Alterations in Natural Killer Cells in Colorectal Cancer Patients with Stroma AReactive Invasion Front Areas (SARIFA)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort, Trial Design, and Ethical Approval
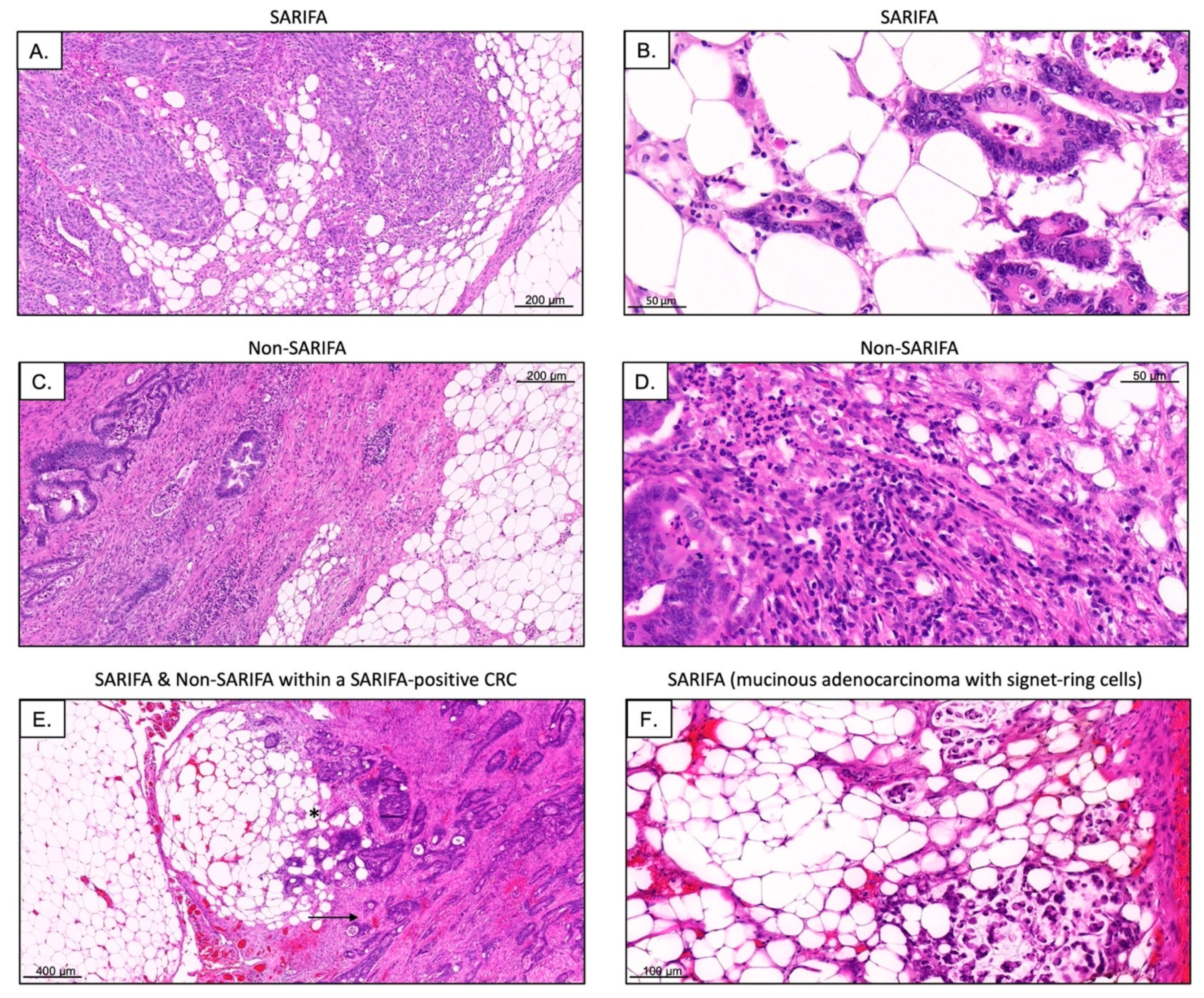
2.2. Definition of SARIFA
2.3. Analysis of Lymphocyte Subsets via Flow Cytometry
2.4. Immunohistochemistry
2.5. dMMR/MSI Testing
2.6. Next-Generation Sequencing
- Analysis of mutations in the following genes: AKT1, ALK, AR, BRAF, CCND1, CDK4, CDK6, CTNNB1, DDR2, EGFR, ERBB2, ERBB3, ERBB4, ESR1, FGFR1, FGFR2, FGFR3, FGFR4, GNA11, GNAQ, HRAS, IDH1, IDH2, JAK1, JAK2, JAK3, KIT, KRAS, MAP2K1, MAP2K2, MET, MTOR, MYC, MYCN, NRAS, PDGFRA, PIK3CA, RAF1, RET, ROS1, and SMO.
- Analysis of fusions in the following genes: ABL1, ALK, AKT3, AXL, BRAF, EGFR, ERBB2, ERG, ETV1, ETV4, ETV5, FGFR1, FGFR2, FGFR3, MET, NTRK1, NTRK2, NTRK3, PDGFRA, PPARG, RAF1, RET, and ROS1.
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics and NGS Analysis
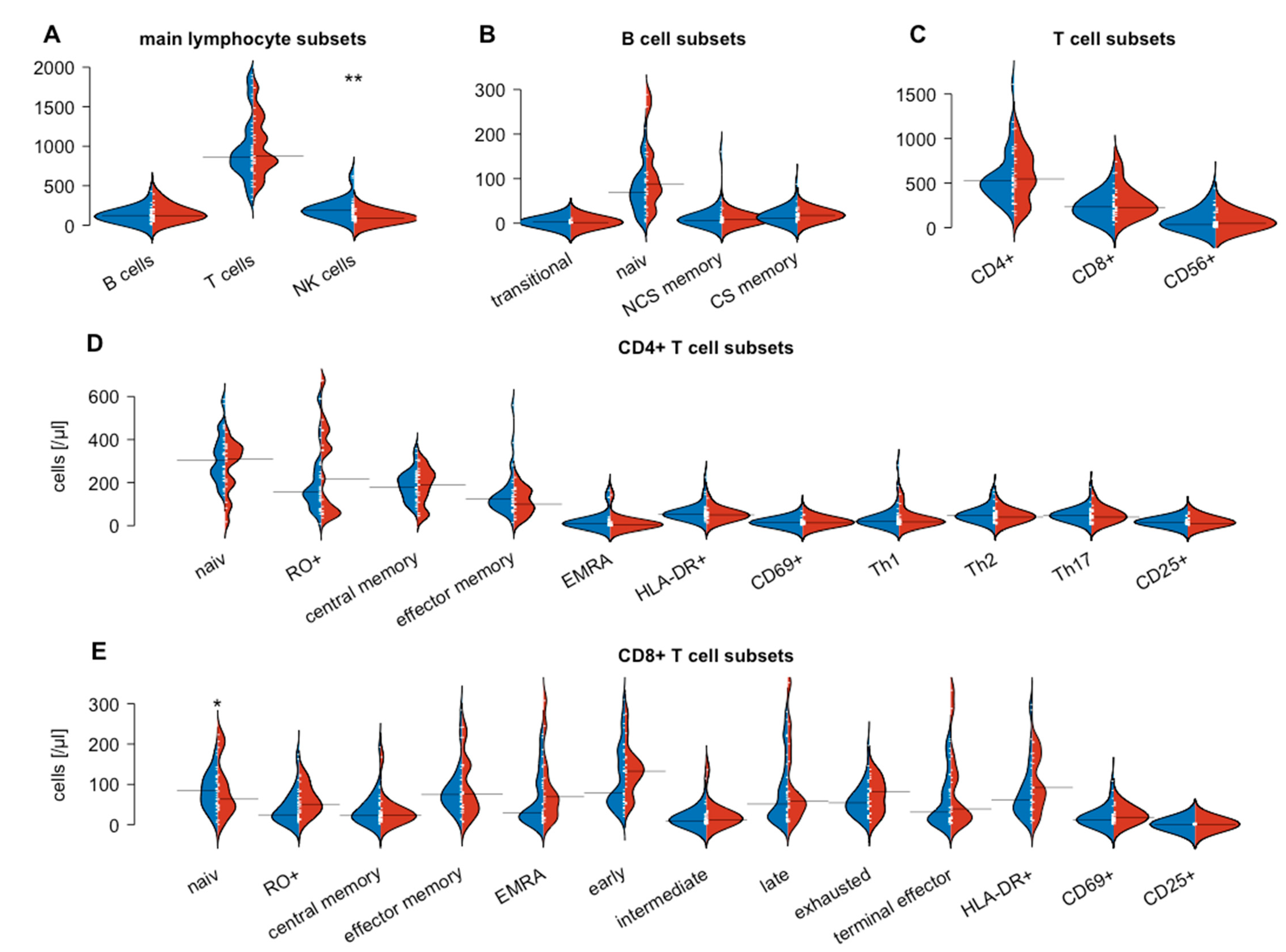
3.2. Flow Cytometry-Based Analysis of Peripheral Blood Lymphocytes
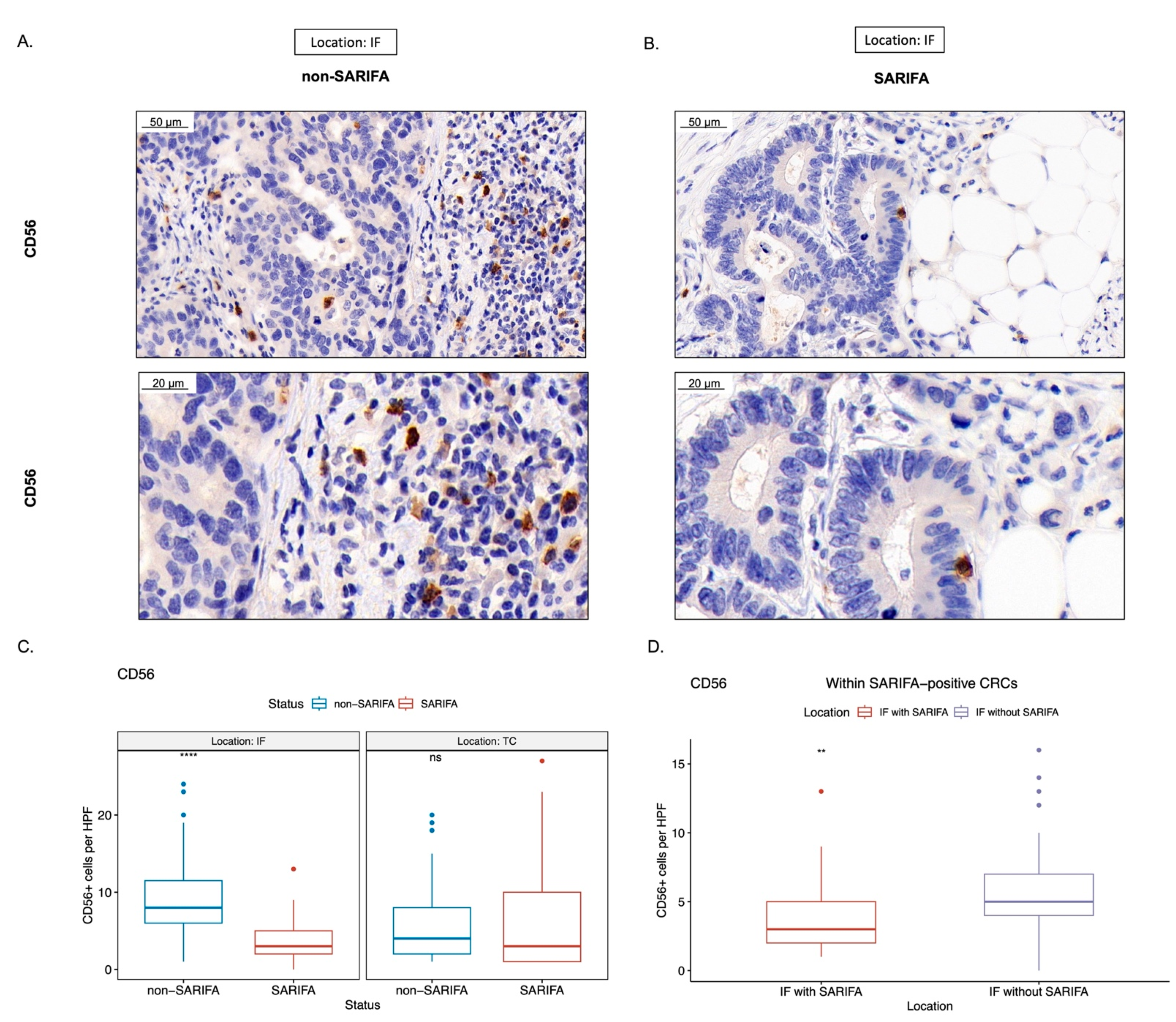
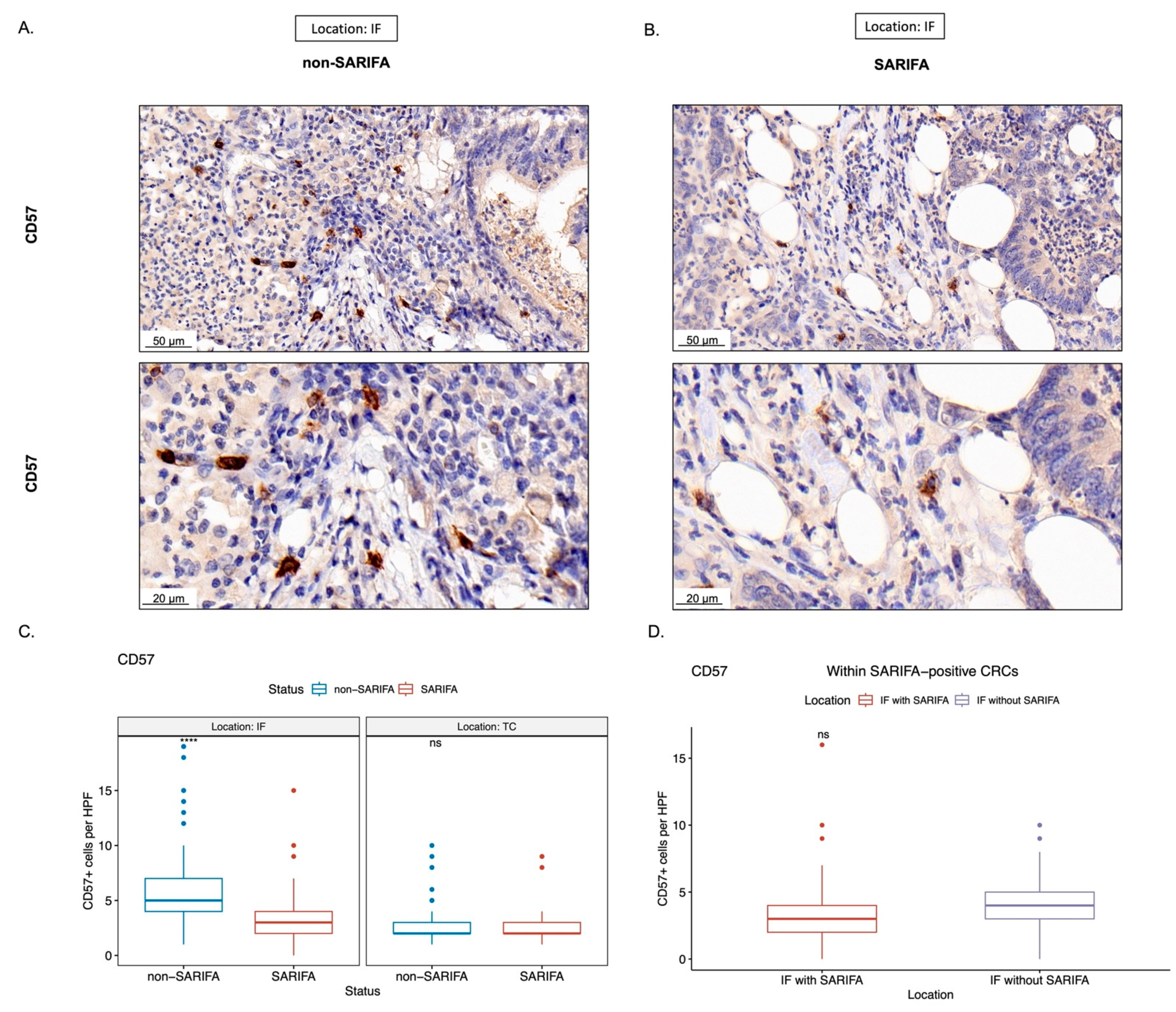
3.3. Immunohistochemical Analysis of Tumor-Infiltrating as well as CD56+ and CD57+ Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer. J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedzwiedzka, E.; Arlukowicz, T.; Przybylowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The Immune Contexture and Immunoscore in Cancer Prognosis and Therapeutic Efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International Validation of the Consensus Immunoscore for the Classification of Colon Cancer: A Prognostic and Accuracy Study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The Immune Contexture in Cancer Prognosis and Treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, C.H.; Jeanmougin, M.; Moosavi, S.H.; Svindland, A.; Bruun, J.; Nesbakken, A.; Sveen, A.; Lothe, R.A. Spatial Analysis and CD25-Expression Identify Regulatory T Cells as Predictors of a Poor Prognosis in Colorectal Cancer. Mod. Pathol. 2022, 35, 1236–1246. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Nakao, S.; Matsutani, S.; Hirakawa, K.; Ohira, M. The Peripheral Monocyte Count is Associated with the Density of Tumor-Associated Macrophages in the Tumor Microenvironment of Colorectal Cancer: A Retrospective Study. BMC Cancer 2017, 17, 404. [Google Scholar] [CrossRef]
- Hamm, A.; Prenen, H.; Van Delm, W.; Di Matteo, M.; Wenes, M.; Delamarre, E.; Schmidt, T.; Weitz, J.; Sarmiento, R.; Dezi, A.; et al. Tumour-Educated Circulating Monocytes are Powerful Candidate Biomarkers for Diagnosis and Disease Follow-Up of Colorectal Cancer. Gut 2016, 65, 990–1000. [Google Scholar] [CrossRef]
- Cavalleri, T.; Greco, L.; Rubbino, F.; Hamada, T.; Quaranta, M.; Grizzi, F.; Sauta, E.; Craviotto, V.; Bossi, P.; Vetrano, S.; et al. Tumor-Associated Macrophages and Risk of Recurrence in Stage III Colorectal Cancer. J. Pathol. Clin. Res. 2022, 8, 307–312. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Xu, G.; Jiang, L.; Ye, C.; Qin, G.; Luo, Z.; Mo, Y.; Chen, J. The Ratio of CD86+/CD163+ Macrophages Predicts Postoperative Recurrence in Stage II-III Colorectal Cancer. Front. Immunol. 2021, 12, 724429. [Google Scholar] [CrossRef]
- Waidhauser, J.; Nerlinger, P.; Arndt, T.T.; Schiele, S.; Sommer, F.; Wolf, S.; Lohr, P.; Eser, S.; Muller, G.; Claus, R.; et al. Alterations of Circulating Lymphocyte Subsets in Patients with Colorectal Carcinoma. Cancer Immunol. Immunother. 2022, 71, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Spacek, J.; Vocka, M.; Netikova, I.; Skalova, H.; Dundr, P.; Konopasek, B.; Zavadova, E.; Lubos, P. Immunological Examination of Peripheral Blood in Patients with Colorectal Cancer Compared to Healthy Controls. Immunol. Investig. 2018, 47, 643–653. [Google Scholar] [CrossRef]
- Jakubowska, K.; Koda, M.; Grudzinska, M.; Kanczuga-Koda, L.; Famulski, W. Monocyte-to-Lymphocyte Ratio as a Prognostic Factor in Peripheral Whole Blood Samples of Colorectal Cancer Patients. World J. Gastroenterol. 2020, 26, 4639–4655. [Google Scholar] [CrossRef] [PubMed]
- Waidhauser, J.; Nerlinger, P.; Sommer, F.; Wolf, S.; Eser, S.; Lohr, P.; Rank, A.; Markl, B. Circulating Lymphocytes Reflect the Local Immune Response in Patients with Colorectal Carcinoma. Diagnostics 2022, 12, 1408. [Google Scholar] [CrossRef]
- Martin, B.; Grosser, B.; Kempkens, L.; Miller, S.; Bauer, S.; Dhillon, C.; Banner, B.M.; Brendel, E.M.; Sipos, E.; Vlasenko, D.; et al. Stroma AReactive Invasion Front Areas (SARIFA)-A New Easily to Determine Biomarker in Colon Cancer-Results of a Retrospective Study. Cancers 2021, 13, 4880. [Google Scholar] [CrossRef]
- Grosser, B.; Gluckstein, M.I.; Dhillon, C.; Schiele, S.; Dintner, S.; VanSchoiack, A.; Kroeppler, D.; Martin, B.; Probst, A.; Vlasenko, D.; et al. Stroma AReactive Invasion Front Areas (SARIFA)-a New Prognostic Biomarker in Gastric Cancer Related to Tumor-Promoting Adipocytes. J. Pathol. 2022, 256, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, S.; Guillaumond, F. Lipids in Cancer: A Global View of the Contribution of Lipid Pathways to Metastatic Formation and Treatment Resistance. Oncogenesis 2022, 11, 46–48. [Google Scholar] [CrossRef]
- Wulczyn, E.; Steiner, D.F.; Moran, M.; Plass, M.; Reihs, R.; Tan, F.; Flament-Auvigne, I.; Brown, T.; Regitnig, P.; Chen, P.C.; et al. Interpretable Survival Prediction for Colorectal Cancer using Deep Learning. NPJ Digit. Med. 2021, 4, 71–72. [Google Scholar] [CrossRef]
- Foersch, S.; Glasner, C.; Woerl, A.; Eckstein, M.; Wagner, D.; Schulz, S.; Kellers, F.; Fernandez, A.; Tserea, K.; Kloth, M.; et al. Multistain Deep Learning for Prediction of Prognosis and Therapy Response in Colorectal Cancer. Nat. Med. 2023. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Flejou, J.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for Reporting Tumor Budding in Colorectal Cancer Based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Lord, A.; Brown, G.; Abulafi, M.; Bateman, A.; Frankel, W.; Goldin, R.; Gopal, P.; Kirsch, R.; Loughrey, M.B.; Markl, B.; et al. Histopathological Diagnosis of Tumour Deposits in Colorectal Cancer: A Delphi Consensus Study. Histopathology 2021, 79, 168–175. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xing, X.; Harris, J.W.; Zaytseva, Y.Y.; Mitov, M.I.; Napier, D.L.; Weiss, H.L.; Evers, B.M.; Gao, T. Adipocytes Activate Mitochondrial Fatty Acid Oxidation and Autophagy to Promote Tumor Growth in Colon Cancer. Cell. Death Dis. 2017, 8, e2593. [Google Scholar] [CrossRef]
- Rank, A.; Lohr, P.; Hoffmann, R.; Ebigbo, A.; Grutzner, S.; Schmid, C.; Claus, R. Sustained Cellular Immunity in Adults Recovered from Mild COVID-19. Cytom. A 2021, 99, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Waidhauser, J.; Schuh, A.; Trepel, M.; Schmalter, A.; Rank, A. Chemotherapy Markedly Reduces B Cells but Not T Cells and NK Cells in Patients with Cancer. Cancer Immunol. Immunother. 2020, 69, 147–157. [Google Scholar] [CrossRef]
- Lohr, P.; Schiele, S.; Arndt, T.T.; Grutzner, S.; Claus, R.; Rommele, C.; Muller, G.; Schmid, C.; Dennehy, K.M.; Rank, A. Impact of Age and Gender on Lymphocyte Subset Counts in Patients with COVID-19. Cytom. A 2021, 1–9. [Google Scholar] [CrossRef]
- Bojarska-Junak, A.; Hus, I.; Sieklucka, M.; Wasik-Szczepanek, E.; Mazurkiewicz, T.; Polak, P.; Dmoszynska, A.; Rolinski, J. Natural Killer-Like T CD3+/CD16+CD56+ Cells in Chronic Lymphocytic Leukemia: Intracellular Cytokine Expression and Relationship with Clinical Outcome. Oncol. Rep. 2010, 24, 803–810. [Google Scholar] [CrossRef]
- Guilmot, A.; Carlier, Y.; Truyens, C. Differential IFN-Gamma Production by Adult and Neonatal Blood CD56+ Natural Killer (NK) and NK-Like-T Cells in Response to Trypanosoma Cruzi and IL-15. Parasite Immunol. 2014, 36, 43–52. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef]
- Kared, H.; Martelli, S.; Ng, T.P.; Pender, S.L.F.; Larbi, A. CD57 in Human Natural Killer Cells and T-Lymphocytes. Cancer Immunol. Immunother. 2016, 65, 441–452. [Google Scholar] [CrossRef]
- Xu, H.; Cha, Y.J.; Clemenceau, J.R.; Choi, J.; Lee, S.H.; Kang, J.; Hwang, T.H. Spatial Analysis of Tumor-Infiltrating Lymphocytes in Histological Sections using Deep Learning Techniques Predicts Survival in Colorectal Carcinoma. J. Pathol. Clin. Res. 2022, 8, 327–339. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Vornanen, J.O.; Sajanti, S.; Bohm, J.P.; Tuomisto, A.; Makinen, M.J. An Improved Image Analysis Method for Cell Counting Lends Credibility to the Prognostic Significance of T Cells in Colorectal Cancer. Virchows Arch. 2012, 460, 455–465. [Google Scholar] [CrossRef]
- Cicek, M.S.; Lindor, N.M.; Gallinger, S.; Bapat, B.; Hopper, J.L.; Jenkins, M.A.; Young, J.; Buchanan, D.; Walsh, M.D.; Le Marchand, L.; et al. Quality Assessment and Correlation of Microsatellite Instability and Immunohistochemical Markers among Population- and Clinic-Based Colorectal Tumors Results from the Colon Cancer Family Registry. J. Mol. Diagn. 2011, 13, 271–281. [Google Scholar] [CrossRef]
- Mojtahed, A.; Schrijver, I.; Ford, J.M.; Longacre, T.A.; Pai, R.K. A Two-Antibody Mismatch Repair Protein Immunohistochemistry Screening Approach for Colorectal Carcinomas, Skin Sebaceous Tumors, and Gynecologic Tract Carcinomas. Mod. Pathol. 2011, 24, 1004–1014. [Google Scholar] [CrossRef]
- Hall, G.; Clarkson, A.; Shi, A.; Langford, E.; Leung, H.; Eckstein, R.P.; Gill, A.J. Immunohistochemistry for PMS2 and MSH6 Alone can Replace a Four Antibody Panel for Mismatch Repair Deficiency Screening in Colorectal Adenocarcinoma. Pathology 2010, 42, 409–413. [Google Scholar] [CrossRef]
- Reitsam, N.G.; Markl, B.; Dintner, S.; Waidhauser, J.; Vlasenko, D.; Grosser, B. Concurrent Loss of MLH1, PMS2 and MSH6 Immunoexpression in Digestive System Cancers Indicating a Widespread Dysregulation in DNA Repair Processes. Front. Oncol. 2022, 12, 1019798. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xie, M.; Li, K.; Li, J.; Cai, Z.; Hu, B. Prognostic Value of Peripheral Blood Natural Killer Cells in Colorectal Cancer. BMC Gastroenterol. 2020, 20, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Marechal, R.; De Schutter, J.; Nagy, N.; Demetter, P.; Lemmers, A.; Deviere, J.; Salmon, I.; Tejpar, S.; Van Laethem, J. Putative Contribution of CD56 Positive Cells in Cetuximab Treatment Efficacy in First-Line Metastatic Colorectal Cancer Patients. BMC Cancer 2010, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Alderdice, M.; Dunne, P.D.; Cole, A.J.; O’Reilly, P.G.; McArt, D.G.; Bingham, V.; Fuchs, M.; McQuaid, S.; Loughrey, M.B.; Murray, G.I.; et al. Natural Killer-Like Signature Observed Post Therapy in Locally Advanced Rectal Cancer is a Determinant of Pathological Response and Improved Survival. Mod. Pathol. 2017, 30, 1287–1298. [Google Scholar] [CrossRef]
- Chaput, N.; Svrcek, M.; Auperin, A.; Locher, C.; Drusch, F.; Malka, D.; Taieb, J.; Goere, D.; Ducreux, M.; Boige, V. Tumour-Infiltrating CD68+ and CD57+ Cells Predict Patient Outcome in Stage II-III Colorectal Cancer. Br. J. Cancer 2013, 109, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Liska, V.; Vycital, O.; Daum, O.; Novak, P.; Treska, V.; Bruha, J.; Pitule, P.; Holubec, L. Infiltration of Colorectal Carcinoma by S100+ Dendritic Cells and CD57+ Lymphocytes as Independent Prognostic Factors After Radical Surgical Treatment. Anticancer Res. 2012, 32, 2129–2132. [Google Scholar] [PubMed]
- Coca, S.; Perez-Piqueras, J.; Martinez, D.; Colmenarejo, A.; Saez, M.A.; Vallejo, C.; Martos, J.A.; Moreno, M. The Prognostic Significance of Intratumoral Natural Killer Cells in Patients with Colorectal Carcinoma. Cancer 1997, 79, 2320–2328. [Google Scholar] [CrossRef]
- Papanikolaou, I.S.; Lazaris, A.C.; Apostolopoulos, P.; Kavantzas, N.; Papas, M.G.; Mavrogiannis, C.; Patsouris, E.S.; Archimandritis, A. Tissue Detection of Natural Killer Cells in Colorectal Adenocarcinoma. BMC Gastroenterol. 2004, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, W.; Hu, B.; Wang, P.; Lv, X.; Chen, S.; Shao, Z. Prognostic Significance of Tumor-Infiltrating Natural Killer Cells in Solid Tumors: A Systematic Review and Meta-Analysis. Front. Immunol. 2020, 11, 1242. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S. Prognostic Role of Tumor-Infiltrating CD57-Positive Lymphocytes in Solid Tumors: A Meta-Analysis. Oncotarget 2017, 9, 8111–8119. [Google Scholar] [CrossRef]
- Vayrynen, J.P.; Haruki, K.; Lau, M.C.; Vayrynen, S.A.; Ugai, T.; Akimoto, N.; Zhong, R.; Zhao, M.; Dias Costa, A.; Borowsky, J.; et al. Spatial Organization and Prognostic Significance of NK and NKT-Like Cells Via Multimarker Analysis of the Colorectal Cancer Microenvironment. Cancer. Immunol. Res. 2022, 10, 215–227. [Google Scholar] [CrossRef]
- Garrido, F.; Ruiz-Cabello, F.; Cabrera, T.; Perez-Villar, J.J.; Lopez-Botet, M.; Duggan-Keen, M.; Stern, P.L. Implications for Immunosurveillance of Altered HLA Class I Phenotypes in Human Tumours. Immunol. Today 1997, 18, 89–95. [Google Scholar] [CrossRef]
- Stabile, H.; Fionda, C.; Gismondi, A.; Santoni, A. Role of Distinct Natural Killer Cell Subsets in Anticancer Response. Front. Immunol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Corvino, D.; Kumar, A.; Bald, T. Plasticity of NK Cells in Cancer. Front. Immunol. 2022, 13, 888313. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The Biology of Human Natural Killer-Cell Subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bilecz, A.J.; Lengyel, E. The Adipocyte Microenvironment and Cancer. Cancer Metastasis Rev. 2022, 41, 575–587. [Google Scholar] [CrossRef]
- Wu, Q.; Li, B.; Li, J.; Sun, S.; Yuan, J.; Sun, S. Cancer-Associated Adipocytes as Immunomodulators in Cancer. Biomark Res. 2021, 9, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Niavarani, S.R.; Lawson, C.; Bakos, O.; Boudaud, M.; Batenchuk, C.; Rouleau, S.; Tai, L. Lipid Accumulation Impairs Natural Killer Cell Cytotoxicity and Tumor Control in the Postoperative Period. BMC Cancer 2019, 19, 823-y. [Google Scholar] [CrossRef]
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-Associated Macrophages are a Distinct M2 Polarised Population Promoting Tumour Progression: Potential Targets of Anti-Cancer Therapy. Eur. J. Cancer 2006, 42, 717–727. [Google Scholar] [CrossRef]
- Brockmoeller, S.; Echle, A.; Ghaffari Laleh, N.; Eiholm, S.; Malmstrom, M.L.; Plato Kuhlmann, T.; Levic, K.; Grabsch, H.I.; West, N.P.; Saldanha, O.L.; et al. Deep Learning Identifies Inflamed Fat as a Risk Factor for Lymph Node Metastasis in Early Colorectal Cancer. J. Pathol. 2022, 256, 269–281. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Lugli, A. The Tumor Border Configuration of Colorectal Cancer as a Histomorphological Prognostic Indicator. Front. Oncol. 2014, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer show Immunoscore is a Stronger Predictor of Patient Survival than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Liu, H.; Wei, C.; Ru, H.; Qin, H.; Lai, H.; Meng, Y.; Wu, G.; Xie, W.; et al. Immune Landscape and Prognostic Immune-Related Genes in KRAS-Mutant Colorectal Cancer Patients. J. Transl. Med. 2021, 19, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, X.; Duanmu, J.; Li, T.; Jiang, Q. KRAS Mutations are Negatively Correlated with Immunity in Colon Cancer. Aging (Albany NY) 2020, 13, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Kocian, P.; Sedivcova, M.; Drgac, J.; Cerna, K.; Hoch, J.; Kodet, R.; Bartunkova, J.; Spisek, R.; Fialova, A. Tumor-Infiltrating Lymphocytes and Dendritic Cells in Human Colorectal Cancer: Their Relationship to KRAS Mutational Status and Disease Recurrence. Hum. Immunol. 2011, 72, 1022–1028. [Google Scholar] [CrossRef]
- Vail, E.; Song, J.; Xu, J.; Frye, J.S.; Kim, J.T.; Pao, A.; Schreck, R.; Aguiluz, A.S.; Zhang, W.; Alkan, S.; et al. Comparison of Large, Medium, and Small Solid Tumor Gene Panels for Detection of Clinically Actionable Mutations in Cancer. Target Oncol. 2020, 15, 523–530. [Google Scholar] [CrossRef]
- Misyura, M.; Zhang, T.; Sukhai, M.A.; Thomas, M.; Garg, S.; Kamel-Reid, S.; Stockley, T.L. Comparison of Next-Generation Sequencing Panels and Platforms for Detection and Verification of Somatic Tumor Variants for Clinical Diagnostics. J. Mol. Diagn. 2016, 18, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pages, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of Cancer-Associated Immune Cells in Human Solid Tumors. Elife 2018, 7, 36967. [Google Scholar] [CrossRef]
- Schurch, C.M.; Bhate, S.S.; Barlow, G.L.; Phillips, D.J.; Noti, L.; Zlobec, I.; Chu, P.; Black, S.; Demeter, J.; McIlwain, D.R.; et al. Coordinated Cellular Neighborhoods Orchestrate Antitumoral Immunity at the Colorectal Cancer Invasive Front. Cell 2020, 182, 1341–1359.e19. [Google Scholar] [CrossRef] [PubMed]
- Nearchou, I.P.; Lillard, K.; Gavriel, C.G.; Ueno, H.; Harrison, D.J.; Caie, P.D. Automated Analysis of Lymphocytic Infiltration, Tumor Budding, and their Spatial Relationship Improves Prognostic Accuracy in Colorectal Cancer. Cancer. Immunol. Res. 2019, 7, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Pelka, K.; Hofree, M.; Chen, J.H.; Sarkizova, S.; Pirl, J.D.; Jorgji, V.; Bejnood, A.; Dionne, D.; Ge, W.H.; Xu, K.H.; et al. Spatially Organized Multicellular Immune Hubs in Human Colorectal Cancer. Cell 2021, 184, 4734–4752.e20. [Google Scholar] [CrossRef]
- Beechem, J.M. High-Plex Spatially Resolved RNA and Protein Detection using Digital Spatial Profiling: A Technology Designed for Immuno-Oncology Biomarker Discovery and Translational Research. Methods Mol. Biol. 2020, 2055, 563–583. [Google Scholar] [PubMed]
- Merritt, C.R.; Ong, G.T.; Church, S.E.; Barker, K.; Danaher, P.; Geiss, G.; Hoang, M.; Jung, J.; Liang, Y.; McKay-Fleisch, J.; et al. Multiplex Digital Spatial Profiling of Proteins and RNA in Fixed Tissue. Nat. Biotechnol. 2020, 38, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N.; Halama, N. Harnessing the Innate Immune System and Local Immunological Microenvironment to Treat Colorectal Cancer. Br. J. Cancer 2019, 120, 871–882. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7-w. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Setti, C.; Giordano, C.; Obino, V.; Greppi, M.; Pesce, S.; Marcenaro, E.; Rutigliani, M.; Provinciali, N.; Paleari, L.; et al. NK Cell-Based Immunotherapy in Colorectal Cancer. Vaccines 2022, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Makowska, A.; Meier, S.; Shen, L.; Busson, P.; Baloche, V.; Kontny, U. Anti-PD-1 Antibody Increases NK Cell Cytotoxicity Towards Nasopharyngeal Carcinoma Cells in the Context of Chemotherapy-Induced Upregulation of PD-1 and PD-L1. Cancer Immunol. Immunother. 2021, 70, 323–336. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J. Clin. Invest. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef]
- Munari, E.; Quatrini, L.; Ciancaglini, C.; Eccher, A.; Bogina, G.; Moretta, L.; Mariotti, F.R. Immunotherapy Targeting Inhibitory Checkpoints: The Role of NK and Other Innate Lymphoid Cells. Semin. Immunol. 2022, 61–64, 101660. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Grossi, F.; Del Zotto, G.; Moretta, L.; Sivori, S.; Genova, C.; Marcenaro, E. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front. Immunol. 2019, 10, 1242. [Google Scholar] [CrossRef]
- Grottoli, M.; Carrega, P.; Zullo, L.; Dellepiane, C.; Rossi, G.; Parisi, F.; Barletta, G.; Zinoli, L.; Coco, S.; Alama, A.; et al. Immune Checkpoint Blockade: A Strategy to Unleash the Potential of Natural Killer Cells in the Anti-Cancer Therapy. Cancers 2022, 14, 5046. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant Immunotherapy Leads to Pathological Responses in MMR-Proficient and MMR-Deficient Early-Stage Colon Cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.I.; Fergadis, E.; Skouteris, N.; Christakos, E.; Tsakatikas, S.A.; Lianos, E.; Kosmas, C. Case Report: Combination of Olaparib with Chemotherapy in a Patient with ATM-Deficient Colorectal Cancer. Front. Oncol. 2021, 11, 788809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, X.; Chen, H.; Hu, Y.; Zhang, H.; Wu, L.; Yang, Y.; Mao, B.; Wang, H. The Mutational Pattern of Homologous Recombination-Related (HRR) Genes in Chinese Colon Cancer and its Relevance to Immunotherapy Responses. Aging (Albany NY) 2020, 13, 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020, 10, 570. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, K.; Arao, T.; Shimoyama, T.; Tamura, T.; Nishio, K. Antibody-Dependent Cellular Cytotoxicity of Cetuximab Against Tumor Cells with Wild-Type Or Mutant Epidermal Growth Factor Receptor. Cancer Sci. 2007, 98, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Wulff, S.; Pries, R.; Borngen, K.; Trenkle, T.; Wollenberg, B. Decreased Levels of Circulating Regulatory NK Cells in Patients with Head and Neck Cancer Throughout all Tumor Stages. Anticancer Res. 2009, 29, 3053–3057. [Google Scholar]
- Bouzidi, L.; Triki, H.; Charfi, S.; Kridis, W.B.; Derbel, M.; Ayadi, L.; Sellami-Boudawara, T.; Cherif, B. Prognostic Value of Natural Killer Cells Besides Tumor-Infiltrating Lymphocytes in Breast Cancer Tissues. Clin. Breast Cancer 2021, 21, e738–e747. [Google Scholar] [CrossRef]
- Levi, I.; Amsalem, H.; Nissan, A.; Darash-Yahana, M.; Peretz, T.; Mandelboim, O.; Rachmilewitz, J. Characterization of Tumor Infiltrating Natural Killer Cell Subset. Oncotarget 2015, 6, 13835–13843. [Google Scholar] [CrossRef]
- Hinterseher, I.; Schworer, C.M.; Lillvis, J.H.; Stahl, E.; Erdman, R.; Gatalica, Z.; Tromp, G.; Kuivaniemi, H. Immunohistochemical Analysis of the Natural Killer Cell Cytotoxicity Pathway in Human Abdominal Aortic Aneurysms. Int. J. Mol. Sci. 2015, 16, 11196–11212. [Google Scholar] [CrossRef]
- Bilal, M.; Nimir, M.; Snead, D.; Taylor, G.S.; Rajpoot, N. Role of AI and Digital Pathology for Colorectal Immuno-Oncology. Br. J. Cancer 2022, 128, 3–11. [Google Scholar] [CrossRef] [PubMed]

| Variable | SARIFA-Positive | SARIFA-Negative | p-Value |
|---|---|---|---|
| n = 15 (33.3%) | n = 30 (67.7%) | ||
| Age: median (range) | 61 (42–77) | 68 (48–84) | 0.043 |
| Gender | |||
| male; n (%) | 10 (66.7) | 8 (26.7) | 0.012 |
| female; n (%) | 5 (33.3) | 22 (73.3) | |
| Stage | |||
| UICC I and II; n (%) | 7 (46.7) | 21 (70.0) | ns |
| UICC III and IV; n (%) | 8 (53.3) | 9 (30.0) | |
| Tumor sidedness | |||
| right n (%) | 11 (73.3) | 20 (66.7) | ns |
| left n (%) | 4 (26.7) | 10 (33.3) | |
| Microsatellite status | |||
| stable n (%) | 12 (80.0) | 22 (73.3) | ns |
| unstable n (%) | 3 (20.0) | 7 (23.3) | |
| information not available | 1 |
| Gene | Mutational Status: wt/mut | SARIFA-Positive n = 15 * | SARIFA-Negative n = 23 * | p-Value |
|---|---|---|---|---|
| KRAS | wt | 6 (40.0%) | 13 (56.5%) | ns |
| mut | 9 (60.0%) | 10 (43.5%) | ||
| BRAF | wt | 14 (93.3%) | 18 (78.3%) | ns |
| mut | 1 (6.7%) | 5 (21.7%) | ||
| NRAS | wt | 14 (93.3%) | 22 (95.7%) | ns |
| mut | 1 (6.7%) | 1 (4.3%) | ||
| PIK3CA | wt | 11 (73.3%) | 18 (78.3%) | ns |
| mut | 4 (26.7%) | 5 (21.7%) | ||
| CTNNB1 | wt | 15 (100%) | 22 (95.7%) | ns |
| mut | 0 (0%) | 1 (4.3%) | ||
| FGFR1 | wt | 15 (100%) | 22 (95.7%) | ns |
| mut | 0 (0%) | 1 (4.3%) | ||
| FGFR3 | wt | 15 (100%) | 22 (95.7%) | ns |
| mut | 0 (0%) | 1 (4.3%) | ||
| ERBB2 | wt | 15 (100%) | 22 (95.7%) | ns |
| mut | 0 (0%) | 1 (4.3%) | ||
| MET | wt | 15 (100%) | 22 (95.7%) | ns |
| mut | 0 (0%) | 1 (4.3%) | ||
| AKT1 | wt | 15 (100%) | 22 (95.7%) | ns |
| mut | 0 (0%) | 1 (4.3%) |
| Healthy Controls n = 27 | SARIFA-Positive n = 15 | SARIFA-Negative n = 30 | p1 SARIFApos-SARIFAneg | p2 Healthy-SARIFAneg | p3 Healthy-SARIFApos | |
|---|---|---|---|---|---|---|
| Total lymphocytes | 1795 (1195–2216) | 1388 (1185–1687) | 1288 (965–1623) | ns | 0.038 | ns |
| CD3+ cells | 1101 (671–1678) | 876 (783–1320) | 866 (714–1142) | ns | ns | ns |
| CD8+ cells | 228 (191–364) | 228 (184–397) | 233 (118–325) | ns | ns | ns |
| Naive | 48 (9–70) | 51 (33–83) | 25 (15–58) | ns | ns | ns |
| Memory | 102 (55–133) | 63 (39–124) | 84 (49–117) | ns | ns | ns |
| CM | 21 (4–40) | 24 (13–33) | 23 (13–55) | ns | ns | ns |
| EM | 79 (47–126) | 76 (35–148) | 72 (47–101) | ns | ns | ns |
| EMRA | 68 (23–134) | 69 (18–148) | 29 (16–110) | ns | ns | ns |
| Early | 160 (89–206) | 133 (94–165) | 78 (56–181) | ns | 0.025 | ns |
| Intermediate | 20 (10–30) | 13 (6–30) | 10 (6–24) | ns | ns | ns |
| Late | 36 (22–87) | 58 (42–201) | 51 (18–126) | ns | ns | ns |
| Exhausted | 81 (51–133) | 82 (29–114) | 53 (23–79) | ns | 0.019 | ns |
| Terminal effector | 21 (12–63) | 39 (15–135) | 31 (9–97) | ns | ns | ns |
| HLA-DR+ | 31 (10–45) | 92 (56–177) | 60 (36–112) | ns | 0.011 | 0.002 |
| CD69+ | 21 (17–46) | 19 (12–32) | 13 (7–24) | ns | 0.037 | ns |
| CD4+ cells | 634 (487–1042) | 547 (268–773) | 524 (406–640) | ns | ns | ns |
| Naive | 282 (143–371) | 218 (59–441) | 157 (117–239) | ns | ns | ns |
| Memory | 411 (255–552) | 311 (182–366) | 303 (234–386) | ns | ns | 0.027 |
| CM | 198 (91- 289) | 190 (109–250) | 190 (139–236) | ns | ns | ns |
| EM | 181 (98–268) | 99 (63–169) | 123 (82–163) | ns | ns | 0.05 |
| EMRA | 14 (1–44) | 4 (2–8) | 8 (1–26) | ns | ns | ns |
| Th1 | 28 (13–61) | 17 (14–33) | 19 (8–44) | ns | ns | ns |
| Th2 | 48 (34–81) | 40 (16–63) | 47 (33–60) | ns | ns | ns |
| Th17 | 67 (36–83) | 40 (17–70) | 48 (33–60) | ns | ns | ns |
| CD25high | 11 (6–18) | 10 (7–32) | 15 (9–24) | ns | ns | ns |
| HLA-DR+ | 39 (27–64) | 50 (34–62) | 51 (44–67) | ns | 0.011 | 0.002 |
| CD69+ | 12 (7–19) | 13 (11–21) | 14 (9–18) | ns | 0.037 | ns |
| NK cells | 189 (125–281) | 87 (59–117) | 187 (118–252) | 0.002 | ns | <0.001 |
| CD56dim CD16bright | 12 (9–19) | 6 (4–12) | 14 (8–25) | 0.004 | ns | 0.004 |
| CD56+ CD16+ | 162 (98–264) | 56 (36–90) | 151 (103–220) | <0.001 | ns | <0.001 |
| CD56bright CD16dim | 15 (10–19) | 10 (9–14) | 11 (8–15) | ns | ns | ns |
| NK-like T cells | 47 (18–83) | 47 (21–67) | 34 (17–134) | ns | ns | ns |
| B cells | 208 (146–236) | 122 (85–205) | 123 (62–165) | ns | <0.001 | ns |
| Naive | 116 (88–164) | 88 (58–152) | 69 (37–99) | ns | 0.006 | ns |
| Memory | 7 (5–9) | 8 (4–12) | 6 (2–15) | ns | ns | ns |
| Class switch | 25 (16–41) | 18 (12–21) | 11 (6–28) | ns | 0.002 | ns |
| Transitory | 7 (5–14) | 1 (1–2) | 2 (1–4) | ns | <0.001 | <0.001 |
| CD4/CD8 Ratio | 3 (2–4) | 2 (1–3) | 3 (2–4) | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitsam, N.G.; Märkl, B.; Dintner, S.; Sipos, E.; Grochowski, P.; Grosser, B.; Sommer, F.; Eser, S.; Nerlinger, P.; Jordan, F.; et al. Alterations in Natural Killer Cells in Colorectal Cancer Patients with Stroma AReactive Invasion Front Areas (SARIFA). Cancers 2023, 15, 994. https://doi.org/10.3390/cancers15030994
Reitsam NG, Märkl B, Dintner S, Sipos E, Grochowski P, Grosser B, Sommer F, Eser S, Nerlinger P, Jordan F, et al. Alterations in Natural Killer Cells in Colorectal Cancer Patients with Stroma AReactive Invasion Front Areas (SARIFA). Cancers. 2023; 15(3):994. https://doi.org/10.3390/cancers15030994
Chicago/Turabian StyleReitsam, Nic G., Bruno Märkl, Sebastian Dintner, Eva Sipos, Przemyslaw Grochowski, Bianca Grosser, Florian Sommer, Stefan Eser, Pia Nerlinger, Frank Jordan, and et al. 2023. "Alterations in Natural Killer Cells in Colorectal Cancer Patients with Stroma AReactive Invasion Front Areas (SARIFA)" Cancers 15, no. 3: 994. https://doi.org/10.3390/cancers15030994
APA StyleReitsam, N. G., Märkl, B., Dintner, S., Sipos, E., Grochowski, P., Grosser, B., Sommer, F., Eser, S., Nerlinger, P., Jordan, F., Rank, A., Löhr, P., & Waidhauser, J. (2023). Alterations in Natural Killer Cells in Colorectal Cancer Patients with Stroma AReactive Invasion Front Areas (SARIFA). Cancers, 15(3), 994. https://doi.org/10.3390/cancers15030994









