Simple Summary
We identified a panel of co-diagnosis occurring at higher or lower frequencies in CRC patients compared to matched controls without CRC. Such data might help optimizing CRC screening and improving the clinical management of CRC patients.
Abstract
Background: The prognosis of colorectal cancer (CRC) patients is determined to a decisive extent by comorbidities. On the other hand, anti-cancer treatments for CRC are associated with relevant toxicities and may therefore cause additional comorbidities. Methods: This retrospective cohort study assessed the prevalence of various diseases in patients 12 months before and 12 months after an initial diagnosis of colorectal cancer (ICD-10: C18, C20) in 1274 general practices in Germany between January 2000 and December 2018. The study is based on the Disease Analyzer database (IQVIA), which contains drug prescriptions, diagnoses, and basic medical and demographic data. Patients with and without CRC were matched by sex, age, and index year. Results: We identified several diagnoses with a significantly higher prevalence among CRC patients 12 months prior to the index date compared to controls. These diagnoses included gastrointestinal hemorrhage, hemorrhoids, perianal venous thrombosis, and abdominal and pelvic pain, as well as functional intestinal disorders. In contrast, the prevalence of lipid metabolism disorder, depression, hypertension, coronary heart disease, or acute bronchitis was significantly lower in CRC cases. After diagnosis of CRC, we found a significantly higher prevalence of anemia, polyneuropathies, functional intestinal disorders, and chronic kidney disease among CRC patients compared to the control group, while the prevalence of acute upper respiratory infections of multiple and unspecified sites and acute bronchitis was significantly lower in CRC patients compared to non-CRC patients. Conclusions: In the present study, we identified a variety of diseases occurring at higher or lower frequencies in CRC patients compared to matched controls without CRC. This might help to select patients for early CRC screening and improve the clinical management of CRC patients.
1. Introduction
Colorectal cancer represents the second most common cause of cancer-related death worldwide [1,2]. In Germany, 62,230 new cases and 25,972 deaths were registered in 2018 [1]. Large epidemiological studies have shown that numerous facets of the “Western lifestyle” such as insufficient physical activity, excessive consumption of animal fats and red meat, and insufficient intake of dietary fiber are important risk factors predisposing to the development of colon cancer [1]. The prognosis for patients with colorectal cancer has improved significantly in recent years after decades of stagnation. This progress has been achieved on the one hand by new targeted systemic therapies and on the other hand by the increasing use of highly active multimodal treatment approaches in the therapeutic course of many patients [3]. However, for multimorbid patients who cannot receive such intensive therapies, or can only receive them to a very limited extent, the prognosis remains poor [3]. Cardiovascular and pulmonary disease play a particular role here, sharing numerous risk factors with colorectal cancer. A more detailed analysis of the spectrum of co-diagnoses of patients with colorectal carcinoma may therefore provide valuable data regarding the possibilities of prevention of the tumor disease itself but also regarding the prevention of diseases that prevent optimal treatment of the tumor. High-intensity multimodal treatment procedures, which are standard today for many patients with colorectal carcinoma, place—as mentioned above—high demands on the physical resilience of the patients. On the other hand, they are also associated with a whole spectrum of side effects that can lead to significant limitations in the quality of life. Examples include oxaliplatin-associated polyneuropathy or chronic gastrointestinal complaints as a consequence of extensive bowel resections [3]. In this context, a more detailed investigation of the spectrum of co-diagnoses of patients after diagnosis of colorectal cancer may help to individualize and further optimize the clinical management of such patients.
2. Materials and Methods
2.1. Database
This study was based on data from the Disease Analyzer database (IQVIA), which contains drug prescriptions, diagnoses, and basic medical and demographic data obtained directly and in anonymous format from computer systems used in the practices of general practitioners and specialists (Rathmann et al., 2018). The database covers approximately 3% of all outpatient practices in Germany. It has previously been shown that the panel of practices included in the Disease Analyzer database is representative of general and specialized practices in Germany [4]. Finally, this database has already been used in previous studies focusing on cancer [5,6,7].
2.2. Study Population
This retrospective cohort study included adult individuals (≥18 years) with an initial diagnosis of colorectal cancer (CRC, ICD-10: C18, C20) in 1274 general practices in Germany between January 2000 and December 2018 (index date; Figure 1). Further inclusion criteria were an observation time of at least 12 months prior to and a follow-up time of at least 12 months after the index date. CRC patients were matched to individuals without CRC (controls) by propensity scores based on age, gender, index year, and consultation frequency within 12 months prior to and 12 months after the index date. For the control group, the index date was defined as a randomly selected visit between January 2000 and December 2018 (Figure 1).
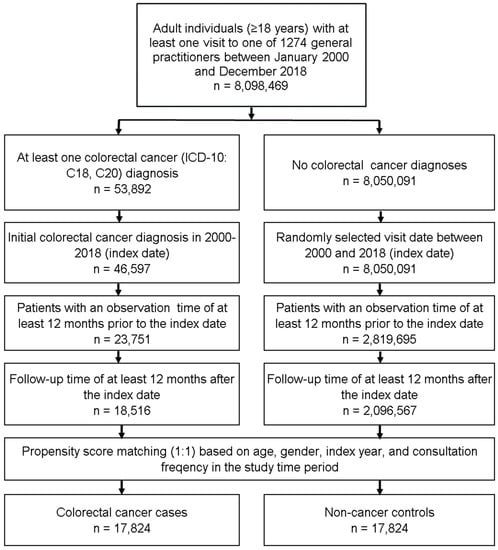
Figure 1.
Selection of study patients.
2.3. Study Outcomes and Statistical Analyses
The outcomes of this study were various co-diagnoses documented within 12 months prior as well as 12 months after the index date (diagnosis of CRC) among CRC patients compared to non-CRC individuals. Differences with respect to the cohort characteristics between CRC patients and controls were tested using Chi-squared tests for categorical variables and Wilcoxon tests for continuous variables. All co-diagnoses with a prevalence of at least 3% in CRC patients or controls were included in the analysis. The index for potential differences between CRC and non-CRC individuals was defined as the prevalence in CRC patients divided by prevalence in controls. Differences of the prevalence of each diagnosis were tested using Chi-squared tests. To counteract the problem of multiple comparisons, p-values < 0.001 were considered statistically significant. Analyses were performed using SAS version 9.4 (SAS institute, Cary, NC, USA).
3. Results
3.1. Basic Characteristics of the Study Sample
The present analysis included a total of 17,824 patients with a documented diagnosis of colorectal cancer (CRC) as well as a propensity score matched cohort of 17,824 individuals without CRC. The basic characteristics of the study cohort are summarized in Table 1. 47.4% of patients were female. The mean age [SD] of the cohort was 69.3 [12.0] years; The average number of annual GP visits was 10 within 12 months prior to the index date (diagnosis of CRC) and 13 within 12 months after the index date.

Table 1.
Basic characteristics of the study sample (after 1:1 propensity score matching).
3.2. Evaluation of Frequently Coded Co-Diagnoses within 12 Months Prior to Diagnosis of Colorectal Cancer
We first assess the prevalence of various co-diagnoses within 12 months prior to the index date. We identified several co-diagnoses with a significantly higher prevalence among CRC patients compared to controls. These co-diagnoses included gastrointestinal hemorrhage (4.7 vs. 0.6%), hemorrhoids and perianal venous thrombosis (3.7 vs. 1.5%), abdominal and pelvic pain (7.8 vs. 3.9%), other functional intestinal disorders (3.9 vs. 2.2%), gastroenteritis and colitis of infectious and unspecified origin (5.8 vs. 3.4%), and gastritis and duodenitis (7.6 vs. 6.5%, Table 2). In contrast, the prevalence of lipid metabolism disorder, depression, hypertension, coronary heart disease, acute bronchitis, or dorsalgia was significantly lower in CRC cases compared to controls (Table 2). The frequency of other co-diagnoses such as arterial fibrillation, COPD, or dizziness were comparable between CRC and non-CRC patients (Table 2).

Table 2.
Prevalence of different co-diagnoses documented within 12 months before the diagnosis of colorectal cancer.
3.3. Evaluation of Frequently Coded Co-Diagnoses within 12 Months after the Diagnosis of Colorectal Cancer
In a next step, we evaluated the prevalence of different co-diagnoses within 12 months following the index date (initial diagnosis of CRC). Here, we observed a significantly higher prevalence of iron deficiency anemia (6.8 vs. 2.2%), other anemias (4.0 vs. 2.1%), other and unspecified polyneuropathies (3.2 vs. 1.8%), diverticular disease of intestine (4.0 vs. 2.4%), other functional intestinal disorders (5.6 vs. 3.5%), chronic kidney disease (3.2 vs. 2.4%), and gastroenteritis and colitis of infectious and unspecified origin (5.8 vs. 4.8%) among CRC patients compared to the control group (Table 3). In contrast, the prevalence of acute upper respiratory infections of multiple and unspecified sites, acute bronchitis, dorsalgia, and osteoarthritis of the knee was significantly lower in CRC patients compared to non-CRC patients (Table 3). Again, we identified some other co-diagnoses such as arterial fibrillation, dizziness, or nontoxic goiter that were comparable between CRC and non-CRC patients (Table 3).

Table 3.
Prevalence of different co-diagnoses documented within 12 months after the diagnosis of colorectal cancer.
4. Discussion
In this study, we performed a comprehensive analysis on the prevalence of a broad panel of co-diagnoses in patients with colorectal cancer. Most importantly we show that gastrointestinal diseases including hemorrhage, abdominal and pelvic pain, and functional intestinal disorders were overrepresented in patients within 12 months before the diagnosis of CRC. Unexpectedly, frequencies of lipid metabolism disorder, depression, hypertension, and coronary heart disease were significantly lower in CRC cases compared to controls. Within 12 months after the diagnosis of CRC, more patients displayed anemia, polyneuropathies, and chronic kidney disease, most likely representing treatment-related complications in these patients. Such data might help to improve the clinical management of patients with CRC.
Gastrointestinal bleedings belong to the most important clinical symptoms occurring in the context of CRC [3]. Likewise, in our analyses, gastrointestinal hemorrhage was coded at significantly higher rates in patients with CRC when compared to controls. Interestingly, the diagnoses “hemorrhoids” and “perianal venous thrombosis” were also significantly overrepresented in the 12 months period before the index date in CRC patients. Similarly, rather unspecific gastrointestinal diseases including abdominal and pelvic pain, functional intestinal disorders (incl. constipation, diarrhea), gastroenteritis, colitis and duodenitis were found at higher frequencies in this group. These results suggest that at least in some patients, complaints caused by a CRC were initially attributed to other gastrointestinal diseases before the final diagnosis of CRC was made. Thus, our data support the role of early colonoscopy in patients with gastrointestinal symptoms and specifically in patients with gastrointestinal bleeding to ensure early CRC as recently described [3,8,9].
In contrast to various digestive tract cancers, the prognosis of CRC patients has constantly improved over the last years [10]. Presently, many different multimodal therapeutic approaches are available even in patients with metastasized disease stages, particularly in case of liver-limited disease [10,11,12]. Most importantly, even extensive tumor resection of liver metastases has not only proven efficacy to reduce the tumor burden, but may even be done with curative intent, potentially providing long-term survival [13,14]. Moreover, novel systemic treatment options were recently introduced into clinical algorithms for CRC patients, including specific antibodies or tyrosine kinase inhibitors, offering several lines of chemotherapy which can also lead to a considerably prolonged survival [15,16,17]. With all these options available, the individual patients’ characteristics such as age or comorbidities increasingly gain attention since they determine the patients’ ability to receive treatment. Here we demonstrate that—unexpectedly—patients with CRC display significantly lower frequencies of lipid metabolism disorders, arterial hypertension, and coronary heart disease all representing diagnoses potentially preventing the patient from receiving aggressive treatment. Nevertheless, it is important to note that the overall prevalence of this diseases in the CRC group is still high (e.g., hypertension with 31.4% and coronary heart disease 9%), clearly demonstrating that such co-diagnoses need to be considered in patients with CRC.
Oxaliplatin represent a cornerstone in the systemic treatment of patients with CRC, both in metastasized and non-metastasized disease stages [18]. Despite having proven efficacy in combination with 5-FU and/or different antibodies, the neurotoxic effect of oxaliplatin limits its use in many patients [19]. In line with these previous data, rates of polyneuropathies (ICD G62) were significantly higher in patients with CRC than in other patients. These data clearly underscore the important role of this toxicity in the context of CRC treatment and simultaneously prove the reliability of our data. Interestingly, next to polyneuropathies, chronic kidney disease was overrepresented in patients after diagnosis of CRC. It seems likely that this represents a direct consequence of anti-cancer treatments including, e.g., nephrotoxic substances or kidney malperfusion during or after surgery. Finally, functional intestinal disorders (obstipation, diarrhea) were coded at higher frequencies in CRC patients than in non-CRC patients after the index date. Of course, they might be related to postoperative gastrointestinal problems but also to psychological stress occurring after a cancer diagnosis.
We acknowledge important limitations of our study. First, our analysis is fully descriptive and does not allow to draw any conclusions on whether a certain disease might represent a risk factor for the development of CRC or was really caused by the presence of CRC or associated anti-cancer treatments. Moreover, database analyses as performed here are generally limited by a lack of completeness of the data on which they are based. For example, we cannot provide information on the individual disease stages or on the guideline-based therapy of the diseases and the course of the disease. As such, we are unable to differentiate between metastasized or non-metastasized CRC. Furthermore, no data were available in the database on the individual tumor localization, which might be of importance since left-sided CRC and right-sided CRC are regarded as fully different diseases in terms of tumor biology, response to treatment, or prognosis. In addition, identification of patients was based on officially coded diagnoses only, most likely resulting in a recording bias. The possibility that diagnoses have been misclassified within the ICD-10 coding system cannot be excluded. Finally, the depth of the analyses is partially limited because of the data source used, and we are therefore unable to comment on, for example, more precise locations of GI bleeding. However, we used data from a large database for our study. We are therefore confident that the data shown are reliable and clinically meaningful.
In summary, we provide a comprehensive and broad picture of the spectrum of co-diagnoses in patients before and after diagnosis of CRC. Co-diagnoses might both represent risk factors for the occurrence of CRC as well as aggravating factors for the course of the disease. Finally, they offer new starting points for multimodal treatment approaches for CRC in the future.
Author Contributions
S.H.L., C.R. and K.K. designed the study; K.K. performed statistical analyses and generated figures and tables; S.H.L., C.R. and K.K. wrote the manuscript, D.S., S.L., A.M., M.L., T.L. and M.S.J. provided intellectual input and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The “Disease Analyzer” database, used for analysis, contains anonymized electronic patient records. Patient data were analyzed in aggregated form without individual data being available. An individual consent form was not obtained following national and European legislation.
Informed Consent Statement
Patient data were analyzed in aggregated form without individual health data being available. Therefore, an individual consent form was not necessary and was not obtained.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hossain, M.d.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Kostev, K.; Luedde, M.; Luedde, T.; Roderburg, C. Low blood levels of high-density lipoprotein (HDL) cholesterol are positively associated with cancer. J. Cancer Res. Clin. Oncol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Loosen, S.H.; Kalder, M.; Luedde, T.; Roderburg, C.; Kostev, K. Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers 2021, 13, 408. [Google Scholar] [CrossRef]
- Loosen, S.H.; Kostev, K.; Keitel, V.; Tacke, F.; Roderburg, C.; Luedde, T. An elevated FIB-4 score predicts liver cancer development: A longitudinal analysis from 29,999 NAFLD patients. J. Hepatol. 2021, 76, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Screening for Colorectal Cancer. Hematol. Oncol. Clin. N. Am. 2022, 36, 393–414. [Google Scholar] [CrossRef]
- Chan, S.C.H.; Liang, J.Q. Advances in tests for colorectal cancer screening and diagnosis. Expert Rev. Mol. Diagn. 2022, 22, 449–460. [Google Scholar] [CrossRef]
- Zarour, L.R.; Anand, S.; Billingsley, K.G.; Bisson, W.H.; Cercek, A.; Clarke, M.F.; Coussens, L.M.; Gast, C.E.; Geltzeiler, C.B.; Hansen, L.; et al. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Hadden, W.J.; de Reuver, P.R.; Brown, K.; Mittal, A.; Samra, J.S.; Hugh, T.J. Resection of colorectal liver metastases and extra-hepatic disease: A systematic review and proportional meta-analysis of survival outcomes. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2016, 18, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Lodewick, T.M.; van den Broek, M.A.J.; de Jong, M.C.; Greve, J.W.; Jansen, R.L.H.; Bemelmans, M.H.A.; Neumann, U.P.; Olde Damink, S.W.M.; Dejong, C.H.C. Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB 2014, 16, 550–559. [Google Scholar] [PubMed] [Green Version]
- Neumann, U.P.; Seehofer, D.; Neuhaus, P. The surgical treatment of hepatic metastases in colorectal carcinoma. Dtsch. Arztebl. Int. 2010, 107, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Kopetz, S. Recent developments in the treatment of metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2017, 9, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Samoon, Z.; Naher, S.K.; Sjoquist, K.M.; Zalcberg, J. Chemotherapy in resectable or potentially resectable colon cancer with liver metastases. Expert Opin. Pharmacother. 2022, 23, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.H.; Hansen, T.F.; Svenningsen, Å.F.; Vilholm, O.J. Oxaliplatin-induced neuropathy in colorectal cancer: Many questions with few answers. Clin. Colorectal Cancer 2014, 13, 73–80. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).