Simple Summary
We investigated whether dietary patterns of insulinemia, inflammation and overall dietary quality are associated with the risk of total cancer, site-specific cancers, and pathological subtypes among postmenopausal women. We followed 112,468 women, 50–79 years of age, in the Women’s Health Initiative for a median of 17.8 years, documenting 18,768 incident invasive cancers. A higher overall dietary quality was associated with lower risk of total cancer and colorectal cancer. The potential of the dietary pattern to contribute to higher insulinemia and inflammation was associated with greater risk of total cancer, colorectal cancer and more strongly associated with risk of endometrial cancer and breast cancer (including triple negative breast cancer) than overall dietary quality. Additionally, a higher score of metabolites reflecting higher dietary quality was associated with lower lung cancer risk. Dietary patterns associated with cancer risk, therefore, warrant testing in clinical trials for cancer prevention among postmenopausal women.
Abstract
We evaluated associations of the Empirical Dietary Index for Hyperinsulinemia (EDIH), Empirical Dietary Inflammatory Pattern (EDIP) and Healthy Eating Index (HEI2015) and their metabolomics profiles with the risk of total and site-specific cancers. We used baseline food frequency questionnaires to calculate dietary scores among 112,468 postmenopausal women in the Women’s Health Initiative. We used multivariable-adjusted Cox regression to estimate hazard ratios (HR) and 95% confidence intervals for cancer risk estimation. Metabolomic profile scores were derived using elastic-net regression with leave-one-out cross validation. In over 17.8 years, 18,768 incident invasive cancers were adjudicated. Higher EDIH and EDIP scores were associated with greater total cancer risk, and higher HEI-2015 with lower risk: HRQ5vsQ1(95% CI): EDIH, 1.10 (1.04–1.15); EDIP, 1.08 (1.02–1.15); HEI-2015, 0.93 (0.89–0.98). The multivariable-adjusted incidence rate difference(Q5vsQ1) for total cancer was: +52 (EDIH), +41 (EDIP) and −49 (HEI-2015) per 100,000 person years. All three indices were associated with colorectal cancer, and EDIH and EDIP with endometrial and breast cancer risk. EDIH was further associated with luminal-B, ER-negative and triple negative breast cancer subtypes. Dietary patterns contributing to hyperinsulinemia and inflammation were associated with greater cancer risk, and higher overall dietary quality, with lower risk. The findings warrant the testing of these dietary patterns in clinical trials for cancer prevention among postmenopausal women.
1. Introduction
Hyperinsulinemia and sustained inflammation are two proposed mechanisms driving cancer risk [1,2,3]. Dietary patterns that promote chronic hyperinsulinemia and chronic systemic inflammation may affect the risk of developing cancers and serve as modifiable risk factors for cancer prevention. We developed and validated two empirical hypothesis-oriented dietary indices: Empirical Dietary Index for Hyperinsulinemia (EDIH) and Empirical Dietary Inflammatory Pattern (EDIP), which predict the ability of the diet to contribute to insulin hypersecretion or chronic systemic inflammation, respectively. These dietary patterns are data-driven yet based on specific biological hypotheses relating diet with chronic disease [4,5].
EDIH and EDIP scores have shown stronger associations with cancer risk [6,7,8,9,10,11] than traditional dietary patterns in both men and women [12,13]. For example, dietary patterns including the Mediterranean diet and alternative healthy eating index, and other patterns, have not been consistently associated with cancer risk among women [14]. However, in the Nurses’ Health Study (NHS), higher EDIH was associated with a 22–47% higher risk of developing digestive system cancers [6,7]. Similarly, higher EDIP was associated with cancer risk among women in the NHS [8,15]. Due to advancing age and higher adiposity, postmenopausal women may represent a higher risk group for cancer related to these underlying mechanisms of malignant progression. However, outside of the NHS, these two dietary patterns have not been investigated in association with cancer risk among women.
Nutritional metabolomics may inform on more specific mechanistic pathways linking diet and cancer. Our metabolomics studies in the Women’s Health Initiative (WHI) suggested that patterns of cholesteryl esters(CEs), phospholipids, acylglycerols and acylcarnitines, may reflect the metabolic impact of insulinemic dietary patterns [16], while metabolites associated with coffee and lipid metabolism may reflect the metabolic potential of an inflammatory dietary pattern [17]. Among these metabolites, some have been evaluated for associations with risk of some cancers [18,19]. Although associations of EDIH and EDIP with cancer risk suggest that hyperinsulinemia and inflammation may broadly underlie these associations, the mechanistic pathways warrant investigation, and metabolomics profiles may provide a link to disease risk. The current study evaluated the etiologic role of EDIH and EDIP in relation to risk of total cancer, site-specific cancers and pathological subtypes, while comparing these associations with an established index of overall dietary quality—Healthy Eating Index-2015 (HEI-2015). We also characterized the plasma metabolomics profiles of each of the three dietary patterns and investigated their associations with cancer risk.
2. Methods
2.1. Study Population
The WHI enrolled 161,808 postmenopausal women aged 50–79 years between 1993 and 1998 in the United States. The study design has been described [20]. Briefly, the WHI study consisted of a three-component Clinical Trial (CT, n = 68,132) and Observational Study (OS, n = 93,676). The CT included a Dietary Modification trial (DM), two Hormone Therapy trials (HT), and a Calcium and Vitamin D trial (CaD). After the exclusions described in Supplementary Figure S1, for each cancer site, we retained 112,468 women for the total cancer analyses. For the metabolomics aim, we used metabolomics data among 2306 participants from a matched case–control study in the WHI (BAA-24)—the Metabolomics of Coronary Heart Disease in the WHI [21]. The WHI protocol was approved by the institutional review boards at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA) and at each clinical center and all women signed informed consent. WHI is registered at clinicaltrials.gov as NCT00000611.
2.2. Dietary Assessment and Calculation of Dietary Indices
Dietary data from baseline—self-administered food frequency questionnaire (FFQ) representing intake in the preceding three months—were used to calculate the dietary indices [22]. The FFQ scanned data were processed with the University of Minnesota Nutrition Coordinating Center food and nutrient database (version 2005) to derive nutrient intakes [22,23]. The development and validation of the EDIH and EDIP scores have been described [4,5], and components of both scores in the WHI FFQ have been described as well [24]. The HEI-2015 measures adherence to the Dietary Guidelines for Americans (DGA) [25]. Supplementary Table S1 shows the food group components of each index.
2.3. Ascertainment of Incident Cancer
Study outcomes included total cancer, invasive breast cancer (overall, ER+, ER-, PR+, PR-, HER2+, HER2-, ER- PR- HER2+, luminal A, luminal B, triple negative, invasive ductal carcinoma, invasive lobular carcinoma), colorectal cancer (colon, proximal colon, distal colon and rectum), non-cancerous intestinal polyps, endometrial cancer (overall, endometrioid, non-endometrioid), ovarian cancer (overall, serous, non-serous) and lung cancer (overall, small-cell, non-small cell). Primarily, CT and OS participants were contacted semi-annually and annually, respectively, to identify cancer diagnoses. Information on cancer incidence was initially verified by medical records and pathology reports and then underwent local and central adjudications by trained physicians [26]. Intestinal polyps were not adjudicated [26]. The definition of each cancer site is included in table footnotes and in Supplementary Table S2.
Time-to-cancer-development was defined as days from enrollment to the return of the follow-up questionnaire in which the event was reported. Participants were followed from enrollment to death, lost to follow-up or to the most recent follow-up (through 1 March 2019), whichever was first.
2.4. Metabolomics Profiling and Derivation of Metabolomics Profile Scores for the Dietary Patterns
The metabolomics profiling method used has previously been described [21]. Briefly, plasma metabolites were measured as peak areas using a targeted liquid chromatography tandem mass spectrometry (LC-MS/MS) metabolomics platform at the Broad Institute (Cambridge, MA, USA). The current study included a total of 509 named metabolites. Forty-five metabolites with >10% missing values were excluded. For 84 metabolites with <10% missing values, we imputed half the sample minimum value for the metabolite [16]. We transformed all metabolites using rank-based inverse normal transformation to achieve normal distribution of the metabolites [27]. We identified metabolomics profiles for adherence to each dietary pattern using elastic-net regression to regress each dietary index on the 464 metabolites, using a 7:3 training-to-testing dataset ratio and obtained the metabolomics signature using a leave-one-out cross-validation approach to avoid overfitting [28]. Metabolomics profile scores for each dietary pattern were derived from a weighted sum of metabolites selected via a series of elastic-net regressions and the weight for each metabolite was the regression coefficient of the selected model. Furthermore, we grouped metabolites into metabolomics class scores for each dietary index, using the pool of metabolites comprising each dietary index metabolomics profile score. Metabolomics class selection was determined non-empirically using information from the Human Metabolome Database (HMDB) to classify the metabolites into weighted metabolomics class scores.
2.5. Statistical Analysis
Each dietary index was adjusted for total energy intake using the residual method [29]. We used Cox proportional hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) of the relative associations of each dietary index and risk of developing total and site-specific cancers using the lowest dietary index quintiles as reference. Covariates included in the Cox models are listed in Supplementary Table S3. The proportional hazard assumption was assessed using Schoenfeld residual method and the time dependent covariate method. Because BMI [30] and type 2 diabetes [24,31] may strongly mediate the association of the dietary patterns and cancer risk, we additionally adjusted for these mediators in separate models.
In addition to the relative risk estimates, we calculated multivariable-adjusted absolute risk (incidence rate) of cancer in quintiles of each dietary index. Using the residual method [29], each dietary index was sequentially adjusted for each of the covariates included in the Cox models, then categorized into quintiles. Incidence rates per 100,000 person years were then calculated in dietary score quintile by dividing the number of cancer cases by the sum of the follow-up time within that dietary score quintile.
Metabolomics profile scores were categorized into tertiles because of the lower sample size and examined in relation to cancer risk (total cancer, colorectal, colon, breast, endometrial, lung cancers and intestinal polyps) using Cox regression and adjusting for the same covariates as in the diet analyses. Furthermore, we used Spearman correlation coefficients to assess correlations between metabolomics classes and dietary index food group components for each dietary pattern, adjusting for BMI, physical activity, and pack years of smoking.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA), and R Studio (2021.09.0) was used for data visualization. Two-sided p < 0.05 was considered statistically significant, and we further adjusted the nominal p-values for potential false discovery rate (FDR) using the Benjamini–Hochberg procedure.
3. Results
3.1. Participant Characteristics (Table 1)
Over a median of 17.8 years of follow-up, 18,768 incident invasive cancers were diagnosed. Participants who consumed the most hyperinsulinemic or most pro-inflammatory dietary pattern (EDIH/EDIP quintiles 5) or the lowest overall dietary quality per DGA (HEI-2015 quintile 1) were more likely to be Black or Hispanic or Latino, have a higher BMI and report lower physical activity and education levels.

Table 1.
Distribution of participant characteristics in dietary index quintiles, using the total cancer analytic sample.
Table 1.
Distribution of participant characteristics in dietary index quintiles, using the total cancer analytic sample.
| Empirical Dietary Index for Hyperinsulinemic (EDIH) Score a,b | Empirical Dietary Inflammatory Pattern (EDIP) Score a,b | Health Eating Index 2015 (HEI-2015) a,b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 |
| n | 22,493 | 22,494 | 22,494 | 22,494 | 22,493 | 22,493 | 22,494 | 22,494 | 22,494 | 22,493 | 22,493 | 22,493 | 22,494 | 22,493 | 22,493 |
| Race/ethnicity | |||||||||||||||
| American Indian or Alaskan Native | 0.3 | 0.3 | 0.5 | 0.5 | 0.5 | 0.4 | 0.3 | 0.4 | 0.5 | 0.6 | 0.6 | 0.4 | 0.4 | 0.3 | 0.3 |
| Asian or Pacific Islander | 2.2 | 2.8 | 3.2 | 3.1 | 2.7 | 1.3 | 1.9 | 2.3 | 3.5 | 5.1 | 2.7 | 3.1 | 2.9 | 2.9 | 2.5 |
| Black | 4.0 | 5.1 | 7.1 | 9.5 | 13 | 3.2 | 4.2 | 6.2 | 9.5 | 16 | 11 | 8.9 | 7.4 | 6.0 | 5.5 |
| Hispanic/Latino | 2.5 | 2.9 | 3.4 | 4.1 | 5.0 | 1.5 | 1.8 | 2.6 | 3.9 | 8.3 | 5.4 | 4.5 | 3.5 | 2.6 | 1.8 |
| Other | 1.4 | 1.5 | 1.3 | 1.4 | 1.5 | 1.2 | 1.2 | 1.4 | 1.6 | 1.8 | 1.6 | 1.5 | 1.4 | 1.3 | 1.3 |
| White | 89 | 87 | 84 | 81 | 77 | 92 | 91 | 87 | 81 | 68 | 78 | 81 | 84 | 87 | 88 |
| Age, years | 63 ± 7 | 64 ± 7 | 64 ± 7 | 63 ± 7 | 62 ± 7 | 63 ± 7 | 63 ± 7 | 64 ± 7 | 64 ± 7 | 62 ± 7 | 62 ± 7 | 63 ± 7 | 63 ± 7 | 64 ± 7 | 64 ± 7 |
| BMI, kg/m2 | 26 ± 5 | 26 ± 5 | 27 ± 5 | 28 ± 6 | 30 ± 6 | 27 ± 5 | 27 ± 5 | 27 ± 5 | 28 ± 6 | 29 ± 6 | 29 ± 6 | 28 ± 6 | 27 ± 6 | 27 ± 5 | 26 ± 5 |
| Under/Normal weight (15 ≤ BMI < 25) | 49 | 44 | 39 | 34 | 24 | 44 | 42 | 39 | 36 | 29 | 29 | 33 | 37 | 42 | 49 |
| Overweight (25 ≤ BMI < 30) | 33 | 35 | 35 | 35 | 32 | 35 | 35 | 35 | 34 | 32 | 33 | 34 | 35 | 35 | 33 |
| Obese (BMI ≥ 30) | 18 | 21 | 25 | 31 | 43 | 21 | 23 | 26 | 29 | 39 | 38 | 32 | 27 | 23 | 18 |
| Physical activity, MET-hours/week | 17 ± 16 | 15± 14 | 13 ± 13 | 11 ± 12 | 9 ± 11 | 16 ± 15 | 14 ± 14 | 13 ± 13 | 12 ± 13 | 10 ± 12 | 8 ± 11 | 11 ± 12 | 13 ± 14 | 15 ± 14 | 17 ± 15 |
| Pack years of smoking | 11 ± 18 | 9 ± 17 | 9 ± 17 | 9 ± 18 | 11 ± 19 | 13 ± 20 | 10 ±18 | 9 ± 17 | 8 ± 17 | 8 ± 17 | 12 ± 21 | 10 ± 19 | 9 ± 18 | 9 ± 16 | 8 ±16 |
| Current smoking | 6 | 5 | 6 | 7 | 9 | 8 | 6 | 6 | 6 | 7 | 13 | 8 | 6 | 4 | 3 |
| Aspirin/NSAIDs use | 14 | 14 | 13 | 13 | 13 | 14 | 14 | 14 | 13 | 12 | 13 | 13 | 14 | 13 | 14 |
| Statin use | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 |
| Hypercholestrolemia | 12 | 14 | 15 | 15 | 15 | 12 | 13 | 15 | 15 | 16 | 12 | 14 | 14 | 15 | 16 |
| Educational level | |||||||||||||||
| <high school | 3 | 4 | 5 | 6 | 8 | 3 | 3 | 4 | 6 | 10 | 9 | 6 | 5 | 4 | 2 |
| High school/GED | 45 | 51 | 55 | 59 | 62 | 50 | 52 | 54 | 57 | 58 | 62 | 58 | 54 | 51 | 46 |
| ≥4 years of college | 51 | 45 | 39 | 35 | 29 | 46 | 43 | 41 | 36 | 31 | 28 | 35 | 41 | 44 | 51 |
| Total alcohol intake, alcohol servings/week c | 4.8 ± 7.5 | 2.4 ± 4.2 | 1.9 ± 3.7 | 1.6 ± 3.6 | 1.5 ± 3.6 | 5.3 ± 7.8 | 2.8 ± 4.4 | 1.9 ± 3.6 | 1.3 ± 3.0 | 0.8 ± 2.6 | 1.7 ± 4.0 | 2.2 ± 4.6 | 2.6 ± 5.0 | 2.8 ± 5.2 | 3.0 ± 5.5 |
| Macronutrients, %kcal/d | |||||||||||||||
| Carbohydrates | 54 ± 10 | 54 ± 9 | 52 ± 8 | 49 ± 8 | 45 ± 9 | 50 ± 10 | 51 ± 9 | 51 ± 9 | 51 ± 9 | 50 ± 9 | 46 ± 9 | 48 ± 9 | 51 ± 9 | 53 ± 9 | 56 ± 8 |
| Total fat | 28 ± 8 | 29 ± 8 | 31 ± 8 | 34 ± 7 | 38 ± 7 | 30 ± 9 | 31 ± 8 | 32 ± 8 | 33 ± 8 | 34 ± 8 | 38 ± 7 | 35 ± 7 | 32 ± 7 | 29 ± 7 | 26 ± 7 |
| Saturated fat | 9 ± 3 | 10 ± 3 | 10 ± 3 | 11 ± 3 | 13 ± 3 | 10 ± 3 | 11 ± 3 | 10 ± 3 | 11 ± 3 | 11 ± 3 | 14 ± 3 | 12 ± 3 | 11 ± 3 | 9 ± 2 | 8 ± 2 |
| Unsaturated fat | 16 ± 5 | 17 ± 5 | 18 ± 5 | 20 ± 5 | 22 ± 5 | 18 ± 5 | 18 ± 5 | 19 ± 5 | 19 ± 5 | 20 ± 5 | 22 ± 5 | 20 ± 5 | 19 ± 5 | 17 ± 5 | 16 ± 5 |
| Total protein | 16 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 4 | 17 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 4 | 16 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 3 | 18 ± 3 |
| Animal/plant protein ratio | 2 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 2 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | 3 ± 2 | 3 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 |
| Micronutrients, per 1000 kcal | |||||||||||||||
| Calcium, mg/d | 577 ± 217 | 560 ± 211 | 528 ± 203 | 484 ± 184 | 410 ± 156 | 533 ± 205 | 531 ± 198 | 525 ± 201 | 511 ± 207 | 459 ± 201 | 419 ± 167 | 470 ± 183 | 508 ± 196 | 549 ± 204 | 614 ± 213 |
| Potassium, mg/d | 1860 ± 427 | 1816 ± 412 | 1728 ± 388 | 1599 ± 353 | 1384 ± 319 | 1882 ± 416 | 1781 ± 383 | 1709 ± 376 | 1616 ± 377 | 1397 ± 371 | 1296 ± 295 | 1523 ± 309 | 1688 ± 335 | 1848 ± 360 | 2030 ± 360 |
| Vitamin D, mcg/d | 3 ± 2 | 3 ± 2 | 3 ± 2 | 3 ± 2 | 2 ± 1 | 3 ± 2 | 3 ± 2 | 3 ± 2 | 3 ± 2 | 3 ± 2 | 2 ± 1 | 2 ± 1 | 3 ± 2 | 3 ± 2 | 3 ± 2 |
| Magnesium, mg/d | 180 ± 35 | 175 ± 35 | 167 ± 33 | 154 ± 30 | 134 ± 28 | 177 ± 34 | 170 ± 33 | 164 ± 34 | 158 ± 34 | 141 ± 35 | 126 ± 24 | 147 ± 25 | 162 ± 26 | 177 ± 28 | 197 ± 30 |
| Iron, mg/d | 8 ± 2 | 8 ± 3 | 8 ± 3 | 8 ± 2 | 7 ± 2 | 8 ± 2 | 8 ± 2 | 8 ± 3 | 8 ± 3 | 8 ± 3 | 7 ± 2 | 8 ± 2 | 8 ± 3 | 8 ± 3 | 9 ± 3 |
| Folate, mcg/d | 184 ± 64 | 183 ± 65 | 175 ± 64 | 161 ± 59 | 136 ± 50 | 184 ± 62 | 177 ± 61 | 171 ± 62 | 164 ± 63 | 142 ± 61 | 129 ± 52 | 152 ± 55 | 169 ± 59 | 185 ± 62 | 202 ± 62 |
| Vitamin A, mcg RAE/d | 483 ± 194 | 493 ± 199 | 486 ± 198 | 467 ± 197 | 423 ± 196 | 490 ± 205 | 485 ± 189 | 480 ± 188 | 470 ± 192 | 426 ± 210 | 385 ± 182 | 438 ± 188 | 469 ± 196 | 506 ± 192 | 553 ± 193 |
| Vitamin C, mg/d | 74 ± 40 | 76 ± 41 | 72 ± 39 | 63 ± 35 | 49 ± 28 | 70 ± 38 | 71 ± 38 | 70 ± 39 | 67 ± 38 | 54 ± 34 | 41 ± 26 | 57 ± 31 | 69 ± 36 | 79 ± 38 | 88 ± 38 |
| Vitamin E, IU/d | 6 ± 3 | 6 ± 4 | 6 ± 4 | 6 ± 3 | 5 ± 3 | 6 ± 3 | 6 ± 3 | 6 ± 4 | 6 ± 4 | 5 ± 3 | 5 ± 3 | 6 ± 3 | 6 ± 4 | 6 ± 4 | 7 ± 4 |
a EDIP, EDIH and HEI2015 scores were adjusted for total energy intake using the residual method. Lower EDIP indicates anti-inflammatory diets, while higher EDIP scores indicate pro-inflammatory diets. Lower EDIH indicates anti-hyperinsulinemic diet while a higher score indicates pro-hyperinsulinemic diet. HEI-2015 measures adherence to the 2015–2020 Dietary Guidelines for Americans—higher HEI-2015 scores are indicative of greater adherence and higher dietary quality. b The values in the table are percentages for categorical variables and mean ± standard deviation for continuous variables. c Alcohol serving was the sum of beer (1 glass, 1 bottle or 1 can), wine (4 oz glass of red wine or white wine) and liquor (1 drink or 1 shot whiskey, gin, etc.).
3.2. Food and Nutrient Profiles of the Dietary Patterns (Supplementary Tables S1 and S4)
There are 27 food groups comprising the EDIH and EDIP and nine are common to both indices, including red meat, processed meat, non-dark (non-fatty) fish, sugar-sweetened beverages (regular sodas), artificially sweetened beverages (diet sodas) and refined grains, contributing higher scores; leafy-green vegetables, wine and coffee contributed lower scores (Supplementary Table S1). Unique to EDIH are French fries, butter, margarine (high scores), whole fruit and whole dairy (low scores). The correlation between EDIH and EDIP was 0.63. In addition to foods, HEI has specific nutrients to reduce, such as saturated fats; therefore, the score only includes low/non-fat dairy foods. Additionally, HEI does not include foods without caloric value such as coffee or diet sodas. HEI-2015 had a correlation of −0.36 with EDIH and −0.26 with EDIP.
Participants consuming a more hyperinsulinemic dietary pattern consumed fewer calories from total carbohydrates and more calories from total fat, saturated fat, added sugar and animal protein. The macronutrient distribution (Supplementary Table S4) for EDIP was similar but with smaller contrasts between high and low EDIP compared to EDIH. Higher overall dietary quality based on higher HEI-2015 was characterized by higher intake of total carbohydrates and lower intake of total fat and saturated fat.
3.3. Metabolomics Profile Scores of the Dietary Patterns (Figure 1)
Of the 464 metabolites retained, elastic-net regression selected 93 for EDIH, 88 for EDIP and 67 for HEI-2015. Correlations among the metabolomics profile scores (m---) were mEDIH-mEDIP (0.73), mEDIH-mHEI-2015 (−0.61) and mEDIP-mHEI-2015 (−0.29). In addition, correlations between the metabolomics profile scores and their corresponding dietary scores were: mEDIH-EDIH (0.45), mEDIP-EDIP (0.33), and mHEI-2015-HEI-2015 (0.50). Higher EDIH was associated with higher amino acids and glycerophosphocholines (glyceroPC), and with lower mono/di-carboxylic acids (MCA, DCA), CEs, phosphosphingolipids (phosphoSL), alkaloids, purines, pyridines and pyrimidines (Figure 1). The metabolite classes were similarly associated with the EDIH food group components, e.g., amino acids correlated positively with red/processed meat and French fries, which contribute to higher EDIH, and inversely with fruit, leafy-greens, coffee and wine, which contribute to lower scores. EDIP had a similar pattern of correlations with the metabolomics classes. Alkaloids were strongly positively correlated with coffee, which contributes to lower scores in both EDIH and EDIP (Figure 1A,B). In contrast, alkaloids were not strongly correlated with HEI-2015, which does not include coffee. Higher HEI-2015 was correlated with higher DCA, indoles, benzoic acids, glyceroPE, glyceroPC, phosphoSL, purines, pyrimidines and pyridines and with lower carnitines, amino acids, TAGs and amines. However, saturated fat, a moderation component of HEI-2015 was also associated with higher carboxylic acids, glycerol-PE/PC/SL and lower carnitines and amino acids (Figure 1C).
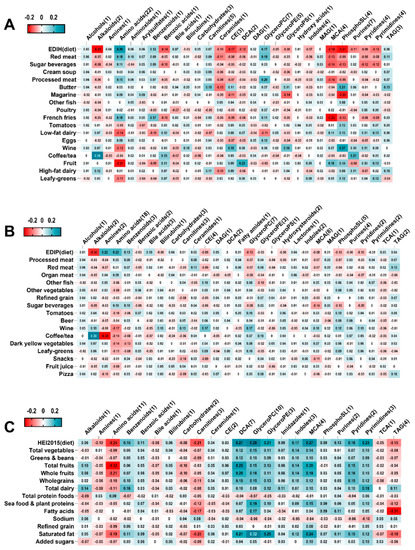
Figure 1.
Correlations between dietary pattern food group components and metabolomics class scores. (A) Correlations between EDIH food group components and EDIH metabolomic class scores. (B), Correlations between EDIP food group components and EDIP metabolomic class scores. (C), Correlations between HEI-2015 food group components and HEI-2015 metabolomics class scores. Values are partial Spearman correlation coefficients adjusted for BMI, physical activity and pack years of smoking. EDIH, empirical dietary index for hyperinsulinemia; EDIP, empirical dietary inflammatory pattern; HEI-2015, healthy eating index-2015; TAG: Triradylcglycerols. MAG: Monoradylglycerols. DAG: Diradylglycerols. GlyceroPS: Glycerophosphoserines. GlyceroPE: Glycerophosphoethanolamines. GlyceroPC: Glycerophosphocholines. CE: Cholesterol esters. PhosphoSL: phosphosphingolipids. MCA: mono-carboxylic acids. DCA: di-carboxylic acids. TCA: Tricarboxylic acids.
3.4. EDIH and Cancer Risk (Table 2, Figure 2)
Women classified in the highest quintile of EDIH had greater risk of total cancer compared to those in the lowest quintile, with a multivariable-adjusted incidence rate difference of 52 per 100,000 person years and corresponding HR (95% CI) of 1.10 (1.04–1.15). Findings from the categorical analyses were aligned with EDIH modelled as a continuous variable (Table 2). A 1 sd increment in EDIH was associated with higher risk of colorectal cancer, colon cancer and proximal colon cancer but not distal colon or rectal cancer. EDIH was also strongly associated with intestinal polyps. Further, higher EDIH was associated with breast cancer and pathological subtypes including ER-negative, luminal B, invasive lobular carcinoma, and triple negative breast cancer. Higher EDIH was associated with a greater risk of endometrial cancer especially the endometrioid subtype, but not ovarian cancer or lung cancer (Figure 2). The EDIH metabolomics score was generally not significantly associated with cancer risk, but a 1 sd increment in the score was associated with elevated risk of endometrial cancer: HR, 3.58; 95% CI, 0.96–13.29 (Table 3).

Table 3.
Dietary pattern-related metabolomics signatures in relation to total cancer and site-specific cancer risks a,b,c,d,e,f.

Table 2.
Multivariable-adjusted absolute and relative risk for the associations of dietary patterns and future development of total cancer, site-specific cancers and pathological subtypes a,b.
Table 2.
Multivariable-adjusted absolute and relative risk for the associations of dietary patterns and future development of total cancer, site-specific cancers and pathological subtypes a,b.
| Dietary Pattern | Cancer Risk Type | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Q5-Q1 (Absolute Risk Difference); c P-Trend. d | FDR-Adjusted p-Value |
|---|---|---|---|---|---|---|---|---|
| Total cancer (except non-melanoma skin cancer) | ||||||||
| EDIH e | Absolute risk/cases | 969/3934 | 962/3861 | 993/3772 | 1015/3594 | 1021/3607 | 52 | |
| EDIH | Relative risk | 1 (reference) | 1.04 (1.00, 1.09) | 1.06 (1.01, 1.11) | 1.04 (1.00, 1.10) | 1.10 (1.04, 1.15) | 0.0008 | 0.0122 |
| EDIP e | Absolute risk/cases | 979/4131 | 970/4027 | 992/3703 | 1000/3608 | 1020/3299 | 41 | |
| EDIP | Relative risk | 1 (reference) | 1.06 (1.01, 1.11) | 1.02 (0.97, 1.08) | 1.07 (1.01, 1.13) | 1.08 (1.02, 1.15) | 0.0163 | 0.0808 |
| HEI-2015 | Absolute risk/cases | 1030/3697 | 1005/3740 | 967/3786 | 977/3750 | 981/3795 | −49 | |
| HEI-2015 e | Relative risk | 1 (reference) | 0.98 (0.93, 1.02) | 0.97 (0.92, 1.01) | 0.94 (0.89, 0.98) | 0.93 (0.89, 0.98) | 0.0008 | 0.0122 |
| Colorectal cancer | ||||||||
| EDIH | Absolute risk/cases | 80/3795 | 80/309 | 89/372 | 85/306 | 91/320 | 11 | |
| EDIH | Relative risk | 1 (reference) | 1.06 (0.90, 1.25) | 1.31 (1.11, 1.54) | 1.08 (0.91, 1.28) | 1.19 (1.00, 1.43) | 0.0658 | 0.2084 |
| EDIP | Absolute risk/cases | 84/315 | 82/333 | 82/338 | 86/319 | 91/303 | 7 | |
| EDIP | Relative risk | 1 (reference) | 1.14 (0.96, 1.35) | 1.19 (0.99, 1.43) | 1.19 (0.98, 1.45) | 1.23 (0.99, 1.52) | 0.0595 | 0.1966 |
| HEI-2015 | Absolute riskc/cases | 96/342 | 86/337 | 86/316 | 76/297 | 82/316 | −14 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.96 (0.82, 1.12) | 0.90 (0.77, 1.06) | 0.83 (0.70, 0.97) | 0.86 (0.72, 1.01) | 0.0158 | 0.0808 |
| Colon cancer | ||||||||
| EDIH | Absolute risk/cases | 67/250 | 66/264 | 74/312 | 72/254 | 76/266 | 9 | |
| EDIH | Relative risk | 1 (reference) | 1.09 (0.91, 1.31) | 1.33 (1.11, 1.60) | 1.09 (0.90, 1.32) | 1.22 (1.00, 1.48) | 0.0755 | 0.2295 |
| EDIP | Absolute risk/cases | 69/260 | 68/286 | 72/279 | 69/266 | 78/255 | 9 | |
| EDIP | Relative risk | 1 (reference) | 1.18 (0.98, 1.43) | 1.20 (0.98, 1.46) | 1.22 (0.98, 1.51) | 1.28 (1.02, 1.62) | 0.0483 | 0.1835 |
| HEI-2015 | Absolute risk/cases | 80/285 | 71/284 | 73/264 | 64/249 | 67/264 | −13 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.97 (0.82, 1.14) | 0.90 (0.76, 1.07) | 0.81 (0.68, 0.97) | 0.83 (0.69, 1.00) | 0.0102 | 0.0808 |
| Proximal colon cancer | ||||||||
| EDIH | Absolute risk/cases | 39/141 | 38/160 | 46/204 | 50/155 | 45/168 | 6 | |
| EDIH | Relative risk | 1 (reference) | 1.16 (0.92, 1.47) | 1.55 (1.23, 1.95) | 1.19 (0.93, 1.52) | 1.41 (1.10, 1.81) | 0.0134 | 0.0808 |
| EDIP | Absolute risk/cases | 42/147 | 41/185 | 47/178 | 44/160 | 44/158 | 2 | |
| EDIP | Relative risk | 1 (reference) | 1.36 (1.07, 1.73) | 1.34 (1.03, 1.73) | 1.29 (0.98, 1.71) | 1.42 (1.05, 1.92) | 0.0587 | 0.1966 |
| HEI-2015 | Absolute risk/cases | 47/173 | 46/176 | 44/169 | 41/151 | 40/159 | −7 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.96 (0.78, 1.19) | 0.92 (0.74, 1.14) | 0.78 (0.62, 0.98) | 0.80 (0.64, 1.01) | 0.0156 | 0.0808 |
| Distal colon and rectal cancer | ||||||||
| EDIH | Absolute risk/cases | 33/127 | 34/116 | 30/132 | 31/119 | 36/133 | 3 | |
| EDIH | Relative risk | 1 (reference) | 0.91 (0.70, 1.19) | 0.97 (0.74, 1.27) | 0.95 (0.72, 1.25) | 1.09 (0.82, 1.44) | 0.4874 | 0.6174 |
| EDIP | Absolute risk/cases | 33/141 | 34/109 | 27/119 | 32/131 | 39/127 | 6 | |
| EDIP | Relative risk | 1 (reference) | 0.80 (0.60, 1.05) | 0.87 (0.65, 1.16) | 1.02 (0.75, 1.39) | 1.03 (0.74, 1.44) | 0.5117 | 0.6375 |
| HEI-2015 | Absolute risk/cases | 39/136 | 30/138 | 33/114 | 31/117 | 31/122 | −8 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.02 (0.80, 1.31) | 0.88 (0.68, 1.14) | 0.86 (0.66, 1.12) | 0.92 (0.70, 1.20) | 0.2774 | 0.4679 |
| Intestinal polyps | ||||||||
| EDIH | Absolute risk/cases | 551/1991 | 571/2030 | 594/2134 | 635/2211 | 649/2308 | 98 | |
| EDIH | Relative risk | 1 (reference) | 1.05 (0.99, 1.12) | 1.14 (1.07, 1.22) | 1.18 (1.11, 1.27) | 1.23 (1.15, 1.32) | <0.0001 | 0.0038 |
| EDIP | Absolute risk/cases | 566/2143 | 604/2144 | 574/2113 | 616/2081 | 639/2193 | 73 | |
| EDIP | Relative risk | 1 (reference) | 1.05 (0.98, 1.12) | 1.07 (1.00, 1.15) | 1.08 (1.01, 1.17) | 1.16 (1.07, 1.26) | 0.0004 | 0.0101 |
| HEI-2015 | Absolute risk/cases | 651/2307 | 626/2223 | 586/2172 | 585/2075 | 553/1896 | −98 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.97 (0.91, 1.03) | 0.95 (0.89, 1.00) | 0.89 (0.84, 0.95) | 0.84 (0.78, 0.89) | <0.0001 | 0.0038 |
| Invasive Breast cancer | ||||||||
| EDIH | Absolute risk/cases | 275/899 | 259/921 | 272/827 | 276/843 | 294/903 | 19 | |
| EDIH | Relative risk | 1 (reference) | 1.09 (0.99, 1.20) | 1.02 (0.92, 1.12) | 1.06 (0.96, 1.18) | 1.19 (1.07, 1.32) | 0.0032 | 0.0405 |
| EDIP | Absolute risk/cases | 264/926 | 266/936 | 285/874 | 285/866 | 275/791 | 11 | |
| EDIP | Relative risk | 1 (reference) | 1.09 (0.99, 1.20) | 1.07 (0.96, 1.19) | 1.13 (1.01, 1.27) | 1.12 (1.00, 1.27) | 0.0561 | 0.1966 |
| HEI-2015 | Absolute risk/cases | 281/872 | 274/867 | 262/847 | 278/869 | 280/938 | −1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.95 (0.86, 1.04) | 0.90 (0.82, 1.00) | 0.92 (0.83, 1.01) | 0.99 (0.90, 1.09) | 0.6135 | 0.6842 |
| ER+ | ||||||||
| EDIH | Absolute risk/cases | 226/745 | 207/747 | 217/685 | 231/688 | 232/714 | 6 | |
| EDIH | Relative risk | 1 (reference) | 1.08 (0.97, 1.20) | 1.04 (0.93, 1.16) | 1.07 (0.96, 1.20) | 1.16 (1.04, 1.30) | 0.017 | 0.0808 |
| EDIP | Absolute risk/cases | 217/769 | 213/768 | 232/726 | 228/704 | 222/612 | 5 | |
| EDIP | Relative risk | 1 (reference) | 1.09 (0.98, 1.22) | 1.09 (0.97, 1.23) | 1.13 (1.00, 1.29) | 1.08 (0.94, 1.24) | 0.1966 | 0.3735 |
| HEI-2015 | Absolute risk/cases | 223/694 | 220/692 | 213/695 | 228/720 | 229/778 | 6 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.95 (0.85, 1.05) | 0.93 (0.84, 1.04) | 0.95 (0.86, 1.06) | 1.03 (0.92, 1.14) | 0.6815 | 0.7117 |
| ER- | ||||||||
| EDIH | Absolute risk/cases | 29/100 | 37/123 | 36/105 | 29/100 | 38/127 | 9 | |
| EDIH | Relative risk | 1 (reference) | 1.22 (0.93, 1.60) | 1.05 (0.79, 1.40) | 1.01 (0.75, 1.36) | 1.31 (0.98, 1.76) | 0.1847 | 0.3640 |
| EDIP | Absolute risk/cases | 31/108 | 35/104 | 35/106 | 37/124 | 31/113 | 0 | |
| EDIP | Relative risk | 1 (reference) | 0.98 (0.73, 1.31) | 1.02 (0.75, 1.39) | 1.23 (0.89, 1.70) | 1.19 (0.84, 1.68) | 0.1796 | 0.3640 |
| HEI-2015 | Absolute risk/cases | 38/123 | 31/110 | 31/107 | 33/104 | 35/111 | −3 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.87 (0.67, 1.12) | 0.83 (0.64, 1.08) | 0.79 (0.61, 1.04) | 0.85 (0.65, 1.12) | 0.1725 | 0.3640 |
| PR+ | ||||||||
| EDIH | Absolute risk/cases | 183/626 | 177/636 | 188/588 | 195/575 | 194/604 | 11 | |
| EDIH | Relative risk | 1 (reference) | 1.10 (0.99, 1.24) | 1.08 (0.96, 1.21) | 1.09 (0.96, 1.23) | 1.20 (1.06, 1.36) | 0.0085 | 0.0808 |
| EDIP | Absolute risk/cases | 184/648 | 177/649 | 197/635 | 195/597 | 184/500 | 0 | |
| EDIP | Relative risk | 1 (reference) | 1.12 (0.99, 1.26) | 1.17 (1.03, 1.33) | 1.18 (1.03, 1.36) | 1.09 (0.94, 1.27) | 0.1438 | 0.3277 |
| HEI-2015 | Absolute risk/cases | 190/597 | 187/580 | 181/593 | 191/615 | 189/644 | −1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.92 (0.82, 1.04) | 0.92 (0.82, 1.03) | 0.94 (0.84, 1.06) | 0.98 (0.87, 1.10) | 0757 | 0.8946 |
| PR- | ||||||||
| EDIH | Absolute risk/cases | 66/206 | 62/222 | 62/195 | 60/204 | 70/225 | 4 | |
| EDIH | Relative risk | 1 (reference) | 1.08 (0.89, 1.31) | 0.96 (0.78, 1.19) | 1.02 (0.83, 1.26) | 1.16 (0.94, 1.43) | 0.2408 | 0.4679 |
| EDIP | Absolute risk/cases | 61/220 | 65/214 | 65/182 | 66/222 | 63/214 | 2 | |
| EDIP | Relative risk | 1 (reference) | 0.96 (0.79, 1.18) | 0.83 (0.66, 1.03) | 1.04 (0.83, 1.31) | 1.06 (0.83, 1.36) | 0.5338 | 0.6579 |
| HEI-2015 | Absolute risk/cases | 68/212 | 59/212 | 60/204 | 65/199 | 69/225 | 1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.96 (0.79, 1.16) | 0.91 (0.74, 1.10) | 0.88 (0.72, 1.07) | 0.99 (0.81, 1.21) | 0.6136 | 0.7117 |
| HER2+ | ||||||||
| EDIH | Absolute risk/cases | 27/86 | 24/85 | 26/86 | 28/85 | 30/100 | 3 | |
| EDIH | Relative risk | 1 (reference) | 0.99 (0.73, 1.35) | 1.03 (0.75, 1.41) | 1.02 (0.74, 1.42) | 1.24 (0.90, 1.71) | 0.1823 | 0.3640 |
| EDIP | Absolute risk/cases | 25/81 | 26/97 | 27/91 | 31/86 | 26/87 | 1 | |
| EDIP | Relative risk | 1 (reference) | 1.25 (0.91, 1.72) | 1.21 (0.86, 1.71) | 1.20 (0.83, 1.74) | 1.29 (0.86, 1.92) | 0.3012 | 0.4766 |
| HEI-2015 | Absolute risk/cases | 28/97 | 26/84 | 25/84 | 28/90 | 28/87 | 0 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.84 (0.63, 1.13) | 0.84 (0.63, 1.14) | 0.91 (0.68, 1.22) | 0.89 (0.66, 1.22) | 0.5863 | 0.6749 |
| HER2- | ||||||||
| EDIH | Absolute risk/cases | 208/698 | 198/708 | 205/639 | 207/647 | 216/660 | 8 | |
| EDIH | Relative risk | 1 (reference) | 1.09 (0.98, 1.22) | 1.03 (0.92, 1.16) | 1.08 (0.96, 1.21) | 1.15 (1.02, 1.29) | 0.0424 | 0.1696 |
| EDIP | Absolute risk/cases | 203/726 | 200/722 | 214/665 | 210/660 | 207/579 | 4 | |
| EDIP | Relative risk | 1 (reference) | 1.09 (0.98, 1.22) | 1.06 (0.94, 1.20) | 1.13 (0.99, 1.29) | 1.09 (0.95, 1.26) | 0.1868 | 0.3640 |
| HEI-2015 | Absolute risk/cases | 212/654 | 202/652 | 198/662 | 208/657 | 214/727 | 2 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.95 (0.85, 1.06) | 0.94 (0.84, 1.05) | 0.92 (0.82, 1.03) | 1.01 (0.90, 1.13) | 0.9486 | 0.9486 |
| ER- PR- HER2+ | ||||||||
| EDIH | Absolute risk/cases | 7/25 | 8/23 | 8/25 | 7/25 | 7/22 | 0 | |
| EDIH | Relative risk | 1 (reference) | 0.92 (0.51, 1.65) | 1.01 (0.56, 1.82) | 1.01 (0.55, 1.84) | 0.89 (0.47, 1.68) | 0.8008 | 0.8008 |
| EDIP | Absolute risk/cases | 6/19 | 8/31 | 8/21 | 9/25 | 5/24 | −1 | |
| EDIP | Relative risk | 1 (reference) | 1.88 (1.00, 3.51) | 1.35 (0.66, 2.77) | 1.71 (0.81, 3.58) | 1.73 (0.78, 3.83) | 0.2972 | 0.4766 |
| HEI-2015 | Absolute risk/cases | 9/30 | 5/22 | 7/23 | 7/23 | 8/22 | −1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.72 (0.42, 1.26) | 0.75 (0.43, 1.31) | 0.76 (0.44, 1.34) | 0.76 (0.42, 1.36) | 0.3750 | 0.5182 |
| Luminal A | ||||||||
| EDIH | Absolute risk/cases | 189/640 | 173/625 | 183/575 | 188/582 | 190/576 | 1 | |
| EDIH | Relative risk | 1 (reference) | 1.06 (0.95, 1.19) | 1.03 (0.91, 1.16) | 1.08 (0.95, 1.22) | 1.11 (0.98, 1.26) | 0.1054 | 0.2762 |
| EDIP | Absolute risk/cases | 182/660 | 178/655 | 190/599 | 185/577 | 188/507 | 6 | |
| EDIP | Relative risk | 1 (reference) | 1.10 (0.98, 1.24) | 1.07 (0.94, 1.22) | 1.11 (0.97, 1.28) | 1.08 (0.93, 1.25) | 0.3097 | 0.4766 |
| HEI-2015 | Absolute risk/cases | 187/579 | 181/582 | 179/593 | 186/593 | 190/651 | 3 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.95 (0.85, 1.07) | 0.95 (0.84, 1.06) | 0.93 (0.83, 1.05) | 1.02 (0.90, 1.14) | 0.9289 | 0.9289 |
| Luminal B | ||||||||
| EDIH | Absolute risk/cases | 19/59 | 16/61 | 18/60 | 20/59 | 22/76 | 3 | |
| EDIH | Relative risk | 1 (reference) | 1.05 (0.72, 1.51) | 1.06 (0.72, 1.54) | 1.05 (0.71, 1.55) | 1.40 (0.96, 2.04) | 0.0885 | 0.2491 |
| EDIP | Absolute risk/cases | 19/61 | 17/65 | 20/69 | 21/59 | 19/61 | 0 | |
| EDIP | Relative risk | 1 (reference) | 1.07 (0.73, 1.56) | 1.15 (0.77, 1.71) | 1.02 (0.66, 1.58) | 1.11 (0.70, 1.77) | 0.7315 | 0.7315 |
| HEI-2015 | Absolute risk/cases | 19/65 | 19/59 | 18/60 | 20/67 | 20/64 | 1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.88 (0.62, 1.26) | 0.90 (0.63, 1.29) | 1.01 (0.71, 1.44) | 0.98 (0.68, 1.41) | 0.9019 | 0.9019 |
| Triple negative | ||||||||
| EDIH | Absolute risk/cases | 18/57 | 24/83 | 21/61 | 17/63 | 25/84 | 7 | |
| EDIH | Relative risk | 1 (reference) | 1.43 (1.01, 2.01) | 1.05 (0.72, 1.54) | 1.09 (0.74, 1.60) | 1.49 (1.02, 2.16) | 0.1386 | 0.3277 |
| EDIP | Absolute risk/cases | 19/66 | 21/65 | 22/65 | 24/81 | 18/71 | −1 | |
| EDIP | Relative risk | 1 (reference) | 0.97 (0.67, 1.40) | 0.98 (0.66, 1.45) | 1.24 (0.82, 1.85) | 1.13 (0.73, 1.76) | 0.3704 | 0.5182 |
| HEI-2015 | Absolute risk/cases | 24/75 | 21/70 | 19/69 | 19/61 | 23/73 | −1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.91 (0.66, 1.27) | 0.89 (0.64, 1.24) | 0.78 (0.55, 1.10) | 0.93 (0.66, 1.31) | 0.4526 | 0.5950 |
| Invasive ductal carcinoma | ||||||||
| EDIH | Absolute risk/cases | 144/463 | 140/482 | 143/474 | 153/478 | 158/500 | 14 | |
| EDIH | Relative risk | 1 (reference) | 1.07 (0.94, 1.22) | 1.08 (0.94, 1.24) | 1.11 (0.97, 1.28) | 1.20 (1.04, 1.38) | 0.0119 | 0.0808 |
| EDIP | Absolute risk/cases | 146/489 | 143/512 | 152/465 | 153/492 | 146/439 | 0 | |
| EDIP | Relative risk | 1 (reference) | 1.08 (0.94, 1.23) | 1.00 (0.87, 1.16) | 1.11 (0.95, 1.30) | 1.06 (0.89, 1.25) | 0.4745 | 0.6112 |
| HEI-2015 | Absolute risk/cases | 149/459 | 140/485 | 142/450 | 154/506 | 154/497 | 6 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.02 (0.89, 1.16) | 0.93 (0.82, 1.06) | 1.05 (0.92, 1.19) | 1.04 (0.91, 1.19) | 0.5326 | 0.6529 |
| Invasive lobular carcinoma | ||||||||
| EDIH | Absolute risk/cases | 25/87 | 20/81 | 23/65 | 21/68 | 28/81 | 3 | |
| EDIH | Relative risk | 1 (reference) | 1.04 (0.76, 1.42) | 0.88 (0.63, 1.24) | 0.97 (0.69, 1.37) | 1.23 (0.87, 1.72) | 0.3275 | 0.4787 |
| EDIP | Absolute risk/cases | 22/96 | 23/62 | 24/89 | 23/64 | 24/71 | 2 | |
| EDIP | Relative risk | 1 (reference) | 0.70 (0.49, 0.98) | 1.05 (0.76, 1.47) | 0.81 (0.55, 1.18) | 1.00 (0.67, 1.48) | 0.8900 | 0.8900 |
| HEI-2015 | Absolute risk/cases | 24/80 | 24/57 | 20/77 | 24/82 | 23/86 | −1 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.66 (0.47, 0.93) | 0.87 (0.63, 1.19) | 0.89 (0.65, 1.23) | 0.91 (0.66, 1.26) | 0.9513 | 0.9513 |
| Localized | ||||||||
| EDIH | Absolute risk/cases | 186/622 | 179/633 | 185/580 | 190/582 | 200/615 | 14 | |
| EDIH | Relative risk | 1 (reference) | 1.08 (0.97, 1.21) | 1.03 (0.92, 1.16) | 1.07 (0.94, 1.21) | 1.18 (1.04, 1.34) | 0.0193 | 0.0863 |
| EDIP | Absolute risk/cases | 176/630 | 190/658 | 193/597 | 196/606 | 185/541 | 9 | |
| EDIP | Relative risk | 1 (reference) | 1.13 (1.01, 1.28) | 1.08 (0.95, 1.23) | 1.17 (1.02, 1.34) | 1.14 (0.98, 1.32) | 0.0836 | 0.2444 |
| HEI-2015 | Absolute risk/cases | 189/584 | 192/595 | 179/597 | 188/598 | 192/658 | 3 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.97 (0.86, 1.09) | 0.95 (0.84, 1.07) | 0.94 (0.83, 1.06) | 1.03 (0.91, 1.16) | 0.8605 | 0.8605 |
| Regional/distant | ||||||||
| EDIH | Absolute risk/cases | 67/219 | 62/230 | 67/199 | 66/215 | 70/229 | 3 | |
| EDIH | Relative risk | 1 (reference) | 1.09 (0.90, 1.32) | 0.98 (0.80, 1.21) | 1.09 (0.89, 1.34) | 1.20 (0.98, 1.48) | 0.1030 | 0.2762 |
| EDIP | Absolute risk/cases | 69/234 | 59/220 | 71/224 | 67/219 | 67/195 | −2 | |
| EDIP | Relative risk | 1 (reference) | 1.00 (0.82, 1.22) | 1.07 (0.86, 1.32) | 1.11 (0.88, 1.39) | 1.07 (0.83, 1.37) | 0.4475 | 0.5950 |
| HEI-2015 | Absolute risk/cases | 71/226 | 60/216 | 66/201 | 67/220 | 69/229 | −2 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.92 (0.77, 1.12) | 0.85 (0.70, 1.03) | 0.92 (0.76, 1.12) | 0.97 (0.80, 1.18) | 0.7019 | 0.7117 |
| Endometrial cancer | ||||||||
| EDIH | Absolute risk/cases | 46/74 | 47/88 | 53/79 | 52/73 | 62/89 | 16 | |
| EDIH | Relative risk | 1 (reference) | 1.30 (0.94, 1.79) | 1.24 (0.89, 1.74) | 1.24 (0.88, 1.76) | 1.63 (1.16, 2.30) | 0.0110 | 0.0808 |
| EDIP | Absolute risk/cases | 50/81 | 49/93 | 49/67 | 55/84 | 58/78 | 8 | |
| EDIP | Relative risk | 1 (reference) | 1.21 (0.88, 1.68) | 0.94 (0.65, 1.36) | 1.27 (0.87, 1.85) | 1.39 (0.92, 2.09) | 0.1466 | 0.3277 |
| HEI-2015 | Absolute risk/cases | 56/67 | 52/84 | 57/98 | 42/81 | 53/73 | −3 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.16 (0.84, 1.60) | 1.27 (0.93, 1.74) | 1.01 (0.72, 1.41) | 0.88 (0.62, 1.24) | 0.3151 | 0.4766 |
| Endometroid | ||||||||
| EDIH | Absolute risk/cases | 32/49 | 22/50 | 35/53 | 38/52 | 42/62 | 10 | |
| EDIH | Relative risk | 1 (reference) | 1.14 (0.76, 1.71) | 1.30 (0.86, 1.96) | 1.38 (0.90, 2.10) | 1.74 (1.15, 2.64) | 0.0058 | 0.0630 |
| EDIP | Absolute risk/cases | 28/54 | 32/59 | 32/42 | 33/55 | 44/56 | 16 | |
| EDIP | Relative risk | 1 (reference) | 1.19 (0.80, 1.77) | 0.90 (0.57, 1.43) | 1.28 (0.80, 2.03) | 1.52 (0.93, 2.50) | 0.117 | 0.2964 |
| HEI-2015 | Absolute risk/cases | 36/48 | 29/53 | 45/66 | 34/54 | 25/45 | −11 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.03 (0.69, 1.52) | 1.21 (0.83, 1.77) | 0.94 (0.63, 1.41) | 0.77 (0.50, 1.18) | 0.2181 | 0.4043 |
| Non-endometroid | ||||||||
| EDIH | Absolute risk/cases | 15/25 | 19/39 | 19/25 | 17/21 | 16/27 | 1 | |
| EDIH | Relative risk | 1 (reference) | 1.57 (0.94, 2.64) | 1.07 (0.60, 1.92) | 0.97 (0.52, 1.80) | 1.36 (0.75, 2.48) | 0.7359 | 0.7359 |
| EDIP | Absolute risk/cases | 20/27 | 13/33 | 19/26 | 18/29 | 16/22 | −4 | |
| EDIP | Relative risk | 1 (reference) | 1.21 (0.70, 2.09) | 1.01 (0.54, 1.88) | 1.18 (0.62, 2.27) | 1.03 (0.50, 2.13) | 0.9443 | 0.9443 |
| HEI-2015 | Absolute risk/cases | 15/19 | 20/31 | 15/31 | 15/27 | 21/29 | 6 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.52 (0.86, 2.70) | 1.43 (0.80, 2.55) | 1.21 (0.66, 2.20) | 1.25 (0.68, 2.28) | 0.7907 | 0.7907 |
| Ovarian cancer | ||||||||
| EDIH | Absolute rate/cases | 35/60 | 34/52 | 25/54 | 36/41 | 37/53 | 2 | |
| EDIH | Relative risk | 1 (reference) | 0.95 (0.65, 1.39) | 1.04 (0.70, 1.53) | 0.85 (0.55, 1.30) | 1.16 (0.77, 1.75) | 0.5950 | 0.6749 |
| EDIP | Absolute rate/cases | 37/64 | 31/63 | 32/44 | 33/46 | 34/43 | −3 | |
| EDIP | Relative risk | 1 (reference) | 1.02 (0.70, 1.49) | 0.76 (0.49, 1.18) | 0.85 (0.54, 1.36) | 0.89 (0.54, 1.47) | 0.4541 | 0.5950 |
| HEI-2015 | Absolute risk/cases | 33/47 | 28/45 | 36/42 | 34/69 | 37/57 | 4 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.93 (0.62, 1.40) | 0.84 (0.55, 1.29) | 1.37 (0.93, 2.01) | 1.09 (0.73, 1.64) | 0.2412 | 0.4365 |
| Serous | ||||||||
| EDIH | Absolute risk/cases | 14/27 | 16/25 | 9/23 | 17/15 | 17/24 | 3 | |
| EDIH | Relative risk | 1 (reference) | 1.06 (0.60, 1.85) | 1.06 (0.59, 1.91) | 0.75 (0.38, 1.47) | 1.26 (0.68, 2.32) | 0.7023 | 0.7117 |
| EDIP | Absolute risk/cases | 18/28 | 12/35 | 14/20 | 15/10 | 14/21 | −4 | |
| EDIP | Relative risk | 1 (reference) | 1.38 (0.80,2.39) | 0.88 (0.46, 1.71) | 0.48 (0.21, 1.11) | 1.41 (0.54, 2.42) | 0.5589 | 0.6637 |
| HEI-2015 | Absolute risk/cases | 16/23 | 11/19 | 15/21 | 16/28 | 14/23 | −2 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.80 (0.44, 1.48) | 0.86 (0.47, 1.57) | 1.13 (0.64, 2.00) | 0.90 (0.49, 1.66) | 0.9040 | 0.9040 |
| Non-Serous | ||||||||
| EDIH | Absolute risk/cases | 20/33 | 18/27 | 16/31 | 18/26 | 20/29 | 0 | |
| EDIH | Relative risk/cases | 1 (reference) | 0.86 (0.51, 1.44) | 1.01 (0.60, 1.70) | 0.92 (0.53, 1.60) | 1.10 (0.63, 1.91) | 0.6922 | 0.7117 |
| EDIP | Absolute risk/cases | 19/36 | 18/28 | 18/24 | 18/36 | 20/22 | 1 | |
| EDIP | Relative risk | 1 (reference) | 0.75 (0.44, 1.28) | 0.66 (0.37, 1.91) | 1.04 (0.58, 1.87) | 0.70 (0.36, 1.39) | 0.5761 | 0.6736 |
| HEI-2015 | Absolute risk/cases | 16/23 | 15/27 | 20/21 | 19/41 | 22/34 | −6 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.14 (0.65, 1.99) | 0.87 (0.48, 1.58) | 1.66 (0.98, 2.80) | 1.32 (0.76, 2.31) | 0.1355 | 0.3277 |
| Lung cancer | ||||||||
| EDIH | Absolute risk/cases | 116/482 | 108/408 | 107/410 | 113/417 | 119/399 | 3 | |
| EDIH | Relative risk | 1 (reference) | 0.94 (0.82, 1.08) | 0.97 (0.84, 1.11) | 1.01 (0.88, 1.16) | 0.89 (0.77, 1.03) | 0.2609 | 0.4507 |
| EDIP | Absolute risk/cases | 110/547 | 109/464 | 114/397 | 117/377 | 113/331 | 3 | |
| EDIP | Relative risk | 1 (reference) | 1.05 (0.92, 1.20) | 0.97 (0.84, 1.13) | 1.03 (0.88, 1.21) | 1.02 (0.85, 1.21) | 0.9331 | 0.9331 |
| HEI-2015 | Absolute risk/cases | 130/520 | 112/439 | 110/415 | 113/387 | 99/354 | −31 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.97 (0.86, 1.11) | 0.99 (0.87, 1.13) | 0.97 (0.84, 1.11) | 0.91 (0.78, 1.05) | 0.2555 | 0.4507 |
| Small cell | ||||||||
| EDIH | Absolute risk/cases | 7/25 | 8/32 | 7/26 | 6/29 | 9/26 | 2 | |
| EDIH | Relative risk | 1 (reference) | 1.43 (0.83, 2.45) | 1.02 (0.57, 1.83) | 1.15 (0.64, 2.04) | 0.81 (0.44, 1.48) | 0.3198 | 0.4766 |
| EDIP | Absolute risk/cases | 8/31 | 5/38 | 6/23 | 10/28 | 8/18 | 0 | |
| EDIP | Relative risk | 1 (reference) | 1.55 (0.92, 2.61) | 0.93 (0.50, 1.72) | 1.28 (0.68, 2.42) | 0.83 (0.40, 1.74) | 0.6212 | 0.6842 |
| HEI-2015 | Absolute risk/cases | 7/33 | 7/27 | 7/32 | 8/23 | 7/23 | 0 | |
| HEI-2015 | Relative risk | 1 (reference) | 1.21 (0.72, 2.04) | 1.81 (1.09, 3.02) | 1.51 (0.86, 2.65) | 1.79 (1.00, 3.23) | 0.028 | 0.1182 |
| Non-small cell | ||||||||
| EDIH | Absolute risk/cases | 57/249 | 59/183 | 51/204 | 55/225 | 59/198 | 2 | |
| EDIH | Relative risk | 1 (reference) | 0.81 (0.67, 0.99) | 0.94 (0.77, 1.14) | 1.06 (0.88, 1.30) | 0.89 (0.72, 1.09) | 0.7986 | 0.7986 |
| EDIP | Absolute risk/cases | 57/275 | 50/218 | 61/205 | 59/192 | 54/169 | −3 | |
| EDIP | Relative risk | 1 (reference) | 0.98 (0.80, 1.18) | 1.00 (0.81, 1.23) | 1.04 (0.83, 1.31) | 1.04 (0.81, 1.34) | 0.6585 | 0.7117 |
| HEI-2015 | Absolute risk/cases | 64/251 | 56/219 | 53/207 | 57/197 | 51/184 | −13 | |
| HEI-2015 | Relative risk | 1 (reference) | 0.98 (0.81, 1.18) | 0.97 (0.80, 1.17) | 0.95 (0.78, 1.16) | 0.90 (0.73, 1.11) | 0.3434 | 0.4924 |
a Values presented are hazard ratios (HR) and 95% confidence intervals (95% CI) for relative risk and incidence rate per 100,000 person years for absolute risk. HRs were derived from multivariable-adjusted Cox proportional hazards regression models adjusted for the following baseline covariates: age at enrollment, physical activity, race and ethnicity, educational level, family history of cancer, number of hormones used, comorbidity score, baseline cardiovascular disease status, baseline lung disease, number of supplements used, non-steroidal anti-inflammatory drug use, hormone therapy study arm, baseline hormone therapy ever, oral contraceptive duration, pack years of smoking, coffee/tea and total alcohol intake. Colorectal cancer and subtype analyses were additionally adjusted for colorectal cancer screening. Invasive breast cancer and subtype analyses were additionally adjusted for months of breast-feeding, age at menopause, mammogram ever, parity, bilateral oophorectomy, passive smoking and Gail 5-year risk score. Endometrial cancer and ovarian cancer analyses were additionally adjusted for age at first birth, age at menarche, age at menopause, months of breast-feeding and parity. Ovarian cancer analyses were further adjusted for tubal ligation. Lung cancer analyses were additionally adjusted for smoking status and passive smoking. b Details of cancer site definition are included in Table S2. c Each dietary pattern was adjusted for the same covariates using the residual method prior to estimating the incidence rates. Incidence rate per 100,000 person years were calculated using the number of cases of each cancer within a quintile of the dietary score divided by the sum of year-to-event within that dietary score quintile and then multiplied by 100,000. The Q5-Q1 incidence rate per 100,000 person years was calculated using the incidence rate per 100,000 person-year of the 5th quintile of a dietary score minus that of the 1st quintile of the dietary score. d The p value for linear trend was estimated in the same multivariable-adjusted models by assigning the quintile-specific median value of each dietary pattern to all participants in the quintile and modelling as an ordinal variable. The p value for linear trend was adjusted for false discovery rate using the Benjamini and Hotchberg approach. e EDIH, empirical dietary index for hyperinsulinemia score assesses the ability of the dietary pattern to contribute to insulin hypersecretion—higher EDIH scores reflect more hyperinsulinemic dietary patterns; EDIP, empirical dietary inflammatory pattern score assesses the ability of the dietary pattern to contribute to chronic systemic inflammation—higher EDIP scores reflect more pro-inflammatory dietary patterns; HEI-2015, healthy eating index-2015 assesses adherence to the 2015–2020 Dietary Guidelines for Americans—higher HEI-2015 scores are indicative of greater adherence and higher dietary quality. EDIH and EDIP are positively correlated, whereas both scores are inversely correlated with HEI-2015, i.e., more hyperinsulinemic or pro-inflammatory dietary patterns are of lower dietary quality.

Figure 2.
Associations between a 1 standard deviation increment in hyperinsulinemic, pro-inflammatory dietary patterns or higher overall dietary quality, and risk of incident total cancer, site-specific cancers, and pathological subtypes. Values are hazard ratios (HR) and 95% confidence intervals (95% CI) for cancer risk per 1 standard deviation increment in dietary score. Values in colored background/asterisk represent elevated risk (EDIH/EDIP), reduced risk (HEI-2015), or significant FDR-adjusted p-value at <0.15. HRs were derived from multivariable-adjusted Cox proportional hazards regression models adjusted for the following baseline covariates: age at enrollment, physical activity, race and ethnicity, educational level, family history of cancer, number of hormones used, comorbidity score, baseline cardiovascular disease status, baseline lung disease, number of supplements used, non-steroidal anti-inflammatory drug use, hormone therapy study arm, baseline hormone therapy ever, oral contraceptive duration, pack years of smoking, coffee/tea, and total alcohol intake. Colorectal cancer and subtype analyses were additionally adjusted for colorectal cancer screening. Invasive breast cancer and subtype analyses were additionally adjusted for months of breast-feeding, age at menopause, mammogram ever, parity, bilateral oophorectomy, passive smoking, Gail 5-year risk score. Endometrial cancer and ovarian cancer analyses were additionally adjusted for age at first birth, age at menarche, age at menopause, months of breast-feeding, and parity.
3.5. EDIP and Cancer Risk (Table 2, Figure 2)
Women in the highest EDIP quintile were at greater risk of total cancer compared to those in the lowest quintile, with a multivariable-adjusted incidence rate difference of 41 per 100,000 person years and corresponding HR (95% CI) of 1.08 (1.02–1.15) (Table 2). A 1 sd increment in EDIP was associated with higher risk of colorectal cancer, colon cancer and marginally with proximal and distal colon or rectal cancer. EDIP was also associated with intestinal polyps. Higher EDIP was associated with greater risk of overall breast cancer and ER-negative cancer. Furthermore, higher EDIP was associated with greater risk for endometrial cancer, but not ovarian cancer or lung cancer (Figure 2). The EDIP metabolomics score was generally not associated with cancer risk (Table 3).
3.6. HEI-2015 and Cancer Risk (Table 2, Figure 2)
Women classified in the highest HEI-2015 quintile were at lower risk of total cancer compared to those in the lowest quintile, with a multivariable-adjusted incidence rate difference of −49 per 100,000 person years and corresponding HR (95% CI) of 0.93 (0.89–0.98) (Table 2). Higher HEI-2015 was associated with lower risk of colorectal cancer, colon cancer and proximal colon cancer but not distal colon or rectal cancer. HEI-2015 was also strongly associated with intestinal polyps. Unlike EDIH and EDIP, HEI-2015 was not associated with overall breast cancer or its subtypes nor with endometrial or ovarian cancers but was marginally inversely associated with overall lung cancer, though positively associated with small-cell lung cancer. Higher HEI-2015 metabolomics profile score was associated with lower risk for overall lung cancer, HR, 0.46 (0.24–0.90) (Table 3).
3.7. Sensitivity Analyses and Subgroup Analyses (Supplementary Tables S5–S8)
Additional adjustment for BMI and type 2 diabetes, major mediators strongly associated with EDIH and EDIP in previous studies, did not materially change the results, though results for endometrial cancer were attenuated and no longer statistically significant (Supplementary Table S5). Findings from subgroup analyses are reported in Supplementary Tables S6 and S7. p values for the interactions of the dietary patterns and the potential effect modifiers were generally not significant. The results for total cancer, breast cancer and endometrial cancer remained robust for EDIH after mutually adjusting for EDIP and HEI-2015 (Supplementary Table S8).
4. Discussion
4.1. Principal Findings, Strengths and Weaknesses in Relation to Other Studies
The present study employed two empirical hypothesis-oriented dietary indices to investigate associations between diets that contribute to chronic hyperinsulinemia (EDIH) or chronic systemic inflammation (EDIP) and risk of developing total cancer and site-specific cancers among postmenopausal women, while also examining these associations with an established index of overall dietary quality (HEI-2015). While all three dietary indices were significantly associated with risk of total cancer, colorectal cancer and intestinal polyps, EDIH and EDIP were further associated with risk of breast cancer and endometrial cancer. In addition, EDIH was associated with multiple breast cancer subtypes including the more aggressive triple negative breast cancer.
Although a previous study found that higher EDIH was associated with higher total cancer mortality in both men and women [32], most previous studies have examined single cancer sites and reported similar findings to ours. For example, in the NHS (another all-female cohort), EDIH and EDIP were associated with higher risk of colorectal cancer and its anatomic subsites except the rectum [6,8]. EDIH was also associated with higher risk of digestive system cancers and accessory organs [7]. In the WHI, dietary inflammatory potential assessed using a literature-derived nutrient-based dietary inflammatory index (DII) was associated with higher risk of colorectal cancer [33] similar to the current study. Given that DII was calculated with total intake (nutrients plus supplements), findings may not be directly comparable to EDIP, which is exclusively food-based. Though different polyp types were not adjudicated in WHI, the strong associations of EDIH and EDIP with intestinal polyps warrant future studies to test if dietary intakes may inform risk stratification in colorectal cancer screening for improved preventive strategies. Associations between EDIH and EDIP with total cancer and colorectal cancer were consistent with findings for HEI-2015. A study in WHI also found that higher HEI-2010 score was associated with lower colorectal cancer risk [34].
Although EDIH and EDIP were associated with higher risk of overall breast cancer and ER-negative subtype, only EDIH was associated with risk of multiple other breast cancer subtypes, while HEI-2015 showed no association. Only one study has examined EDIH in relation to breast cancer risk, and found higher risk for overall breast cancer with stronger associations for ER- and HER2+ tumors [35]. Two studies in the WHI found no associations between the DII and risk of overall breast cancer/subtypes [36,37]. The HEI and most other dietary indices have not been consistently associated with breast cancer risk [38]. The EDIH and EDIP dietary patterns are more strongly related to obesity and type 2 diabetes than most traditional dietary patterns [24,30,31,39]. Obesity and diabetes drive risk of the same cancers including colorectum, postmenopausal breast, endometrium and ovary, among other sites [3,40]. In the current study, both EDIH and EDIP were strongly associated with endometrial cancer risk, which attenuated after adjusting for obesity as a mediator. Similarly, a study in NHS and NHS-II cohorts applied the EDIH and EDIP scores and found strong associations with endometrial cancer, and showed that BMI mediated 84% and 93% of the associations, respectively [9]. Obesity is linked to inflammation and insulin resistance [41], and a study that analyzed data on 1.2 million women, found that each 10-kg/m2 increment in BMI was associated with a nearly 3-fold increase in endometrial cancer risk [42]. While EDIH has not been studied in relation to ovarian cancer risk, the null association with EDIP was consistent with a study in NHS and NHS-II [43].
The metabolomic profile of HEI-2015 had 23 metabolites that overlapped with the EDIH, and had 17 metabolites overlapped with the EDIP, which may partly explain the higher correlation between the HEI-2015 related metabolomics score is and EDIH-related metabolomics score, compared with the EDIP-related metabolomics score. We observed no associations between the metabolomics profile scores of the three dietary patterns and cancer risk, except for the associations between HEI-2015 score and overall lung cancer. We had 441 cancer cases in the metabolomics sample compared to 18,768 in the overall sample. It is therefore possible that the lack of associations in contrast to the dietary analysis is indicative of the low statistical power in our metabolomics sample. The association observed had wide 95% CIs, reflecting potentially unstable point estimates. Nevertheless, we characterized the correlations of metabolomics classes with the food group components of the dietary scores, which yielded novel and confirmatory findings. In the two previous metabomomics studies [16,17], higher levels of nine CEs, one glycerophosphoserine, trigonelline and eicosapentanoate were associated with lower EDIH score [16]. In the current study, EDIH showed inverse associations with three CEs and one glycerophosphoserine, though using a different method to derive metabolomic profile scores. Higher CE levels were associated with higher intake of wine and fruit and with lower intake of red meat, sugar-sweetened beverages, and processed meat. It is therefore possible that a low EDIH diet may reduce disease risks via CE’s greater efficiency in clearing blood remnants of lipid metabolism [16,44]. We found that higher plasma levels of alkaloids and purines were associated with lower EDIH/EDIP and with higher coffee/tea which contribute to lower EDIH/EDIP scores. Laboratory studies suggest that specific alkaloids can intervene in the insulin signal transduction pathway, and reverse molecular defects that could otherwise lead to insulin resistance and glucose intolerance [45]. These alkaloids may be involved in pathophysiological processes associated with insulin resistance, β-cell failure, oxidative stress and inflammation [45].
4.2. Strengths and Weaknesses of the Study
Our study has several strengths. We investigated EDIH, EDIP and HEI-2015 in relation to multiple cancers, and dietary pattern-related metabolomics profiles and their association with cancer risk. We estimated multivariable-adjusted incidence rates in addition to the usual relative risk estimates, providing better clinical and public health context for interpreting the relative risk estimates, e.g., incidence rate among the non-exposed (reference—quintile 1). We adjusted p-values to minimize the potential for false discovery. Potential limitations include using self-reported dietary intake though the FFQ was evaluated for bias and precision [22]. Although the FFQ and metabolites were single measurements, previous studies have shown that diet in adults and plasma metabolites remain stable overtime [46,47]. Though the sample sizes for the cancer risk analyses were large, the number of cancer cases in the metabolomics sample was small, precluding robust associations. Additionally, though we adjusted for numerous potential confounding factors, residual confounding may persist [48,49].
4.3. Possible Implications and Conclusions
In summary, our findings suggest that hyperinsulinemic and pro-inflammatory dietary patterns, as well as overall dietary quality, are associated with risk for several cancers among postmenopausal women, supporting further investigation of these dietary patterns in relation to cancer risk in dietary intervention studies to modify cancer risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15061756/s1, Figure S1: Workflow to derive the final analytic datasets. Table S1: Food group components of the empirical dietary index for hyperinsulinemia (EDIH) score, empirical dietary inflammatory pattern (EDIP) score and Health Eating Index (HEI) 2015. Table S2: Cancer sites definitions. Table S3: Description of covariates used in the current study. Table S4: Distribution of all nutrients available in the WHI by score quintiles in the total cancer analytic dataset. Table S5: Hazard ratios (95% CI) for the associations of dietary patterns with total and site-specific cancers, further adjusted for body mass index and type 2 diabetes. Table S6: Multivariable-adjusted associations of dietary patterns with specific cancers in body mass index (kg/m2) subgroups. Table S7: Multivariable-adjusted associations of dietary patterns with specific cancers in type 2 diabetes subgroups. Table S8: Multivariable-adjusted associations of dietary patterns with specific cancers including mutual adjustment.
Author Contributions
Q.J. and F.K.T. designed research; Q.J. conducted research and performed statistical analysis; N.S. conducted data reviews for accuracy; Q.J. wrote initial drafts of the manuscript; N.S., D.H.L., K.M.R., J.E.M., R.B., X.Z., M.L.N., M.L.-P., C.A.T., S.M.Z., A.S.F., D.G.S., S.D.S., A.E., X.M., S.K.C. and F.K.T. analyzed and interpreted the data and provided critical input; F.K.T. provided study oversight; all authors read and approved final content. The authors assume full responsibility for analyses and interpretation of these data. All authors have read and agreed to the published version of the manuscript.
Funding
National Cancer Institute (NCI) grant # R00207736 (FKT, QJ, DHL), American Cancer Society Research Scholar Grant # RSG2012401CCE (FKT, NS). National Cancer Institute Cancer Center support grant #P30 CA016058 (FKT, XM, SKC), The WHI program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. Metabolomic analysis in the WHI was funded by the NHLBI through contract HHSN268201300008C.
Institutional Review Board Statement
Our study was a secondary data analysis using data provided by the Women’s Health Initiative (WHI). The WHI protocol was approved by the institutional review boards at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA) and at each clinical center and all women signed informed consent. WHI is registered at clinicaltrials.gov as NCT00000611.
Informed Consent Statement
The WHI obtained written informed consent from all study participants.
Data Availability Statement
The data used in this project were provided by the Women’s Health Initiative. Data will be made available on request via the Women’s Health Initiative manuscript proposal process available at: https://www.whi.org/md/working-with-whi-data, accessed on 6 January 2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brucher, B.L.; Jamall, I.S. Epistemology of the origin of cancer: A new paradigm. BMC Cancer 2014, 14, 331. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, X.; Ma, Y.; Yuan, C.; Wang, M.; Wu, K.; Tabung, F.K.; Tobias, D.; Hu, F.B.; Giovannucci, E.; et al. Incident Type 2 Diabetes Duration and Cancer Risk: A Prospective Study in Two US Cohorts. J. Natl. Cancer Inst. 2020, 113, 381–389. [Google Scholar] [CrossRef]
- Tabung, F.K.; Wang, W.; Fung, T.T.; Hu, F.B.; Smith-Warner, S.A.; Chavarro, J.E.; Fuchs, C.S.; Willett, W.C.; Giovannucci, E.L. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br. J. Nutr. 2016, 116, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- Tabung, F.K.; Wang, W.; Fung, T.T.; Smith-Warner, S.A.; Keum, N.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Giovannucci, E.L. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am. J. Clin. Nutr. 2018, 108, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fung, T.T.; Wang, M.; Smith-Warner, S.A.; Giovannucci, E.L.; Tabung, F.K. Association of the Insulinemic Potential of Diet and Lifestyle With Risk of Digestive System Cancers in Men and Women. JNCI Cancer Spectr. 2018, 2, pky080. [Google Scholar] [CrossRef]
- Tabung, F.K.; Liu, L.; Wang, W.; Fung, T.T.; Wu, K.; Smith-Warner, S.A.; Cao, Y.; Hu, F.B.; Ogino, S.; Fuchs, C.S.; et al. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMA Oncol. 2018, 4, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Romanos-Nanclares, A.; Tabung, F.K.; Sinnott, J.A.; Trabert, B.; De Vivo, I.; Playdon, M.C.; Eliassen, A.H. Inflammatory and insulinemic dietary patterns and risk of endometrial cancer among US women. J. Natl. Cancer Inst. 2022, 115, 311–321. [Google Scholar] [CrossRef]
- Romanos-Nanclares, A.; Tabung, F.K.; Willett, W.C.; Rosner, B.; Holmes, M.D.; Chen, W.Y.; Tamimi, R.M.; Eliassen, A.H. Insulinemic potential of diet and risk of total and subtypes of breast cancer among US females. Am. J. Clin. Nutr. 2022, 116, 1530–1539. [Google Scholar] [CrossRef]
- Sasamoto, N.; Wang, T.; Townsend, M.K.; Eliassen, A.H.; Tabung, F.K.; Giovannucci, E.L.; Matulonis, U.A.; Terry, K.L.; Tworoger, S.S.; Harris, H.R. Pre-diagnosis and post-diagnosis dietary patterns and survival in women with ovarian cancer. Br. J. Cancer 2022, 127, 1097–1105. [Google Scholar] [CrossRef]
- Petimar, J.; Smith-Warner Stephanie, A.; Fung Teresa, T.; Rosner Bernard Chan Andrew, T.; Hu Frank, B.; Giovannucci Edward, L.; Tabung Fred, K. Recommendation-based dietary indices and risk of colorectal cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Am. J. Clin. Nutr. 2018, 108, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Petimar, J.; Smith-Warner, S.A.; Rosner, B.A.; Chan, A.T.; Giovannucci, E.L.; Tabung, F.K. Adherence to The World Cancer Research Fund/American Institute for Cancer Research 2018 Recommendations for Cancer Prevention and Risk of Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Brown Lisa, S.; Fung Teresa, T. Dietary Patterns and Colorectal Cancer Risk: A Review of 17 Years of Evidence (2000–2016). Curr. Color. Cancer Rep. 2017, 13, 440–454. [Google Scholar] [CrossRef]
- Liu, L.; Nishihara, R.; Qian, Z.R.; Tabung, F.K.; Nevo, D.; Zhang, X.; Song, M.; Cao, Y.; Mima, K.; Masugi, Y.; et al. Association Between Inflammatory Diet Pattern and Risk of Colorectal Carcinoma Subtypes Classified by Immune Responses to Tumor. Gastroenterology 2017, 153, 1517–1530.e1514. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Balasubramanian, R.; Liang, L.; Clinton, S.K.; Cespedes Feliciano, E.M.; Manson, J.E.; Van Horn, L.; Wactawski-Wende, J.; Clish, C.B.; Giovannucci, E.L.; et al. Identifying Metabolomic Profiles of Insulinemic Dietary Patterns. Metabolites 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Liang, L.; Huang, T.; Balasubramanian, R.; Zhao, Y.; Chandler, P.D.; Manson, J.E.; Cespedes Feliciano, E.M.; Hayden, K.M.; Van Horn, L.; et al. Identifying metabolomic profiles of inflammatory diets in postmenopausal women. Clin. Nutr. 2020, 39, 1478–1490. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Mukherjee, S.; Malick, C.; Kundu, R. Trigonelline inhibits Nrf2 via EGFR signalling pathway and augments efficacy of Cisplatin and Etoposide in NSCLC cells. Toxicol. In Vitro 2020, 70, 105038. [Google Scholar] [CrossRef]
- Chandler, P.D.; Song, Y.; Lin, J.; Zhang, S.; Sesso, H.D.; Mora, S.; Giovannucci, E.L.; Rexrode, K.E.; Moorthy, M.V.; Li, C.; et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am. J. Clin. Nutr. 2016, 103, 1397–1407. [Google Scholar] [CrossRef]
- The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control. Clin. Trials 1998, 19, 61–109. [Google Scholar] [CrossRef]
- Paynter, N.P.; Balasubramanian, R.; Giulianini, F.; Wang, D.D.; Tinker, L.F.; Gopal, S.; Deik, A.A.; Bullock, K.; Pierce, K.A.; Scott, J.; et al. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation 2018, 137, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Kristal, A.R.; Tinker, L.F.; Carter, R.A.; Bolton, M.P.; Agurs-Collins, T. Measurement Characteristics of the Women’s Health Initiative Food Frequency Questionnaire. Ann. Epidemiol. 1999, 9, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F.; Sievert, Y.A.; Buzzard, I.M. Sources of data for developing and maintaining a nutrient database. J. Am. Diet. Assoc. 1988, 88, 1268–1271. [Google Scholar] [CrossRef]
- Jin, Q.; Shi, N.; Aroke, D.; Lee, D.H.; Joseph, J.J.; Donneyong, M.; Conwell, D.L.; Hart, P.A.; Zhang, X.; Clinton, S.K.; et al. Insulinemic and Inflammatory Dietary Patterns Show Enhanced Predictive Potential for Type 2 Diabetes Risk in Postmenopausal Women. Diabetes Care 2021, 44, 707–714. [Google Scholar] [CrossRef]
- Healthy Eating Index. U.S. Department of Agriculture Food and Nutrition Service. Available online: https://www.fns.usda.gov/healthy-eating-index-hei (accessed on 17 February 2023).
- Curb, J.; Mctiernan, A.; Heckbert, S.R.; Kooperberg, C.; Stanford, J.; Nevitt, M.; Johnson, K.C.; Proulx-Burns, L.; Pastore, L.; Criqui, M.; et al. Outcomes Ascertainment and Adjudication Methods in the Women’s Health Initiative. Ann. Epidemiol. 2003, 13, S122–S128. [Google Scholar] [CrossRef]
- Yang, J.; Loos, R.J.; Powell, J.E.; Medland, S.E.; Speliotes, E.K.; Chasman, D.I.; Rose, L.M.; Thorleifsson, G.; Steinthorsdottir, V.; Magi, R.; et al. FTO genotype is associated with phenotypic variability of body mass index. Nature 2012, 490, 267–272. [Google Scholar] [CrossRef]
- Li, J.; Guasch-Ferre, M.; Chung, W.; Ruiz-Canela, M.; Toledo, E.; Corella, D.; Bhupathiraju, S.N.; Tobias, D.K.; Tabung, F.K.; Hu, J.; et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020, 41, 2645–2656. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Tabung, F.K.; Satija, A.; Fung, T.T.; Clinton, S.K.; Giovannucci, E.L. Long-Term Change in both Dietary Insulinemic and Inflammatory Potential Is Associated with Weight Gain in Adult Women and Men. J. Nutr. 2019, 149, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Li, J.; Li, Y.; Liu, G.; Wu, K.; Bhupathiraju, S.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; et al. Dietary Inflammatory and Insulinemic Potential and Risk of Type 2 Diabetes: Results From Three Prospective U.S. Cohort Studies. Diabetes Care 2020, 43, 2675–2683. [Google Scholar] [CrossRef]
- Wan, Y.; Tabung, F.K.; Lee, D.H.; Fung, T.T.; Willett, W.C.; Giovannucci, E.L. Dietary Insulinemic Potential and Risk of Total and Cause-Specific Mortality in the Nurses’ Health Study and the Health Professionals Follow-up Study. Diabetes Care 2022, 45, 451–459. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Caan, B.; Hou, L.; Johnson, K.C.; Mossavar-Rahmani, Y.; Shivappa, N.; et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the Women’s Health Initiative. Cancer Causes Control 2015, 26, 399–408. [Google Scholar] [CrossRef]
- Vargas, A.J.; Neuhouser, M.L.; George, S.M.; Thomson, C.A.; Ho, G.Y.; Rohan, T.E.; Kato, I.; Nassir, R.; Hou, L.; Manson, J.E. Diet Quality and Colorectal Cancer Risk in the Women’s Health Initiative Observational Study. Am. J. Epidemiol. 2016, 184, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Romanos-Nanclares, A.; Willett, W.C.; Rosner, B.A.; Tamimi, R.M.; Tabung, F.K.; Holmes, M.D.; Chen, W.Y.; Eliassen, A.H. Abstract P1-09-06: Insulinemic potential of diet and risk of total and subtypes of breast cancer among US women. Cancer Res. 2022, 82, P1-09-06. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Liese, A.D.; Zhang, J.; Ma, Y.; Caan, B.; Chlebowski, R.T.; Freudenheim, J.L.; Hou, L.; Mossavar-Rahmani, Y.; et al. Association between dietary inflammatory potential and breast cancer incidence and death: Results from the Women’s Health Initiative. Br. J. Cancer 2016, 114, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Liese, A.D.; Zhang, J.; Ma, Y.; Johnson, K.C.; Lane, D.S.; Qi, L.; Snetselaar, L.; Vitolins, M.Z.; et al. Patterns of change over time and history of the inflammatory potential of diet and risk of breast cancer among postmenopausal women. Breast Cancer Res. Treat. 2016, 159, 139–149. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Shi, N.; Aroke, D.; Jin, Q.; Lee, D.H.; Hussan, H.; Zhang, X.; Manson, J.E.; LeBlanc, E.S.; Barac, A.; Arcan, C.; et al. Proinflammatory and Hyperinsulinemic Dietary Patterns Are Associated With Specific Profiles of Biomarkers Predictive of Chronic Inflammation, Glucose-Insulin Dysregulation, and Dyslipidemia in Postmenopausal Women. Front. Nutr. 2021, 8, 690428. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef]
- Dashti, S.G.; Simpson, J.A.; Viallon, V.; Karahalios, A.; Moreno-Betancur, M.; Brasky, T.; Pan, K.; Rohan, T.E.; Shadyab, A.H.; Thomson, C.A.; et al. Adiposity and breast, endometrial, and colorectal cancer risk in postmenopausal women: Quantification of the mediating effects of leptin, C-reactive protein, fasting insulin, and estradiol. Cancer Med. 2022, 11, 1145–1159. [Google Scholar] [CrossRef]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; Million Women Study, C. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef]
- Tabung, F.K.; Huang, T.; Giovannucci, E.L.; Smith-Warner, S.A.; Tworoger, S.S.; Poole, E.M. The inflammatory potential of diet and ovarian cancer risk: Results from two prospective cohort studies. Br. J. Cancer 2017, 117, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Cesar, T.B.; Oliveira, M.R.; Mesquita, C.H.; Maranhao, R.C. High cholesterol intake modifies chylomicron metabolism in normolipidemic young men. J. Nutr. 2006, 136, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.I.; Tchoumtchoua, J.; Skaltsounis, A.L.; Scorilas, A.; Halabalaki, M. Natural Alkaloids Intervening the Insulin Pathway: New Hopes for Anti-Diabetic Agents? Curr. Med. Chem. 2019, 26, 5982–6015. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, O.A.; Wittenbecher, C.; Deik, A.; Jeanfavre, S.; Avila-Pacheco, J.; Rosner, B.; Rexrode, K.M.; Clish, C.B.; Hu, F.B.; Eliassen, A.H. Intrapersonal Stability of Plasma Metabolomic Profiles over 10 Years among Women. Metabolites 2022, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Casso, D.; White, E.; Patterson, R.E.; Agurs-Collins, T.; Kooperberg, C.; Haines, P.S. Correlates of serum lycopene in older women. Nutr. Cancer 2000, 36, 163–169. [Google Scholar] [CrossRef]
- Kapala, A.; Szlendak, M.; Motacka, E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).