Schulman-Green, D.; Schilling, L.S.; Lorig, K.; Wagner, E.H. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: A Cancer J. Clin. 2011, 61, 50–62. [Google Scholar] [CrossRef]
- D’egidio, V.; Sestili, C.; Mancino, M.; Sciarra, I.; Cocchiara, R.; Backhaus, I.; Mannocci, A.; De Luca, A.; Frusone, F.; Monti, M. Counseling interventions delivered in women with breast cancer to improve health-related quality of life: A systematic review. Qual. Life Res. 2017, 26, 2573–2592. [Google Scholar] [CrossRef]
- Sweegers, M.G.; Altenburg, T.M.; Chinapaw, M.J.; Kalter, J.; Verdonck-de Leeuw, I.M.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; Brug, J. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sport. Med. 2018, 52, 505–513. [Google Scholar] [CrossRef]
- Rummans, T.A.; Clark, M.M.; Sloan, J.A.; Frost, M.H.; Bostwick, J.M.; Atherton, P.J.; Johnson, M.E.; Gamble, G.; Richardson, J.; Brown, P. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: A randomized controlled trial. J. Clin. Oncol. 2006, 24, 635–642. [Google Scholar] [CrossRef]
- Howell, D.; Mayer, D.K.; Fielding, R.; Eicher, M.; Verdonck-de Leeuw, I.M.; Johansen, C.; Soto-Perez-de-Celis, E.; Foster, C.; Chan, R.; Alfano, C.M. Management of cancer and health after the clinic visit: A call to action for self-management in cancer care. JNCI: J. Natl. Cancer Inst. 2021, 113, 523–531. [Google Scholar] [CrossRef]
- Cooley, M.E.; Nayak, M.M.; Abrahm, J.L.; Braun, I.M.; Rabin, M.S.; Brzozowski, J.; Lathan, C.; Berry, D.L. Patient and caregiver perspectives on decision support for symptom and quality of life management during cancer treatment: Implications for e H ealth. Psycho-Oncol. 2017, 26, 1105–1112. [Google Scholar] [CrossRef]
- Bertucci, F.; Corroller-Soriano, L.; Monneur-Miramon, A.; Moulin, J.-F.; Fluzin, S.; Maraninchi, D.; Gonçalves, A. Outpatient cancer care delivery in the context of e-oncology: A french perspective on “cancer outside the hospital walls”. Cancers 2019, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Pappot, H.; Taarnhøj, G.A.; Elsbernd, A.; Hjerming, M.; Hanghøj, S.; Jensen, M.; Boisen, K.A. Health-related quality of life before and after use of a smartphone app for adolescents and young adults with cancer: Pre-post interventional study. JMIR Mhealth Uhealth 2019, 7, e13829. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Weber, D.; Bougatf, N.; Sprave, T.; Oetzel, D.; Huber, P.E.; Debus, J.; Nicolay, N.H. Supportive care in radiotherapy based on a mobile app: Prospective multicenter survey. JMIR Mhealth Uhealth 2018, 6, e10916. [Google Scholar] [CrossRef] [PubMed]
- Faria, E.T.; Ghobad, P.C.; Alves, L.Y.M.; Dos Reis, P.E.D. A Mobile App (AMOR Mama) for Women With Breast Cancer Undergoing Radiation Therapy: Functionality and Usability Study. J. Med. Internet Res. 2021, 23, e24865. [Google Scholar]
- Davis, S.W.; Oakley-Girvan, I. mHealth education applications along the cancer continuum. J. Cancer Educ. 2015, 30, 388–394. [Google Scholar] [CrossRef]
- Uhm, K.E.; Yoo, J.S.; Chung, S.H.; Lee, J.D.; Lee, I.; Kim, J.I.; Lee, S.K.; Nam, S.J.; Park, Y.H.; Lee, J.Y. Effects of exercise intervention in breast cancer patients: Is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res. Treat. 2017, 161, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Im, E.-O.; Ji, X.; Kim, S.; Chee, E.; Bao, T.; Mao, J.J.; Chee, W. Challenges in a technology-based cancer pain management program among Asian American breast cancer survivors. Comput. Inform. Nurs. CIN 2019, 37, 243. [Google Scholar] [CrossRef]
- Egbring, M.; Far, E.; Roos, M.; Dietrich, M.; Brauchbar, M.; Kullak-Ublick, G.A.; Trojan, A. A mobile app to stabilize daily functional activity of breast cancer patients in collaboration with the physician: A randomized controlled clinical trial. J. Med. Internet Res. 2016, 18, e238. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.I.; Showalter, S.L.; Gerber, M.; Kennedy, E.M.; Brenin, D.; Mohr, D.C.; Lattie, E.G.; Gupta, A.; Ocker, G.; Cohn, W.F. Use of mental health apps by patients with breast cancer in the united states: Pilot pre-post study. JMIR Cancer 2020, 6, e16476. [Google Scholar] [CrossRef]
- Botha, A.; Weiss, M.; Herselman, M. Towards a taxonomy of mHealth. In Proceedings of the 2018 International Conference on Advances in Big Data, Computing and Data Communication Systems (icABCD), Durban, South Africa, 6–7 August 2018; pp. 1–9. [Google Scholar]
- Bashshur, R.; Shannon, G.; Krupinski, E.; Grigsby, J. The taxonomy of telemedicine. Telemed. E-Health 2011, 17, 484–494. [Google Scholar] [CrossRef]
- Abraham, C.; Michie, S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008, 27, 379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fadhil, A.; Lange, J.P.; Reiterer, H. Integrating Taxonomies Into Theory-Based Digital Health Interventions for Behavior Change: A Holistic Framework. JMIR Res. Protoc. 2019, 8, e8055. [Google Scholar] [CrossRef]
- Dahlke, D.V.; Fair, K.; Hong, Y.A.; Beaudoin, C.E.; Pulczinski, J.; Ory, M.G. Apps seeking theories: Results of a study on the use of health behavior change theories in cancer survivorship mobile apps. JMIR Mhealth Uhealth 2015, 3, e3861. [Google Scholar]
- Munn, Z.; Peters, M.D.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Mendoza, J.A.; Baker, K.S.; Moreno, M.A.; Whitlock, K.; Abbey-Lambertz, M.; Waite, A.; Colburn, T.; Chow, E.J. A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study. Pediatr. Blood Cancer 2017, 64, e26660. [Google Scholar] [CrossRef] [PubMed]
- Jibb, L.A.; Stevens, B.J.; Nathan, P.C.; Seto, E.; Cafazzo, J.A.; Johnston, D.L.; Hum, V.; Stinson, J.N. Implementation and preliminary effectiveness of a real-time pain management smartphone app for adolescents with cancer: A multicenter pilot clinical study. Pediatr. Blood Cancer 2017, 64, e26554. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.; Okuyama, H.; Yamamoto, H.; Nakamura, S.; Kato, Y. Effectiveness of a smartphone application as a support tool for patients undergoing breast cancer chemotherapy: A randomized controlled trial. Clin. Breast Cancer 2020, 20, 201–208. [Google Scholar] [CrossRef]
- Poort, H.; Ryan, A.; MacDougall, K.; Malinowski, P.; MacDonald, A.; Markin, Z.; Pirl, W.; Greer, J.; Fasciano, K. Feasibility and Acceptability of a Mobile Phone App Intervention for Coping With Cancer as a Young Adult: Pilot Trial and Thematic Analysis. J. Med. Internet Res. 2021, 23, e25069. [Google Scholar] [CrossRef]
- Livingston, P.M.; Heckel, L.; Orellana, L.; Ashley, D.; Ugalde, A.; Botti, M.; Pitson, G.; Woollett, A.; Chambers, S.K.; Parente, P. Outcomes of a randomized controlled trial assessing a smartphone Application to reduce unmet needs among people diagnosed with CancEr (ACE). Cancer Med. 2020, 9, 507–516. [Google Scholar] [CrossRef]
- Gustavell, T.; Sundberg, K.; Segersvärd, R.; Wengström, Y.; Langius-Eklöf, A. Decreased symptom burden following surgery due to support from an interactive app for symptom management for patients with pancreatic and periampullary cancer. Acta Oncol. 2019, 58, 1307–1314. [Google Scholar] [CrossRef]
- Merz, A.; Mohamed, A.; Corbett, C.; Herring, K.; Hildenbrand, J.; Locke, S.C.; Patierno, S.; Troy, J.; Wolf, S.; Zafar, S.Y. A single-site pilot feasibility randomized trial of a supportive care mobile application intervention for patients with advanced cancer and caregivers. Support. Care Cancer 2022, 30, 7853–7861. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Doody, D.R.; Di, C.; Armenian, S.H.; Baker, K.S.; Bricker, J.B.; Gopal, A.K.; Hagen, A.M.; Ketterl, T.G.; Lee, S.J. Feasibility of a behavioral intervention using mobile health applications to reduce cardiovascular risk factors in cancer survivors: A pilot randomized controlled trial. J. Cancer Surviv. 2021, 15, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Sanapala, C.; Watson, E.E.; Jensen-Battaglia, M.; Janelsins, M.C.; Klepin, H.D.; Schnall, R.; Culakova, E.; Vertino, P.; Susiarjo, M. A single-arm pilot study of a mobile health exercise intervention (GO-EXCAP) in older patients with myeloid neoplasms. Blood Adv. 2022, 6, 3850–3860. [Google Scholar] [CrossRef]
- Yang, K.; Oh, D.; Noh, J.M.; Yoon, H.G.; Sun, J.-M.; Kim, H.K.; Zo, J.I.; Shim, Y.M.; Ko, H.; Lee, J. Feasibility of an interactive health coaching mobile app to prevent malnutrition and muscle loss in esophageal cancer patients receiving neoadjuvant concurrent chemoradiotherapy: Prospective pilot study. J. Med. Internet Res. 2021, 23, e28695. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Lozano, M.; Cantarero-Villanueva, I.; Martin-Martin, L.; Galiano-Castillo, N.; Sanchez, M.-J.; Fernández-Lao, C.; Postigo-Martin, P.; Arroyo-Morales, M. A mobile system to improve quality of life via energy balance in breast cancer survivors (BENECA mHealth): Prospective test-retest Quasiexperimental feasibility study. JMIR Mhealth Uhealth 2019, 7, e14136. [Google Scholar] [CrossRef] [PubMed]
- Allicock, M.; Kendzor, D.; Sedory, A.; Gabriel, K.P.; Swartz, M.D.; Thomas, P.; Yudkin, J.S.; Rivers, A. A Pilot and Feasibility Mobile Health Intervention to Support Healthy Behaviors in African American Breast Cancer Survivors. J. Racial Ethn. Health Disparities 2021, 8, 157–165. [Google Scholar] [CrossRef]
- Yanez, B.; Oswald, L.B.; Baik, S.H.; Buitrago, D.; Iacobelli, F.; Perez-Tamayo, A.; Guitelman, J.; Penedo, F.J.; Buscemi, J. Brief culturally informed smartphone interventions decrease breast cancer symptom burden among Latina breast cancer survivors. Psycho -Oncol. 2020, 29, 195–203. [Google Scholar] [CrossRef]
- Çınar, D.; Karadakovan, A.; Erdoğan, A.P. Effect of mobile phone app-based training on the quality of life for women with breast cancer. Eur. J. Oncol. Nurs. 2021, 52, 101960. [Google Scholar] [CrossRef]
- Xu, Y.F.; Xu, X.F.; Song, K.; Qiu, C.; Zhang, X.; Di, H. Effects of Extended Care Based on the WeChat Platform on Self-Efficacy and Quality of Life of Postoperative Breast Cancer Patients. Indian J. Pharm. Sci. 2021, 83, 23–30. [Google Scholar] [CrossRef]
- Ghanbari, E.; Yektatalab, S.; Mehrabi, M. Effects of Psychoeducational Interventions Using Mobile Apps and Mobile-Based Online Group Discussions on Anxiety and Self-Esteem in Women With Breast Cancer: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e19262. [Google Scholar] [CrossRef]
- Sheean, P.; Matthews, L.; Visotcky, A.; Banerjee, A.; Moosreiner, A.; Kelley, K.; Chitambar, C.R.; Papanek, P.E.; Stolley, M. Every Day Counts: A randomized pilot lifestyle intervention for women with metastatic breast cancer. Breast Cancer Res. Treat. 2021, 187, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, C.G.; Cepeda, T.G.; Kovač, T.; Kukar, M.; Gorenjec, N.R. Mobile app for symptom management and associated quality of life during systemic treatment in early stage breast cancer: Nonrandomized controlled prospective cohort study. JMIR Mhealth Uhealth 2020, 8, e17408. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Lozano, M.; Martín-Martín, L.; Galiano-Castillo, N.; Fernández-Lao, C.; Cantarero-Villanueva, I.; López-Barajas, I.B.; Arroyo-Morales, M. Mobile health and supervised rehabilitation versus mobile health alone in breast cancer survivors: Randomized controlled trial. Ann. Phys. Rehabil. Med. 2020, 63, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.M.; Shin, H.; Jang, J.-S.; Kim, Y.I.; Han, D.H. A mobile game for patients with breast cancer for chemotherapy self-management and quality-of-life improvement: Randomized controlled trial. J. Med. Internet Res. 2018, 20, e9559. [Google Scholar] [CrossRef]
- Rosen, K.D.; Paniagua, S.M.; Kazanis, W.; Jones, S.; Potter, J.S. Quality of life among women diagnosed with breast Cancer: A randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psycho-oncology 2018, 27, 2023–2030. [Google Scholar] [CrossRef]
- Lengacher, C.A.; Reich, R.R.; Ramesar, S.; Alinat, C.B.; Moscoso, M.; Cousin, L.; Marino, V.R.; Elias, M.N.; Paterson, C.L.; Pleasant, M.L. Feasibility of the mobile mindfulness-based stress reduction for breast cancer (mMBSR (BC)) program for symptom improvement among breast cancer survivors. Psycho -Oncol. 2018, 27, 524–531. [Google Scholar] [CrossRef]
- Ferrante, J.M.; Devine, K.A.; Bator, A.; Rodgers, A.; Ohman-Strickland, P.A.; Bandera, E.V.; Hwang, K.O. Feasibility and potential efficacy of commercial mHealth/eHealth tools for weight loss in African American breast cancer survivors: Pilot randomized controlled trial. Transl. Behav. Med. 2020, 10, 938–948. [Google Scholar] [CrossRef]
- Hou, I.-C.; Lin, H.-Y.; Shen, S.-H.; Chang, K.-J.; Tai, H.-C.; Tsai, A.-J.; Dykes, P.C. Quality of Life of Women After a First Diagnosis of Breast Cancer Using a Self-Management Support mHealth App in Taiwan: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e17084. [Google Scholar] [CrossRef]
- Fjell, M.; Langius-Eklöf, A.; Nilsson, M.; Wengström, Y.; Sundberg, K. Reduced symptom burden with the support of an interactive app during neoadjuvant chemotherapy for breast cancer–A randomized controlled trial. Breast 2020, 51, 85–93. [Google Scholar] [CrossRef]
- Öztürk, E.S.; Kutlutürkan, S. The Effect of the Mobile Application-Based Symptom Monitoring Process on the Symptom Control and Quality of Life in Breast Cancer Patients. Semin. Oncol. Nurs. 2021, 37, 151161. [Google Scholar] [CrossRef]
- Bandani-Susan, B.; Montazeri, A.; Haghighizadeh, M.H.; Araban, M. The effect of mobile health educational intervention on body image and fatigue in breast cancer survivors: A randomized controlled trial. Irish J. Med. Sci. (1971) 2021, 191, 1599–1605. [Google Scholar] [CrossRef]
- Blair, C.K.; Harding, E.; Wiggins, C.; Kang, H.; Schwartz, M.; Tarnower, A.; Du, R.; Kinney, A.Y. A Home-Based Mobile Health Intervention to Replace Sedentary Time With Light Physical Activity in Older Cancer Survivors: Randomized Controlled Pilot Trial. JMIR Cancer 2021, 7, e18819. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Kurtovich, E.; McGinnis, M.; Aghaee, S.; Altschuler, A.; Quesenberry, C., Jr.; Kolevska, T.; Avins, A.L. A randomized controlled trial of mHealth mindfulness intervention for cancer patients and informal cancer caregivers: A feasibility study within an integrated health care delivery system. Integr. Cancer Ther. 2019, 18, 1534735419850634. [Google Scholar] [CrossRef] [PubMed]
- Puszkiewicz, P.; Roberts, A.L.; Smith, L.; Wardle, J.; Fisher, A. Assessment of cancer survivors’ experiences of using a publicly available physical activity mobile application. JMIR Cancer 2016, 2, e5380.1. [Google Scholar] [CrossRef]
- Yang, J.; Weng, L.; Chen, Z.; Cai, H.; Lin, X.; Hu, Z.; Li, N.; Lin, B.; Zheng, B.; Zhuang, Q. Development and testing of a mobile app for pain management among cancer patients discharged from hospital treatment: Randomized controlled trial. JMIR Mhealth Uhealth 2019, 7, e12542. [Google Scholar] [CrossRef]
- Mikolasek, M.; Witt, C.M.; Barth, J. Effects and implementation of a mindfulness and relaxation App for patients with cancer: Mixed methods feasibility study. JMIR Cancer 2021, 7, e16785. [Google Scholar] [CrossRef]
- Walsh, J.C.; Richmond, J.; Mc Sharry, J.; Groarke, A.; Glynn, L.; Kelly, M.G.; Harney, O.; Groarke, J.M. Examining the Impact of an mHealth Behavior Change Intervention With a Brief In-Person Component for Cancer Survivors With Overweight or Obesity: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e24915. [Google Scholar] [CrossRef]
- Robertson, M.C.; Lyons, E.J.; Liao, Y.; Baum, M.L.; Basen-Engquist, K.M. Gamified text messaging contingent on device-measured steps: Randomized feasibility study of a physical activity intervention for cancer survivors. JMIR Mhealth Uhealth 2020, 8, e18364. [Google Scholar] [CrossRef]
- Børøsund, E.; Varsi, C.; Clark, M.M.; Ehlers, S.L.; Andrykowski, M.A.; Sleveland, H.R.S.; Bergland, A.; Nes, L.S. Pilot testing an app-based stress management intervention for cancer survivors. Transl. Behav. Med. 2020, 10, 770–780. [Google Scholar] [CrossRef]
- Ham, K.; Chin, S.; Suh, Y.J.; Rhee, M.; Yu, E.-S.; Lee, H.J.; Kim, J.-H.; Kim, S.W.; Koh, S.-J.; Chung, K.-M. Preliminary results from a randomized controlled study for an app-based cognitive behavioral therapy program for depression and anxiety in cancer patients. Front. Psychol. 2019, 10, 1592. [Google Scholar] [CrossRef] [PubMed]
- Benze, G.; Nauck, F.; Alt-Epping, B.; Gianni, G.; Bauknecht, T.; Ettl, J.; Munte, A.; Kretzschmar, L.; Gaertner, J. PROutine: A feasibility study assessing surveillance of electronic patient reported outcomes and adherence via smartphone app in advanced cancer. Ann. Palliat Med. 2019, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Jacobs, J.; Pensak, N.; MacDonald, J.J.; Fuh, C.X.; Perez, G.K.; Ward, A.; Tallen, C.; Muzikansky, A.; Traeger, L. Randomized trial of a tailored cognitive-behavioral therapy mobile application for anxiety in patients with incurable cancer. The Oncologist 2019, 24, 1111. [Google Scholar] [CrossRef]
- Maguire, R.; McCann, L.; Kotronoulas, G.; Kearney, N.; Ream, E.; Armes, J.; Patiraki, E.; Furlong, E.; Fox, P.; Gaiger, A. Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ 2021, 374, n1647. [Google Scholar] [CrossRef]
- Ormel, H.L.; van der Schoot, G.G.; Westerink, N.-D.L.; Sluiter, W.J.; Gietema, J.A.; Walenkamp, A.M. Self-monitoring physical activity with a smartphone application in cancer patients: A randomized feasibility study (SMART-trial). Support. Care Cancer 2018, 26, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Krebs, P.; Burkhalter, J.; Fiske, J.; Snow, H.; Schofield, E.; Iocolano, M.; Borderud, S.; Ostroff, J.S. The QuitIT coping skills game for promoting tobacco cessation among smokers diagnosed with cancer: Pilot randomized controlled trial. JMIR Mhealth Uhealth 2019, 7, e10071. [Google Scholar] [CrossRef] [PubMed]
- Casillas, J.N.; Schwartz, L.F.; Crespi, C.M.; Ganz, P.A.; Kahn, K.L.; Stuber, M.L.; Bastani, R.; Alquaddomi, F.; Estrin, D.L. The use of mobile technology and peer navigation to promote adolescent and young adult (AYA) cancer survivorship care: Results of a randomized controlled trial. J. Cancer Surviv. 2019, 13, 580–592. [Google Scholar] [CrossRef]
- Rico, T.M.; dos Santos Machado, K.; Fernandes, V.P.; Madruga, S.W.; Santin, M.M.; Petrarca, C.R.; Dumith, S.C. Use of Text Messaging (SMS) for the Management of Side Effects in Cancer Patients Undergoing Chemotherapy Treatment: A Randomized Controlled Trial. J. Med. Syst. 2020, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-M.; Suh, Y.J.; Chin, S.; Seo, D.; Yu, E.-S.; Lee, H.J.; Kim, J.-H.; Kim, S.W.; Koh, S.-J. A Pilot Study Testing the Efficacy of dCBT in Patients With Cancer Experiencing Sleep Problems. Front. Psychol. 2022, 13, 699168. [Google Scholar] [CrossRef]
- Sundberg, K.; Wengström, Y.; Blomberg, K.; Hälleberg-Nyman, M.; Frank, C.; Langius-Eklöf, A. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support. Care Cancer 2017, 25, 2195–2204. [Google Scholar] [CrossRef]
- Lee, B.J.; Park, Y.H.; Lee, J.Y.; Kim, S.J.; Jang, Y.; Lee, J.I. Smartphone application versus pedometer to promote physical activity in prostate cancer patients. Telemed. E-Health 2019, 25, 1231–1236. [Google Scholar] [CrossRef]
- Ji, W.; Kwon, H.; Lee, S.; Kim, S.; Hong, J.S.; Park, Y.R.; Kim, H.R.; Lee, J.C.; Jung, E.J.; Kim, D. Mobile health management platform–based pulmonary rehabilitation for patients with non–small cell lung cancer: Prospective clinical trial. JMIR Mhealth Uhealth 2019, 7, e12645. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.Y.; Lee, J.C.; Kim, H.R.; Song, S.; Kwon, H.; Ji, W.; Choi, C.M. Mobile phone app–based pulmonary rehabilitation for chemotherapy-treated patients with advanced lung cancer: Pilot study. JMIR Mhealth Uhealth 2019, 7, e11094. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-F.; Huang, R.-C.; Yang, S.-C.; Chou, C.; Chen, L.-C. Evaluating the Effects of a Mobile Health App on Reducing Patient Care Needs and Improving Quality of Life After Oral Cancer Surgery: Quasiexperimental Study. JMIR Mhealth Uhealth 2020, 8, e18132. [Google Scholar] [CrossRef]
- De Tommasi, C.; Richardson, E.; Reale, M.; Jordan, J. Evaluation of a novel application of a mindfulness phone application for patients with brain tumours: A feasibility study. J. Neuro-Oncol. 2020, 149, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; Fakhry, C.; Hales, R.K.; Kisuule, F.; Quon, H.; Kiess, A.P.; Yin, L.X.; Zhang, Y.; Blackford, A.L.; Drummond, M.B. Pilot randomized controlled trial of a comprehensive smoking cessation intervention for patients with upper aerodigestive cancer undergoing radiotherapy. Head Neck 2018, 40, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Keum, J.; Chung, M.J.; Kim, Y.; Ko, H.; Sung, M.J.; Jo, J.H.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y. Usefulness of smartphone apps for improving nutritional status of pancreatic cancer patients: Randomized controlled trial. JMIR Mhealth Uhealth 2021, 9, e21088. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Ravi, S.; Arnolda, L.; Feng, X.; Maberly, G.; Astell-Burt, T. Rates of attrition and dropout in app-based interventions for chronic disease: Systematic review and meta-analysis. J. Med. Internet Res. 2020, 22, e20283. [Google Scholar] [CrossRef]
- Melissant, H.C.; Verdonck-de Leeuw, I.M.; Lissenberg-Witte, B.I.; Konings, I.R.; Cuijpers, P.; Van Uden-Kraan, C.F. ‘Oncokompas’, a web-based self-management application to support patient activation and optimal supportive care: A feasibility study among breast cancer survivors. Acta Oncol. 2018, 57, 924–934. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Chen, J.; Wang, C.; Hu, R.; Wu, Y. Effects of Internet-based psycho-educational interventions on mental health and quality of life among cancer patients: A systematic review and meta-analysis. Support. Care Cancer 2020, 28, 2541–2552. [Google Scholar] [CrossRef] [PubMed]
- Buneviciene, I.; Mekary, R.A.; Smith, T.R.; Onnela, J.-P.; Bunevicius, A. Can mHealth interventions improve quality of life of cancer patients? A systematic review and meta-analysis. Crit. Rev. Oncol. /Hematol. 2021, 157, 103123. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Jia, Z.; Zhu, R.; Ding, Y.; Wang, Q.; Han, S. Effect and feasibility of gamification interventions for improving physical activity and health-related outcomes in cancer survivors: An early systematic review and meta-analysis. Support. Care Cancer 2022, 31, 92. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, S.; Kim, S.H.; Yoo, S.-H.; Sung, J.H.; Oh, E.G.; Kim, N.; Lee, J. Digital Health Interventions for Adult Patients With Cancer Evaluated in Randomized Controlled Trials: Scoping Review. J. Med. Internet Res. 2023, 25, e38333. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Digital Health Interventions v1. 0: A Shared Language to Describe the Uses of Digital Technology for Health; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Wald, D.S.; Butt, S.; Bestwick, J.P. One-way versus two-way text messaging on improving medication adherence: Meta-analysis of randomized trials. Am. J. Med. 2015, 128, 1139.e1131–1139.e1135. [Google Scholar] [CrossRef]
- Rincon, E.; Monteiro-Guerra, F.; Rivera-Romero, O.; Dorronzoro-Zubiete, E.; Sanchez-Bocanegra, C.L.; Gabarron, E. Mobile phone apps for quality of life and well-being assessment in breast and prostate cancer patients: Systematic review. JMIR mHealth uHealth 2017, 5, e8741. [Google Scholar] [CrossRef]
- Cheung, A.T.; Li, W.H.C.; Ho, L.L.K.; Ho, K.Y.; Chan, G.C.F.; Chung, J.O.K. Physical activity for pediatric cancer survivors: A systematic review of randomized controlled trials. J. Cancer Surviv. 2021, 15, 876–889. [Google Scholar] [CrossRef]
- Escriva Boulley, G.; Leroy, T.; Bernetière, C.; Paquienseguy, F.; Desfriches-Doria, O.; Préau, M. Digital health interventions to help living with cancer: A systematic review of participants’ engagement and psychosocial effects. Psycho-Oncol. 2018, 27, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; Johnston, M. Health Behaviour Change Competency Framework: Competences to Deliver Interventions to Change Lifestyle Behaviours that Affect Health; Division of Health Psychology, The British Psychological Association, The Scottish Government: Edinburgh, Scotland, 2010.
- Michie, S.; Hyder, N.; Walia, A.; West, R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict. Behav. 2011, 36, 315–319. [Google Scholar] [CrossRef]
- McGrady, M.E.; Schwartz, L.E.; Noser, A.E.; Klages, K.L.; Sweenie, R.; Breen, G.; Ramsey, R.R. Systematic Evaluation of the Behavior Change Techniques and Quality of Commercially Available Cancer Self-Management Apps. JCO Oncol. Pract. 2022, 19, e228–e237. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Gentile, D.; Markham, M.J.; Eaton, T. Patients with cancer and social media: Harness benefits, avoid drawbacks. J. Oncol. Pract. 2018, 14, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Pope, Z.; Lee, J.E.; Zeng, N.; Lee, H.Y.; Gao, Z. Feasibility of smartphone application and social media intervention on breast cancer survivors’ health outcomes. Transl. Behav. Med. 2019, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Attai, D.J.; Cowher, M.S.; Al-Hamadani, M.; Schoger, J.M.; Staley, A.C.; Landercasper, J. Twitter social media is an effective tool for breast cancer patient education and support: Patient-reported outcomes by survey. J. Med. Internet Res. 2015, 17, e4721. [Google Scholar] [CrossRef] [PubMed]
- Dugas, M.; Gao, G.; Agarwal, R. Unpacking mHealth interventions: A systematic review of behavior change techniques used in randomized controlled trials assessing mHealth effectiveness. Digit. Health 2020, 6, 2055207620905411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).