Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics
Abstract
Simple Summary
Abstract
1. Introduction
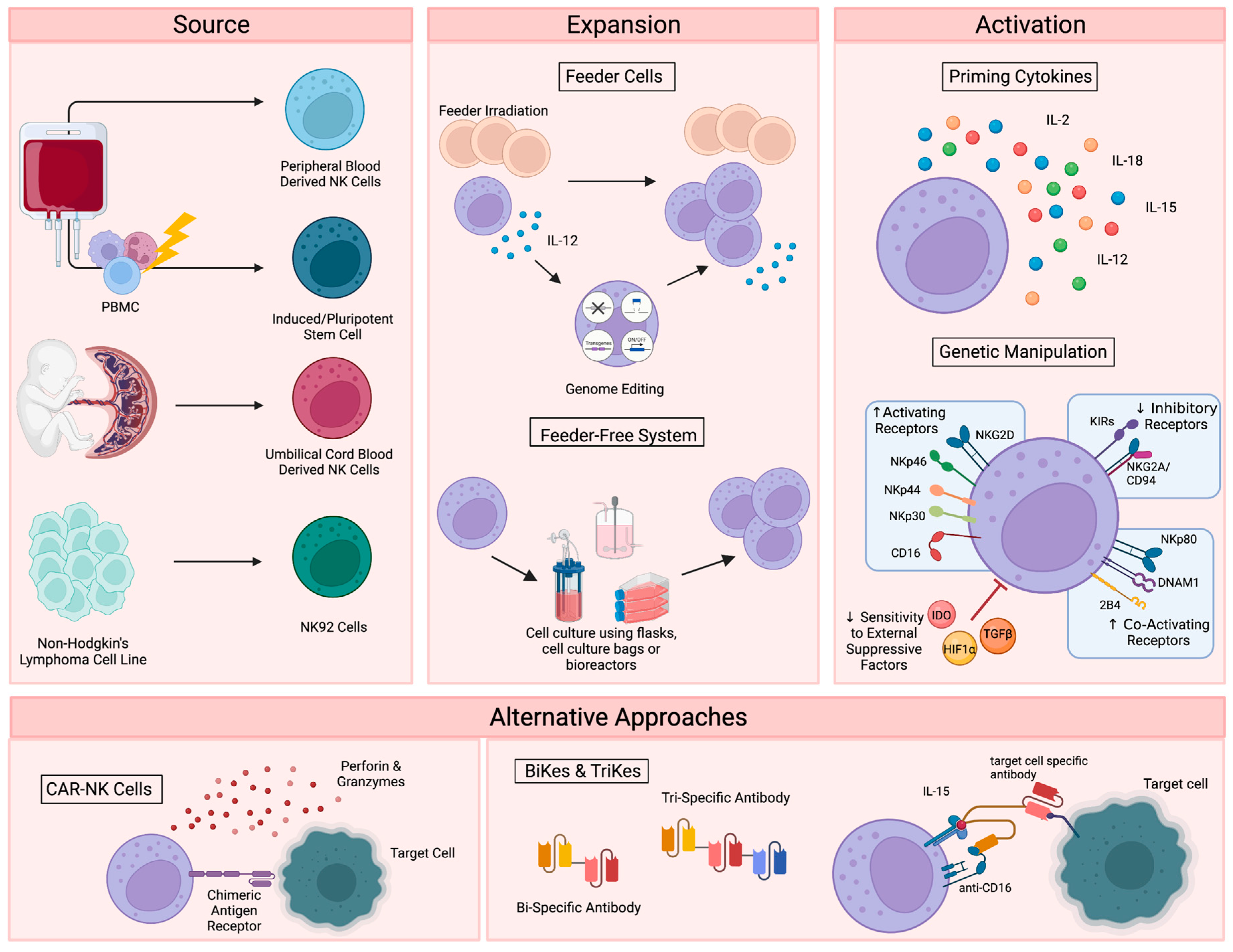
2. NK Cell Therapies
2.1. Primary NK Cell Sources
2.2. Immortalized NK Cell Lines
2.3. NK Cell Expansion and Activation
2.4. Alternative Approaches for NK Cell Activation
3. NK Cell Impairment in Cancer
NK Cell Plasticity
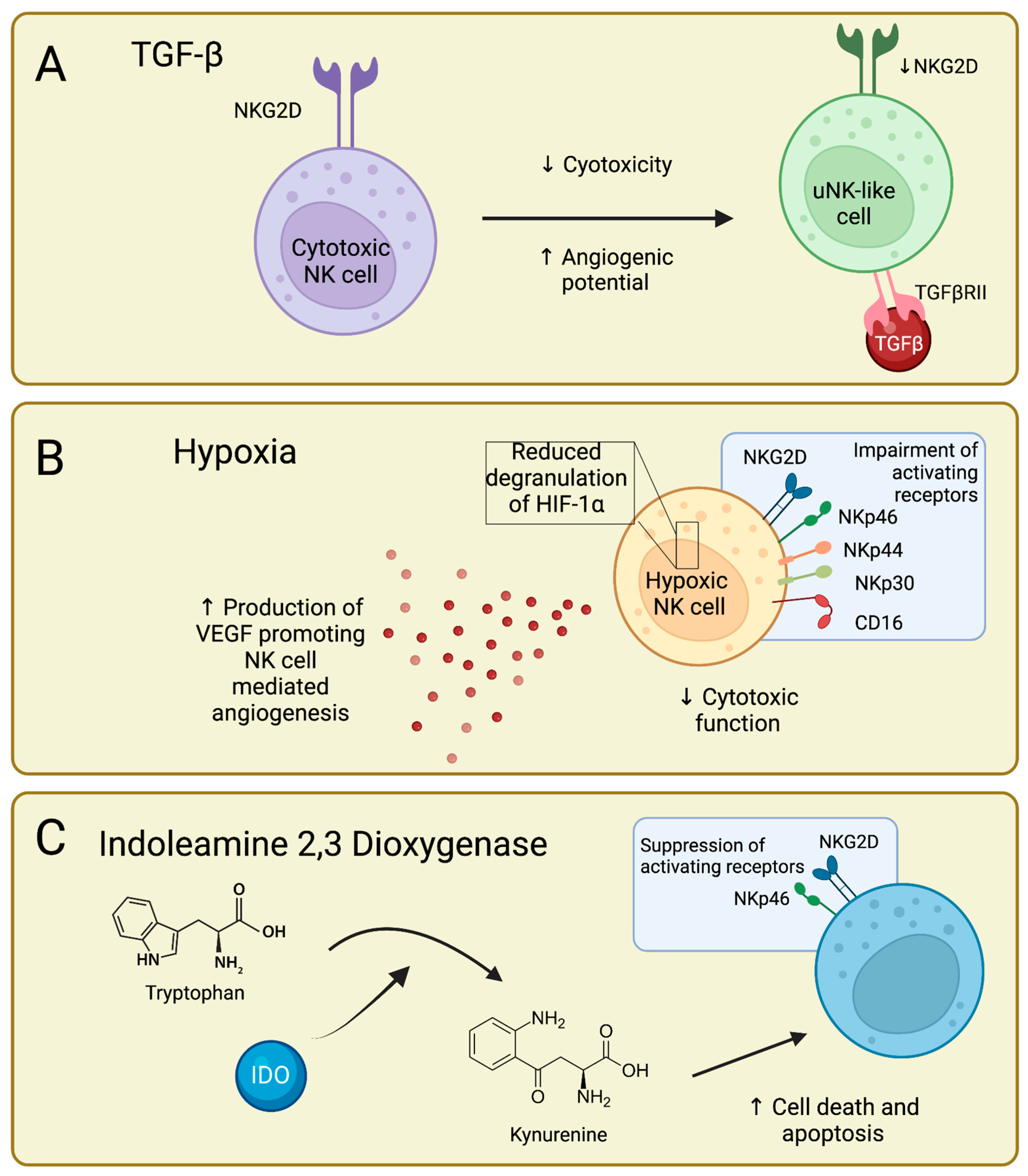
4. TGFβ-Mediated NK Cell Impairment
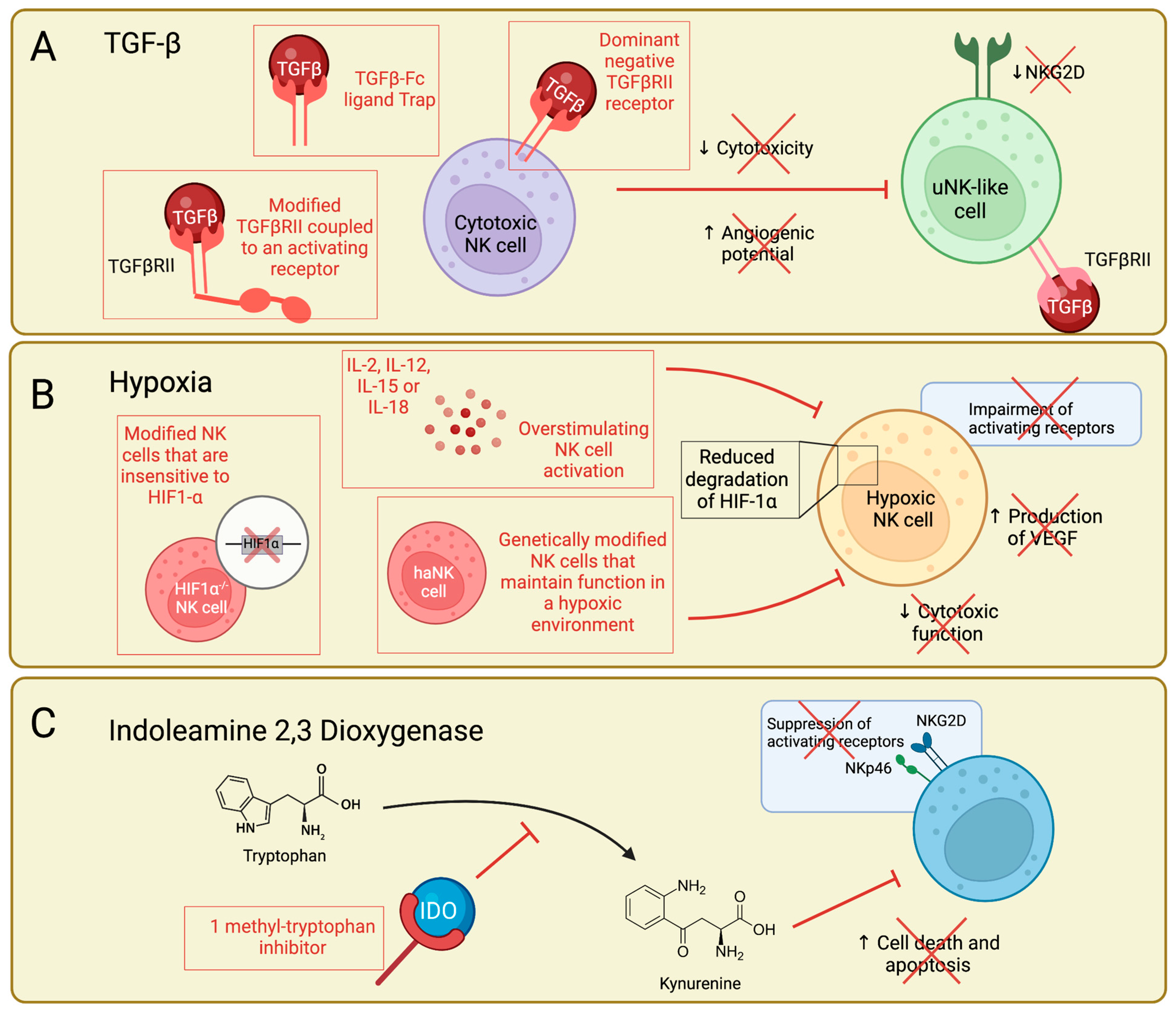
Current Work Targeting the TGFβ-NK Cell Axis
5. Hypoxia-Mediated NK Cell Impairment
Current Work Targeting Hypoxic NK Cells
6. IDO-Mediated NK Cell Impairment
Current Work Targeting the IDO-NK Cell Pathway
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Caligiuri, M.A. Human Natural Killer Cells. Blood J. Am. Soc. Hematol. 2008, 112, 461–469. [Google Scholar] [CrossRef]
- Moretta, A.; Marcenaro, E.; Parolini, S.; Ferlazzo, G.; Moretta, L. NK Cells at the Interface between Innate and Adaptive Immunity. Cell Death Differ. 2008, 15, 226–233. [Google Scholar] [CrossRef]
- Fauriat, C.; Long, E.O.; Ljunggren, H.-G.; Bryceson, Y.T. Regulation of Human NK-Cell Cytokine and Chemokine Production by Target Cell Recognition. Blood J. Am. Soc. Hematol. 2010, 115, 2167–2176. [Google Scholar] [CrossRef]
- Bluman, E.M.; Bartynski, K.J.; Avalos, B.R.; Caligiuri, M.A. Human Natural Killer Cells Produce Abundant Macrophage Inflammatory Protein-1 Alpha in Response to Monocyte-Derived Cytokines. J. Clin. Investig. 1996, 97, 2722–2727. [Google Scholar] [CrossRef]
- Ljunggren, H.-G.; Kärre, K. In Search of the ‘Missing Self’: MHC Molecules and NK Cell Recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Kärre, K. NK Cells, MHC Class I Molecules and the Missing Self. Scand. J. Immunol. 2002, 55, 221–228. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of Natural Killer Cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Ramírez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Pérez, A.; Oñate, C.; Andrés-Tovar, A.; Garzón-Tituaña, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef]
- Paul, S.; Kulkarni, N.; Shilpi; Lal, G. Intratumoral Natural Killer Cells Show Reduced Effector and Cytolytic Properties and Control the Differentiation of Effector Th1 Cells. Oncoimmunology 2016, 5, e1235106. [Google Scholar] [CrossRef]
- Chaix, J.; Tessmer, M.S.; Hoebe, K.; Fuséri, N.; Ryffel, B.; Dalod, M.; Alexopoulou, L.; Beutler, B.; Brossay, L.; Vivier, E. Cutting Edge: Priming of NK Cells by IL-18. J. Immunol. 2008, 181, 1627–1631. [Google Scholar] [CrossRef]
- Ortaldo, J.R.; Winkler-Pickett, R.; Wigginton, J.; Horner, M.; Bere, E.W.; Mason, A.T.; Bhat, N.; Cherry, J.; Sanford, M.; Hodge, D.L. Regulation of ITAM-Positive Receptors: Role of IL-12 and IL-18. Blood 2006, 107, 1468–1475. [Google Scholar] [CrossRef]
- Cai, G.; Kastelein, R.A.; Hunter, C.A. IL-10 Enhances NK Cell Proliferation, Cytotoxicity and Production of IFN-γ When Combined with IL-18. Eur. J. Immunol. 1999, 29, 2658–2665. [Google Scholar] [CrossRef]
- Billiau, A. Interferon-γ: Biology and Role in Pathogenesis. In Advances in Immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 62, pp. 61–130. ISBN 0065-2776. [Google Scholar]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. CELLULAR RESPONSES TO INTERFERON-γ. Annu. Rev. Immunol. 1997, 15, 749–795. [Google Scholar] [CrossRef]
- Hercend, T.; Farace, F.; Baume, D.; Charpentier, F.; Droz, J.-P.; Triebel, F.; Escudier, B. Immunotherapy with Lymphokine-Activated Natural Killer Cells and Recombinant Interleukin-2: A Feasibility Trial in Metastatic Renal Cell Carcinoma. J. Biol. Response Mod. 1990, 9, 546–555. [Google Scholar]
- Miller, J.S.; Tessmer-Tuck, J.; Pierson, B.A.; Weisdorf, D.; McGlave, P.; Blazar, B.R.; Katsanis, E.; Verfaillie, C.; Lebkowski, J.; Radford, J., Jr. Low Dose Subcutaneous Interleukin-2 after Autologous Transplantation Generates Sustained In Vivo Natural Killer Cell Activity. Biol. Blood Marrow Transpl. 1997, 3, 34–44. [Google Scholar]
- Burns, L.J.; Weisdorf, D.J.; DeFor, T.E.; Vesole, D.H.; Repka, T.L.; Blazar, B.R.; Burger, S.R.; Panoskaltsis-Mortari, A.; Keever-Taylor, C.A.; Zhang, M.J. IL-2-Based Immunotherapy after Autologous Transplantation for Lymphoma and Breast Cancer Induces Immune Activation and Cytokine Release: A Phase I/II Trial. Bone Marrow Transpl. 2003, 32, 177–186. [Google Scholar] [CrossRef]
- Liang, S.; Xu, K.; Niu, L.; Wang, X.; Liang, Y.; Zhang, M.; Chen, J.; Lin, M. Comparison of Autogeneic and Allogeneic Natural Killer Cells Immunotherapy on the Clinical Outcome of Recurrent Breast Cancer. Onco Targets Ther. 2017, 10, 4273. [Google Scholar] [CrossRef]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; Defor, T.E.; et al. First-in-Human Phase 1 Clinical Study of the IL-15 Superagonist Complex ALT-803 to Treat Relapse after Transplantation. Blood J. Am. Soc. Hematol. 2018, 131, 2515–2527. [Google Scholar] [CrossRef]
- Rhode, P.R.; Egan, J.O.; Xu, W.; Hong, H.; Webb, G.M.; Chen, X.; Liu, B.; Zhu, X.; Wen, J.; You, L.; et al. Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol. Res. 2016, 4, 49–60. [Google Scholar] [CrossRef]
- Koehl, U.; Brehm, C.; Huenecke, S.; Kloess, S.; Bremm, M.; Zimmermann, S.Y.; Ullrich, E.; Soerensen, J.; Quaiser, A.; Erben, S.; et al. Clinical Grade Purification and Expansion of NK Cell Products for an Optimized Manufacturing Protocol. Front. Oncol. 2013, 3, 118. [Google Scholar] [CrossRef]
- Harris, D.T.; Schumacher, M.J.; Locascio, J.; Besencon, F.J.; Olson, G.B.; DeLuca, D.; Shenker, L.; Bard, J.; Boyse, E.A. Phenotypic and Functional Immaturity of Human Umbilical Cord Blood T Lymphocytes. Proc. Natl. Acad. Sci. 1992, 89, 10006–10010. [Google Scholar] [CrossRef]
- Luevano, M.; Daryouzeh, M.; Alnabhan, R.; Querol, S.; Khakoo, S.; Madrigal, A.; Saudemont, A. The Unique Profile of Cord Blood Natural Killer Cells Balances Incomplete Maturation and Effective Killing Function upon Activation. Hum. Immunol. 2012, 73, 248–257. [Google Scholar] [CrossRef]
- Dolstra, H.; Roeven, M.W.H.; Spanholtz, J.; Hangalapura, B.N.; Tordoir, M.; Maas, F.; Leenders, M.; Bohme, F.; Kok, N.; Trilsbeek, C. Successful Transfer of Umbilical Cord Blood CD34+ Hematopoietic Stem and Progenitor-Derived NK Cells in Older Acute Myeloid Leukemia PatientsHSPC-NK Cell Adoptive Transfer in Older AML Patients. Clin. Cancer Res. 2017, 23, 4107–4118. [Google Scholar] [CrossRef]
- Spanholtz, J.; Tordoir, M.; Eissens, D.; Preijers, F.; van der Meer, A.; Joosten, I.; Schaap, N.; de Witte, T.M.; Dolstra, H. High Log-Scale Expansion of Functional Human Natural Killer Cells from Umbilical Cord Blood CD34-Positive Cells for Adoptive Cancer Immunotherapy. PLoS ONE 2010, 5, e9221. [Google Scholar] [CrossRef]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-Scale Derivation of Natural Killer Cells from Human Pluripotent Stem Cells for Cancer Therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Woan, K.V.; Kim, H.; Bjordahl, R.; Davis, Z.B.; Gaidarova, S.; Goulding, J.; Hancock, B.; Mahmood, S.; Abujarour, R.; Wang, H. Harnessing Features of Adaptive NK Cells to Generate IPSC-Derived NK Cells for Enhanced Immunotherapy. Cell Stem Cell 2021, 28, 2062–2075. [Google Scholar] [CrossRef]
- FT538 in Subjects with Advanced Hematologic Malignancies—Full Text View ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04614636. (accessed on 10 March 2023).
- Safety and Efficacy of Allogeneic NK Cell Infusions in Patients with Relapsed/Refractory AML and High Risk MDS—Full Text View ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04901416 (accessed on 10 March 2023).
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a Human Cell Line (NK-92) with Phenotypical and Functional Characteristics of Activated Natural Killer Cells. Leukemia 1994, 8, 652–658. [Google Scholar]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef]
- Tarn, Y.K.; Martinson, J.A.; Doligosa, K.; Klingernann, H.-G. Ex Vivo Expansion of the Highly Cytotoxic Human Natural Killer Cell Line NK-92 under Current Good Manufacturing Practice Conditions for Clinical Adoptive Cellular Immunotherapy. Cytotherapy 2003, 5, 259–272. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Agha, M.; Redner, R.L.; Sehgal, A.; Im, A.; Hou, J.-Z.; Farah, R.; Dorritie, K.A.; Raptis, A.; Lim, S.H.; et al. Phase 1 Clinical Trial of Adoptive Immunotherapy Using “off-the-Shelf” Activated Natural Killer Cells in Patients with Refractory and Relapsed Acute Myeloid Leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of Patients with Advanced Cancer with the Natural Killer Cell Line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Maurice Morillon, Y.I.; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Yok Tsang, K.; Campbell, K.S.; et al. An NK Cell Line (HaNK) Expressing High Levels of Granzyme and Engineered to Express the High Affinity CD16 Allele. Oncotarget 2016, 7, 86359. [Google Scholar] [CrossRef] [PubMed]
- Fabian, K.P.; Hodge, J.W. The Emerging Role of Off-the-Shelf Engineered Natural Killer Cells in Targeted Cancer Immunotherapy. Mol. Ther. Oncolytics 2021, 23, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.S.; Lee, J.K. Development of NK Cell Expansion Methods Using Feeder Cells from Human Myelogenous Leukemia Cell Line. Blood Res. 2014, 49, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.; Phan, M.T.T.; Chun, S.; Yu, H.B.; Kim, J.; Kim, S.; Lee, J.; Ali, A.K.; Lee, S.H.; Kim, S.K.; et al. Expansion of Human NK Cells Using K562 Cells Expressing OX40 Ligand and Short Exposure to IL-21. Front. Immunol. 2019, 10, 879. [Google Scholar] [CrossRef]
- Lim, S.A.; Kim, T.J.; Lee, J.E.; Sonn, C.H.; Kim, K.; Kim, J.; Choi, J.G.; Choi, I.K.; Yun, C.O.; Kim, J.H.; et al. Ex Vivo Expansion of Highly Cytotoxic Human NK Cells by Cocultivation with Irradiated Tumor Cells for Adoptive Immunotherapy. Cancer Res. 2013, 73, 2598–2607. [Google Scholar] [CrossRef]
- Gurney, M.; Kundu, S.; Pandey, S.; O’Dwyer, M. Feeder Cells at the Interface of Natural Killer Cell Activation, Expansion and Gene Editing. Front. Immunol. 2022, 13, 802906. [Google Scholar] [CrossRef]
- Sutlu, T.; Stellan, B.; Gilljam, M.; Quezada, H.C.; Nahi, H.; Gahrton, G.; Alici, E. Clinical-Grade, Large-Scale, Feeder-Free Expansion of Highly Active Human Natural Killer Cells for Adoptive Immunotherapy Using an Automated Bioreactor. Cytotherapy 2010, 12, 1044–1055. [Google Scholar] [CrossRef]
- Wagner, J.; Pfannenstiel, V.; Waldmann, A.; Bergs, J.W.J.; Brill, B.; Huenecke, S.; Klingebiel, T.; Rödel, F.; Buchholz, C.J.; Wels, W.S. A Two-Phase Expansion Protocol Combining Interleukin (IL)-15 and IL-21 Improves Natural Killer Cell Proliferation and Cytotoxicity against Rhabdomyosarcoma. Front. Immunol. 2017, 8, 676. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Liu, C.; Ma, J.; Ma, P.; Cui, H.; Tao, H.; Gao, B. Expansion of NK Cells from PBMCs Using Immobilized 4-1BBL and Interleukin-21. Int. J. Oncol. 2015, 47, 335–342. [Google Scholar] [CrossRef]
- Gras Navarro, A.; Kmiecik, J.; Leiss, L.; Zelkowski, M.; Engelsen, A.; Bruserud, Ø.; Zimmer, J.; Enger, P.Ø.; Chekenya, M. NK Cells with KIR2DS2 Immunogenotype Have a Functional Activation Advantage to Efficiently Kill Glioblastoma and Prolong Animal Survival. J. Immunol. 2014, 193, 6192–6206. [Google Scholar] [CrossRef] [PubMed]
- Oyer, J.L.; Igarashi, R.Y.; Kulikowski, A.R.; Colosimo, D.A.; Solh, M.M.; Zakari, A.; Khaled, Y.A.; Altomare, D.A.; Copik, A.J. Generation of Highly Cytotoxic Natural Killer Cells for Treatment of Acute Myelogenous Leukemia Using a Feeder-Free, Particle-Based Approach. Biol. Blood Marrow Transplant. 2015, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.D.L.; Zale, N.E.; Frary, E.D.; Lomakin, J.A. Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate. Front. Immunol. 2022, 13, 803380. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-S.; Lai, M.-C.; Shih, H.-A.; Lin, S. A Robust Platform for Expansion and Genome Editing of Primary Human Natural Killer Cells. J. Exp. Med. 2021, 218, e20201529. [Google Scholar] [CrossRef]
- Felices, M.; Lenvik, A.J.; McElmurry, R.; Chu, S.; Hinderlie, P.; Bendzick, L.; Geller, M.A.; Tolar, J.; Blazar, B.R.; Miller, J.S. Continuous Treatment with IL-15 Exhausts Human NK Cells via a Metabolic Defect. JCI. Insight. 2018, 3, e96219. [Google Scholar] [CrossRef]
- Suen, W.C.-W.; Lee, W.Y.-W.; Leung, K.-T.; Pan, X.-H.; Li, G. Natural Killer Cell-Based Cancer Immunotherapy: A Review on 10 Years Completed Clinical Trials. Cancer Investig. 2018, 36, 431–457. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Ni, J.; Miller, M.; Stojanovic, A.; Garbi, N.; Cerwenka, A. Sustained Effector Function of IL-12/15/18–Preactivated NK Cells against Established Tumors. J. Exp. Med. 2012, 209, 2351–2365. [Google Scholar] [CrossRef]
- Lehmann, C.; Zeis, M.; Uharek, L. Activation of Natural Killer Cells with Interleukin 2 (IL-2) and IL-12 Increases Perforin Binding and Subsequent Lysis of Tumour Cells. Br. J. Haematol. 2001, 114, 660–665. [Google Scholar] [CrossRef]
- Bhat, R.; Watzl, C. Serial Killing of Tumor Cells by Human Natural Killer Cells—Enhancement by Therapeutic Antibodies. PLoS ONE 2007, 2, e326. [Google Scholar] [CrossRef]
- Sarkar, S.; Germeraad, W.T.V.; Rouschop, K.M.A.; Steeghs, E.M.P.; van Gelder, M.; Bos, G.M.J.; Wieten, L. Hypoxia Induced Impairment of NK Cell Cytotoxicity against Multiple Myeloma Can Be Overcome by IL-2 Activation of the NK Cells. PLoS ONE 2013, 8, e64835. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Lee, A.J.; Nham, T.; Lusty, E.; Larché, M.J.; Lee, D.A.; Ashkar, A.A. Combined Stimulation with Interleukin-18 and Interleukin-12 Potently Induces Interleukin-8 Production by Natural Killer Cells. J. Innate Immun. 2017, 9, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Urlaub, D.; Höfer, K.; Müller, M.-L.; Watzl, C. LFA-1 Activation in NK Cells and Their Subsets: Influence of Receptors, Maturation, and Cytokine Stimulation. J. Immunol. 2017, 198, 1944–1951. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Khawar, M.B.; Sun, H. CAR-NK Cells: From Natural Basis to Design for Kill. Front. Immunol. 2021, 12, 5157. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat4, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Albinger, N.; Hartmann, J.; Ullrich, E. Current Status and Perspective of CAR-T and CAR-NK Cell Therapy Trials in Germany. Gene Ther. 2021, 28, 513–527. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Daher, M.; Melo Garcia, L.; Li, Y.; Rezvani, K. CAR-NK Cells: The next Wave of Cellular Therapy for Cancer. Clin. Transl. Immunology. 2021, 10, e1274. [Google Scholar] [CrossRef]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric Antigen Receptor-Engineered Natural Killer Cells for Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Felices, M.; Lenvik, T.R.; Davis, Z.B.; Miller, J.S.; Vallera, D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods. Mol. Biol. 2016, 1441, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Felices, M.; McElmurry, R.; McCullar, V.; Zhou, X.; Schmohl, J.U.; Zhang, B.; Lenvik, A.J.; Panoskaltsis-Mortari, A.; Verneris, M.R.; et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, in Vivo Expansion, and En-hanced Function. Clin. Cancer. Res. 2016, 22, 3440–3450. [Google Scholar] [CrossRef]
- Arvindam, U.S.; van Hauten, P.M.M.; Schirm, D.; Schaap, N.; Hobo, W.; Blazar, B.R.; Vallera, D.A.; Dolstra, H.; Felices, M.; Miller, J.S. A Trispecific Killer Engager Molecule against CLEC12A Effectively Induces NK-Cell Mediated Killing of AML Cells. Leukemia 2021, 35, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- GTB-3550 Tri-Specific Killer Engager (TriKE ®) for High Risk Hematologic Malignancies—Full Text View Clini-calTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03214666 (accessed on 10 March 2023).
- Williams, S.M.; Sumstad, D.; Kadidlo, D.; Curtsinger, J.; Luo, X.; Miller, J.S.; McKenna Jr, D.H. Clinical-scale Production of CGMP Compliant CD3/CD19 Cell-depleted NK Cells in the Evolution of NK Cell Immunotherapy at a Single Institution. Transfusion 2018, 58, 1458–1467. [Google Scholar] [CrossRef]
- Passweg, J.R.; Tichelli, A.; Meyer-Monard, S.; Heim, D.; Stern, M.; Kühne, T.; Favre, G.; Gratwohl, A. Purified Donor NK-Lymphocyte Infusion to Consolidate Engraftment after Haploidentical Stem Cell Transplantation. Leukemia 2004, 18, 1835–1838. [Google Scholar] [CrossRef]
- Shankar, K.; Capitini, C.M.; Saha, K. Genome Engineering of Induced Pluripotent Stem Cells to Manufacture Natural Killer Cell Therapies. Stem Cell Res. Ther. 2020, 11, 234. [Google Scholar] [CrossRef]
- Yoon, S.R.; Lee, Y.S.; Yang, S.H.; Ahn, K.H.; Lee, J.-H.; Lee, J.-H.; Kim, D.Y.; Kang, Y.A.; Jeon, M.; Seol, M. Generation of Donor Natural Killer Cells from CD34+ Progenitor Cells and Subsequent Infusion after HLA-Mismatched Allogeneic Hematopoietic Cell Transplantation: A Feasibility Study. Bone Marrow Transpl. 2010, 45, 1038–1046. [Google Scholar] [CrossRef]
- Mark, C.; Czerwinski, T.; Roessner, S.; Mainka, A.; Hörsch, F.; Heublein, L.; Winterl, A.; Sanokowski, S.; Richter, S.; Bauer, N. Cryopreservation Impairs 3-D Migration and Cytotoxicity of Natural Killer Cells. Nat. Commun. 2020, 11, 5224. [Google Scholar] [CrossRef]
- Matosevic, S. Viral and Nonviral Engineering of Natural Killer Cells as Emerging Adoptive Cancer Immunotherapies. J. Immunol. Res. 2018, 2018, 4054815. [Google Scholar] [CrossRef]
- Somanchi, S.S.; Somanchi, A.; Cooper, L.J.N.; Lee, D.A. Engineering Lymph Node Homing of Ex Vivo–Expanded Human Natural Killer Cells via Trogocytosis of the Chemokine Receptor CCR7. Blood J. Am. Soc. Hematol. 2012, 119, 5164–5172. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef] [PubMed]
- Colomar-Carando, N.; Gauthier, L.; Merli, P.; Loiacono, F.; Canevali, P.; Falco, M.; Galaverna, F.; Rossi, B.; Bosco, F.; Caratini, M.; et al. Exploiting Natural Killer Cell Engagers to Control Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. Cancer Immunol. Res. 2022, 10, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Virone-Oddos, A.; Beninga, J.; Rossi, B.; Nicolazzi, C.; Amara, C.; Blanchard-Alvarez, A.; Gourdin, N.; Courta, J.; Basset, A.; et al. Control of Acute Myeloid Leukemia by a Trifunctional NKp46-CD16a-NK Cell Engager Targeting CD123. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Vetizou, M.; Blanchard Alvarez, A.; Vagne, C.; Habif, G.; Batista, L.; Baron, W.; Belaïd, N.; Girard-Madoux, M.; et al. Antitumor Immunity Induced by Antibody-Based Natural Killer Cell Engager Therapeutics Armed with Not-Alpha IL-2 Variant. Cell Rep. Med. 2022, 3, 100783. [Google Scholar] [CrossRef]
- Chretien, A.-S.; Devillier, R.; Fauriat, C.; Orlanducci, F.; Harbi, S.; Le Roy, A.; Rey, J.; Bouvier Borg, G.; Gautherot, E.; Hamel, J.-F. NKp46 Expression on NK Cells as a Prognostic and Predictive Biomarker for Response to Allo-SCT in Patients with AML. Oncoimmunology 2017, 6, e1307491. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S. Cytokine-Induced Memory-like Natural Killer Cells Exhibit Enhanced Responses against Myeloid Leukemia. Sci. Transl. Med. 2016, 8, ra123–ra357. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting Natural Killer Cells in Cancer Immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Mamessier, E.; Sylvain, A.; Thibult, M.L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human Breast Cancer Cells Enhance Self Tolerance by Promoting Evasion from NK Cell Antitumor Immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- O’Sullivan, T.; Saddawi-Konefka, R.; Gross, E.; Tran, M.; Mayfield, S.P.; Ikeda, H.; Bui, J.D. Interleukin-17D Mediates Tumor Rejection through Recruitment of Natural Killer Cells. Cell Rep. 2014, 7, 989–998. [Google Scholar] [CrossRef]
- Platonova, S.; Cherfils-Vicini, J.; Damotte, D.; Crozet, L.; Vieillard, V.; Validire, P.; André, P.; Dieu-Nosjean, M.-C.; Alifano, M.; Régnard, J.-F. Profound Coordinated Alterations of Intratumoral NK Cell Phenotype and Function in Lung Carcinoma. Cancer Res. 2011, 71, 5412–5422. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H. High NKG2A Expression Contributes to NK Cell Exhaustion and Predicts a Poor Prognosis of Patients with Liver Cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Luo, G.; Zhou, J.; Wang, X.; Hu, J.; Cui, Y.; Li, X.C.; Tan, J.; Yang, S.; Zhan, R. CD86 Is an Activation Receptor for NK Cell Cytotoxicity against Tumor Cells. PLoS ONE 2013, 8, e83913. [Google Scholar] [CrossRef]
- Sun, C.; Fu, B.; Gao, Y.; Liao, X.; Sun, R.; Tian, Z.; Wei, H. TGF-Β1 down-Regulation of NKG2D/DAP10 and 2B4/SAP Expression on Human NK Cells Contributes to HBV Persistence. PLoS Pathog. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Lakshmikanth, T.; Johansson, S.; Kärre, K.; Höglund, P. The Strength of Inhibitory Input during Education Quantitatively Tunes the Functional Responsiveness of Individual Natural Killer Cells. Blood 2009, 113, 2434–2441. [Google Scholar] [CrossRef]
- Fernandez, N.C.; Treiner, E.; Vance, R.E.; Jamieson, A.M.; Lemieux, S.; Raulet, D.H. A Subset of Natural Killer Cells Achieves Self-Tolerance without Expressing Inhibitory Receptors Specific for Self-MHC Molecules. Blood 2005, 105, 4416–4423. [Google Scholar] [CrossRef]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.J.; Yang, L.; French, A.R.; Sunwoo, J.B.; Lemieux, S.; Hansen, T.H.; et al. Licensing of Natural Killer Cells by Host Major Histocompatibility Complex Class I Molecules. Nature 2005, 436, 709–713. [Google Scholar] [CrossRef]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell. Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.M.; Rothman, P.B.; Yu, J.; et al. Tissue-Resident Natural Killer (NK) Cells Are Cell Lineages Distinct from Thymic and Conventional Splenic NK Cells. eLife 2014, 2014, e01659. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, B.; Gertz, E.M.; Schäffer, A.A.; Craig, A.; Ruf, B.; Subramanyam, V.; McVey, J.C.; Diggs, L.P.; Heinrich, S.; Rosato, U. The Tumour Microenvironment Shapes Innate Lymphoid Cells in Patients with Hepatocellular Carcinoma. Gut 2022, 71, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Verrier, T.; Satoh-Takayama, N.; Serafini, N.; Marie, S.; di Santo, J.P.; Vosshenrich, C.A.J. Phenotypic and Functional Plasticity of Murine Intestinal NKp46+ Group 3 Innate Lymphoid Cells. J. Immunol. 2016, 196, 4731–4738. [Google Scholar] [CrossRef]
- Cella, M.; Gamini, R.; Sécca, C.; Collins, P.L.; Zhao, S.; Peng, V.; Robinette, M.L.; Schettini, J.; Zaitsev, K.; Gordon, W. Subsets of ILC3− ILC1-like Cells Generate a Diversity Spectrum of Innate Lymphoid Cells in Human Mucosal Tissues. Nat. Immunol. 2019, 20, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Bernink, J.H.; Krabbendam, L.; Germar, K.; de Jong, E.; Gronke, K.; Kofoed-Nielsen, M.; Munneke, J.M.; Hazenberg, M.D.; Villaudy, J.; Buskens, C.J. Interleukin-12 and-23 Control Plasticity of CD127+ Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 2015, 43, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.N.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef]
- Daussy, C.; Faure, F.; Mayol, K.; Viel, S.; Gasteiger, G.; Charrier, E.; Bienvenu, J.; Henry, T.; Debien, E.; Hasan, U.A.; et al. T-Bet and Eomes Instruct the Development of Two Distinct Natural Killer Cell Lineages in the Liver and in the Bone Marrow. J. Exp. Med. 2014, 211, 563–577. [Google Scholar] [CrossRef]
- Gao, F.; Chambon, P.; Tellides, G.; Kong, W.; Zhang, X.; Li, W. Disruption of TGF-β Signaling in Smooth Muscle Cell Prevents Flow-Induced Vascular Remodeling. Biochem. Biophys. Res. Commun. 2014, 454, 245–250. [Google Scholar] [CrossRef]
- Cortez, V.S.; Ulland, T.K.; Cervantes-Barragan, L.; Bando, J.K.; Robinette, M.L.; Wang, Q.; White, A.J.; Gilfillan, S.; Cella, M.; Colonna, M. SMAD4 Impedes the Conversion of NK Cells into ILC1-like Cells by Curtailing Non-Canonical TGF-β Signaling. Nat. Immunol. 2017, 18, 995–1003. [Google Scholar] [CrossRef]
- Hawke, L.G.; Mitchell, B.Z.; Ormiston, M.L. TGF-β and IL-15 Synergize through MAPK Pathways to Drive the Conversion of Human NK Cells to an Innate Lymphoid Cell 1–like Phenotype. J. Immunol. 2020, 204, 3171–3181. [Google Scholar] [CrossRef]
- Lee, J.-C.; Lee, K.-M.; Kim, D.-W.; Heo, D.S. Elevated TGF-Β1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J. Immunol. 2004, 172, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.A.; Han, S.J.; Barry, J.J.; Ahn, B.J.; Lanier, L.L.; Parsa, A.T. TGF-β Downregulates the Activating Receptor NKG2D on NK Cells and CD8+ T Cells in Glioma Patients. Neuro Oncol. 2010, 12, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-Expanded Myeloid-Derived Suppressor Cells Induce Anergy of NK Cells through Membrane-Bound TGF-1 1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Jun, E.; Song, A.Y.; Choi, J.W.; Lee, H.H.; Kim, M.Y.; Ko, D.H.; Kang, H.J.; Kim, S.W.; Bryceson, Y.; Kim, S.C.; et al. Progressive Impairment of NK Cell Cytotoxic Degranulation Is Associated with TGF-Β1 Deregulation and Disease Progression in Pancreatic Cancer. Front. Immunol. 2019, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Chiesa, M.D.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming Growth Factor 1 Inhibits Expression of NKp30 and NKG2D Receptors: Consequences for the NK-Mediated Killing of Dendritic Cells. Natl. Inst. Health 2003, 100, 4120–4125. [Google Scholar] [CrossRef]
- Viel, S.; Marçais, A.; Souza-Fonseca Guimaraes, F.; Loftus, R.; Rabilloud, J.; Grau, M.; Degouve, S.; Djebali, S.; Sanlaville, A.; Charrier, E.; et al. TGF-b Inhibits the Activation and Functions of NK Cells by Repressing the MTOR Pathway. Sci. Signal. 2016, 9, ra19. [Google Scholar] [CrossRef]
- Xu, L.; Ma, Q.; Zhu, J.; Li, J.; Xue, B.; Gao, J.; Sun, C.; Zang, Y.; Zhou, Y.; Yang, D. Combined Inhibition of JAK1, 2/Stat3-PD-L1 Signaling Pathway Suppresses the Immune Escape of Castration-resistant Prostate Cancer to NK Cells in Hypoxia. Mol. Med. Rep. 2018, 17, 8111–8120. [Google Scholar]
- Ashkar, A.A.; Di Santo, J.P.; Croy, B.A. Interferon Contributes to Initiation of Uterine Vascular Modification, Decidual Integrity, and Uterine Natural Killer Cell Maturation during Normal Murine Pregnancy. J. Exp. Med. 2000, 192, 259–269. [Google Scholar] [CrossRef]
- Montaldo, E.; Vacca, P.; Chiossone, L.; Croxatto, D.; Loiacono, F.; Martini, S.; Ferrero, S.; Walzer, T.; Moretta, L.; Mingari, M.C. Unique Eomes+ NK Cell Subsets Are Present in Uterus and Decidua during Early Pregnancy. Front. Immunol. 2016, 6, 646. [Google Scholar] [CrossRef]
- Parr, E.L.; Parr, M.B.; Zheng, L.M.; Young, J.D.E. Mouse Granulated Metrial Gland Cells Originate by Local Activation of Uterine Natural Killer Lymphocytes. Biol. Reprod. 1991, 44, 834–841. [Google Scholar] [CrossRef]
- Yadi, H.; Burke, S.; Madeja, Z.; Hemberger, M.; Moffett, A.; Colucci, F. Unique Receptor Repertoire in Mouse Uterine NK Cells. J. Immunol. 2008, 181, 6140–6147. [Google Scholar] [CrossRef]
- Mallidi, T.V.; Craig, L.E.; Schloemann, S.R.; Riley, J.K. Murine Endometrial and Decidual NK1.1+ Natural Killer Cells Display a B220+CD11c+ Cell Surface Phenotype. Biol. Reprod. 2009, 81, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Moffett, A.; Loke, C. Immunology of Placentation in Eutherian Mammals. Nat. Rev. Immunol. 2006, 6, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Hatta, K.; Macleod, R.J.; Gerber, S.A.; Croy, B.A. Emerging Themes in Uterine Natural Killer Cell Heterogeneity and Function. Am. J. Reprod. Immunol. 2012, 68, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.; Whitley, G.S.J.; Thilaganathan, B.; Cartwright, J.E. Decidual Natural Killer Cells Regulate Vessel Stability: Implications for Impaired Spiral Artery Remodelling. J. Reprod. Immunol. 2015, 110, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Cantoni, C.; Vitale, M.; Prato, C.; Canegallo, F.; Fenoglio, D.; Ragni, N.; Moretta, L.; Mingari, M.C. Crosstalk between Decidual NK and CD14+ Myelomonocytic Cells Results in Induction of Tregs and Immunosuppression. Proc. Natl. Acad. Sci. USA 2010, 107, 11918–11923. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J.; et al. Tumor Immunoevasion by the Conversion of Effector NK Cells into Type 1 Innate Lymphoid Cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef]
- Keskin, D.B.; Allan, D.S.J.; Rybalov, B.; Andzelm, M.M.; Stern, J.N.H.; Kopcow, H.D.; Koopman, L.A.; Strominger, J.L. TGF Promotes Conversion of CD16 Peripheral Blood NK Cells into CD16 NK Cells with Similarities to Decidual NK Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3378–3383. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of Peripheral Blood NK Cells to a Decidual NK-like Phenotype by a Cocktail of Defined Factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef]
- Hawke, L.G.; Whitford, M.K.M.; Ormiston, M.L. The Production of Pro-Angiogenic VEGF-A Isoforms by Hypoxic Human NK Cells Is Independent of Their TGF-β-Mediated Conversion to an ILC1-Like Phenotype. Front. Immunol. 2020, 11, 1903. [Google Scholar] [CrossRef]
- Bruno, A.; Focaccetti, C.; Pagani, A.; Imperatori, A.S.; Spagnoletti, M.; Rotolo, N.; Cantelmo, A.R.; Franzi, F.; Capella, C.; Ferlazzo, G.; et al. The Proangiogenic Phenotype of Natural Killer Cells in Patients with Non-Small Cell Lung Cancer. Neoplasia 2013, 15, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Trifinopoulos, J.; Sexl, V.; Putz, E.M. JAK/STAT Cytokine Signaling at the Crossroad of NK Cell Development and Maturation. Front. Immunol. 2019, 10, 2590. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Shinto, O.; Yashiro, M.; Yamazoe, S.; Iwauchi, T.; Muguruma, K.; Kubo, N.; Ohira, M.; Hirakawa, K. Transforming Growth Factor β Signaling Inhibitor, SB-431542, Induces Maturation of Dendritic Cells and Enhances Anti-Tumor Activity. Oncol. Rep. 2010, 24, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Abe, M.; Hiasa, M.; Oda, A.; Amou, H.; Kido, S.; Harada, T.; Tanaka, O.; Miki, H.; Nakamura, S. TGF-β Inhibition Restores Terminal Osteoblast Differentiation to Suppress Myeloma Growth. PLoS ONE 2010, 5, e9870. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, F.; Zheng, X.; Katakowski, M.; Buller, B.; To, S.-S.T.; Chopp, M. TGF-Β1 Promotes Motility and Invasiveness of Glioma Cells through Activation of ADAM17. Oncol. Rep. 2011, 25, 1329–1335. [Google Scholar] [PubMed]
- Biswas, S.; Guix, M.; Rinehart, C.; Dugger, T.C.; Chytil, A.; Moses, H.L.; Freeman, M.L.; Arteaga, C.L. Inhibition of TGF-β with Neutralizing Antibodies Prevents Radiation-Induced Acceleration of Metastatic Cancer Progression. J. Clin. Investig. 2007, 117, 1305–1313. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Zhou, Q.; Weiss, J.M.; Howard, O.Z.; McPherson, J.M.; Wakefield, L.M.; Oppenheim, J.J. Effective Chemoimmunotherapy with Anti-TGFβ Antibody and Cyclophosphamide in a Mouse Model of Breast Cancer. PLoS ONE 2014, 9, e85398. [Google Scholar] [CrossRef]
- Nam, J.-S.; Terabe, M.; Mamura, M.; Kang, M.-J.; Chae, H.; Stuelten, C.; Kohn, E.; Tang, B.; Sabzevari, H.; Anver, M.R. An Anti–Transforming Growth Factor β Antibody Suppresses Metastasis via Cooperative Effects on Multiple Cell Compartments. Cancer Res. 2008, 68, 3835–3843. [Google Scholar] [CrossRef]
- Otegbeye, F.; Ojo, E.; Moreton, S.; Mackowski, N.; Lee, D.A.; De Lima, M.; Wald, D.N. Inhibiting TGF-Beta Signaling Preserves the Function of Highly Activated, in Vitro Expanded Natural Killer Cells in AML and Colon Cancer Models. PLoS ONE 2018, 13, e0191358. [Google Scholar] [CrossRef]
- Anderton, M.J.; Mellor, H.R.; Bell, A.; Sadler, C.; Pass, M.; Powell, S.; Steele, S.J.; Roberts, R.R.A.; Heier, A. Induction of Heart Valve Lesions by Small-Molecule ALK5 Inhibitors. Toxicol. Pathol. 2011, 39, 916–924. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.L.; Flavell, R.A. Transforming Growth Factor-β Regulation of Immune Responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef] [PubMed]
- Burga, R.A.; Yvon, E.; Chorvinsky, E.; Fernandes, R.; Cruz, C.R.; Bollard, C.M. Engineering the TGFb Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma. Clin. Cancer Res. 2019, 25, 4400–4412. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, L.; Song, Y.; Zhang, Y.; Lin, D.; Hu, B.; Mei, Y.; Sandikin, D.; Liu, H. Augmented Anti-Tumor Activity of NK-92 Cells Expressing Chimeric Receptors of TGF-ΒR II and NKG2D. Cancer Immunol. Immunother. 2017, 66, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Shi, W.; Wang, Z.; Sun, S.; Zhang, G.; Hu, Y.; Liu, T.; Jiao, S. Blocking Transforming Growth Factor-β Signaling Pathway Augments Antitumor Effect of Adoptive NK-92 Cell Therapy. Int. Immunopharmacol. 2013, 17, 198–204. [Google Scholar] [CrossRef]
- Lim, S.A.; Kim, J.; Jeon, S.; Shin, M.H.; Kwon, J.; Kim, T.-J.; Im, K.; Han, Y.; Kwon, W.; Kim, S.-W.; et al. Defective Localization with Impaired Tumor Cytotoxicity Contributes to the Immune Escape of NK Cells in Pancreatic Cancer Patients. Front. Immunol. 2019, 10, 496. [Google Scholar] [CrossRef]
- Klokker, M.; Kharazmi, A.; Galbo, H.; Bygbjerg, I.; Pedersen, B.K. Influence of in Vivo Hypobaric Hypoxia on Function of Lymphocytes, Neutrocytes, Natural Killer Cells, and Cytokines. J. Appl. Physiol. 1993, 74, 1100–1106. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Heppner, G.H.; Juneau, P.L. Natural Killer-cell Activity under Conditions Reflective of Tumor Micro-environment. Int. J. Cancer 1991, 48, 895–899. [Google Scholar] [CrossRef]
- Balsamo, M.; Manzini, C.; Pietra, G.; Raggi, F.; Blengio, F.; Mingari, M.C.; Varesio, L.; Moretta, L.; Bosco, M.C.; Vitale, M. Hypoxia Downregulates the Expression of Activating Receptors Involved in NK-Cell-Mediated Target Cell Killing without Affecting ADCC. Eur. J. Immunol. 2013, 43, 2756–2764. [Google Scholar] [CrossRef]
- Shohet, R.V.; Garcia, J.A. Keeping the Engine Primed: HIF Factors as Key Regulators of Cardiac Metabolism and Angiogenesis during Ischemia. J. Mol. Med. 2007, 85, 1309–1315. [Google Scholar] [CrossRef]
- Halligan, D.N.; Murphy, S.J.E.; Taylor, C.T. The Hypoxia-Inducible Factor (HIF) Couples Immunity with Metabolism. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 28, pp. 469–477. [Google Scholar]
- Parodi, M.; Raggi, F.; Cangelosi, D.; Manzini, C.; Balsamo, M.; Blengio, F.; Eva, A.; Varesio, L.; Pietra, G.; Moretta, L.; et al. Hypoxia Modifies the Transcriptome of Human NK Cells, Modulates Their Immunoregulatory Profile, and Influences NK Cell Subset Migration. Front. Immunol. 2018, 9, 2358. [Google Scholar] [CrossRef]
- Velásquez, S.Y.; Killian, D.; Schulte, J.; Sticht, C.; Thiel, M.; Lindner, H.A. Short Term Hypoxia Synergizes with Interleukin 15 Priming in Driving Glycolytic Gene Transcription and Supports Human Natural Killer Cell Activities. J. Biol. Chem. 2016, 291, 12960–12977. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hu, J.; Sun, W.; Duan, X.; Chen, X. Hypoxia-Mediated Immune Evasion of Pancreatic Carcinoma Cells. Mol. Med. Rep. 2015, 11, 3666–3672. [Google Scholar] [CrossRef]
- Ou, Z.L.; Luo, Z.; Wei, W.; Liang, S.; Gao, T.L.; Lu, Y. Bin Hypoxia-Induced Shedding of MICA and HIF1A-Mediated Immune Escape of Pancreatic Cancer Cells from NK Cells: Role of Circ_0000977/MiR-153 Axis. RNA Biol. 2019, 16, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Yamanegi, K.; Ohyama, H.; Hata, M.; Nakasho, K.; Futani, H.; Okamura, H.; Terada, N. Hypoxia Downregulates the Expression of Cell Surface MICA without Increasing Soluble MICA in Osteosarcoma Cells in a HIF-1α-Dependent Manner. Int. J. Oncol. 2012, 41, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.B.; Hamilton, T.K.; Li, X.; Cotechini, T.; Miles, E.A.; Siemens, D.R.; Graham, C.H. Hypoxia Induces Escape from Innate Immunity in Cancer Cells via Increased Expression of ADAM10: Role of Nitric Oxide. Cancer Res. 2011, 71, 7433–7441. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Putz, E.M.; Grundschober, E.; Prchal-Murphy, M.; Straka, E.; Kudweis, P.; Heller, G.; Bago-Horvath, Z.; Witalisz-Siepracka, A.; Cumaraswamy, A.A.; et al. STAT5 Is a Key Regulator in NK Cells and Acts as a Molecular Switch from Tumor Surveillance to Tumor Promotion. Cancer Discov. 2016, 6, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Krzywinska, E.; Kantari-Mimoun, C.; Kerdiles, Y.; Sobecki, M.; Isagawa, T.; Gotthardt, D.; Castells, M.; Haubold, J.; Millien, C.; Viel, T.; et al. Loss of HIF-1α in Natural Killer Cells Inhibits Tumour Growth by Stimulating Non-Productive Angiogenesis. Nat. Commun. 2017, 8, 1597. [Google Scholar] [CrossRef]
- Ni, J.; Wang, X.; Stojanovic, A.; Zhang, Q.; Wincher, M.; Bühler, L.; Arnold, A.; Correia, M.P.; Winkler, M.; Koch, P.S.; et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals That Inhibition of Transcription Factor HIF-1α Unleashes NK Cell Activity. Immunity 2020, 52, 1075–1087.e8. [Google Scholar] [CrossRef]
- Sun, X.; Kanwar, J.R.; Leung, E.; Lehnert, K.; Wang, D.; Krissansen, G.W. Gene Transfer of Antisense Hypoxia Inducible Factor-1 Enhances the Therapeutic Efficacy of Cancer Immunotherapy. Gene Ther. 2001, 8, 638–645. [Google Scholar] [CrossRef]
- Solocinski, K.; Padget, M.R.; Fabian, K.P.; Wolfson, B.; Cecchi, F.; Hembrough, T.; Benz, S.C.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. Overcoming Hypoxia-Induced Functional Suppression of NK Cells. J. Immunother. Cancer 2020, 8, e000246. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-Derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Kaser, S.; Ledochowski, M.; Neurauter, G.; Mur, E.; Herold, M.; Fuchs, D. Increased Degradation of Tryptophan in Blood of Patients with Rheumatoid Arthritis. J. Rheumatol. 2003, 30, 1935–1939. [Google Scholar]
- Brown, R.R.; Ozaki, Y.; Datta, S.P.; Borden, E.C.; Sondel, P.M.; Malone, D.G. Implications of Interferon-Induced Tryptophan Catabolism in Cancer, Autoimmune Diseases and Aids BT—Kynurenine and Serotonin Pathways: Progress in Tryptophan Research; Schwarcz, R., Young, S.N., Brown, R.R., Eds.; Springer: New York, NY, USA; Boston, MA, USA, 1991; pp. 425–435. ISBN 978-1-4684-5952-4. [Google Scholar]
- Yufit, T.; Vining, V.; Brown, R.R.; Varga, J.; Wang, L. Inhibition of Type I Collagen MRNA Expression Independent of Tryptophan Depletion in Interferon-γ-Treated Human Dermal Fibroblasts. J. Investig. Dermatol. 1995, 105, 388–393. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. Ido Expression by Dendritic Cells: Tolerance and Tryptophan Catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-Dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.D.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The Tryptophan Catabolite L-Kynurenine Inhibits the Surface Expression of NKp46- and NKG2D-Activating Receptors and Regulates NK-Cell Function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Terness, P.; Bauer, T.M.; Röse, L.; Dufter, C.; Watzlik, A.; Simon, H.; Opelz, G. Inhibition of Allogeneic T Cell Proliferation by Indoleamine 2,3-Dioxygenase–Expressing Dendritic Cells: Mediation of Suppression by Tryptophan Metabolites. J. Exp. Med. 2002, 196, 447–457. [Google Scholar] [CrossRef]
- Song, H.; Park, H.; Kim, Y.-S.; Kim, K.D.; Lee, H.-K.; Cho, D.-H.; Yang, J.-W.; Hur, D.Y. L-Kynurenine-Induced Apoptosis in Human NK Cells Is Mediated by Reactive Oxygen Species. Int. Immunopharmacol. 2011, 11, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Saga, Y.; Mizukami, H.; Sato, N.; Nonaka, H.; Fujiwara, H.; Takei, Y.; Machida, S.; Takikawa, O.; Ozawa, K.; et al. Indoleamine-2,3-Dioxygenase, an Immunosuppressive Enzyme That Inhibits Natural Killer Cell Function, as a Useful Target for Ovarian Cancer Therapy. Int. J. Oncol. 2012, 40, 929–934. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Hu, X.; Jin, F.; Gao, Z.; Yin, L.; Qin, J.; Yin, F.; Li, C.; Wang, Y. IDO1 Impairs NK Cell Cytotoxicity by Decreasing NKG2D/NKG2DLs via Promoting MiR-18a. Mol. Immunol. 2018, 103, 144–155. [Google Scholar] [CrossRef]
- Komiya, T.; Huang, C.H. Updates in the Clinical Development of Epacadostat and Other Indoleamine 2,3-Dioxygenase 1 Inhibitors (IDO1) for Human Cancers. Front. Oncol. 2018, 8, 423. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of Indoleamine 2,3-Dioxygenase, an Immunoregulatory Target of the Cancer Suppression Gene Bin1, Potentiates Cancer Chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Yen, M.C.; Lin, C.C.; Lin, C.M.; Chen, Y.L.; Weng, T.Y.; Huang, T.T.; Wu, C.L.; Lai, M.D. A Combination of the Metabolic Enzyme Inhibitor APO866 and the Immune Adjuvant L-1-Methyl Tryptophan Induces Additive Antitumor Activity. Exp. Biol. Med. 2010, 235, 869–876. [Google Scholar] [CrossRef]
- Günther, J.; Däbritz, J.; Wirthgen, E. Limitations and Off-Target Effects of Tryptophan-Related IDO Inhibitors in Cancer Treatment. Front. Immunol. 2019, 10, 1801. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-Y.; Muller, A.J.; Sharma, M.D.; DuHadaway, J.; Banerjee, T.; Johnson, M.; Mellor, A.L.; Prendergast, G.C.; Munn, D.H. Inhibition of Indoleamine 2, 3-Dioxygenase in Dendritic Cells by Stereoisomers of 1-Methyl-Tryptophan Correlates with Antitumor Responses. Cancer Res. 2007, 67, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Villella, J.; Wallace, P.K.; Mhawech-Fauceglia, P.; Tario, J.D.; Andrews, C.; Matsuzaki, J.; Valmori, D.; Ayyoub, M.; Frederick, P.J.; et al. Efficacy of Levo-1-Methyl Tryptophan and Dextro-1-Methyl Tryptophan in Reversing Indoleamine-2,3-Dioxygenase-Mediated Arrest of T-Cell Proliferation in Human Epithelial Ovarian Cancer. Cancer Res. 2009, 69, 5498–5504. [Google Scholar] [CrossRef] [PubMed]
- Perera Molligoda Arachchige, A.S. NK Cell-Based Therapies for HIV Infection: Investigating Current Advances and Future Possibilities. J. Leukoc. Biol. 2022, 111, 921–931. [Google Scholar] [CrossRef]
- Zhen, A.; Kamata, M.; Rezek, V.; Rick, J.; Levin, B.; Kasparian, S.; Chen, I.S.Y.; Yang, O.O.; Zack, J.A.; Kitchen, S.G. HIV-Specific Immunity Derived from Chimeric Antigen Receptor-Engineered Stem Cells. Mol. Ther. 2015, 23, 1358–1367. [Google Scholar] [CrossRef]
- Yang, H.L.; Zhou, W.J.; Chang, K.K.; Mei, J.; Huang, L.Q.; Wang, M.Y.; Meng, Y.; Ha, S.Y.; Li, D.J.; Li, M.Q. The Crosstalk between Endometrial Stromal Cells and Macrophages Impairs Cytotoxicity of NK Cells in Endometriosis by Secreting IL-10 and TGF-β. Reproduction 2017, 154, 815–825. [Google Scholar] [CrossRef]
- Liu, X.T.; Sun, H.T.; Zhang, Z.F.; Shi, R.X.; Liu, L.B.; Yu, J.J.; Zhou, W.J.; Gu, C.J.; Yang, S.L.; Liu, Y.K.; et al. Indoleamine 2,3-Dioxygenase Suppresses the Cytotoxicity of NK Cells in Response to Ectopic Endometrial Stromal Cells in Endometriosis. Reproduction 2018, 156, 397–404. [Google Scholar] [CrossRef]
- Ormiston, M.L.; Chang, C.; Long, L.L.; Soon, E.; Jones, D.; Machado, R.; Treacy, C.; Toshner, M.R.; Campbell, K.; Riding, A.; et al. Impaired Natural Killer Cell Phenotype and Function in Idiopathic and Heritable Pulmonary Arterial Hypertension. Circulation 2012, 126, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Rätsep, X.T.M.; Moore, S.D.; Jafri, S.; Mitchell, M.; Brady, H.J.M.; Mandelboim, O.; Southwood, M.; Morrell, N.W.; Colucci, F.; Ormiston, M.L.; et al. Spontaneous Pulmonary Hypertension in Genetic Mouse Models of Natural Killer Cell Deficiency. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2018, 315, 977–990. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Limitations | |
|---|---|---|
| Source | ||
| Peripheral Blood NK Cells | Reliable source of CD34 progenitor cells [64] | NK cells make up only ~10% of all lymphocytes in peripheral blood |
| High expression of CD16+ | Extensive purification is required to reduce contamination [21] | |
| Clinical studies have shown success with these cells after extensive enrichment and purification [69] | Isolating large amounts of PB NK cells is difficult [70,71,72] | |
| Cryopreservation has been shown to reduce cytotoxicity [71,73] | ||
| Umbilical Cord NK Cells | Greater abundance than PB NK cells (15–30% of total lymphocytes) [23] | UCB NK cells are immature |
| Fewer contaminating T cells in UCB than PB, reducing the risk of graft-versus-host disease [64] | ||
| Associated with good tolerance | May have reduced cytotoxic function [22] | |
| Minimal graft-vs-host-disease or toxicity [24] | ||
| Induced Pluripotent NK Cell | Easily genetically modified | Limited clinical success to date |
| High availability | Complex differentiation steps | |
| Ability to generate multiple doses from a single healthy donor [31,71] | Safety concerns regarding toxicity | |
| Commercial NK Cell Lines | Easy to obtain | Must undergo irradiation to prevent malignant expansion, which could limit persistence. |
| Highly cytotoxic | ||
| Easily expandable [32] | ||
| NK92 cells are the only cell line that has shown success in pre-clinical studies [31] | Efficiency of cells after expansion is variable (4–95%) [74] | |
| Expansion | ||
| Feeder Cells | Effective expansion of large numbers of NK cells [75]. | Difficult to maintain cytotoxic function after expansion [49]. |
| Feeder-Free Expansion | Large amounts of highly active NK cells have been produced | Cytotoxic function after expansion has not been well reported |
| Activation | ||
| IL-2 | Ability to restore NK cell cytotoxicity after exposure to various stressors [54]. | Systemic IL-2 leads to significant toxicity |
| Other Activating Cytokines | Less toxic than IL-2 | Thought to provide only minimal clinical benefit |
| Many combination therapies are required to provide a therapeutic benefit | ||
| Genetic Manipulation | Ability to target specific pathways of interest | Relatively newer area of study |
| Ability to avoid toxic effects associated with global therapies | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coyle, K.M.; Hawke, L.G.; Ormiston, M.L. Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics. Cancers 2023, 15, 1743. https://doi.org/10.3390/cancers15061743
Coyle KM, Hawke LG, Ormiston ML. Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics. Cancers. 2023; 15(6):1743. https://doi.org/10.3390/cancers15061743
Chicago/Turabian StyleCoyle, Kassandra M., Lindsey G. Hawke, and Mark L. Ormiston. 2023. "Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics" Cancers 15, no. 6: 1743. https://doi.org/10.3390/cancers15061743
APA StyleCoyle, K. M., Hawke, L. G., & Ormiston, M. L. (2023). Addressing Natural Killer Cell Dysfunction and Plasticity in Cell-Based Cancer Therapeutics. Cancers, 15(6), 1743. https://doi.org/10.3390/cancers15061743








