Simple Summary
Neurokinins A and B, adrenomedullin, adrenomedullin 2, amylin, and calcitonin gene-related peptide are essential in different tumors. These peptides are involved in tumor cell proliferation and migration, metastasis, angiogenesis, and lymphangiogenesis. Accordingly, several antitumor therapeutic strategies, including peptide receptor antagonists, can be developed. This review highlights the essential roles played by both tachykinin and calcitonin/calcitonin gene-related peptide families in cancer progression, which support the application of promising clinical antitumor therapeutic strategies.
Abstract
The roles played by the peptides belonging to the tachykinin (neurokinin A and B) and calcitonin/calcitonin gene-related peptide (adrenomedullin, adrenomedullin 2, amylin, and calcitonin gene-related peptide (CGRP)) peptide families in cancer development are reviewed. The structure and dynamics of the neurokinin (NK)-2, NK-3, and CGRP receptors are studied together with the intracellular signaling pathways in which they are involved. These peptides play an important role in many cancers, such as breast cancer, colorectal cancer, glioma, lung cancer, neuroblastoma, oral squamous cell carcinoma, phaeochromocytoma, leukemia, bladder cancer, endometrial cancer, Ewing sarcoma, gastric cancer, liver cancer, melanoma, osteosarcoma, ovarian cancer, pancreatic cancer, prostate cancer, renal carcinoma, and thyroid cancer. These peptides are involved in tumor cell proliferation, migration, metastasis, angiogenesis, and lymphangiogenesis. Several antitumor therapeutic strategies, including peptide receptor antagonists, are discussed. The main research lines to be developed in the future are mentioned.
Keywords:
neurokinin; NKA; NKB; CGRP; amylin; adrenomedullin; adrenomedullin 2; peptide; peptide receptor 1. Introduction
To attain a better quality of life, with higher cure rates and fewer sequelae in cancer patients, new molecular targets and compounds that specifically destroy tumor cells must be urgently investigated. One of these promising targets could be peptidergic systems, i.e., peptides and their receptors, which play a crucial role in cell communication. Peptidergic systems have opened up new research lines and possibilities to improve cancer diagnosis and explore new antitumor strategies [1,2,3,4]. The involvement of peptidergic systems in cancer has attracted increasing interest in the last few years. Peptides such as substance P (a full review focused on its participation in cancer was recently published [5]), neurotensin, orexin, angiotensin II, neuropeptide Y, vasoactive intestinal peptide, calcitonin gene-related peptide, adrenomedullin, adrenomedullin 2 or intermedin, and amylin contribute to cancer development [1,2,6]. These peptides promote the mitogenesis/migration of tumor cells, exert an antiapoptotic action, and stimulate the growth of blood vessels and lymphangiogenesis. However, some peptides such as neuropeptide Y, orexin, and vasoactive intestinal peptide also exert an anticancer effect [7]. Furthermore, tumor cells release peptides acting through autocrine, paracrine, and endocrine (tumor mass) mechanisms [1,2,3,5,6,8,9,10,11]. While some peptides (e.g., the heptapeptide angiotensin (1-7)) exert an anticancer effect, others (e.g., galanin) promote both proliferative and antiproliferative actions on tumor cells [3].
Tumor cells also overexpress peptide receptors, allowing for a specific treatment against cancer cells with peptide antagonists. In addition, the overexpression of these receptors can be used as a prognostic biomarker [1]. The overexpression of peptidergic systems has been associated with higher tumor aggressiveness, tumor size, poor prognosis, worse sensitivity to chemotherapy agents, and increased relapse risk [1,5]. Peptide antagonists promote apoptosis in tumor cells, block the migration of cancer cells, and inhibit angiogenesis. In combination therapy with chemotherapy, peptide antagonists decrease the side-effects promoted by cytostatics and exert a synergic effect [1,4]. Because peptide receptors are potential new targets in cancer treatment, peptide antagonists are promising antitumor drugs. Because the involvement of substance P in cancer through the neurokinin-1 receptor is widely known [5], this review aims to update the findings regarding the involvement of the main peptides belonging to the tachykinin (neurokinin A and B) and calcitonin/calcitonin gene-related peptide (adrenomedullin, adrenomedullin 2, amylin, and calcitonin gene-related peptide) systems in the mitogenesis, migration, and invasion of tumor cells, angiogenesis, and lymphangiogenesis; moreover, according to the data reported, this review suggests anticancer therapeutic strategies targeting tachykinin and calcitonin peptidergic systems. The signal transduction pathways, mediated by the different tachykinin/calcitonin receptors involved, are reviewed, along with the structure and dynamics of these receptors.
Currently, it is considered that the tumor microenvironment is composed of tumor cells and cancer stem cells, as well as normal stromal cells. Hence, tumors are not currently regarded as a simple mass of cancer cells [12,13]. Updated hallmarks of cancer cells escaping from normal behavior are the following: (1) growth suppressor evasion; (2) proliferative signaling maintenance; (3) replicative immortality; (4) cell death resistance; (5) invasion/metastasis activation; (6) angiogenesis promotion; (7) inflammation; (8) genome instability; (9) energy metabolism reprogramming; (10) immune destruction evasion; (11) senescent cells; (12) polymorphic microbiomes; (13) non-mutational epigenetic reprogramming; (14) unlocking phenotype plasticity [12,13] (Figure 1). Tachykinin peptides are involved in five of these hallmarks, whereas those belonging to the calcitonin/calcitonin gene-related peptide (CGRP) family are involved in nine. This comment shows the essential roles of both peptidergic systems in cancer development and the numerous research lines that can be developed.
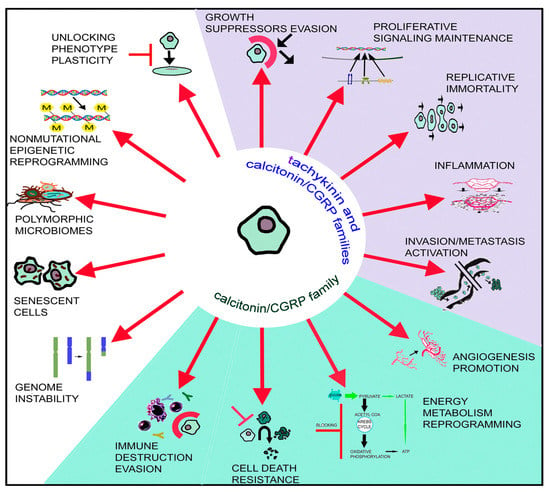
Figure 1.
The 14 hallmarks currently considered to be responsible for the development of cancer are indicated. Five hallmarks (blue background) are mediated by peptides belonging to the tachykinin and calcitonin/CGRP peptide families, and four hallmarks (green background) are mediated by peptides belonging to the calcitonin/CGRP peptide family.
2. Tachykinin and Calcitonin Peptide Families
2.1. Tachykinin Peptide Family
The tachykinin family of peptides includes kassinin, ranakinin, eledoisin, neuropeptide K, hemokinin-1, substance P (SP), neurokinin B (NKB), and neurokinin A (NKA) [14]. Activated metabotropic neurokinin receptors (neurokinin-1 receptor (NK-1R), NK-2R, and NK-3R), widely distributed by the central and peripheral nervous systems, exert many physiological actions and determine many pathophysiological [15,16,17]. NKA and NKB mediate many physiological effects and are involved in several pathologies [16,18,19,20,21,22].
2.1.1. Genes and Products of Human Tachykinins
Tachykinin is the general name for a large family of peptides (hundreds) found in all bilaterians (from insects to amphibians and humans) [23,24]. In humans, three principal tachykinins, SP, NKA, and NKB, participate in cellular mechanisms responsible for physiological and pathological outcomes. Three human genes named TAC1, TAC3, and TAC4 encode the peptides mentioned above, plus hemokinin1 (HK-1) and endokinins [17,25,26,27]. Tachykinin genes transcribe into splice variants [28,29,30]. Gene TAC1 with seven exons is located on chromosome 7 (7q21.3). It gives rise to four different splice mRNA variants, α, β, γ, and δ, that encode four protachykinin peptide isoforms of various sizes, α (111 residues), β (129 residues), γ (114 residues), and δ (96 residues). Exon 3 of TAC1 appears transcribed in all four mRNA variants. It encodes the sequence of the undecapeptide SP. The exon 6 sequence transcript is only present in the β and γ splice variants and encodes the decapeptide NKA. Gene TAC3, with seven exons, is on chromosome 12 (12q13.3) and generates two alternative coding transcript variants, isoforms 1 and 2. Isoform 1 translates into a peptide precursor of 121 residues, and isoform 2 translates into a peptide precursor of 103 amino acids. Exon 5 of both mRNAs encodes the decapeptide NKB. Gene TAC4 sits on chromosome17 (17q21.33) and encodes five splice variants, α, α-2, β, γ, and δ, translating into five peptide precursors that provide functional HK-1 and endokinins after post-translational processing (Figure 2).
Tachykinin peptide precursors undergo post-translational modifications, generating functional peptides [31]. Endopeptidase processing at a specific pair of basic residues liberates the N-terminal ends of SP, NKA, and NKB from their respective precursors. SP occupies amino-acid positions 58–68 in all precursor peptide variants encoded by TAC1. NKA corresponds to amino-acid positions 98–107 in the precursor variants β and γ. Lastly, precursor peptides 1 and 2 encoded by the TAC3 gene contain NKB (amino-acid positions 81–90). Splitting the C-terminal ends of the three neurokinins from their protachykinins follows a shared mechanism required for functional activity, consisting of a PAM (peptidyl glycine α-amidating monooxygenase, EC 1.14.17.3)-catalyzed reaction cleaving the N–Cα bond between methionine and neighboring glycine [32,33] (Figure 2). The reaction’s products are the C-terminal amidated active neurokinin and an N-glyoxylated peptide.
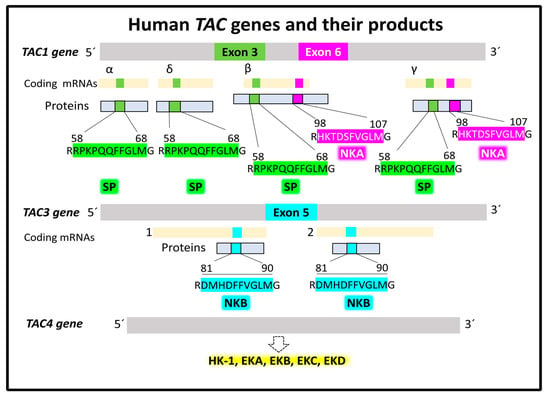
Figure 2.
Human tachykinin genes and their main products, SP, NKA, and NKB. Alternative splicing generates several mRNA isoforms transcribed into protein products that undergo post-translational modifications (protein hydrolysis and amination of C-terminal methionine), giving rise to functional tachykinins. Data supporting this scheme are from the UNIPROT [25] and Entrez Gene (National Library of Medicine) [34] databases.
2.1.2. Structure of NK-2R and NK-3R
Three mammalian neurokinin receptors convey the activity of tachykinins. NK-1R binds with high-affinity SP and HK-1, NK-2R preferentially binds NKA, and NKB is the natural agonist of NK-3R. All three neurokinins exhibit full agonist capacity in all three receptors, albeit with different rank order potency [35]. Figure 3 shows the primary and general tertiary serpentine structure of NK-2R and NK-3R, together with the amino-acid sequence of their preferred endogenous agonist ligands. Sequence homology between NK-2R and NK-3R is 57%. NK-2R has a sequence homology of 54% with NK-1R; NK-3R shares a sequence homology of 61% with NK-1R (according to the SIM alignment tool [36]). The tertiary structure of the NK-2R and NK-3R is very similar when predicted with Alpha-Fold (Figure 4 and Figure 5). However, specific residue positions and transmembrane segment displacements make interactions with agonists and antagonists different. Neurokinin receptors belong to class A of the large family of G-protein-coupled receptors (GPCR), membrane-bound proteins sharing a 3D structure arranged in seven transmembrane domains linked with extra and intracellular loops [37,38]. Structural and functional studies of these membrane proteins have provided broad information on the structure–activity relationships that explain their role in cell function and their relevance in drug discovery with therapeutic applications [38,39,40,41,42]. Some recent research and reviews provided detailed and sound information on the structure and dynamics of NK-1R [43,44,45,46]. This section focuses on the architecture of the human NK-2R and NK 3R.
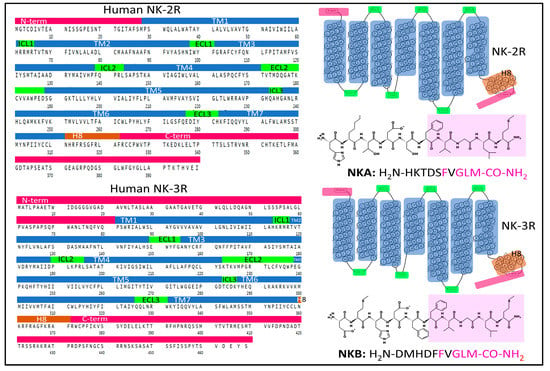
Figure 3.
Complete sequences and snake plots of the neurokinin receptors 2 and 3 and their preferential endogenous agonists, NKA and NKB. The domains of NK-2R and NK-3R (N- and C-terminal regions, transmembrane helices (TM), Helix 8, and intra- and extracellular loops) are highlighted in color. Color areas illuminate the amino-acid sequence of the C-terminal end of neurokinins. The sequences and structures of the receptors are from the GPCR database [47], and the peptide structures were drawn with KingDraw software (Version 1.1.0) [48].
The Structure of NK-2R
NK-2R is a monomeric integral membrane protein (UNIPROT, P-21452) [25] made of 398 amino-acids that expand through the plasma membrane lipid bilayers with seven domains (Figure 3). The human gene TACR1 (Gene ID 6865 from the Entrez Gene database, National Library of Medicine [34]) has five exons with chromosomal localization 10q22.1 and encodes the protein NK-2R [49]. Post-translational modifications of NK-2R include glycosylation of asparagines 11 and 19 (N-terminal region), a disulfide bridge between cysteines 106 (in TM3) and 181 (in ECL2), and the palmitoylation of Cys324 (intracellular C-terminal domain). The preferred endogenous ligand of NK-2R is NKA (Figure 3 and Figure 4).
Early pharmacological studies in isolated organs revealed the functional importance of the NKA sequence from amino acids 4–10. This sequence retained the activity of full NKA (1-10) [50]. Additionally, the substitution of Gly8 for Ala8 increased the selectivity and potency of the short form of NKA [51] Radioligand binding and functional experiments in human specimens showed the importance of amino acids Asp4, Phe6, Val7, Leu9, and Met10 for binding selectivity [52]. The position of Phe6 within the C-terminal pentapeptide determines the binding of NKA to all neurokinin receptors [53]. It makes important contact with the protein through aromatic (π–π) and amino–aromatic (N–π) interactions [50]. Site-directed mutagenesis bestowed crucial amino-acid positions in transmembrane helices 3, 5, and 7 as part of the binding site for NKA and different interactions with SP and NKB agonists, and with NK-2R antagonist SR-48968 [54]. Homology modeling analysis implemented indirect data to attain forms of minimal energy to understand the coupling of agonists, antagonists, and cellular signaling proteins. Molecular modeling and docking of the NKA within NK-2R, using rhodopsin as a structural template and the three-dimensional NKA structure determined with NMR [55] (Figure 4) resulted in the definition of the site where NKA contacts the receptor protein. Within a distance of less than 3 Å, predicted hydrogen bonds (residues His1, Lys2, Thr3, Asp4, Gly8, Leu9, and Met10) stabilize the agonist and receptor interaction. Furthermore, residues Phe6, Leu9, and Met10 are relevant in biological activity. Furthermore, in the established model, some interactions anchor the binding pocket for the agonist. Figure 4 depicts the atomic contacts between the C-terminal portion of NKA and NK-2R. For example, the amidated methionine at the NKA’s C-terminal end contacts several residues of the NK-2R (Ala116, Trp263, Met117, and Ser298). The phenyl ring of Phe6 sits in a hydrophobic cavity employing π–π interactions with residues bearing aromatic rings (Tyr266 and Phe270) and other groups; Leu9 connects through weak interactions with the lateral chains of Ile114, Met117, Ala116, Trp263, and Tyr266. The conserved C-terminal pentapeptide sits in a hydrophobic cavity built by transmembrane helices TM2, TM3, TM6, and TM7 [56] (Figure 4C). The building of a model of NK-2R bound to NKA by comparing sequences with the rhodopsin receptor and analysis of fluorescence resonance energy transfer (FRET) with fluorescent NKA revealed that the N-terminal end of NKA expands to the extracellular medium. In contrast, the C-terminal amidated end is buried in the TM-spanning domains [57]. Site-directed mutagenesis analysis of the antagonist binding site of NK-2R showed that nepadutant (a peptide) partially overlapped with the aperture occupied by the non-peptide antagonist SR-48968. Aromatic residues in TM5, TM6, and TM7 play a fundamental anchor function to accommodate the antagonists studied [58].
NK-2R may adopt different active conformations where NKA adapts to generate different intracellular responses (biased agonism). Different receptor conformations linked to cellular responses facilitate the design of allosteric modulators that may help to control specific signaling by affecting the affinity of NKA to the receptor’s conformations [59]. Binding selectivity depends on the C-terminal end and the interactions of amino acids of the rest of the molecule with the receptor. A recent cryo-EM structure determination of NK-2R-Gq protein complex bound to NKA [60] underlies the importance of several residues that secure the position of NKA within de receptor pocket (see Figure 4D). Lateral chains of residues Y93 and N90 and N97 in TM2 interact with the backbone carbonyl oxygens of Val7 and Leu9. The backbone nitrogen atom of Gly8 forms a hydrogen bond with the hydroxyl group of Y289 in TM7.
Further stabilization of M10 occurs with I114 in TM3 through hydrophobic interaction. Additionally, the N-terminus of NKA contacts the ECL2 region of NK-2R (D175 forms a salt bridge with Lys2 carbonyl oxygen), and Phe6 further contacts Met28 in the N-terminal part of NK-2R and I285 in TM7 (Figure 4D). When Sun et al. [60] compared NK-2R with NK-1R (PDB ID 7RMG, [61]), they found very similar structures, except for an outward shift of the TM5 in NK-2R of 2.4 Å. Moreover, they identified significant differences in the arrangement of extracellular loops, specially ECL2. The differences may explain endogenous ligand selectivities for the three receptors, notably concerning the interaction of the N-terminal sequence of neurokinins with the extracellular loop 2. Nevertheless, we need additional architectural and structure dynamics studies (X-ray crystallography, cryo-electron microscopy, and NMR) to ascertain the atomic environment that accommodates agonists, antagonists, and allosteric modulators. The nuanced structural analysis will unravel the role of these receptors in physiological and pathological outcomes and their collaboration with other NK receptors responding to the three agonists, NKA, SP, and NKB, with different selectivity.
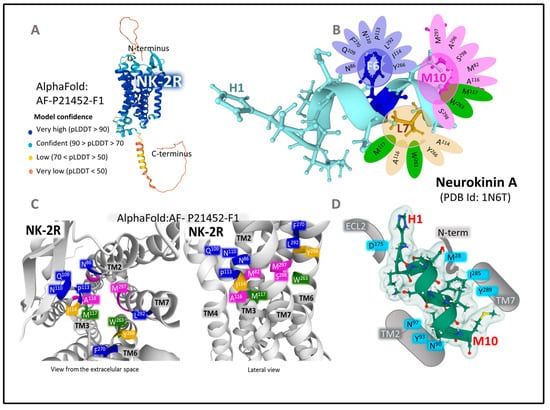
Figure 4.
(A) represents the predicted structure of NK-2R (UNIPROT P21452) according to the Alpha-Fold prediction [62,63,64]. The parameter pLDDT (predicted local distance difference test) assessed the model confidence. (B) depicts the 3D structure of NKA determined by NMR obtained from the Protein Data Bank [65] (PDB ID 1N6T [55]) drawn with Mol* free web-based software [48]. According to the model, the contacts of NKA’s C-terminal amino acids with the receptors’ amino acids are depicted. (C) shows two views of a three-dimensional representation of NK-2R, obtained from modeling and docking studies [56], indicating the situation of principal amino-acid positions in the Alpha-Fold model of NK-2R closely contacting the C-terminal region of NKA. Residues in green contact more than one pharmacophore of NKA. (D) represents further contacts of NKA (PDB ID 1N6T, [56]) with NK-2R according to data from the cryo-EM structure provided by Sun et al. [60].
The Structure of NK-3R
NK-3R is a monomeric membrane protein made (UNIPROT P29371) [25] of 465 amino acids that expand through the plasma membrane lipid bilayers with seven domains (Figure 3). The human gene TACR3 (Gene ID 6870), [34] has five exons with chromosomal localization 4q24 and encodes the protein NK-3R [66]. Main post-translational modifications of the receptor include the glycosylation of asparagines 23, 50, and 73 (in the N-terminal region), a disulfide bridge between cysteines 158 (in TM3) and 233 (in ECL2), and the palmitoylation of cysteine at position 374 in the intracellular C-terminal domain. The preferred endogenous ligand of NK-3R is NKB (Figure 3 and Figure 5).
Molecular modeling studies using the structure of rhodopsin receptors and manual docking of NKB (PDB ID 1P9F [67] within the NK-3R model (Figure 5) resulted in the definition of the site where the NKB C-terminal region contacts the receptor protein [68]. According to the refined model, the C-terminal region of NKB lodges three pharmacophores (Phe6, Leu9, and Met10) that stabilize closely interacting with three hydrophobic holes in the NK-3R. The aromatic ring of Phe6 favors contacts with residues S130, P165, I166, A168, V169, F170, and W312. Interactions π–π between the phenyl group of F6 and the aromatic rings of F170 and W312 orientate the peptide in an optimal position within the receptor cleft. The isobutyl l chain of L9 contacts with residues C311, W312, P314, L344, A345, M346, and S347. The binding pocket for Met10 includes residues F123, V169, S172, M176, V304, F308, C311, S347, S348, and M350. (Figure 5B,C).
Docking analysis performed in a model of NK-3R based on the 3D structure of bovine rhodopsin reported different binding sites for the NKB in TM domains 2, 6, and 7, as well as ECL2. Additionally, the antagonists metaltenant and osatenant occupied different positions within the receptor, indicating that the binding site for the antagonists greatly coincided but did not entirely overlap [69]. Using a model based on the structure of bovine rhodopsin, Geldenhuys et al. [70] proposed a pharmacophore for quinoline derivatives with antagonistic properties formed by three groups: two aromatic rings, two hydrogen donors, and one aromatic acceptor that would anchor in a cleft within the receptor structure.
Further refined structural analysis will unravel the atomic setting accommodating agonists, antagonists, and receptor modulators. The structure dynamics analysis will contribute essential information supporting the physiological and pathological contribution of NK-3R and their collaboration with other NK receptors responding to the three agonists, NKA, SP, and NKB.
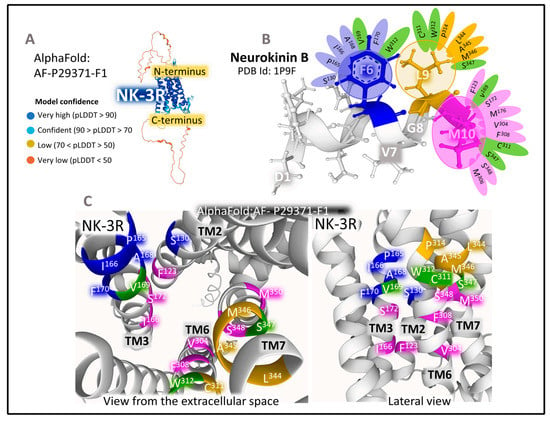
Figure 5.
(A) represents the predicted structure of NK-2R (UNIPROT P29371) [25] according to the prediction Alpha-Fold model [62,63,64]. The parameter pLDDT (predicted local distance difference test) assessed the model confidence. (B) depicts the 3D structure of NKB determined by NMR obtained from the Protein Data Bank [65] (PDB ID 1P9F, [67]) and drawn with Mol* free web-based software [71]. The panel also shows the contacts of NKB’s C-terminal amino acids with the receptors’ amino acids. (C) illustrates two views of a three-dimensional representation of NK-2R, obtained from modeling and docking studies [56], indicating the principal amino-acid positions in the Alpha-Fold model of NK-2R closely contacting the C-terminal region of NKA. Residues in green contact more than one pharmacophore of NKB.
2.1.3. Intracellular Signaling of NK-2R and NK-3R
NKA and NKB favorably bind and activate NK-2R and NK-3R, respectively, but they can also bind and activate NK-1R. Consequently, when studying the intracellular signaling of NK-2R and NK-3R, one should consider the possible activation of NK-1R by both neurokinins. The presence, absence, and abundance of the three neurokinin receptors and their endogenous agonists result in a possible explanation for the multiple outcomes and physiological and pathological consequences in various tissues and organs. Redundance of tachykinin receptors may be a compensatory mechanism [72]. It, however, may also serve excessive stimulation leading to malfunction. For example, NKA commits NK-1R, not NK-2R, in mouse macrophages to activate cellular events dependent on transcription factor NF-κB [72]. Basic information on neurokinin signaling comes from experimental analysis in cell lines and cell expression systems where a total determination of intracellular second messengers points to the activation of specific biochemical cascades. However, the study of signaling endosomes and compartmentalized intracellular signals should also be considered [17]. This section describes the central signaling cascades activated through NK-2R and NK-3R in different tissues. Figure 6 represents how NK-2R and NK-3R recruit the transducers Gαs, Gαq/11, and Gα12/13 protein subunits. As a result, phospholipase C, adenylyl cyclase, and phospholipase A2 turn on, respectively. Downstream reactions include numerous protein kinases that control cellular function through transient phosphorylation of their respective substrates. Therefore, neurokinin signaling results in a pleiotropic display of multiple players that kindle rapid metabolic adaptations and short- and long-term control of gene expression. The effects vary between cells and tissues and depend on the presence and abundance of receptors. The concentrations and the agonists’ availability also affect the receptor protein’s functionality. Deregulation of these signaling systems causes severe alterations in cell proliferation, cell migration, and inflammation.
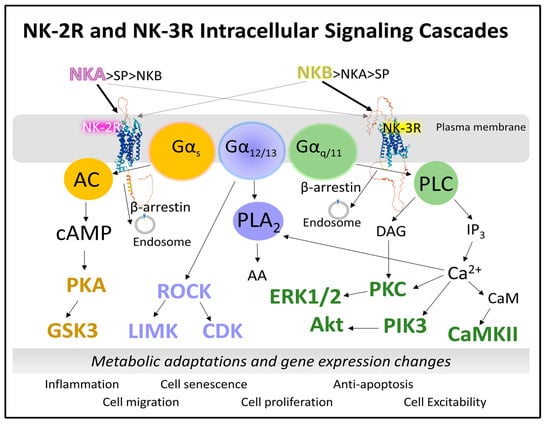
Figure 6.
Schematic representation of signaling cascades triggered by NK-2R and NK-3R. Both receptors may recruit Gα subunits to activate phospholipase C, adenylyl cyclase, or phospholipase A2. Consequently, phosphorylation reactions lead to rapid metabolic changes, the appearance of inflammatory messengers, cell proliferation, cell migration, and the arrangement of cytoskeletal proteins or cell excitability (see text for details). Abbreviations: AA, arachidonic acid; AC, adenylyl cyclase; Akt, Ak strain transforming protein kinase; cAMP, cyclic 3′-5′ adenosine monophosphate; CDK, cyclin-dependent kinase; ERK, extracellular signal-regulated receptor kinase; DAG, diacylglycerol; GSK3 (glycogen synthase kinase); IP3, inositol 1,4,5-trisphosphate; LIMK, LIM protein domains (LIN-11, Isl-1, and MEC-3) kinase; NKA, neurokinin A; NKB, neurokinin B; PLA2, phospholipase A2; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; ROCK, Rho (Ras-homologous)-associated coiled-coil kinase.
Briefly, we describe representative signaling pathways susceptible to activation by NK-2R and NK-3R that may play a role in cell malfunction leading to cancer development.
Activation of adenylyl cyclase generates cAMP, which triggers protein kinase A (PKA) [73,74]. The nucleotide cAMP, independently of PKA, also activates Epac (guanosine exchange proteins directly activated by cAMP) and nonselective cation channels [75]. Numerous substrates of PKA include phosphorylase kinase, GSK3 (glycogen synthase kinase), CaMKII (calcium–calmodulin kinase), and the transcription factor CREB (cAMP-responsive element binding protein), to mention a few [74]. The aberrant function of the signaling axis cAMP/PKA/CREB and the abnormal behavior of other PKA substrates may cause altered cell metabolism and gene expression affecting cell growth, proliferation, migration, and cell adhesion, driving the development of tumors in different tissues [76].
The PLC/IP3/intracellular calcium axis activates PKC (protein kinase C) and other calcium-dependent kinases: CaMKII or PI3K (phosphatidylinositol 3-kinase). PKC is a serine–threonine kinase with copious potential substrates, including, for example, GSK3, histones, integrins, dynamins, the apoptosis regulator Bcl2 (B-cell lymphoma), Bad (Bcl-associated death protein), and the proto-oncogene transcription factor cMyc [77]. PKC, therefore, is a crucial enzyme with a regulatory role associated with many aspects of cell survival and proliferation.
Heterotrimeric Gα12/13 proteins regulate signaling pathways that modulate cell functioning through several targets. One primary target, through Rho GTPase, is the protein kinase ROCK (Rho-associated coiled-coil kinase) [78]. ROCK modulates cytoskeleton organization and assembly by controlling the activity of LIMK (LIM protein domains kinase) and MLC (myosin light chain) phosphatase. In endothelial cells, ROCK regulates angiogenesis by activating transcription factors, such as c-Fos, c-Jun, or HIF-1α (hypoxia-inducible factor) [79]. ROCK proteins also maintain cell proliferation by directing cell-cycle cyclins (cyclin A) and cyclin-dependent kinases (CDK1) [80]. ROCK phosphorylation is associated with the induction of cancer stem cells, cell invasion and metastasis, angiogenesis, dysregulated energy metabolism, and altered cell proliferation. Therefore, its participation in cancer deserves attention [79].
The hydrolytic activity of phospholipase A2 (PLA2) (EC 3.1.1.4) on membrane phospholipids generates two products: mainly arachidonic acid (AA), esterified to the sn-2 position of glycerol, and lysophospholipids [81]. Lysophospholipids regulate cell function by activating specific GPCRs and other effector proteins [82]. The other product of the reaction catalyzed by PLA2 is AA. This fatty acid is the metabolic precursor, through different enzyme-catalyzed reactions, of prostaglandins, leukotrienes, eicosanoids, and prostacyclins. Metabolic products of AA are involved in multiple cellular responses responsible for inflammation, cell survival, cell proliferation, and cell migration [83].
The activation of NK receptors by neurokinins depends on several factors, from receptor number, activated states, receptor occupancy, recruited effectors, and allosteric regulators to neurokinin concentrations and their competitivity for the three receptors NK-1R, NK-2R, and NK-3R. The intricacy of signaling pathways and their fine-tuned regulation protect the cell by employing redundant reactions and controls. Cancer development requires the malfunction of several biochemical mechanisms that make it difficult for the cell to overcome. Not only can all activate signaling pathways triggered by tachykinins crosstalk, but also those dependent on the activity of agonists acting on other co-existing GPCR receptors [84].
2.1.4. The Expression of NK-2R and NK-3R
The NK receptors appear in the central and peripheral nervous system, endothelial cells, smooth muscle, or blood cells (lymphocytes, neutrophils, and macrophages), where they control multiple biochemical mechanisms attending endocrine, inflammation, or smooth muscle activity regulation [85,86].
According to the human protein atlas [87], the expression of NK-2R, measured as mRNA, distributes in different tissues, but the higher presence appears in the gastrointestinal tract, kidney, urinary bladder, muscle tissues (smooth, heart, and skeletal), monocytes, and female and male tissues. Its detection in the brain and other tissues is scarce. The mRNA coding for the NK-3R is detected in the brain, eye, kidney, and urinary bladder with a weak signal in other tissues (lung, pancreas, or gastrointestinal tract). The mRNA coding for the NK-2R increased in human cancer samples from the gastrointestinal tract, testis, breast, endometrial, cervical, and urothelial tissues. The upregulation of mRNA coding for the NK-3R was higher in human lung, breast, urothelial, endometrium, testis, and ovarian cancer than in normal tissues [17,88,89].
Alterations in the expression profiles of TACR2 and TACR3 genes have been reported for different pathologies, e.g., polycystic ovarian syndrome [90], leiomyoma [91], oral carcinoma [21], or breast cancer [92]. However, further studies will reveal why, how, and to what extent dysregulation of NK-2R and NK-3R affects cell function and may provoke abnormal cell growth and development. The altered activity of NK-2R and NK-3R has to be considered together with the modified action of NK-1R.
2.2. Calcitonin/Calcitonin Gene-Related Peptide Family
The peptides of this family (adrenomedullin, adrenomedullin 2, amylin, and calcitonin gene-related peptide) regulate the secretion of hormones and are widely distributed by the body [93,94]. Many tumor types have reported AM expression and secretion [95]. AM exerts an antiapoptotic effect in endothelial cells, and cancer cells promote angiogenesis, regulate the permeability of endothelial cells, and contribute to the differentiation of bone marrow-derived mononuclear cells into endothelial progenitor cells. It is also involved in several pathologies [95,96,97,98,99,100,101,102,103,104,105,106]. Amylin, an islet amyloid polypeptide, regulates insulin secretion/glucose homeostasis and is a crucial constituent of the amyloid in insulinomas [107,108,109,110]. Widespread distribution of calcitonin gene-related peptide (CGRP) throughout the peripheral and central nervous systems has been reported; CGRP has been located in myelinated A gamma fibers, small-diameter sensory C fibers, and unmyelinated fibers [93].
2.2.1. Genes and Products of Human Calcitonin
The human calcitonin family of peptides includes calcitonin (CT), α and β forms of CGRP, amylin (AMY or islet amyloid polypeptide (IAPP)), AM, and AM2 or intermedin. Their amino-acid sequence length varies from 32 in CT to 52 for ADM [47] (Figure 7). Although the amino-acid sequence among the six peptides is variable, they share common architectural traits essential for functionality, including a coil structure, a central area showing a helical or disordered structure, and an amidated C-terminal amino acid [111,112,113]. These peptides activate a family of receptors belonging to class B GPCR to serve numerous and diversified functions, such as plasma calcium reduction (CT), food intake modulation (AMY), and vasodilatation and inflammation (CGRPs) in different tissues [112,113,114].
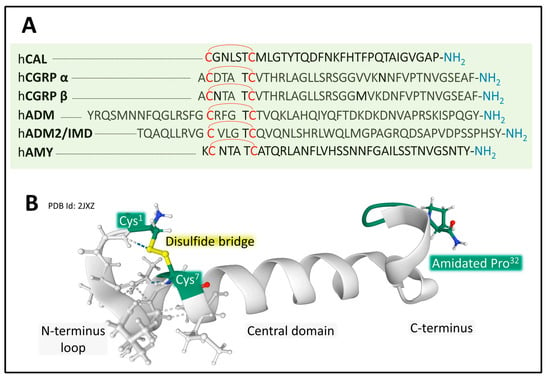
Figure 7.
(A) shows the amino-acid sequences of the calcitonin/CGRP family. All six peptides present a disulfide bridge between two cysteines in the N-terminal region. Amino-acid sequences are from the GPCR database GPCR database [47]. (B) depicts the NMR structure of a human analog of human calcitonin in sodium dodecyl sulfate micelle [115]. Shared structural features include a disulfide bride at the N-terminal region, a helix structure in the central domain, and an amidated C-terminal residue. The configuration corresponding to PDB ID 2JXZ is from the Protein Data Bank [65], drawn with free web-based Mol* software [71].
Five human genes encode the calcitonin/CGRP family of peptides. CALCA, CALCB, and AM are on chromosome 11, IAPP is on chromosome 12, and AM2 is on chromosome 22 [34,113]. The peptide products synthesized are tissue-specific in physiological and pathological situations because spliced variants transcripts encode different products. For example, a CALCA gene transcript variant encodes calcitonin in the thyroid gland in physiological conditions. Another variant of the CALCA gene encodes the neuropeptide CGRPα in the neural tissues [116] (Figure 8).
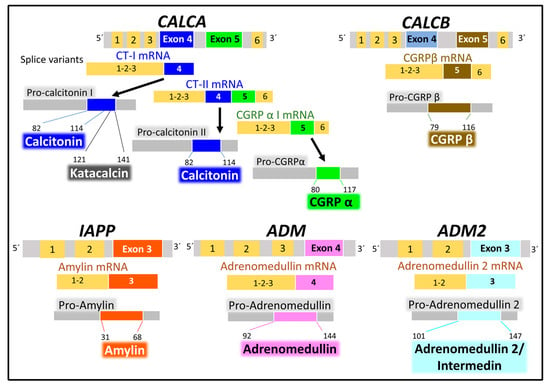
Figure 8.
The figure represents the human calcitonin/CGRP family genes and their main peptide products, calcitonin, CGRP (calcitonin gene-related peptide) isoforms α and β, amylin, adrenomedullin, and adrenomedullin 2/intermedin. Alternative splicing generates mRNA isoforms transcribed into peptides that undergo post-translational modifications (protein hydrolysis, disulfide bridge formation, and amination of C-terminal amino acid), giving rise to functional peptides. Data supporting this scheme are from the UNIPROT [25] and Entrez Gene (National Library of Medicine) [34] databases. Numbers indicate the residue position within the peptide sequences.
All six genes encode precursor peptides (pro-peptides) that undergo post-translational modifications (hydrolysis on a pair of aromatic residues, disulfide bridge formation at the N-terminal region, and amidation of the C-terminal amino acid) to attain a functional structure. The CALCA gene encodes CT and the α form of CGRP, and the CALCB gene encodes the β form of CGRP. The IAPP, AM, and AM2 genes encode AMY, AM, and AM2/intermedin peptides [113,114,116,117]. In addition to the peptides enumerated, this family of genes generates other peptides with biological activity. One of them is katacalcin [118], a calcium-lowering hormone that occupies the pro-calcitonin I precursor’s amino acids from 121 to 141.
2.2.2. Structure and Dynamics of the CGRP Receptor: A Three-Component Complex
The structural states of CGRP receptors (CGRPR), in both their apo and their ligand-bound forms, are essential to understand how structural dynamics direct the recruitment of intracellular proteins responsible for signaling mechanisms leading to molecular changes and cellular adaptations [119]. Defining the functional architecture of protein receptors requires fine studies at the atomic level that unveil how ligands (agonists and antagonists) interact with the protein. Combining CLR (calcitonin receptor-like receptor) and RAMPs homologous (receptor activity modifying protein 1) 1, 2, and 3 generates different receptors with variable affinities for endogenous ligands. The CGRPR (calcitonin-gene-related peptide receptor) is a heterodimer formed by CLR and RAMP1 exhibiting a high affinity for CGRP. CLR-RAMP2 (AM-1-receptor) and CLR-RAMP3 (AM-2-receptor) bind with higher affinity to AM, and CTR (calcitonin receptor) associated with RAMP1 is the AMY-1-receptor [120,121]. CLR belongs to class B1 (secretin), subfamily CT-like [122] of GPCR (G-protein-coupled receptors). The human CLR protein (UNIPROT Q16602) comprises 461 amino acids and exhibits post-translational modifications affecting the amino- and carboxy-terminal ends (Figure 9).
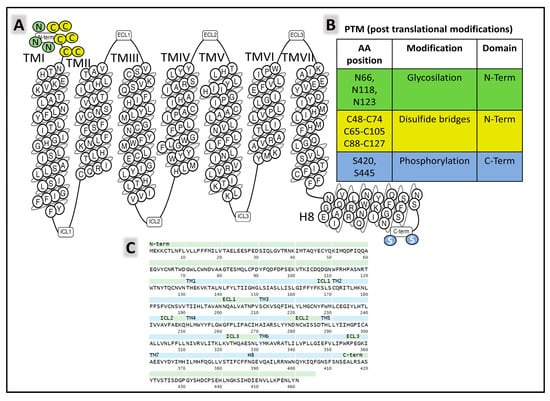
Figure 9.
(A) is a snake plot [47] of the general architecture of CLR (calcitonin receptor-like receptor), indicating transmembrane segments and intra- (ICL) and extracellular (ECL) loops. (B) displays amino acids undergoing post-translational modifications [25]. (C) shows the primary structure of the receptor and the sequences forming different protein domains [47], TM (transmembrane), ECL (extracellular loops), ICL (intracellular loops), and H8 (helix 8).
RAMP1 is a chaperone protein with a single transmembrane domain that allosterically modulates CLR function [123]. It sits within transmembrane (TM) helices TM3, TM4, and TM5 of CLR (Figure 10). The signaling efficiency of the receptor complex CLR-RAMP activated with different affinities by endogenous ligands CGRP, AM, AMY, and CT improves with a third proteinaceous component directly interacting with CLR, the RCP (receptor component protein) [124,125,126].
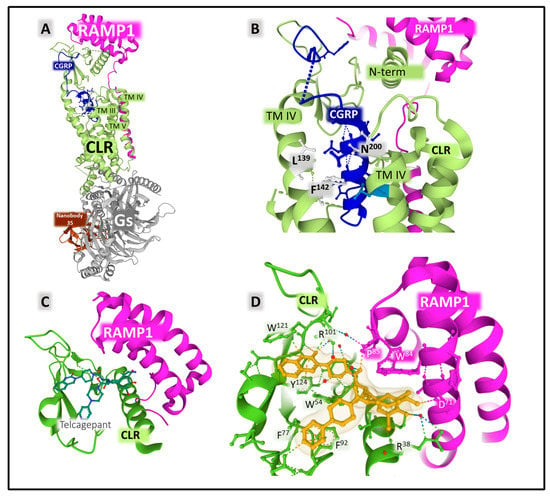
Figure 10.
(A) shows the crystal complex formed by CLR (calcitonin receptor-like receptor in green) bound to protein RAMP1 (receptor activity modifying protein, in pink), Gs heterotrimeric protein (gray), and nanobody 35 (brown). The interaction of RAMP 1 with the transmembrane segments III, IV, and V and the N-terminal portion of CLR are indicated in the figure. (B) depicts the binding site of CGRP (calcitonin gene-related peptide, blue), showing the amino-acid positions of CLR contacting the agonist peptide. (C) shows the crystal structure of an ectodomain complex of the CGRP receptor bound to the antagonist telcagepant (MK0974). (D) highlights the contacts between the antagonist and the receptor structure. The figures mark the position of transmembrane (TM) helices. Dashed lines indicate weak interactions stabilizing peptide structure. The representations in (A,B) corresponding to PDB ID 6E3Y [123], and those in (C,D) corresponding to PDB ID 3N7S [127] were obtained from the Protein Data Bank (PDB [65]) and colored with Mol* free web-based software [71].
A crystal structure of the ectodomain of the CGRP receptor bound to the selective antagonist telcagepant revealed the interactions between the antagonist compound and the lateral chains of amino acids from RAMP1 and CLR (Figure 10C,D) [127]. Telcagepant and olcegepant exert their antagonistic effect by disrupting the access of the endogenous peptide agonist to the binding site located in the interphase between CLR and RAMP1 [127].
Structural studies also revealed the binding of antibodies to halt CGRPR action. Erenubab is a monoclonal IgG antibody approved by the US FDA (Federal Drug Administration) for the treatment of migraine. The antibody is highly selective and potent, and it exhibits full antagonism for the CGRPR [128]. X-ray crystallography and functional studies [129] determined the interaction of erenumab with the receptor complex (Figure 11). Several regions of the light and heavy chain of the antibody, named CDRs (complementary determining regions), are in close contact with the interface between CLR and RAMP1. Given its conformational plasticity, region CDRH3 in the heavy chain, including residues 99 to 119, represents a key sequence responsible for receptor recognition and selectivity. Eventually, the interaction of the antibody with the receptor complex impedes the access of the agonist to its binding site and, consequently, exerts its antagonist effect [129].
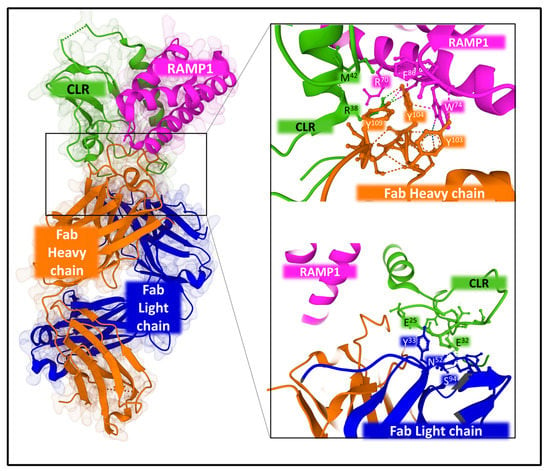
Figure 11.
Structure of the complex CLR-RAMP1 co-crystalized with monoclonal antibody 6UMG. The figure on the right-hand side depicts some atomic contacts of the antibody heavy chain (upper panel) and the antibody light chain (lower panel) with the interface of the complex CLR-RAMP. The structures corresponding to PDB ID 6UMG [129] were obtained from the Protein Data Bank (PDB [65]) and designed with Mol* free web-based software [71].
Distinctive coupling of antibodies and small non-peptide antagonists to the receptors may explain differences in selectivity, efficacy, and potency with important consequences in designing molecules that control the CGRP receptor’s activity [130].
2.2.3. Mechanisms of Signaling of the CGRP Receptor
The heterodimer CLR-RAMP1 shapes the fully functional CGRPR. RAMP1 serves several functions, from determining, together with CLR, the peptide binding site to helping the glycosylation of the N-terminal segment of CLR and the intracellular trafficking of the receptor [131,132]. However, the high-affinity state of the receptor requires the recruitment of RCP, a peripheral membrane protein, acting as an allosteric modulator that augments the effective functioning of the receptor complex but does not modify agonist binding [126]. In cell cultures, eliminating RCP by knockout significantly reduced cAMP production by stimulating CGRP receptors [126]. Interestingly, this protein does not favor the recruitment of Gq protein, does not participate in Gq-conveyed events [133], and may contribute to CGRPR bias signaling. The precise mechanism explaining the influence of RCP on CGRP receptor signaling is not fully understood [132].
Like many other GPCRs, the CGRP receptor signals through several kinase cascades, which amplify the signal to modulate many intracellular components, from metabolic enzymes to transcription factors [38,84]. As CGRP may signal through CGRP receptors, AM, and AMY receptors, delimiting the activity of CGRP only through CGRP receptors may be difficult [132]. However, signaling profiles depend on the selectivity of the agonist-receptor binding, the availability of signal transducers, and intracellular kinase targets.
Figure 12 summarizes the principal routes of intracellular signaling activated by the heterodimer CLR-RAMP1 with the allosteric aid of RCP. CGRP receptors recruit Gαs subunits to activate adenylyl cyclase and set PKA in action. Various PKA substrates (from K+ channels to transcription factors and enzymes) amplify and diversify the primary signal [134,135,136,137] CGRP receptors may also trigger Gαq subunits, leading to PKC activation.
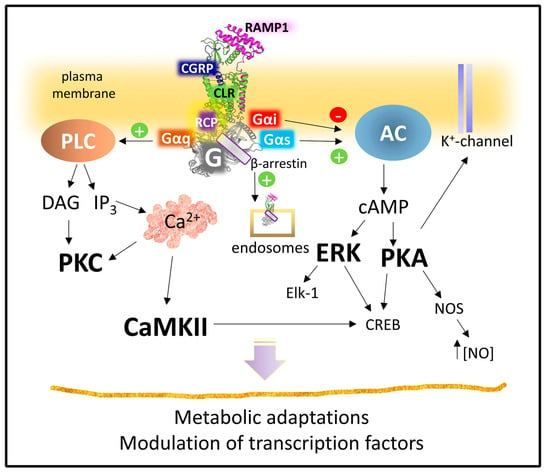
Figure 12.
Signaling pathways activated by CGRP receptor. Abbreviations: CaMKII, calcium-calmodulin kinase II; cAMP, cyclic adenosine 5′monophosphate; CLR, calcitonin receptor-like receptor; CREB, cAMP-response element binding protein; DAG, diacylglycerol; Elk-1, ETS-erythroblast transformation specific-like protein; ERK, extracellular receptor kinase; IP3, inositol 1,4,5-trisphosphate; NOS, nitric oxide synthase; PKA, protein kinase A; PKC, protein kinase C; RAMP1, receptor activity modifying protein 1; RCP, receptor component protein (see text for details).
However, CGRP receptors do not only signal from the plasma membrane settlement. Upon activation, the CLR component suffers phosphorylation and β-arrestin recruitment leading to internalization [132]. A clathrin and dynamin-dependent endocytosis can transport the receptor complex to the cell’s interior. Once in the cytoplasm, the complex may locally signal to regulate different pathophysiological outcomes [138].
It is necessary to consider that most experiments and data concerning CGRP receptor signaling come from studies on cell cultures or cells transfected with the receptor complex. In vivo studies are necessary to corroborate the receptor’s role in a more physiological context.
3. Involvement of the Tachykinin and Calcitonin/Calcitonin Gene-Related Peptide Families in Cancer
3.1. Tachykinin Peptide Family
SP is the most studied tachykinin, including its implication in cancer [1,5]. However, other main tachykinins are less studied; thus, the participation of both NKA and NKB in cancer is reviewed (Table 1).

Table 1.
Involvement of NKA/NKB in cancer.
3.1.1. Breast Cancer
NKA exerts a proliferative action on breast carcinoma tumor cells expressing NK-2R [139]. Compared to non-metastatic breast cancer cells, overexpression of NK-2R/NK-1R was reported in metastatic breast cancer cell lines [140]. Malignant breast biopsies and breast cancer cells lines showed an increased expression of both NK-1R and pre-protachykinin A compared with benign breast biopsies and normal mammary epithelial cells; the level of NK-2R was high in both malignant and normal cells [92]. NK-2R and NK-1R antagonists blocked the proliferation of breast cancer cells, and this suggests that an autocrine stimulation of these cells occurs via pre-protachykinin A peptides (NKA, SP) [92]. Importantly, NK-2R mediated the proliferation of breast cancer cells but not that of normal cells [92].
NKA and SP increased the aggressiveness of a metastatic breast cancer cell line by increasing its ability to migrate and invade tissues [141]. Both peptides augmented the expression of NK-2R and NK-1R on the metastatic breast cancer cell line and, in addition, promoted the release from these cells of the high-molecular-weight kininogen molecule (bradykinin precursor) which exerted tumorigenic and pro-nociceptive actions [141]. Significantly, SP only increased the expression of the NK-1R, but NKA increased the expression of both NK-2R and NK-1R in the mentioned breast cancer cell line [141].
3.1.2. Colorectal Cancer
A high expression of the NK-2R gene has been related to poor survival in patients with colorectal cancer; this expression was increased by interferon-α/β in a Janus kinase 1/2-dependent manner [143]. NK-2R rs4644560 GC polymorphism alone or in combination with NK-1R rs10198644 GC is a prognostic marker for lymph node metastasis in patients with colorectal cancer [142]. Patients with NK-2R rs4644560 CC showed lesser positive lymph nodes than those with rs4644560 GC, and the number of positive lymph nodes was increased in NK-2R rs4644560 GC/NK-1R rs10198644 GG patients compared to NK-2R rs4644560 GG/NK-1R rs10198644 GG individuals.
Colorectal tumor cells overexpressing NK-2R showed increased tumorigenesis and metastatic colonization in vivo experiments; this expression has been associated with the malignancy of colon cancer cells [143]. SP reduced the invasive potential of colon carcinoma cells, but NKA had no effect [167]. NKA increased the viability and proliferation of colon cancer cells and the phosphorylation of extracellular-signal-regulated kinase 1/2 levels in interferon-α/β-treated colon cancer cells, whereas NK-2R antagonists reduced the proliferation of these cells [143]. However, NKA did not exert growth-regulatory actions on a human colon cancer cell line (HT 29) and a non-transformed small-intestinal cell line from the rat (IEC-6) [168].
3.1.3. Glioma
NKA promoted the proliferation and release of interleukin-6 from glioma cells expressing NK-1R; these actions mediated by NKA were entirely blocked with a specific NK-1R antagonist (MEN-11467) [144]. The above means that NKA exerted its actions not only via NK-2R but also through NK-1R.
SP binding sites but not NKA binding sites have been reported in an astrocytoma cell line (U 373) [169]. NKA and NKB promoted taurine release from astrocytoma cells. NKA induced a greater release than NKB; however, SP promoted the most significant release of taurine from these cells [170]. Astrocytoma cells express NK-3R, and endocytosis of copper-bound NKB occurs through a trafficking pathway that includes early endosomes [145].
3.1.4. Insulinoma
RIN5mF cells (rat insulinoma cells) express the pre-protachykinin A gene and release NKA and SP [146,147]. No change in the concentration of NKA/SP was observed in the small intestine and stomach of insulinoma rats [171].
3.1.5. Lung Cancer
Pulmonary carcinoid tumors express mRNA pre-protachykinin A; in some, NKA/SP has been reported [148]. NKA and NKB inhibited the growth of small-cell lung cancer cells [20].
3.1.6. Medullary Thyroid Carcinoma
NKA has not been detected in medullary thyroid carcinoma [172].
3.1.7. Midgut Carcinoid Tumor
Pre-pro-tachykinin A mRNA expression and the presence of NKA/SP have been reported in midgut carcinoid tumors and in the plasma of patients with midgut carcinoids [148,149]. These tumors show an unpredictable clinical behavior; hence, specific biomarkers are needed to detect the disease early [150]. Plasma NKA level is an excellent biomarker of prognosis; patients with a high NKA level showed worse survival than those with a decreased or stabilized level [150]. Notably, the most recent plasma NKA level predicted a better survival than the initial value of such a level. Moreover, increased luminal content of tachykinins (e.g., NKA and NKB) has been reported in malignant midgut carcinoids compared with that reported in healthy individuals [151].
3.1.8. Neuroblastoma
NKB but not NKA/SP has been located in neuroblastoma [152]. NK-2R and NK-1R mediate the proliferation exerted by pre-protachykinin A peptides on neuroblastoma cells [153]. The murine neuroblastoma C1300 cell line expresses mRNA NK-2R/NK-3R, but not mRNA NK-1R and NKA; NK-3R increased its cytosolic Ca++ concentration, which was inhibited with the NK-2R antagonist SR-48968 [154,155]. NK-2R and NK-3R are activated independently by NKA; the activation of both receptors promoted not only a Ca++ increase but also the formation of inositol trisphosphate, whereas both mechanisms were blocked with phospholipase C inhibitors [154]. The results demonstrated that NK-2R/NK-3R-mediated Ca++ increase was due to the activation of phospholipase C and, in addition, it was dependent on the entry of extracellular Ca++ via voltage-independent channels and the mobilization of internal Ca++ stores [154].
3.1.9. Oral Squamous Cell Carcinoma
NK-3R expression was very high in oral squamous tumor cells, whereas, in normal epithelial cells, NK-3R was not observed [21]. Moreover, those squamous cells that invaded the mandible bone matrix showed a higher expression of NK-3R and using the selective NK-3R antagonist SB-222,200 significantly inhibited tumorigenesis and the osteolytic lesion [21,156]. Cancer cells did not express NKB, but this peptide was observed in sensory nerves in the mandible. This discovery suggests that the release of NKB from these nerves could regulate the proliferation of tumor cells.
3.1.10. Phaeochromocytoma
NKB has only been detected in one of the 10 phaeochromocytomas studied; in the same study, NKB was not detected in carcinoid tumors [157]. Moreover, in a human phaeochromocytoma extract, NKA/SP was detected [31].
3.1.11. Schwannoma-Derived Cells
NKA and SP were located in the cytoplasm of malignant schwannoma-derived cells [158]. Both peptides seem to play a role in the tumor microenvironment; however, future studies must elucidate this.
3.1.12. Small Bowel Neuroendocrine Tumors
Small bowel neuroendocrine tumors are difficult to diagnose; thus, it is important to determine specific biomarkers associated with the disease. NKA is a specific blood biomarker because high plasma NKA levels (≥50 ng/L) have been associated with poor prognosis in patients with these tumors. Monitoring such levels could be useful in selecting patients with a poor prognosis; hence, a high NKA level means that an urgent therapeutic intervention is needed [159,160,161,162,163]. Thus, plasma NKA levels predict survival in patients with small bowel neuroendocrine tumors. An improved prognosis was reported by lowering the plasma NKA level below 50 ng/L [161,163].
Compared with healthy individuals, a rise in circulating NKA/SP has been reported in patients suffering from ileal metastatic carcinoid tumors showing cutaneous flushing; the release of both peptides from carcinoid tumors was partially blocked after the administration of a somatostatin analog [164]. The presence of NKA, NKA3-10, and NKA4-10 has been detected in ileal metastatic carcinoid tumors [165]. Therefore, NKA shows an N-terminal heterogeneity in these tumors, and carcinoid tumors can release different amounts of several tachykinins contributing to individual differences.
3.1.13. Uterine Leiomyomata
Leiomyoma, a benign smooth muscle tumor, showed an upregulation of NK-2R mRNA compared with normal myometrium; however, the levels of NK-2R protein were similar in both tumor and normal cells [166]. Moreover, leiomyomas express NKB and NK-3R, which were significantly more highly expressed in this benign tumor than in normal myometrium [91]. NKB was observed in the nuclei of smooth muscle cells in normal myometrium, whereas leiomyoma cells showed a predominant cytoplasmic expression of the peptide [91]. Estrogens control NK-3R, and the activation of this receptor promoted nuclear translocation affecting gene expression and chromatin structure [91]. These results suggest that the NKB/NK-3R system is involved in the pathological events observed in women suffering from leiomyomata.
Figure 13 shows the main findings mentioned in this section regarding the implication of NKA/NKB in cancer.
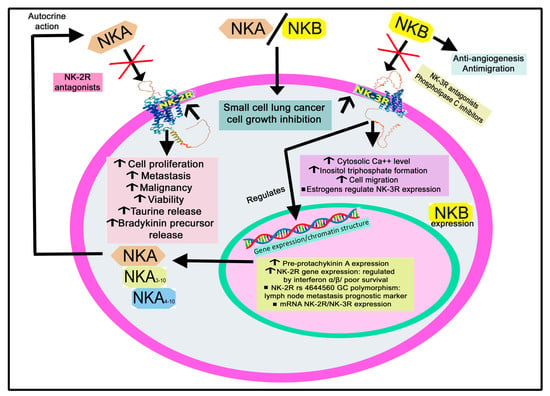
Figure 13.
Summary of the mechanisms mediated by NKA/NKB via NK-2R/NK-3R in tumor cells. NKA: neurokinin A; NKB: neurokinin B; NK-2R: neurokinin-2 receptor; NK-3R: neurokinin-3 receptor. ↑: increase; ↓: decrease.
3.2. Calcitonin/Calcitonin Gene-Related Peptide Family
Extensive data have shown the involvement of this peptide family in cancer. For example, adrenomedullin (AM) is released from choroid plexus carcinoma [173]; AM released from tumor cells drives both tumor and lymph node lymphangiogenesis, and AM gene dosage has been related to both mechanisms [174]. Tumor cells express/overexpress AM (e.g., ovary, prostate, kidney, skin, endometrial, liver, pancreatic, brain, and breast cancer), and the level of AM has been correlated with cancer severity [104,175].
AM acts as a mitogenic agent on tumor cells and favors a more aggressive tumor phenotype. Under hypoxic conditions that occur in the proximity of solid tumors, the peptide is upregulated via an HIF 1-dependent pathway in normal and tumor cells, promoting angiogenesis [176,177,178,179]. AM also prevents apoptosis, suppresses the immune system, and is involved in bone metastasis [180]. Adrenomedullin 2 (AM2/intermedin) and CGRP act as tumor survival/growth factors promoting lymphangiogenesis and angiogenesis [175]. Lastly, the infection of dermal endothelial cells with live and UV-inactivated Kaposi’s sarcoma-associated herpesvirus (KSHV) demonstrated that the viral gene expression was responsible for the upregulation of the AM2 gene [181]. The implication of AM, AM2, AMY, and CGRP in 29 tumors is reviewed in Table 2.

Table 2.
Participation of adrenomedullin (AM), adrenomedullin 2 (AM2), amylin (AMY), and calcitonin gene-related peptide (CGRP) in cancer.
3.2.1. Acute Myeloid Leukemia
Adrenomedullin
AM, CLR, and RAMP2 and 3 have been reported in acute myeloid leukemia. A high level of AM has been associated with low overall survival and disease-free survival [182,183]. AM and AM22–52 fragment regulate the growth of leukemia cells through autocrine and paracrine mechanisms [184]. The expression of AM in acute myeloid leukemia has been associated with genes related to immunosuppression and a stem cell phenotype, leading to disease relapse and therapy resistance [185]. This observation is vital since drug-tolerant and -resistant leukemia stem cells have been associated with relapses in acute myeloid leukemia. AM via CLR has been correlated with adverse outcomes of acute myeloid leukemia [183]. Depleting this receptor decreased leukemia stem cell frequency of relapse-initiating cells post chemotherapy in vivo. CLR knockdown also decreased leukemia stem cell frequency and impaired leukemia cell growth [183]. This finding suggests that AM is involved in the post-chemotherapy persistence of these cells, and that targeting CLR could prevent relapse in acute myeloid leukemia [183].
Calcitonin Gene-Related Peptide
A possible origin of leukemogenesis is the disruption of DNA methylation patterns, and, in acute leukemias, the CT gene plays a crucial role in gene hypermethylation [186]. The CT gene methylation pattern is an independent prognostic factor in pediatric acute leukemia that could characterize a group of patients with enhanced risk of relapse and death [186]. This gene was found to be hypermethylated in children with acute myeloblastic leukemia (54.3%) or with acute lymphoblastic leukemia (65.7%) [186]. This hypermethylation was not associated with response to induction therapy, standard prognostic factors, and clinicopathologic characteristics.
A high CT receptor expression has been related to a poor prognosis in acute myeloid leukemia. This receptor was upregulated in leukemic stem cells and at relapse of acute myeloid leukemia [182]. Moreover, CT receptor expression was correlated with chemotherapy resistance [182]. In experimental animal models of acute myeloid leukemia, olcegepant (CGRP antagonist) increased cell differentiation and decreased leukemic burden and stem-cell characteristics [182]. Moreover, MK0974 (CGRP antagonist) blocked the CLR/RAMP1 complex and promoted apoptosis in the EVI1 acute myeloid leukemia cell line, and this antagonist also attenuated p38 and ERK phosphorylation in this cell line [136]. Thus, blocking the CT receptor/CGRP system could be an antitumor strategy to treat this disease.
3.2.2. Adrenocortical Tumor
Adrenomedullin
AM is synthesized and released from adrenocortical tumors and phaeochromocytomas [189], with the release of AM controlled by cytokines [286]. It has been suggested that the low/undetectable level of AM immunoreactivity in adrenocortical tumors is due to a rapid release of AM from tumor cells [189]. mRNA expression of AM and its receptor RAMP2/CLR has been reported in phaeochromocytoma tissues and the normal adrenal medulla. Nevertheless, this expression was higher in phaeochromocytomas than in normal medulla [190]. The RDC1 receptor (an AM putative receptor) is expressed differently in benign and malignant phaeochromocytomas. The authors suggested that AM/RDC1 is involved in chromaffin cell tumorigenesis through pro-survival actions [287].
AM plays a part in the pathogenesis of phaeochromocytoma; the peptide is increased in this disease, and its level has been correlated with plasma noradrenaline levels [288,289]. AM blocked the proliferation of human phaeochromocytoma cells [190], and the level of AM has been suggested to be a biomarker in patients with phaeochromocytoma [191]. Circulating AM levels increased in patients with phaeochromocytoma compared with patients with nonfunctional adrenocortical adenomas and healthy individuals [289]. Moreover, plasma chromogranin levels correlated with plasma AM/metanephrine concentrations in phaeochromocytoma patients [289]. AM expression was controlled by the nerve growth factor in phaeochromocytoma PC12 cells, but its expression was inhibited during the neuronal differentiation of these cells [290].
Adrenomedullin 2
Phaeochromocytomas and aldosterone-secreting adenomas express AM2 [187]. This expression has been reported in human adrenal tumors of both medullary or cortical origins and in attached non-neoplastic adrenal tissues [192]. However, this expression was lower in the non-neoplastic portion of attached adrenal cortices than in adrenocortical tumor cells [192]. Moreover, AM2, CLR, RAMP1, RAMP2, and RAMP3 mRNA expressions were also observed in both adrenal tumors and attached adrenal tissues [192]. Thus, it seems that AM2 is involved in tumor growth.
Calcitonin Gene-Related Peptide
A high CGRP level has been reported in tissues from phaeochromocytomas, and plasma CGRP level was slightly increased. Nevertheless, it did not change significantly with tumor manipulation or early after tumor resection [188].
3.2.3. Bladder Cancer
Adrenomedullin
Patients with bladder urothelial cell carcinoma showed a higher presence of AM in the tumor than in adjacent nontumor bladder regions. Under hypoxic conditions, AM expression increased in human bladder cancer cell lines [193]. Moreover, compared to the control group, AM knockdown in T24 cells promoted apoptosis, and the combination of AM knockdown and cisplatin decreased tumor growth when compared with cisplatin treatment alone or AM knockdown alone [193]. This finding indicates that AM is involved in the development of bladder cancer, and that AM may represent a potential antitumor target.
3.2.4. Breast Cancer
Adrenomedullin
Overall, 82% of the breast cancer samples studied showed a moderate to strong staining for AM, and this expression was associated with axillary lymph node metastasis [194]. AM plasma level reflects the primary tumor size in breast cancer patients. Nevertheless, no difference was reported between the AM plasma levels of healthy individuals and patients with breast cancer [194]. Thus, the level of circulating AM cannot be used as a tumor marker in breast cancer, but it could be a predictor for lymph node metastasis.
Human breast cancer cell lines (T47D and MCF7) express a low level of AM; however, when they were transfected with an expression AM construct, both cell types expressed a high level of AM (protein and AM mRNA) [99]. T47D and MCF7 cells overexpressing AM increased their angiogenic potential and showed less apoptotic mechanisms after serum deprivation and a more pleiotropic morphology [99]. Both cell types did not show motility, but ECV ovarian tumor cells treated with AM showed higher motility than saline-treated ECV cancer cells [99]. Moreover, compared with control cells (T47D cells transfected with empty vector), T47D cancer cells overexpressing AM showed a higher level of proteins involved in oncogenic signal pathways (e.g., mitogen-activated protein kinase (MAPK) p49, protein kinase C, Raf, and Ras) and a lower level of proapoptotic proteins (e.g., caspase 8, Bid, and Bax) [99]. Importantly, animals (three of 10) xenografted with T47D cells overexpressing AM developed tumors, whereas none of the animals xenografted with cells carrying the empty plasmid developed tumors [99]. The data show that AM is a tumor survival factor and that the blockade of AM could be a promising antitumor strategy.
It is known that the lysyl oxidase (LOX) L2 enzyme plays an important role in tumor expression since it induces the epithelial-to-mesenchymal transition (EMT) in cells, which is an important mechanism for tumors to become metastatic. In the MDA-MB-231 metastatic breast cancer cell line, a link between RAMP3 and LOXL2 has been reported; the blockade of LOXL2 promoted a mesenchymal-to-epithelial transition in breast cancer cells, a decreased invasive phenotype, and RAMP3 expression, leading to a reduced tumor development and tumor microvessel density when compared with controls [291]. Therefore, RAMP3 is involved in cancer metastasis. Breast tumor cells, expressing and releasing AM, favor cell proliferation, breast cancer bone metastasis, and angiogenesis, as well as stimulate bone formation and osteoblast activity [195]. This discovery suggests that the peptide is involved in skeletal metastases. Significantly, AM antagonists blocked bone tumor growth and decreased the markers for osteoclast activity [195]. AM expression diminished in triple-negative breast cancer samples and cell lines. This low expression has been related to poor prognosis and an increased risk of tumor recurrence and metastasis [196]. AM could be used as a biomarker for triple-negative breast cancer prognosis. The peptide could act as an antimetastatic agent in this disease because AM, via its effect on cancer cell EMT, decreased tumor cell invasion [196].
Tumor cells interact with cells located in their environment to favor tumor growth and invasion. Cancer-associated fibroblasts are involved in tumorigenesis and angiogenesis. The importance played by the cancer-associated fibroblast-derived AM system in neovascularization and breast carcinoma growth has been demonstrated [197]. AM22–52 treatment disrupted the vasculature of tumors, depleted vascular endothelial cells, decreased tumor cell proliferation, and induced apoptosis [197]. Moreover, breast cancer cells release AM, which regulates the activity of cancer-associated adipocytes, promoting delipidation and metabolic changes [292].
Adrenomedullin 2
Pre-operative plasma AM2 level has been related to poor outcomes in breast cancer patients; hence, it can be used as a prognostic biomarker for these patients [198]. AM2 expression is increased in breast cancer samples. Its level was positively correlated with both Ki67 expression and lymph node metastasis and promoted the growth, migration, and invasion of breast cancer cells; all these actions were blocked with a monoclonal anti-AM2 antibody [199]. AM2 increased the malignancy of breast cancer cells, upregulated the expression of ribosomal component genes by activating the Src/c-Myc signaling pathway, improved tumor blood perfusion, and was involved in vascular remodeling [199].
Calcitonin Gene-Related Peptide
Neoangiogenesis and CGRP expression are increased in mixed invasive–preinvasive breast lesions [200], and CGRP is involved in breast cancer metastasis [201]. CGRP regulates osteoclast coupling genes in the MG-63 osteoblast cell line by decreasing RANKL/Runx2 expressions, increasing OPG expression, and blocking the osteolytic factors induced by the interaction between osteoblasts and breast cancer cells [201]. This blockade was reverted with a CGRP antagonist.
3.2.5. Choriocarcinoma
Adrenomedullin
AM is synthesized and released from cytotrophoblastic cells expressing the AM receptor [202]. AM mRNA expression was reported in human placental trophoblastic tissues and choriocarcinoma JAr cells and AM receptors in trophoblastic cells [202].
3.2.6. Colon Cancer
Adrenomedullin
CLR, RAMP2, and RAMP3 expressions have been reported in colorectal cancer; high levels of AM, CLR, RAMP2, and RAMP3 correlate with lymph nodes and distant metastasis, and a high level of AM correlates with a low disease-free survival [203,204]. AM concentration is higher in colorectal cancer tissues than in adjacent normal tissues, and the presence of AM has been reported in inflammatory bowel disease-derived colorectal cancer [179,205]. A higher AM mRNA expression was observed in patients with colorectal cancer (clinical stages I, III, and IV) than in healthy individuals [204]. AM expression has been associated with vascular endothelial growth factor (VEGF) and HIF-1α in colorectal cancer; these molecules are involved in angiogenesis, and the authors of the study suggested that the degree of AM expression could be used as a marker for the prediction of cancer-related death and high risk of relapse in colorectal cancer patients with a curative resection [203]. DLD-1, a human colorectal carcinoma cell line, increased AM immunoreactivity and the level of mRNA AM under hypoxic conditions; this was also found when these cells were treated with cobalt chloride, a compound that mimics hypoxic states [293]. The conclusion is that AM is an important agent in ischemic states.
HT-29 human colon carcinoma cells synthesized and released AM, and this effect increased under hypoxic conditions. Treating these cells with AM promoted cell proliferation and invasion [204]. By activating the phosphatidylinositol 3-kinase/Akt pathway or upregulating B-cell lymphoma (Bcl)-2, AM exerts an antiapoptotic effect. The AM expression level has been associated with clinical survival rate and cancer stage in colon cancer [206]. This level was higher in colon cancer than in normal tissues, and a relation between AM expression and clinical or pathological parameters was not found in stomach cancers [206]. AM is an upregulated gene in the colon cancer cell line DKs5, which expresses the KRAS oncogene (involved in metastasis, angiogenesis, and chemoresistance) under hypoxia [207]. Knockdown of AM in colon tumor xenografts promoted apoptosis and inhibited angiogenesis, leading to the suppression of tumors [207]. Thus, AM is involved in colon cancer development. A mouse model of colon cancer treatment with the AM positive modulator, 145425, showed a lower number of tumors when compared to the control; this modulator controlled the expression of the Lgr5 proliferation marker [208].
Adrenomedullin 2
Colorectal adenocarcinomas showed a higher expression of pre-proAM, pre-proAM2, CLR, RAMP2, RAMP3, metalloproteinase (MMP)-9, and VEGF-A mRNAs than the adjacent normal tissues [205]. AM and AM2 were mainly detected in cancer cells, along with MMP-9 in the adjacent stroma [205]. Moreover, a positive correlation between MMP-9 gene expression and pre-proAM was observed, but not with pre-proAM2 [205].
Amylin
AMY is rarely expressed in intestine endocrine tumors [294].
Calcitonin Gene-Related Peptide
The number of nerve fibers and neurons containing CGRP was studied in the submucous/myenteric plexuses located in the transitional zone between cancer-invaded areas and morphologically unchanged regions: a decrease in the number of neurons and fibers was observed in both plexuses [295].
3.2.7. Cutaneous Nerve Neuromas
Calcitonin Gene-Related Peptide
CGRP release from nerve fibers was observed in saphenous nerve neuromas [209].
3.2.8. Endometrial Cancer
Adrenomedullin
Cobalt chloride increased the release of AM in endometrial cancer, favoring angiogenic and tumorigenic activities and the secretion of VEGF from tumor cells [210]. AM, by upregulating the Bcl-2 antiapoptotic protein expression, blocks cell death by hypoxia in endometrial cancer cells [211]. AM is upregulated in endometrial cells by tamoxifen, a nonsteroidal antiestrogen; AM also promotes the growth of carcinoma [101,211]. In the endometrium, the level of AM increased in progression in cells from normal, simple, or complex hyperplasia with or without atypia to grade 1 adenocarcinoma, but Bcl-2 expression decreased [212]. Another study confirmed this observation in which AM expression increased from benign endometrium to endometrial intraepithelial neoplasia and type-1 adenocarcinoma, but Bcl-2 expression decreased in the transition from endometrial intraepithelial neoplasia to carcinoma [296]. Lastly, no correlation between AM, HIF-1α, or Bcl-2 expressions and SUVmax (maximum standardized uptake value) was observed in endometrial cancer [297].
3.2.9. Ewing Sarcoma
Calcitonin Gene-Related Peptide
CGRP is expressed in Ewing sarcoma and promotes the proliferation of Ewing sarcoma cells [213]. CGRPβ mRNA is transcribed from the CT II gene in humans, and its expression has been reported in Ewing sarcoma [298].
3.2.10. Gastric Cancer
Adrenomedullin
BGC-823 gastric cancer cells showed a high expression of AM under hypoxic conditions; the association of AM in angiogenesis and its release under hypoxia in solid tumors have also been reported [299]. AM knockdown expression decreased the levels of B-cell lymphoma 2 and phosphoprotein kinase B and increased the levels of Bcl-2 associated x protein (Bax) and cleaved-caspase 3 [299].
Mast cells observed in solid tumors show different phenotypes in the tumor microenvironment, and patients with gastric cancer show a high mast cell infiltration into the tumor [214]. Tumor-derived AM promoted, via PI3K/Akt signaling pathway, mast cell degranulation, as well as favored tumor cell proliferation and the blockade of apoptosis in gastric cancer cells; these effects were reverted by blocking the release of interleukin-17A from mast cells [214]. These observations indicate that mast cells play an essential role in the development of gastric cancer.
3.2.11. Glioma
Adrenomedullin
CLR expression was observed in 95 biopsies of human gliomas of varying grades; astrocytic cancer cells and endothelial cells showed a high immunoreactivity for the receptor [300]. AM receptors were also reported in glioblastoma cells and glioma tissue [217]. AM also exerts an antiapoptotic action in glioma; hence, the blockade of this effect mediated by AM is a promising antitumor strategy [215]. AM has also been suggested as a tumor angiogenic factor in glioblastoma [216]. Exogenously added AM promoted the growth of these cells; the inhibition of AM produced by tumor cells suppressed tumor growth and decreased the density of tumor vessels [217]. It seems then that AM is involved in developing glioblastoma by promoting the mitogenesis of tumor cells and an angiogenic effect. AM mRNA has been associated with tumor type and grade in glioblastoma; a high expression was reported in all glioblastomas studied, but a low expression was observed in anaplastic astrocytomas, with barely detectable AM mRNA levels in oligodendrogliomas and low-grade astrocytomas [218]. The above indicates that AM has a role in the progression of gliomas.
The expression of AM is highly induced during hypoxia in human T98G glioblastoma cells [301]. Moreover, treating these cells with interleukin-1β or interferon γ increased mRNA AM expression and the release of AM in the culture media, whereas tumor necrosis factor α decreased both effects dose-dependently [217,302]. The data show that AM is released from glioblastoma cells and that the peptide is involved in tumor pathophysiology. Dexamethasone increased AM mRNA levels in T98G cells and AM immunoreactive levels in the culture medium but decreased immunoreactive-endothelin-1 levels [303]. Treatment with tumor necrosis factor α, interleukin-1β, and interferon γ promoted the expression of both AM and endothelin-1 in T98G cells [303]. The cAMP level was also increased in glioblastoma cells when they were treated with synthetic AM1-52 [302]; AM favored c-Jun and c-Jun N-terminal kinase (JNK) phosphorylation in glioblastoma cells, and the suppression of c-Jun expression or the inhibition of JNK activation impaired the actions mediated by AM on cyclin D1 and cell proliferation [219]. Thus, the c-Jun/JNK pathway is involved in the growth-regulatory activity mediated by AM in glioblastoma cells.
AM expression was upregulated in temozolomide (TMZ)-resistant glioma samples [221]. miR-1297 targeted AM, blocked its expression, and sensitized glioma cells to TMZ treatment; this could be mediated by the Bax/Bcl-2, Akt, and extracellular signal-regulated protein kinase (ERK)1/2 signaling pathways [221].
The cytokine oncostatin M promoted the expression of AM in astroglioma cells favoring the phosphorylation of activator of transcription-3 (STAT-3), nuclear translocation, and DNA binding to AM promoter. The expression of AM is controlled by STAT-3 in these cells, and AM also increases the migration of astroglioma cells [220]. Therefore, the activation of STAT-3, which is, for example, observed in malignant brain tumors, is crucial for AM expression and glioma invasion and metastasis.
Adrenomedullin 2
AM2 expression increased and correlated with higher-grade gliomas [222]. AM2 promotes filopodia formation, increasing the invasive capacity of glioma cells, and the activation of ERK1/2 has been involved in the proliferation, invasion, and malignancy of glioblastoma cells [222]. AM2 improves tumor blood and has also been engaged in hypoxia-induced responses and mitochondrial functions (e.g., regulating the critical components of respiratory complex I) in glioblastoma cells [222].
Amylin
AMY promoted the release of inflammatory cytokines (interleukin-6, interleukin-8) from U-373 MG human astrocytoma cells and the production of interleukin-1β in these cells [223].
3.2.12. Head and Neck Squamous Cell Carcinoma
Calcitonin Gene-Related Peptide
Head and neck squamous cell carcinoma is highly innervated by peripheral sensory neurons releasing CGRP; the peptide has been involved in oral cancer progression [304]. CGRP secreted from peripheral nerve terminals exerts a paracrine action on oral squamous carcinoma cells, and it has been suggested that, in this disease, CGRP is a bridge target between cancer-associated pain and cancer development because CGRP promotes the algesia transmission to pain centers [225]. CGRP also links perineural invasion and lymph node metastasis in oral squamous cell carcinoma. The pre-operative plasma CGRP level has been suggested to predict lymph node metastasis in this disease [224].
3.2.13. Liver Cancer
Adrenomedullin
Hepatocellular carcinoma cells express AM and its receptor and, under hypoxic conditions, favor AM expression [226]; in addition, AM mRNA, HIF-1α and VEGF levels were increased under the same conditions in human hepatocellular carcinoma cell lines [229]. CLR, RAMP2, and RAMP3 have been reported in liver cancer, and a high AM level has been related to increased intrahepatic metastasis [226,227]. AM is upregulated in human intrahepatic cholangiocellular carcinoma tissues (73/133; the second most common type of primary liver cancer) compared with healthy individuals [228]. AM regulates ZEB1 activation, which mediates EMT [228]. This observation means that AM-mediated activation could be a promising antitumor target. AM, through Akt activation, promoted the growth of hepatocellular carcinoma cells, which was inhibited with AM inhibitors [226]. Knockdown of AM expression promoted apoptotic mechanisms in hepatocellular carcinoma cells and, combined with cisplatin, significantly decreased tumor growth when compared with knockdown of AM expression or cisplatin alone [229]. The data show the association of AM with hepatocellular carcinoma development.
Microvessel density and mRNA AM and erythropoietin receptor levels were higher in hepatocellular carcinoma than in nontumor tissues [230]. Both levels correlated with tumor metastasis, pathological differentiation, and capsule invasion in hepatocellular carcinoma [230]. AM expression was also associated with the erythropoietin receptor and microvessel density in the same carcinoma [230]. Thus, AM and erythropoietin receptors may induce angiogenesis in hepatocellular carcinoma. AM is also associated with N-cadherin intensity, vascular invasion, and poor prognosis [226]. Consequently, AM level could be a prognostic factor in hepatocellular carcinoma. Additionally, in patients with this disease, AM mRNA levels were higher in tumor tissues than in adjacent nontumor liver tissues [229].
Adrenomedullin 2
A high AM2 mRNA expression was found in human hepatocellular carcinoma tumors, even in the early stage; tumors showed a higher level of AM2 than that observed in adjacent benign liver tissues [231]. A high expression of AM2 has been observed in the mesenchymal area of human hepatocellular carcinoma, with this expression being observed in stromal (e.g., vascular smooth muscle cells, fibroblasts) and endothelial cells [305]. AM2 increased hepatic carcinoma cell proliferation and survival [231] and favored HepG2 hepatic carcinoma cell proliferation; the AM2 receptor antagonist AM217-47 blocked this proliferation [232]. Moreover, the proliferation action mediated by AM2 in these cells was via the activation of the Wnt signaling cascade [232]. AM2 also promoted the proliferation of SMMC7721 hepatic carcinoma cells, as well as upregulated miR-155 expression, and the blockade of miR-155 inhibited the proliferation of SMMC7721 cells mediated by AM2 [306]. The data suggest that AM2 is involved in angiogenesis in human hepatocellular carcinoma, and that the blockade of AM2 activity may represent an antitumor treatment against this carcinoma [233].
Amylin
AMY binding sites were observed in the human hepatoblastoma cell line HepG2. The peptide favored adenylate cyclase activity [234].
3.2.14. Lung Cancer
Adrenomedullin
Lung cancer cells express CLR and RAMP2/3 [235,307]. AM appeared expressed in most non-small-cell and half of the small-cell lung carcinomas studied [308]. However, AM expression did not correlate with the overall survival of patients, cancer stage, and tumor differentiation. The results reported by the authors do not support the role of AM as an independent survival agent for lung cancer [235]. The aryl hydrocarbon receptor is partially responsible for tobacco-induced carcinogenesis, and it has been reported that AM contributes to the carcinogenicity of tobacco-activated aryl hydrocarbon receptor products [236]. Thus, the AM/aryl hydrocarbon receptor axis seems to play an important role in lung cancer development.
Calcitonin Gene-Related Peptide
CGRP gene expression has been reported in lung cancer, and patients with small-cell lung carcinomas showed a high serum CGRP, but only 27% showed values above the normal upper range [237,238]. CGRP was observed in a few of the tumors studied; thus, the high serum CGRP level observed was from an extratumoral origin [238]. CGRP expression was similar in normal adult lungs, pulmonary tumorlets, hyperplastic bronchial neuroendocrine cells, and, to some extent, in small-cell lung carcinomas [309]. Moreover, CGRP expression in bronchial carcinoids was similar to that observed in late fetal and neonatal lungs [309].
3.2.15. Melanoma
Adrenomedullin
AM, CLR, and RAMP2/3 expressions have been reported in melanoma [239,310] and it has been demonstrated that tumor-associated macrophages, through AM, favor melanoma growth and angiogenesis [239]. The number of tumor-associated macrophages (which express and release AM) in the tumor microenvironment has been correlated with poor prognosis in melanoma [239]. In addition, the secretion of AM by macrophages was upregulated when these cells were cocultured with melanoma cells or with melanoma cells conditioned media [239]. Tumor-associated macrophages favored the migration of endothelial cells, as well as increased B16/F10 tumor growth [239]. AM stimulated melanoma growth and angiogenesis through a paracrine effect (mediated by the endothelial nitric oxide synthase signaling pathway) and an autocrine effect (favoring the polarization of macrophages toward an alternatively activated phenotype) [239]. Human melanomas exhibited higher expressions of AM and AM receptors in tumor-associated macrophages than those found in adjacent normal skins. It is also known that antibodies against AM or AM receptor antagonists decreased tumor-associated macrophages-induced angiogenesis and melanoma growth [239].
3.2.16. Nasopharyngeal Carcinoma
Adrenomedullin
It has been mentioned that plasma AM levels could be a biomarker for predicting the long-term prognosis of patients with nasopharyngeal carcinoma [240].
3.2.17. Neuroblastoma
Adrenomedullin
Human neuroblastoma SK-N-MC cells express AM receptors, and AM’s N-terminal ring structure is essential for binding to the receptor. AM mRNA expression has been associated with tumor differentiation but not to prognostic markers in neuroblastoma [241,242]. In human IMR-32 and NB69 neuroblastoma cell lines, AM expression appeared augmented under hypoxic conditions, but RAMP2 expression was suppressed under the same conditions [311]. In SK-N-SH human neuroblastoma cells, AM favors, probably via cAMP-protein kinase A-dependent mechanisms, Ca++ influx via L-type Ca++ channels and ryanodine-sensitive Ca++ release from the endoplasmic reticulum; Ca++ concentration increase activated neuronal nitric oxide synthase and nitric oxide release [312].
3.2.18. Neuroendocrine Tumors
Adrenomedullin
Plasma and tissue AM expressions predict tumor progression in patients with neuroendocrine carcinomas of the gastroenteropancreatic and bronchial systems [243]. High AM plasma level or tumor AM mRNA expression correlates with a worsened prognosis in neuroendocrine, renal, prostate, or ovarian cancer patients [243,313].
3.2.19. Oropharyngeal Squamous Cell Carcinoma
Adrenomedullin
It has been reported that Jumonji domain-containing 1A (JMJD1A favors histone demethylation). Moreover, H3K9me1/2 and AM expression predicts progression and prognosis in patients with oral and oropharynx cancers [244]. Moreover, the JMJD1A gene targeting AM has been shown to favor tumorigenesis and cell growth [244].
3.2.20. Osteosarcoma
Adrenomedullin
AM, CLR, and RAMP2/3 expressions have been reported in osteosarcoma, and a high AM expression has been associated with the degree of metastasis and malignancy [245,246]. A correlation between VEGF and AM expression in the plasma of osteosarcoma patients has been reported. Hypoxia stimulated the expression of both VEGF and AM in MG-63 osteosarcoma cells, whose proliferation decreased when AM was inhibited by AM shRNA [314]. AM was overexpressed in human osteosarcoma tissue, but a low expression was observed in adjacent tissues, and a shallow expression was found in normal tissues [245]. MG-63 cell proliferation increased when AM was added but VEGF inhibition attenuated this action. The decrease in AM blocked CD31 expression, VEGF, and the growth of cancer cells in osteosarcoma experimental animal models [314]. Thus, it seems that the VEGF pathway mediated these mechanisms. mRNA and protein levels of AM increased in human osteosarcoma SOSP-F5M2 cells under hypoxic conditions [246]. AM blunted hypoxic-induced apoptosis via the CLR-RAMP receptor; this antiapoptotic effect was due to the upregulation of the Bcl-2 expression, which partially occurred via the activation of the MEK/ERK1/2 signaling pathway [246]. The increased Bcl-2 expression mediated by AM was inhibited with U0126, a selective inhibitor of MEK or AM22–52 (an AM-specific receptor antagonist) [246]. Thus, AM is a survival agent in osteosarcoma cells, and the targeting of this peptide could be a valuable antitumor strategy to promote apoptosis in these cells.
3.2.21. Ovarian Cancer
Adrenomedullin
AM, CLR, and RAMP2/3 are present in ovarian cancer, and a high AM level has been related to the tumor stage [247,248]. AM gene expression has been studied in 60 cases of epithelial ovarian cancer. A correlation between this expression and the histological grade, lymph node metastasis, and prognosis has been reported. This correlation was not observed between AM gene expression and the disease stage, histological subtype, residual tumor mass after initial surgery, and patient’s age at diagnosis [249,252]. AM gene expression may define a more aggressive tumor phenotype. Moreover, AM was observed in carcinoma cells (cell membrane, cytoplasm) and endothelial cells of the tumor stroma [252]. Ovarian tumor cells synthesize AM, as well as express AM mRNA for both ligand and receptor, and the estrogen receptor alpha/beta ratio was higher in tumors than in other tissues; a correlation was observed between estrogen receptor alpha and estrogen receptor beta mRNA and AM mRNA expression in tumors [247]. It is known that AM metabolism (mRNA expression and secretion) is not under estradiol control and that AM and anti-AM antibodies show no growth actions in vitro. Nevertheless, the adjunction of anti-AM antibodies to estradiol exerted inhibitory effects on cells showing a high mitogenic activity [315]. AM mRNA expression was higher in granulosa tumor cells than in fibrothecomas and normal human ovaries. Activin A, prostaglandin E, follicle-stimulating hormone, and cAMP blocked AM gene expression in granulosa-luteal cells [316]. AM (mRNA and protein) was observed in ovarian epithelial carcinoma tissue, and the basic fibroblast growth factor, via the JNK-activator protein (AP)1 pathway, promoted AM expression in the CAOV(3) ovarian epithelial carcinoma cell line [317].
AM gene silencing blocked cell proliferation, increased the chemosensitivity in HO-8910 cells, decreased AM mRNA/protein expression, and downregulated p-ERK and Bcl-2 expressions [250]. AM is involved in the progression of epithelial ovarian cancer and cell migration by activating the integrin α5β1 signaling pathway [248]. AM also upregulated focal adhesion kinase (FAK)/paxillin phosphorylation. Cancer patients with a high AM expression showed a larger residual size of tumors after cytoreduction, a shorter disease-free and overall survival time, and a higher incidence of metastasis [248]. AM can polarize macrophages which favor, via activation of the RhoA signaling pathway, the migration of HO8910 ovarian cancer cells [318]. FAK and AM expressions were higher in epithelial ovarian cancer than in benign tumors or normal ovarian tissues. FAK can upregulate AM during the migration and invasion of epithelial ovarian cancer cells [249]. FAK and AM have been suggested as biomarkers to evaluate the prognosis and malignant potential in human ovarian cancer [249].
VEGF expression induced by AM was mediated through the JNK/AP1 pathway in HO-8910 epithelial ovarian carcinoma cells [319]. AM expression positively correlated with HIF-1α, VEGF, or microvessel density in epithelial ovarian cancer [251]. AM is an upstream molecule of VEGF/HIF-1α that favored angiogenesis after upregulating both factors in CAOV3 epithelial ovarian cancer cells [251].
3.2.22. Pancreatic Cancer
Adrenomedullin
The expression of AM, CLR, and RAMP2/3 has been reported in pancreatic cancer, and a high level of AM is associated with a decrease in disease-free survival [253,254]. AM blocked glucose-stimulated insulin secretion from β-cells, and this effect was attenuated by AM knockdown [255]. AM overexpression led to glucose intolerance, and plasma AM level was higher in pancreatic cancer patients than in control patients or patients with diabetes [255]. Moreover, AM level was higher in pancreatic cancer patients with diabetes than in pancreatic cancer patients without diabetes [255]. The increased circulating AM in insulinoma is related to the neoplastic phenotype [256]. Pancreatic cancer promotes a paraneoplastic β-cell dysfunction by shedding AM(+)/CA19-9(+) exosomes into circulation, which block, via AM-induced endoplasmic reticulum stress, insulin release [320]. Moreover, pancreatic cancer exosomes induced lipolysis in subcutaneous adipose tissue [321].
AM and its receptor are expressed in pancreatic cancer cells, and AM is involved in the proliferation of these cells and metastasis [263]. AM increased the invasiveness of some pancreatic cancer cells, as well as regulated angiogenesis; AM is induced under hypoxic conditions, slightly upregulates VEGF release, and is overexpressed in pancreatic ductal adenocarcinoma, which could serve as a potential tumor marker [253]. AM mRNA/protein expressions increased in human pancreatic cancer samples compared to healthy individuals [255]. AM has been observed in the neoplastic epithelium of human pancreatic adenocarcinomas (43 of 48), and it was expressed in all eight pancreatic cancer cell lines studied; in addition, AM receptor silencing in pancreatic cancer cells inhibited AM-induced cell growth and invasion [257]. Thus, AM increases pancreatic cancer cell aggressiveness. AM antagonists decreased the growth of pancreatic cancer cells, and the diameter of blood vessels was smaller in tumors treated with AM antagonists than in those treated with AM N-terminal fragment (AM1-25) [254].
AM receptors mediate the AM stimulatory action on pancreatic cancer cells and endothelial and stellate cells. The silencing of AM receptors decreased both the growth of pancreatic ductal adenocarcinoma cells and the polygon formation of endothelial cells in vitro [263]. Pancreatic ductal adenocarcinoma is characterized by the stromal infiltration of myelomonocytic cells (which express all components of the AM receptor). This infiltration is associated with a poor prognosis [259]. Pancreatic cancer cells release AM, which, after activating the eNOS, PI3K/Akt, and MAPK signaling pathways and raising the expression of MMP-2, increased the migration/invasion of myelomonocytic cells [259]. AM also favored the migration of myelomonocytic cells by increasing ICAM-1 and VCAM-1 expression in endothelial cells and promoted the expression of protumor phenotypes in myeloid-derived suppressor cells and macrophages [259].
Desmoplasia is a prominent pathological characteristic of pancreatic cancer regulated by MYB [322]. MYB, a cellular progenitor of the v-Myb oncogene, encodes an oncogenic transcription factor that regulates gene expression (e.g., AM gene) [322]. AM controlled the growth of pancreatic tumor cells and pancreatic stellate cells in this disease [322]. Lastly, when PAN02 pancreatic cancer cells were injected into the spleens of mice, spontaneous liver metastasis occurred [258]. In this model, selective activation of RAMP2 and inhibition of RAMP3 could suppress tumor metastasis [258].
Adrenomedullin 2
AM2 is a tumor angiogenic factor in pancreatic cancer, regulating MAPK, ERK, Akt, PI3K, and VEGF pathways. The total cellular AM2 level is a poorer predictor of survival in this disease (patients with pancreatic cancer and a high expression of AM2 showed a median survival of 10 months shorter than the remainder of the cohort studied). Hence, this level could be a biomarker for predicting survival [260].
Amylin
AMY level is an early feature of pancreatic cancer [323] and has been suggested as a marker for neuroendocrine tumor growth [324]. However, later studies reported that a high plasma AMY level is not a good marker for detecting pancreatic cancer [325,326,327]. AMY, a glycolysis inhibitor, prevents BRAF and RAS oncogene-induced senescence in human cells. This connection between oncogene-induced senescence and AMY is an important link between metabolism and cancer since metabolic reprogramming is a cancer hallmark [328].
A relationship between insulin and AMY has been suggested regarding human AMY toxicity in pancreatic β-cells [329]. AMY overexpression seems to cause amyloid deposition in the islets of type 2 (non-insulin-dependent) diabetic patients and insulinomas [330]. In type 2 diabetes, the deposit of β-cell-toxic islet amyloid is a characteristic feature. The main amyloid component is AMY, but heparan sulfate proteoglycan is also present [331]. Heparan sulfate binds to AMY, promoting AMY conformational changes and accelerating the formation of fibrils [331]. This interaction could be a therapeutic target to increase β cell survival. Type 3 diabetes mellitus is a harbinger of pancreatic cancer in at least 30% of patients [332].
Insulinomas express AMY [264], and AMY or islet amyloid polypeptide (IAPP) has been detected in endocrine tumors (e.g., pancreatic tumors producing insulin or glucagon) and human pancreas [333]. This result means that AMY could be related to amyloid deposition in endocrine tumors. Silibinin inhibited the cytotoxic effects mediated by AMY on the INS-1 insulinoma cell line [334]. Glucose increases AMY expression in normal pancreatic β-cells [329], and an inhibitor of β-cell glucokinase mannoheptulose and glucose analog 2-DG inhibited the effect of glucose on AMY mRNA; this means that glycolysis is necessary for AMY mRNA accumulation [329]. Long-standing diabetes increases the risk of pancreatic cancer, and recent-onset diabetes is also associated with a risk increase [332]. The plasma AMY level is augmented in nondiabetic patients with pancreatic cancer, but this level is low in patients with diabetes [261]. AMY promotes apoptosis in human pancreatic islet β-cells via the stimulation of c-Jun expression/activation [335]. It seems that dimerization and co-expression of c-Jun and c-fos or ATF-2 are essential to induce apoptotic mechanisms.
Calcitonin Gene-Related Peptide
CALCA (αCGRP) and CALCB (βCGRP) methylation increased in pancreatic adenocarcinoma cells, and, under that condition, p-CREB and p-AKT levels decreased [262].
3.2.23. Pituitary Adenoma
Adrenomedullin
AM expression was lower in anterior pituitary tumors than in normal glands [265].
Amylin
A study reported that AMY is not a suitable marker for pituitary adenomas; however, this must be confirmed in future studies [325].
3.2.24. Prostate Cancer
NKX3.1 is a prostate-specific transcription factor related to prostate development and tumor progression. NKX3.1 deletion leads to premalignant prostate lesions [180]. RAMP1 is an NKX3.1 target gene, upregulated in prostate cancer, and RAMP1 knockdown in prostate cancer cell lines showed a decrease in colony formation, numbers of cells in the S phase, and cell proliferation [180]. Moreover, ERK1/2 phosphorylated level was reduced, linking RAMP1 to the MAPK pathway [180].
Adrenomedullin
CLR and RAMP2/3 have been reported in prostate cancer, and a high level of AM has been associated with a high Gleason score [266,267]. Plasma AM2 level has been associated with prognostic factors (tumor node metastasis, 5 year metastasis) in prostate cancer patients [180]. AM is widely expressed in prostate carcinomas and normal prostates, and the peptide was observed in neurons of the prostate ganglia and endothelial, stroma, and secretory cells [268]. Prostate cancer cells are derived from prostatic epithelial cells in which the expression of AM has been observed. AM, through autocrine and paracrine mechanisms, suppressed prostate cancer cell malignancy [336]. AM (overexpressed or exogenously added) inhibited prostate cancer cell growth. This blockade was due to ERK1/2 activation and not cAMP intracellular level changes; this study was performed in PC-3, DU 145, and LNCaP prostate cancer cells expressing the CLR/RAMP-2 receptor complex [269]. This overexpression resulted in the dysregulation of approximately 100 genes involved in apoptosis, cell cycle, extracellular matrix, cell adhesion, and cytoskeleton [337]. For example, AM upregulated genes (e.g., RUNX-3, IGF-BP6, and GADD45) are involved in cell growth arrest [337]. However, other studies showed that AM, mediated by the AM2 receptor (CLR/RAMP3) subtype, promoted the growth of the human prostate, being the peptide involved in developing epithelial cell malignant phenotype. After serum deprivation, AM blocked apoptosis in DU 145 and PC-3 cells, but not in LNCaP cells [270]. Moreover, an AM-induced mechanism of tumor sensitization has been reported, upregulating the interleukin-13R alpha 2 chain, a target for the specific recombinant chimeric cytotoxin interleukin-13-PE38 [337]. Interleukin-13 receptor alpha 2 (IL-13Ralpha2) is a tumor antigen, and a high-affinity interleukin-13-binding subunit is known to be amplified in human tumor cell lines and tumors [338]. AM upregulated IL-13Ralpha2 in a human prostate tumor cell line, indicating that the peptide and interleukin-13 are closely related to each other. AM is involved in sensitizing prostate tumors to the interleukin-13R-directed therapeutic agent.
AM was located in the carcinomatous epithelial compartment, and androgen-independent PC-3 and DU145 cell lines derived from human prostate cancer synthesized and released AM, which acted as a growth factor on DU145 cells [266]. Advanced and metastatic prostate carcinomas treated with antiandrogenic therapy become, in general, refractory to the treatment. It has been reported that pre-proAM and peptidyl glycine alpha-amidating monooxygenase expressions are androgen-independent in the human prostate [339]. The previous enzyme is involved in AM amidation, which must be fully active. No relation between anti-androgenic treatment or Gleason score and AM expression has been reported [339]. AM, upon androgen ablation, is involved in hormone-independent tumor growth and lymphangiogenesis and neoangiogenesis [267]. Neuroendocrine differentiation is an early marker associated with developing androgen independence in prostate cancer. It has been reported that AM is a mediator in such differentiation and that its production is vital for tumor resurgence following androgen ablation [340].
AM is involved in the spread of prostate tumors to the bone and can also influence localized levels of RANKL in the bone to favor tumorigenesis [180]. AM, via transient receptor potential channel (TRPV2) translocation to the plasma membrane, promoted prostate and urothelial cancer cell migration and invasion, increasing resting Ca++ level [271]. Moreover, AM induced the phosphorylation of FAK and β1 integrin in prostate cancer cell lines, which raised these cells’ invasive and migratory capacity [180]. Smooth muscle cells migrated when these cells were cultured with conditioned media from lymphatic endothelial cells; this effect and the formation of lymphatic vasculature were inhibited when the action of AM was blocked [267]. This observation means that AM promotes tumor lymphangiogenesis [180].
Adrenomedullin 2
AM2, upregulated under hypoxic conditions, is involved in prostate cancer progression by stimulating cancer cell migration and angiogenesis [305,341]. Plasma AM2 levels were higher in patients with prostate cancer than in healthy individuals, and those patients showing a Gleason score ≥7, organ unconfined, seminal vesicle invasion, tumor node metastasis stage T2, positive lymph node, or extra-prostatic extension showed a higher level of AM2 [272]. The elevated plasma AM2 level is independently associated with long-term recurrence and distant metastasis in prostate cancer [272]. AM2 has been suggested as a prognostic, predictive biomarker for 5 year metastasis and 5 year progression.
Calcitonin Gene-Related Peptide
CGRP is expressed in the prostate’s neuroendocrine cells and sensory nerves [180]. Neuroendocrine cells frequently differentiate in prostate cancer, the peptide increases prostate cancer cells’ invasive and migratory capacity, CGRP serum level correlates with prostate cancer progression in humans, and CGRP receptor mediates prostate cancer cell metastasis to the bone [274,342,343]. Neuronal CGRP promotes prostate tumor growth in the bone microenvironment and the femur of experimental animals; this effect was blocked with CGRP antagonists [275]. Moreover, CGRP activated the signal transducer and activator of transcription 3 (STAT3) and ERK signaling pathways in prostate cancer cells [275]. Serum CGRP level was higher in patients with higher histological grade and clinical stages than in patients with lower grade and stages [273,274]. Thus, in untreated prostate cancer patients, serum CGRP level could be a predictor of grading and staging; this does not occur in patients with prostate cancer who received hormonal treatment since this treatment influences the level of CGRP [274].
3.2.25. Renal Carcinoma
Adrenomedullin
AM, CLR, RAMP2, and RAMP3 have been reported in renal cancer, and a high level of CLR has been associated with a high tumor grade [276,277,313]. Both VEGF and AM were observed in the cytosol of human renal carcinoma cells, while VEGF and AM mRNAs were also detected in this carcinoma [278]. This outcome suggests that AM facilitates tumor angiogenesis in conjunction with VEGF and favors renal carcinoma cell growth. Human clear cell renal cell carcinoma displays mutations in the tumor suppressor von Hippel–Lindau protein, leading to a high expression of HIF-1 [276]. Higher AM tissue levels have been reported in human clear cell renal cell carcinomas than in other kidney tumors, but plasma AM levels cannot be used as a tumor marker for this disease [276]. AM, CLR, and RAMP2 were observed in the carcinomatous epithelial compartment of chromophobe renal cell carcinoma (CRCC), and RAMP3 was only found in the inflammatory cells that infiltrated tumors [313]. AM mRNA level was higher in CRCC than in normal renal tissue, and AM mRNA expression correlated with VEGF-A mRNA expression in CRCC [313]. CRCC BIZ and 786-O cells expressed and released AM, the peptide promoted cell proliferation, migration, and invasion, and these actions were mediated by CLR/RAMP2 and CLR/RAMP3 [313]. Moreover, a high AM mRNA level has been related to an increased risk of relapse after curative nephrectomy for CRCC.
3.2.26. Thymic Lymphomas
Amylin
Pramlintide, a synthetic analog of AMY, promoted tumor regression in p53-deficient thymic lymphomas [279]. AMY, via CT receptor and RAMP3, blocks glycolysis, promotes the formation of reactive oxygen species, and induces apoptosis [279]. Thus, an antitumor strategy targeting p53-deficient cancers could be developed.
3.2.27. Thyroid Cancer
Adrenomedullin 2
AM2 is secreted from tumor cells under mitochondrial stress resulting from overnutrition, and it is involved in developing thyroid cancer [280]. AM2 upregulation was increased after the activation of the mitochondrial stress response pathway in tumor cells, and AM2 expression was increased in obese patients with thyroid cancer showing locoregional recurrence, a high prevalence of lymph node metastasis, and larger tumor size [280]. High levels of circulating AM2 and tumor cell AM2 expression have been associated with aggressive pathological parameters (e.g., body mass index) in patients with thyroid cancer. AM2 plays an essential role in the nexus of obesity and thyroid cancer, and the level of AM2 could be used as a biomarker for predicting thyroid tumor progression [280].
Amylin
Medullary thyroid carcinoma patients (seven of 10) showed high levels of AMY, and the peptide was observed by immunohistochemistry in four of 12 medullary thyroid carcinomas and two of five lymph-node metastases [281]. Serum insulin and plasma AMY levels were correlated in both controls and patients with medullary thyroid carcinoma. However, the slope of the regression line was significantly higher for medullary thyroid carcinoma patients [281].
Calcitonin Gene-Related Peptide
CGRP gene expression has been reported in thyroid carcinoma; CGRP is released from medullary thyroid carcinoma cells, and plasma CGRP level has been suggested as a marker for medullary thyroid carcinoma [237,282,283].
3.2.28. Uterine Cervical Carcinoma
Adrenomedullin
High AM and AM mRNA expressions were observed in the cytoplasm of invasive cervical squamous carcinoma cells. Both expressions were more prominent in the stromal cells adjacent to early invasive carcinoma cells than in the carcinoma cells [284]. Consequently, the peptide appears involved in the growth and invasion of uterine cervix squamous carcinoma cells. Another study reported that, in invasive uterine cervix squamous carcinoma, AM and Bcl-2 were involved in promoting malignant progression and in selecting carcinoma cells resistant to apoptosis [285].
3.2.29. Vascular Tumors
Adrenomedullin
The proliferation of endothelial cells characterizes vascular tumors (e.g., capillary haemangioma and Kaposi’s sarcoma), and these tumors express more CLR than that expressed in the adjacent normal endothelium [344]. Therefore, the proliferation of endothelial cells and the neoplastic vascular growth seem to be mediated by AM.
Figure 14, Figure 15 and Figure 16 summarize the main findings regarding the involvement of AM, AM2, AMY, and CGRP in cancer.
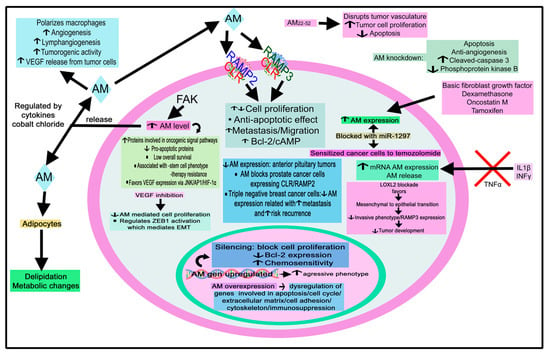
Figure 14.
Summary of the mechanisms mediated by adrenomedullin (AM) in tumor cells. AP1: activator protein 1; cAMP: cyclic 3′-5′ adenosine monophosphate; CLR: calcitonin receptor-like receptor; EMT: epithelial-to-mesenchymal transition; FAK: focal adhesion kinase; JNK: c-Jun N-terminal kinase; INF γ: interferon γ; IL-1β: interleukin 1β; RAMP2/3: receptor activity modifying protein 2 and 3; VEGF: vascular endothelial growth factor. ↑: increase; ↓: decrease.
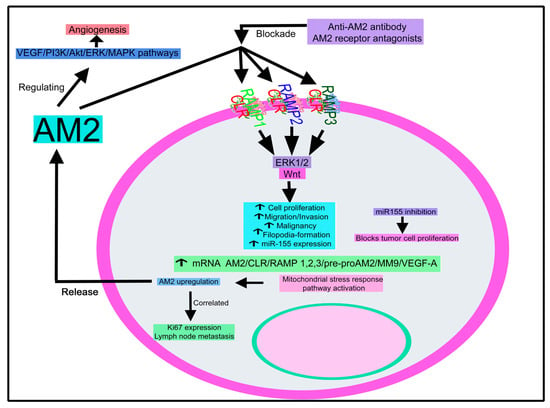
Figure 15.
Summary of the mechanisms mediated by adrenomedullin 2 (AM2) in tumor cells. Akt: Ak strain transforming protein kinase; CLR: calcitonin receptor-like receptor; ERK 1/2: extracellular signal-regulated protein kinase; MAPK: mitogen-activated protein kinase; MMP9: matrix metalloproteinase 9; PI3K: phosphatidylinositol 3-kinase; RAMP1/2/3: receptor activity modifying protein 1, 2 and 3; VEGF-A: vascular endothelial growth factor A. ↑: increase; ↓: decrease.
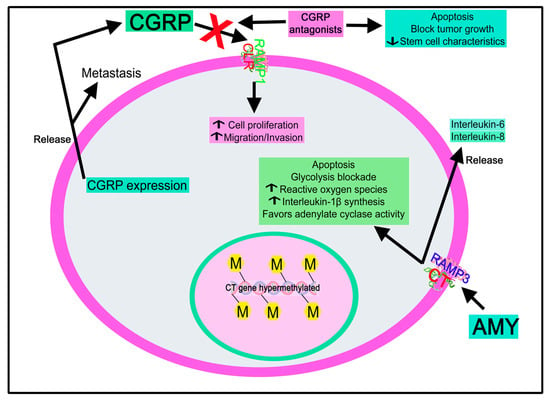
Figure 16.
Summary of the mechanisms mediated by amylin (AMY) and calcitonin gene-related peptide (CGRP) in tumor cells. CLR: calcitonin receptor-like receptor; RAMP1/3: receptor activity modifying proteins 1 and 3. ↑: increase; ↓: decrease.
4. Antitumor Therapeutic Strategies Based on the Modulation of Peptidergic Systems
4.1. Tachykinin Peptide Family
No NK-2R or NK-3R antagonist has been approved for clinical practice [14], although several NK-2R or NK-3R antagonists have been tested in clinical trials [16]. Unfortunately, although NK-2R/NK-3R antagonists were safe, these trials were abandoned due to the lack of efficacy. The ineffective results could also have been due to a non-appropriate selection of patients and endpoints in clinical trials and a lack of knowledge regarding the molecular interaction between tachykinin ligands and NK receptors [17]. Moreover, the ineffective effects could also be due to a non-appropriate selection of patients and endpoints in clinical trials and to a lack of knowledge regarding the molecular interaction between tachykinin ligands and NK receptors [17]. NK-2R antagonists (e.g., nepadutant (MEN-11,420) blocked tumor cell proliferation promoted by NKA in breast carcinoma [139]. Metastatic and non-metastatic breast cancer cell proliferation was blocked by inhibiting the action of SP, at NK-2R, with NK-2R antagonists (GR-159,897); however, this action was less prominent than that observed with NK-1R antagonists (RP-67,580) [140]. This finding suggests that breast cancer cells respond differently to NK-2R and NK-1R antagonists. Moreover, these cells reacted differently to NK-2R and NK-1R agonists (e.g., SP) [140]. NK-2R antagonists enhanced the secretion of macrophage inflammatory protein-2 and increased p38 phosphorylation [140]. This study also reported that SP altered neither breast cancer cell proliferation nor the effects mediated by NK-2R and NK-1R antagonists on these cells [140]. The latter does not agree with the results reported in most of the performed studies. This discrepancy must be investigated. SB-222,200, a selective NK-3R antagonist, inhibited tumorigenesis and osteolytic lesions promoted by the oral squamous tumor cells that invade the bone matrix, inducing bone destruction [156]. GR-138,676 shows little activity at NK-2R. It can be used as an NK-3R antagonist, despite its activity at NK-1R [345]. NK-3R has been suggested as a potential antiangiogenic target because NKB exerted anti-angiogenesis effects. NKB analogs have shown antimigration and antitumor activities in vivo with no significant side-effects [346].
4.2. Calcitonin/Calcitonin Gene-Related Peptide Family
4.2.1. Adrenomedullin
AM ligand/receptor overexpression occurs in colonic cancers, and antibodies directed against both targets decreased tumor growth [204,205,206,347,348,349,350]. The peptide fragment AM22–52 [254], polyclonal antibodies against AM [217,308] or its receptor [350], monoclonal antibodies [351], and small interfering RNAs [257] regulate AM expression and actions [208]. Small molecules (e.g., 16311, a negative AM modulator; 145425, a positive AM modulator) also regulate the effects mediated by AM. For example, 145425 reduced tumor burden, colon weight/length ratio, and tumor growth in mice [208]. AM promotes cell proliferation and survival, alters the cell phenotype more aggressively, and increases vascularization [352]. Tumors are, in general, the main source of excessive AM production since AM levels returned to normal levels when the tumor was removed after surgery [353]. The blockade of AM receptors or lowering AM amounts are antitumor strategies to decrease the tumor mass [175]. Many preclinical studies have shown reduced tumor cell proliferation, metastasis, and angiogenesis after applying AM receptor antagonists, AM receptor interference, or AM-neutralizing antibodies [352]. Thus, a cocktail of antibodies directed against CLR and RAMP2/3 exerted an antitumor effect against glioblastoma, mesothelioma, and lung and colon cancer, and peptide antagonists such as AM22–52 also showed an antitumor action against melanoma, mesothelioma, and renal, ovarian, breast and pancreatic cancer [217,350,354]. Moreover, small-molecule antagonists targeting the AM ligand (NSC 16311) or AM2 receptors (2, 2-dimethyl-N-((2-(methylaminomethyl) phenyl) methyl)-N-(2-oxo-2-(((2 R)-2′-oxospirol (1, 3-dihydroindene-2, 3′-1 H-pyrrolo (2, 3-b) pyredine)-5-yl) amino) ethyl) propanamide) respectively exerted antitumor effects against breast cancer and breast/pancreatic cancer [236,355,356]. Cancer patients show, in general, a high level of circulating AM, and this high level has been associated with the worst prognosis [353]. AM-1 and AM-2 receptors are involved in cancer development; the former also regulates blood pressure. Thus, using AM-1 receptor antagonists as antitumor agents is not possible since these antagonists increase blood pressure [357]. However, AM-2 receptor antagonists are promising agents as antitumor drugs because they show good pharmacokinetic properties, no serious side-effects, and 1000-fold selectivity over the AM-1 receptor [355,357]. In this sense, it is crucial to know which receptors are involved in the effects mediated by the CT/CGRP peptide family in cancer. This knowledge will help to develop selective receptor antagonists [175]. Potent small-molecule AM-2 receptor antagonists have been synthesized but retain activity against the CGRP receptor [355]. These compounds blocked tumor growth and extended life in an experimental animal model of pancreatic cancer [355]. This finding is important and must be developed as an anticancer strategy, in addition to increasing the knowledge of AM receptor pharmacology. The importance of investigating the residue differences between the AM receptors has also been highlighted to develop selective AM-2 receptor antagonists [357].
AM antagonist peptide (AM22–52) decreased the growth of pancreatic cancer cells in vivo. It has been reported that intramuscular or intratumoral transfer of naked DNA encoding AM22–52 might be a promising strategy for treating human cancers [358]. This strategy eliminates CD31-positive cells from the tumor, which means that neo-vascularization is completely inhibited [358]. A constructed AM antagonist expression vector decreased tumor growth and microvessel density in renal cell carcinoma [359]. Moreover, AM inhibition blocked sunitinib-resistant renal cell carcinoma growth by targeting the ERK/MAPK pathway; this means that combination therapy with AM receptor antagonists and sunitinib is a promising strategy against renal cell carcinoma [360].
Tumor development is highly dependent on the formation of both lymphatic (lymphangiogenesis) and blood (angiogenesis) vessels from pre-existing ones; these mechanisms are regulated by AM and VEGF, which are released from cancer and tumor stroma cells (e.g., endothelial cells, pericytes, fibroblasts, and macrophages) [352]. Thus, the inhibition of both angiogenesis and lymphangiogenesis mechanisms is a sound antitumor strategy. The AM/RAMP2 system controls vascular integrity. It has been reported that the deletion of RAMP2 favored vascular permeability and the formation of pre-metastatic niches in distant organs by altering the vascular structure and promoting inflammation [361]. As a result, the AM/RAMP2 system regulates vascular integrity, and this system could be a promising antitumor therapeutic target to block metastasis. AM plays an essential role as a crosstalk agent integrating mast and tumor cell communication [362]. AM favors the release of beta-hexosaminidase or histamine from human mast cells, which are also involved in angiogenesis; this was inhibited with anti-AM monoclonal antibodies [362]. Alpha-AMR (an antibody against the AM receptor) decreased the growth of HT-29 colorectal cancer cells and U87 glioblastoma cells in vitro, as well as the growth of colon, lung, and glioblastoma tumors in vivo [338]. Alpha-AMR increased apoptosis in cancer cells, depleted endothelial and pericyte cells, disrupted tumor vascularity, and decreased microvessel density [338]. Thus, alpha-AMR blocked angiogenesis and tumor growth. Moreover, the use of a polyclonal antibody designed to block all the receptor components of AM (RAMP2, RAMP3, and CLR) decreased both proliferation and invasion of prostate cancer cells, tumor weight, metastasis, and lymphatic vasculature [180]. Notably, the reduction in lymphatic vasculature was observed in the tumor, not in the tissue surrounding the tumor [180].
AM gene silencing decreased the expression of both AM mRNA and protein, blocked cell proliferation, and increased the chemosensitivity of HO8910 ovarian cancer cells via downregulation of Bcl-2/p-ERK expressions [250]. Anti-AM treatment and Bcl-2/ERK inhibited expressions are promising strategies to treat ovarian cancer [250]. Carriers of a single-nucleotide polymorphism (rs4910118) in the proximity of the AM gene showed lower levels of circulating AM; patients with cancer (breast, lung) showed a lower frequency than healthy donors, and carriers of the minor allele showed a lower risk of developing cancer than those homozygotes for the major allele [363]. The authors concluded that carriers of rs4910118 close to the AM gene are protected against cancer.
The AM/RAMP2 system is involved in tumor angiogenesis; liver metastasis (PAN02 pancreatic cancer cells were administered into the spleen) increased in vascular endothelial cell-specific RAMP2 knockout mice, and liver metastasis was suppressed in RAMP3−/− animals in which the number of podoplanin-positive cancer-associated fibroblasts decreased in the periphery of tumors at metastatic sites [258]. RAMP3 deficiency cancer-associated fibroblasts inhibited cell proliferation/migration and metastasis, and the activation of RAMP2 in RAMP3−/− mice blocked tumor growth and metastasis [258]. Moreover, podoplanin upregulation in RAMP2−/− animals augmented malignancy, and podoplanin downregulation in RAMP3−/− mice decreased malignancy [258]. The observation means that RAMP2 activation and RAMP3 inhibition can suppress metastasis, and that deficiency of the AM/RAMP3 system inhibited metastasis via the modification of cancer-associated fibroblasts. Acylated truncated AM/AM2 analogs of 27–31 residues showed a potent antagonistic action toward CLR/RAMP1, and non-acylated analogs showed minimal activity [364]. The authors of the study reported that a lysine residue in a 17-amino-acid analog, named antagonist 2–4, is important for increasing its antagonistic activity; they also showed that the analog, showing a 12-amino-acid binding domain of CGRP and AM sequence motif, exerted a potent CLR/RAMP1-inhibitory effect, and that a chimeric analog, constituted by an AM antagonist and a somatostatin analog, exerted a dual effect on CLR/RAMP and somatostatin receptors [364]. The blockage of CLR/RAMP inhibited tumor growth and metastasis, and it can be concluded that somatostatin-AM antagonist analogs could be applied to treat tumors [364].
In sum, current AM-based antitumor therapeutic strategies have been focused on targeting AM with blocking antibodies (e.g., adrecizumab, mAb-C1, and MoAb-G6) or AM receptor antagonists (e.g., NSC-16311 and NSC-37133, both small molecules with antitumor properties). They show more favorable pharmacokinetic characteristics than antitumor peptides such as AM22–52; this is important since using specific anti-RAMP2 or anti-RAMP3 antibodies is crucial to selectively inhibit AM-1 or AM-2 receptors [352]. Moreover, it has been reported that a ribozyme diminished the expression of AM, although this strategy shows significant disadvantages from a pharmacokinetic point of view [352].
4.2.2. Adrenomedullin 2
AM2 is involved in vascular remodeling processes and angiogenesis; hence, the peptide is an important target for developing angiogenesis-based antitumor strategies [305]. AM2 increases tumor blood perfusion and promotes quiescent endothelial cells to proliferate by restraining endothelial cell response to VEGF; hence, the excessive vessel sprouting is blocked, and the vascular lumen is increased [365]. AM2 also favors the formation of a signaling complex containing CLR/β-arrestin1/Src in endothelial cells and promotes its internalization into the cytoplasm via a clathrin-dependent manner to activate downstream ERK1/2 pathway; this action was not inhibited by endothelial cell contact [365].
AM2 blockade with neutralizing antibodies/antagonist peptides inhibited the growth of tumor cells by promoting apoptosis, Bcl2/Gli1 downregulation, and caspase-8 cleavage [231]. Significantly, administering anti-AM2 monoclonal antibodies not only inhibited tumor growth but also increased the antitumor activity of temozolomide [222]. By using anti-AM2 antibodies, ERK1/2 phosphorylation was inhibited in endothelial cells and, in the same cells, a decreased expression of vascular endothelial cadherin and dissociation of vascular endothelial cadherin/vascular endothelial growth factor receptor 2/phosphoinositide 3-kinase complex occurred, leading to the internalization and phosphorylation of vascular endothelial growth factor receptor 2 and the blockade of the PI3K/Akt pathway [305]. Moreover, anti-AM2 monoclonal antibodies blocked glioblastoma multiforme growth and significantly enhanced the activity of temozolomide [222].
4.2.3. Amylin
The p53 family promotes tumor suppression, and deletion of the ΔN isoforms of p63 or p73 led to metabolic reprogramming and regression of p53-deficient tumors via upregulation of the AMY gene [279]. AMY is involved in tumor regression and, via the CT receptor/receptor activity modifying protein 3, promotes apoptosis, induces reactive oxygen species, and blocks glycolysis [279]. Pramlintide, a synthetic analog of AMY, stimulated tumor regression in p53-deficient thymic lymphomas, representing a novel strategy to target p53-deficient cancers [279]. Pramlintide also exerted an antiproliferative action against colorectal cancer cells, and its coadministration with classic chemotherapeutics increased cytotoxicity [366].
4.2.4. Calcitonin Gene-Related Peptide
CGRP signaling, through the CT receptor, increased chemotherapy resistance and stem cell properties in acute myeloid leukemia, and olcegepant, a CGRP antagonist, decreased key stem cell properties and leukemic burden [182]. The CGRP/CT receptor system could be an antitumor target against acute myeloid leukemia. Moreover, the CGRP8-37 peptide antagonist exerted an antitumor action against prostate cancer [367].
CGRP augmented cytotoxic CD8+ T cell exhaustion, limiting their capacity to eliminate melanoma cells [368]. CGRP antagonism of the receptor RAMP1 or pharmacological silencing of nociceptors decreased tumor-infiltrating leukocyte exhaustion and B16F10 melanoma cell growth [368]. Thus, reducing CGRP release from tumor-innervating nociceptors is a valuable strategy to improve antitumor immunity via eliminating the immunomodulatory actions exerted by CGRP on these cytotoxic T cells [368].
5. Future Research
Previous sections showed how both peptide families reviewed here are involved in cancer progression and how several promising antitumor strategies could be developed. This knowledge is crucial, highlighting that the same peptide favors the development of many different tumors and that a common antitumor approach could be possible. Tumor size, higher tumor aggressiveness, malignancy, increased relapse risk, worse sensitivity to chemotherapy agents, and poor prognosis are associated with the peptidergic systems reviewed here. Peptides are widely involved in cancer development (mitogenesis, migration, invasion, metastasis, angiogenesis, lymphangiogenesis, and antiapoptotic effect). Some peptides exert an antiproliferative action against cancer cells, while others promote proliferative and antiproliferative effects. Consequently, peptidergic systems are promising antitumor targets to open up new cancer research lines, select new molecular targets (e.g., receptors), improve cancer diagnosis (peptidergic systems as prognostic biomarkers), and explore new antitumor strategies using new compounds (e.g., peptide antagonists) that could specifically destroy cancer cells and block angiogenesis alone or in combination with radiotherapy/chemotherapy. In the SP/NK-1R system, it has been demonstrated that the combination therapy of NK-1R antagonists with chemotherapy/radiotherapy increased the antitumor activity by chemosensitization and radiosensitization, as well as decreased the serious side-effects mediated by chemotherapy/radiotherapy (e.g., neurogenic inflammation, nephrotoxicity, neurotoxicity, hepatotoxicity, and cardiotoxicity) [4].
The involvement of NKB/NK-3R in cancer has been much less studied than NKA/NK-2R. Thus, information is lacking regarding the NKB/NK-3R system (e.g., NKB/NK-3R expression and mitogenesis, migration, and metastasis mediated by NKB in many tumors, as well as involvement of NK-3R in the viability of these cells). This critical research line must be developed in the following years to know which type of tachykinin receptor (NK-1R, NK-2R, and NK-3R) is expressed in the same tumor cell, and how SP, NKA, and NKB interact and regulate tumor cell mitogenesis and migration. This knowledge is also crucial to develop effective antitumor treatments. In most tumors (e.g., astrocytoma, insulinoma, neuroblastoma, phaeochromocytoma, schwannoma-derived cells, lung cancer, and leiomyoma), knowledge on the involvement of NKA/NKB is lacking, scarce, or incomplete. For example, NK-3R expression has been reported in astrocytoma cells [145]; however, it is not known whether NKB exerts a proliferative action in these cells.
Regarding insulinoma, it is only known that cancer cells release NKA and express the pre-protachykinin A gene [146,147]; in the pathological events observed in women suffering from leiomyomata, the involvement of NKB/NK-3R must be established [91]. However, in a few cancers (e.g., breast cancer and colorectal cancer), the involvement of NKA has been better studied. For example, in breast cancer, NKA promotes the migration of cells and exerts a proliferative action on tumor cells that are blocked with NK-2R antagonists [92,139,140,141]. In colorectal cancer, an important point must be investigated [142,143], i.e., why NKA did not exert a growth-regulatory action on the colon cancer cell line HT 29 [168]. The answer may be that the tachykinin receptor type and the activated signal transduction pathways depend on the coupled G protein type.
Moreover, NKB/NKB analogs exerted anti-angiogenesis and antimigration/antitumor actions [346]. It must be investigated whether these actions mediated by NKB are observed in different tumors since NKA favored through NK-1R the proliferation of glioma cells [144]. This is a crucial point because NKA exerted its physiological actions not exclusively via NK-2R but also via NK-1R. Moreover, it is essential to remark that NKA, NKA3-10, and NKA4-10 have been reported in ileal metastatic carcinoid tumors; these tumors can release different amounts of several tachykinins contributing to individual differences [165]. Knowing the physiological actions mediated by the unmodified peptide and its fragments is an important point that must be studied in other tumors.
Studies have reported that NKA/NKB blocked the growth of small-cell lung cancer cells [20]. This is a significant point that must also be investigated in depth to develop new antitumor strategies since NKA exerts a proliferative action on other tumor cells, and it is widely known that SP, another member of the tachykinin peptide family, promotes the proliferation of many different human cancer cell lines [5]. Oral squamous tumor cells do not express NKB; however, NKB appears in sensory nerves in the mandible. The finding suggests the release of the peptide from these nerves and that NKB could regulate the proliferation of tumor cells. The above must be confirmed to establish a clear relationship between tumor cells and the nervous system. A crucial point is the use of peptidergic systems as predictive biomarkers. In this line, elevated plasma NKA concentration has been suggested as a biomarker of prognosis in patients suffering from midgut carcinoid tumors [150] and to predict survival in patients with small bowel neuroendocrine tumors [161,163]. The result must be confirmed in future studies. Cancer biomarkers are crucial since some tumors are challenging to diagnose and because monitoring peptide plasma levels could be handy in selecting patients showing a poor prognosis and requiring urgent therapeutic intervention.
Compared with NKA/NKB, many more studies have been focused on the involvement of the CT/CGRP peptide family in cancer. It is established that members of this family favor tumor cell proliferation, metastasis, angiogenesis, and lymphangiogenesis, prevent apoptosis, and suppress the immune system [175,211,369,370]. AM level has been correlated with cancer severity [6,104,175]. AM is the most studied member of the CT/CGRP peptide family, followed by AM2, whereas AMY and CGRP are less studied. Thus, much effort must be performed to increase the knowledge of this family in cancer and, in particular, to decipher the roles played by AMY and CGRP. An important point that must be developed and clarified is the involvement of AM in the post-chemotherapy persistence of solid tumor cells; in leukemia, whether targeting CLR prevents relapse or not, and the methylation patterns of certain genes in this disease must be investigated [183,186]. Additional studies must be performed to confirm that the blockade of the CT receptor/CGRP system is a useful antitumor strategy to treat leukemia [136]. Two important findings have been reported in phaeochromocytoma: AM inhibited the proliferation of tumor cells, and circulating AM was increased in patients suffering from the disease [190]. Is this increase an endogenous antitumor strategy directed against the tumor? Moreover, in phaeochromocytoma, the involvement of AM2 in tumor growth must be elucidated, and the role of STAT-3 in invasion and metastasis must be investigated in tumors other than astroglioma [192,220]. The knowledge on the involvement of AM in bladder cancer is scarce [193]. Hence, additional experiments must be developed to confirm its involvement and fully demonstrate that AM is a potential antitumor target in this disease. AM is a tumor survival factor in breast cancer; therefore, the blockade of AM could be a promising antitumor strategy [99]. This must be confirmed, in addition to whether the level of circulating AM can be used or not as a predictor for lymph node metastasis in this disease. By contrast, AM22–52 decreased tumor cell proliferation, induced apoptosis, and disrupted tumor vasculature in breast carcinoma [197]. These findings are important since they highlight that tumor development was mediated by the unmodified peptide, whereas the contrary effect, i.e., an antitumor action, was favored by its fragment AM22–52 in breast cancer. Thus, the role played in cancer by peptide fragments belonging to the two peptide families studied here must also be elucidated. Moreover, AM, through its action on cancer cell EMT, decreased tumor cell invasion [196]; this finding means that AM could act as an antimetastatic agent in triple-negative breast cancer; however, the effect must be investigated and confirmed. AM mRNA expression has been described in choriocarcinoma [202]; however, it is unknown whether AM or other peptides belonging to the CT/CGRP peptide family exert a proliferative action on choriocarcinoma cells. Like choriocarcinoma, much information is lacking regarding the involvement of AM, AM2, AMY, and CGRP in certain tumors such as astroglioma, cutaneous nerve neuromas, neuroendocrine tumors, oropharyngeal squamous cell carcinoma, thymic lymphomas, Ewing sarcoma, uterine cervical carcinoma, and vascular tumors. It is important to remark that peptides belonging to the CT/CGRP peptide family favor the growth of different tumors; however, it is currently unknown whether CT/CGRP receptor antagonists exert an antitumor effect against these tumors. It is probably the case, but it is a crucial piece of information currently missing that deserves investigation. AM expression has been suggested as a predictive factor for tumor progression in patients with cancer; this is an important point that must be confirmed in the future. For example, AM expression has been suggested as a marker for predicting cancer-related death and a high risk of relapse in colorectal cancer patients with a curative resection [203]. It must also be confirmed whether plasma AM level can be used as a biomarker for predicting the long-term prognosis of patients suffering from nasopharyngeal carcinoma, whether AM expression can be used to evaluate the prognosis and malignant potential in human ovarian cancer, and whether AM2 expression can be used to predict survival in pancreatic cancer [240,249,260]. Additional experiments must be performed to confirm that plasma AM2 level is a prognostic biomarker for breast cancer patients [198]. Another important point is to know how the tumor microenvironment is involved in tumor progression. In this sense, tumor-associated macrophages, through AM, favor melanoma growth and angiogenesis, and the number of tumor-associated macrophages expressing and releasing AM in the tumor microenvironment has been associated with poor prognosis in this disease [239]. However, this knowledge is lacking in many other tumors and should be investigated in depth. It is also important to remark that AM inhibits prostate cancer cell growth [269], but it has been demonstrated that AM, through the AM-2 receptor, promotes the growth of the human prostate. This contradictory finding highlights that the physiological actions mediated by peptides could depend on the receptor type expressed by tumor cells. For this reason, it is crucial to know which receptors are expressed in cancer cells to develop specific antitumor strategies. After targeting AM, an important antitumor strategy has been demonstrated in glioma: miR-1297 blocked its expression and sensitized glioma cells to temozolomide treatment [221]. The aforementioned is an important finding and research field that must be developed in other tumors. AMY induces apoptosis in thymic lymphoma via the RAMP3/CT receptor [279]. Moreover, pramlintide, a synthetic analog of AMY, exerted an antiproliferative action against tumor cells, and its coadministration with chemotherapeutics increased cytotoxicity [366]. This knowledge is important for developing new antitumor strategies.
Extensive experimental evidence implicating peptidergic systems in cancer appearance, development, or treatment failure derives from studies in cancer cell lines isolated and maintained in culture. Furthermore, the literature provides numerous reports establishing the abnormal presence or absence of peptides and their receptors or mRNAs in different types of cancer cells extracted from tumors. Without a doubt, this information is relevant. However, new experimental approaches centered on analyzing dynamic and complex systems would provide relevant data concerning mechanisms triggered by peptidergic systems and their crosstalk signaling pathways under stress, eventually provoking cellular changes leading to uncontrolled growth and survival. Furthermore, determination of the influence of the peptidergic systems on the cell microenvironment would be ideal to unravel unknown factors that may explain the appearance and maintenance of cancer cells.
6. Conclusions
Due to the peptidergic systems’ crucial roles in cancer, a promising line of research is to study the involvement of peptides and their receptors in tumor progression to develop specific antitumor therapeutic strategies. Unfortunately, pharmaceutical companies generally have no interest in this promising research line. However, many data suggest that peptides play a crucial role in cancer development, and that peptide receptor antagonists or peptides coupled to cytotoxic agents could be used as antitumor agents opening up new clinical applications in oncology. In this sense, the use of peptides as radiopharmaceuticals for the diagnosis/treatment of NKA-, NKB-, AM-, AM2-, AMY-, and CGRP-positive tumors must be developed. Henceforth, targeted radionuclide cancer therapy is a promising line of research; in fact, peptide receptor-targeted radioligand molecules are being used in research or clinical trials [371,372,373].
The knowledge of the involvement of the SP/NK-1R system in cancer development has notably increased in the last years, and, thanks to this knowledge, the repurposing of the NK-1R antagonist aprepitant has been proposed as an antitumor agent [1,5]. By contrast, there is much to investigate regarding the involvement of NKA, NKB, AM, AM2, AMY, and CGRP in cancer progression. Regarding these peptides, basic information is lacking, and it is scarce or incomplete in many tumors. Peptide receptor antagonists could be used as an antitumor strategy; however, although NK-1R/NK-2R/NK-3R antagonists were safe in clinical trials, they were abandoned due to a lack of efficacy. The ineffectiveness of these antagonists could be due to the low dose used and to a lack of knowledge regarding the molecular interaction between NK receptors and their tachykinin ligands. More systematic research must be developed, and much more basic information is required to attain a similar degree of knowledge regarding the NKA/NK-2R and NKB/NK-2R systems currently on SP/NK1. For example, in vivo studies must be carried out, in vitro investigations combining chemotherapeutic agents and NK-2R/NK-3R antagonists must be performed, the use of NK-2R/NK-3R antagonists as antitumor agents must be widely developed, the roles played by NKA/NKB in the tumor microenvironment must be elucidated, the signaling pathways involved in tumor cell proliferation must be studied in depth, it must be demonstrated whether NK-2R and NK-3R are involved or not in the viability of tumor cells, the use of NKA/NKB and their receptors as cancer predictive factors must be established, and the structure–function relationships between NKA-NK-2R and NKB-NK-3R for the design of new broad-spectrum antitumor drugs must be determined. In this sense, the inhibition of signaling pathways common to several peptides can be a proper antitumor strategy, and it will help to develop broad-spectrum antagonists. Knowing which cancer receptors are involved is crucial to creating specific antitumor ligands and drug-design studies. Thus, understanding how SP, NKA, and NKB and their receptors interact and regulate tumor cells will help to establish specific antitumor strategies.
The involvement of the CT/CGRP peptide family in cancer is more studied than that of NKA/NK-2R and NKB/NK-3R systems; however, it can be said that the research lines that must be developed in the future are, in general, similar to those indicated above for the neurokinin system. More systematic research must be designed to avoid the incomplete knowledge that currently is known regarding the involvement of some peptides belonging to the CT/CGRP peptide family in cancer. There is so much to do. In particular, research lines using peptide receptor antagonists (e.g., AM2 receptor antagonists, NSC-16311 and NSC-37133), peptide receptor interference, peptide fragments (e.g., AM22–52), small molecules (e.g., 145425), or neutralizing antibodies against peptides or their receptors (e.g., adrecizumab, mAb-C1, and MoAb-G6) must be potentiated. Treatments against metastatic, angiogenic, and lymphangiogenic mechanisms must also be developed, as well as those increasing the chemosensitivity of tumor cells. Studies focused on the pathological significance of single-nucleotide polymorphisms must also be performed in patients with cancer, since carriers showing the single-nucleotide polymorphism rs4910118 close to the AM gene were protected against cancer.
In sum, the involvement of the peptidergic systems in cancer reviewed here must be reopened, developed, and potentiated in the future to attract more researchers to this exciting field and increase interest in the pharmaceutical industry. The data reported in this review indicate that NKA, NKB, AM, AM2, AMY, and CGRP and their receptors are promising antitumor targets, and that the study of the participation of these peptides in cancer development must be significantly increased in future studies.
Author Contributions
Conceptualization, M.L.S., F.D.R. and R.C.; sources, M.L.S., F.D.R. and R.C.; writing—original draft preparation, M.L.S., F.D.R. and R.C.; writing—review and editing, M.L.S., F.D.R. and R.C.; supervision, M.L.S., F.D.R. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coveñas, R.; Muñoz, M. Involvement of the Substance P/Neurokinin-1 Receptor System in Cancer. Cancers 2022, 14, 3539. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Coveñas, R. The Neurotensinergic System: A Target for Cancer Treatment. Curr. Med. Chem. 2022, 29, 3231–3260. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Coveñas, R. The Galaninergic System: A Target for Cancer Treatment. Cancers 2022, 14, 3755. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination Therapy of Chemotherapy or Radiotherapy and the Neurokinin-1 Receptor Antagonist Aprepitant: A New Antitumor Strategy? Curr. Med. Chem. 2023, 30, 1798–1812. [Google Scholar] [CrossRef]
- Coveñas, R.; Rodríguez, F.D.; Muñoz, M. The Neurokinin-1 Receptor: A Promising Antitumor Target. Receptors 2022, 1, 5. [Google Scholar] [CrossRef]
- Kastin, A.J. Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wu, Y.; Berisha, A.; Borniger, J.C. Neuropeptides in Cancer: Friend and Foe? Adv. Biol. 2022, 6, 2200111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, T.; Liu, Q.; Cummins, S. Copy Number Alteration of Neuropeptides and Receptors in Multiple Cancers. Sci. Rep. 2017, 7, 4598. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. The Neuropeptide System and Colorectal Cancer Liver Metastases: Mechanisms and Management. Int. J. Mol. Sci. 2020, 21, 3494. [Google Scholar] [CrossRef]
- Colucci, R.; Blandizzi, C.; Ghisu, N.; Florio, T.; Del Tacca, M. Somatostatin Inhibits Colon Cancer Cell Growth through Cyclooxygenase-2 Downregulation: Somatostatin and Cyclooxygenase-2 in Colon Cancer. Br. J. Pharmacol. 2008, 155, 198–209. [Google Scholar] [CrossRef]
- Godlewski, J.; Kmiec, Z. Colorectal Cancer Invasion and Atrophy of the Enteric Nervous System: Potential Feedback and Impact on Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3391. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Coveñas, R. Neurokinin Receptor Antagonism: A Patent Review (2014-Present). Expert Opin. Ther. Pat. 2020, 30, 527–539. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Involvement of Substance P and the NK-1 Receptor in Human Pathology. Amino Acids 2014, 46, 1727–1750. [Google Scholar] [CrossRef]
- Ebner, K.; Sartori, S.; Singewald, N. Tachykinin Receptors as Therapeutic Targets in Stress-Related Disorders. Curr. Pharm. Des. 2009, 15, 1647–1674. [Google Scholar] [CrossRef]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and Their Receptors: Contributions to Physiological Control and the Mechanisms of Disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef]
- Coveñas, R.; Mangas, A.; Narvaez, J.A. Introduction to Neuropeptides. In Focus on Neuropeptide Research; Transworld Research Network: Trivandrum, India, 2007; pp. 1–26. [Google Scholar]
- Onaga, T. Tachykinin: Recent Developments and Novel Roles in Health and Disease. Biomol. Concepts 2014, 5, 225–243. [Google Scholar] [CrossRef]
- Bepler, G.; Rotsch, M.; Jaques, G.; Haeder, M.; Heymanns, J.; Hartogh, G.; Kiefer, P.; Havemann, K. Peptides and Growth Factors in Small Cell Lung Cancer: Production, Binding Sites, and Growth Effects. J. Cancer Res. Clin. Oncol. 1988, 114, 235–244. [Google Scholar] [CrossRef]
- Obata, K.; Shimo, T.; Okui, T.; Matsumoto, K.; Takada, H.; Takabatake, K.; Kunisada, Y.; Ibaragi, S.; Nagatsuka, H.; Sasaki, A. Tachykinin Receptor 3 Distribution in Human Oral Squamous Cell Carcinoma. Anticancer Res. 2016, 36, 6335–6342. [Google Scholar] [CrossRef]
- Muñoz, M.; Coveñas, R. Neurokinin/Tachykinin Receptors. In Ecyclopedia of Molecular Pharmacology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1093–1103. [Google Scholar]
- Severini, C. The Tachykinin Peptide Family. Pharmacol. Rev. 2002, 54, 285–322. [Google Scholar] [CrossRef]
- Nässel, D.R.; Zandawala, M.; Kawada, T.; Satake, H. Tachykinins: Neuropeptides That Are Ancient, Diverse, Widespread and Functionally Pleiotropic. Front. Neurosci. 2019, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- UNIPROT. Database. Available online: https://www.uniprot.org/uniprot/P25103 (accessed on 25 October 2022).
- MacDonald, M.R.; McCourt, D.W.; Krause, J.E. Posttranslational Processing of Alpha-, Beta-, and Gamma-Preprotachykinins. Cell-Free Translation and Early Posttranslational Processing Events. J. Biol. Chem. 1988, 263, 15176–15183. [Google Scholar] [CrossRef]
- Shimizu, Y.; Matsuyama, H.; Shiina, T.; Takewaki, T.; Furness, J.B. Tachykinins and Their Functions in the Gastrointestinal Tract. Cell. Mol. Life Sci. 2008, 65, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Nawa, H.; Kotani, H.; Nakanishi, S. Tissue-Specific Generation of Two Preprotachykinin MRNAs from One Gene by Alternative RNA Splicing. Nature 1984, 312, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Nawa, H.; Doteuchi, M.; Igano, K.; Inouye, K.; Nakanishi, S. Substance K: A Novel Mammalian Tachykinin That Differs from Substance P in Its Pharmacological Profile. Life Sci. 1984, 34, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J.; Armstrong, A.; Pascall, J.C.; Chapman, K.; Rosie, R.; Curtis, A.; Going, J.; Edwards, C.R.W.; Fink, G. CDNA Sequence of Human β-Preprotachykinin, the Common Precursor to Substance P and Neurokinin A. FEBS Lett. 1986, 208, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kage, R.; Thim, L.; Creutzfeldt, W.; Conlon, J.M. Post-Translational Processing of Preprotachykinins. Isolation of Protachykinin-(1-37)-Peptide from Human Adrenal-Medullary Phaeochromocytoma Tissue. Biochem. J. 1988, 253, 203–207. [Google Scholar] [CrossRef]
- Prigge, S.T.; Mains, R.E.; Eipper, B.A.; Amzel, L.M. New Insights into Copper Monooxygenases and Peptide Amidation: Structure, Mechanism and Function. Cell. Mol. Life Sci. 2000, 57, 1236–1259. [Google Scholar] [CrossRef]
- Wong, M.; Jeng, A.Y. Posttranslational Modification of Glycine-Extended Substance P by an Alpha-Amidating Enzyme in Cultured Sensory Neurons of Dorsal Root Ganglia. J. Neurosci. Res. 1994, 37, 97–102. [Google Scholar] [CrossRef]
- Entrez-Gene. National Library of Medicine. Gene Database. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 20 October 2022).
- Maggi, C.A. The Mammalian Tachykinin Receptors. Gen. Pharmacol. Vasc. Syst. 1995, 26, 911–944. [Google Scholar] [CrossRef]
- EXPASY. SIM Alignment Tool for Protein Sequences. Available online: https://web.expasy.org/sim/ (accessed on 20 October 2022).
- Lefkowitz, R.J. The Superfamily of Heptahelical Receptors. Nat. Cell Biol. 2000, 2, E133–E136. [Google Scholar] [CrossRef] [PubMed]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and Dynamics of GPCR Signaling Complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wootten, D.; Christopoulos, A.; Sexton, P.M. Emerging Paradigms in GPCR Allostery: Implications for Drug Discovery. Nat. Rev. Drug Discov. 2013, 12, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Hilger, D. The Role of Structural Dynamics in GPCR-mediated Signaling. FEBS J. 2021, 288, 2461–2489. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, T.; Yun, Y.; Xie, X. Recent Progress in Assays for GPCR Drug Discovery. Am. J. Physiol.-Cell Physiol. 2022, 323, C583–C594. [Google Scholar] [CrossRef] [PubMed]
- Schöppe, J.; Ehrenmann, J.; Klenk, C.; Rucktooa, P.; Schütz, M.; Doré, A.S.; Plückthun, A. Crystal Structures of the Human Neurokinin 1 Receptor in Complex with Clinically Used Antagonists. Nat. Commun. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Valentin-Hansen, L.; Frimurer, T.M.; Mokrosinski, J.; Holliday, N.D.; Schwartz, T.W. Biased Gs Versus Gq Proteins and β-Arrestin Signaling in the NK1 Receptor Determined by Interactions in the Water Hydrogen Bond Network. J. Biol. Chem. 2015, 290, 24495–24508. [Google Scholar] [CrossRef]
- Yin, J.; Chapman, K.; Clark, L.D.; Shao, Z.; Borek, D.; Xu, Q.; Wang, J.; Rosenbaum, D.M. Crystal Structure of the Human NK 1 Tachykinin Receptor. Proc. Natl. Acad. Sci. USA 2018, 115, 13264–13269. [Google Scholar] [CrossRef]
- Thom, C.; Ehrenmann, J.; Vacca, S.; Waltenspühl, Y.; Schöppe, J.; Medalia, O.; Plückthun, A. Structures of Neurokinin 1 Receptor in Complex with G q and G s Proteins Reveal Substance P Binding Mode and Unique Activation Features. Sci. Adv. 2021, 7, eabk2872. [Google Scholar] [CrossRef]
- Rodríguez, F.D.; Coveñas, R. The Neurokinin-1 Receptor: Structure Dynamics and Signaling. Receptors 2022, 1, 4. [Google Scholar] [CrossRef]
- GPCR. Database. Available online: https://gpcrdb.org/protein/nk1r_human (accessed on 25 October 2022).
- KingDraw. Free Software. Available online: http://www.kingdraw.cn/en/index.html (accessed on 20 October 2022).
- Gerard, N.P.; Bao, L.; Xiao-Ping, H.; Gerard, C. Molecular Aspects of the Tachykinin Receptors. Regul. Pept. 1993, 43, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Gembitsky, D.S.; Murnin, M.; Ötvös, F.L.; Allen, J.; Murphy, R.F.; Lovas, S. Importance of the Aromatic Residue at Position 6 of [Nle10] Neurokinin A (4−10) for Binding to the NK-2 Receptor and Receptor Activation. J. Med. Chem. 1999, 42, 3004–3007. [Google Scholar] [CrossRef] [PubMed]
- Rovero, P.; Pestellini, V.; Rhaleb, N.-E.; Dion, S.; Rouissi, N.; Tousignant, C.; Télémaque, S.; Drapeau, G.; Regoli, D. Structure-Activity Studies of Neurokinin A. Neuropeptides 1989, 13, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Warner, F.J.; Mack, P.; Comis, A.; Miller, R.C.; Burcher, E. Structure–Activity Relationships of Neurokinin A (4–10) at the Human Tachykinin NK2 Receptor: The Role of Natural Residues and Their Chirality. Biochem. Pharmacol. 2001, 61, 55–60. [Google Scholar] [CrossRef]
- Regoli, D.; Boudon, A.; Fauchére, J.L. Receptors and Antagonists for Substance P and Related Peptides. Pharmacol. Rev. 1994, 45, 551–599. [Google Scholar]
- Bhogal, N.; Donnelly, D.; Findlay, J.B. The Ligand Binding Site of the Neurokinin 2 Receptor. Site-Directed Mutagenesis and Identification of Neurokinin A Binding Residues in the Human Neurokinin 2 Receptor. J. Biol. Chem. 1994, 269, 27269–27274. [Google Scholar] [CrossRef]
- Chandrashekaran, I.R.; Rao, G.S.; Cowsik, S.M. Molecular Modeling of the Peptide Agonist-Binding Site in a Neurokinin-2 Receptor. J. Chem. Inf. Model. 2009, 49, 1734–1740. [Google Scholar] [CrossRef]
- Chandrashekar, I.R.; Cowsik, S.M. Three-Dimensional Structure of the Mammalian Tachykinin Peptide Neurokinin A Bound to Lipid Micelles. Biophys. J. 2003, 85, 4002–4011. [Google Scholar] [CrossRef]
- Zoffmann, S.; Bertrand, S.; Do, Q.-T.; Bertrand, D.; Rognan, D.; Hibert, M.; Galzi, J.-L. Topological Analysis of the Complex Formed between Neurokinin A and the NK2 Tachykinin Receptor. J. Neurochem. 2007, 101, 506–516. [Google Scholar] [CrossRef]
- Meini, S.; Bellucci, F.; Catalani, C.; Cucchi, P.; Patacchini, R.; Rotondaro, L.; Altamura, M.; Giuliani, S.; Giolitti, A.; Maggi, C.A. Mutagenesis at the Human Tachykinin NK2 Receptor to Define the Binding Site of a Novel Class of Antagonists. Eur. J. Pharmacol. 2004, 488, 61–69. [Google Scholar] [CrossRef]
- Maillet, E.L.; Pellegrini, N.; Valant, C.; Bucher, B.; Hibert, M.; Bourguignon, J.-J.; Galzi, J.-L. A Novel, Conformation-specific Allosteric Inhibitor of the Tachykinin NK2 Receptor (NK2R) with Functionally Selective Properties. FASEB J. 2007, 21, 2124–2134. [Google Scholar] [CrossRef]
- Sun, W.; Yuan, Q.; Zhang, H.; Yang, F.; Ling, S.; Luo, Y.; Lv, P.; Eric Xu, H.; Tian, C.; Yin, W.; et al. Structural Insights into the Activation of Neurokinin 2 Receptor by Neurokinin A. Cell Discov. 2022, 8, 72. [Google Scholar] [CrossRef]
- Harris, J.A.; Faust, B.; Gondin, A.B.; Dämgen, M.A.; Suomivuori, C.-M.; Veldhuis, N.A.; Cheng, Y.; Dror, R.O.; Thal, D.M.; Manglik, A. Selective G Protein Signaling Driven by Substance P–Neurokinin Receptor Dynamics. Nat. Chem. Biol. 2022, 18, 109–115. [Google Scholar] [CrossRef]
- AlphaFold. AlphaFold Protein Structure Database. Available online: https://alphafold.ebi.ac.uk/ (accessed on 15 October 2022).
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- PDB. Protein Data Bank (PDB). Available online: https://pdb101.rcsb.org (accessed on 25 October 2022).
- Huang, R.-R.C.; Cheung, A.H.; Mazina, K.E.; Strader, C.D.; Fong, T.M. CDNA Sequence and Heterologous Expression of the Human Neurokinin-3 Receptor. Biochem. Biophys. Res. Commun. 1992, 184, 966–972. [Google Scholar] [CrossRef]
- Mantha, A.K.; Chandrashekar, I.R.; Baquer, N.Z.; Cowsik, S.M. Three Dimensional Structure of Mammalian Tachykinin Peptide Neurokinin B Bound to Lipid Micelles. J. Biomol. Struct. Dyn. 2004, 22, 137–147. [Google Scholar] [CrossRef]
- Ganjiwale, A.D.; Rao, G.S.; Cowsik, S.M. Molecular Modeling of Neurokinin B and Tachykinin NK3 Receptor Complex. J. Chem. Inf. Model. 2011, 11, 2932–2938. [Google Scholar] [CrossRef]
- Malherbe, P.; Bissantz, C.; Marcuz, A.; Kratzeisen, C.; Zenner, M.-T.; Wettstein, J.G.; Ratni, H.; Riemer, C.; Spooren, W. Me-Talnetant and Osanetant Interact within Overlapping but Not Identical Binding Pockets in the Human Tachykinin Neurokinin 3 Receptor Transmembrane Domains. Mol. Pharmacol. 2008, 73, 1736–1750. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Simmons, M.A. 3D-Quantitative Structure–Activity Relationship and Docking Studies of the Tachykinin NK3 Receptor. Bioorg. Med. Chem. Lett. 2011, 21, 7405–7411. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ramnath, R.D.; Tamizhselvi, R.; Bhatia, M. Neurokinin A Engages Neurokinin-1 Receptor to Induce NF-ΚB-Dependent Gene Expression in Murine Macrophages: Implications of ERK1/2 and PI 3-Kinase/Akt Pathways. Am. J. Physiol.-Cell Physiol. 2008, 295, C679–C691. [Google Scholar] [CrossRef] [PubMed]
- Khannpnavar, B.; Mehta, V.; Qi, C.; Korkhov, V. Structure and Function of Adenylyl Cyclases, Key Enzymes in Cellular Signaling. Curr. Opin. Struct. Biol. 2020, 63, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, S.; Wang, J.; Zhu, G. CAMP-PKA Cascade: An Outdated Topic for Depression? Biomed. Pharmacother. 2022, 150, 113030. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, P. The Cyclic AMP Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex Roles of CAMP–PKA–CREB Signaling in Cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- He, S.; Li, Q.; Huang, Q.; Cheng, J. Targeting Protein Kinase C for Cancer Therapy. Cancers 2022, 14, 1104. [Google Scholar] [CrossRef]
- Maekawa, M.; Ishizaki, T.; Boku, S.; Watanabe, N.; Fujita, A.; Iwamatsu, A.; Obinata, T.; Ohashi, K.; Mizuno, K.; Narumiya, S. Signaling from Rho to the Actin Cytoskeleton Through Protein Kinases ROCK and LIM-Kinase. Science 1999, 285, 895–898. [Google Scholar] [CrossRef]
- Lee, M.-H.; Kundu, J.K.; Chae, J.-I.; Shim, J.-H. Targeting ROCK/LIMK/Cofilin Signaling Pathway in Cancer. Arch. Pharm. Res. 2019, 42, 481–491. [Google Scholar] [CrossRef]
- Kümper, S.; Mardakheh, F.K.; McCarthy, A.; Yeo, M.; Stamp, G.W.; Paul, A.; Worboys, J.; Sadok, A.; Jørgensen, C.; Guichard, S.; et al. Rho-Associated Kinase (ROCK) Function Is Essential for Cell Cycle Progression, Senescence and Tumorigenesis. Elife 2016, 5, e12203. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 Biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, L.; Araújo, T.G.; Azevedo, F.V.P.d.V.; Mota, S.T.S.; Ávila, V.d.M.R.; Ribeiro, M.A.; Goulart, L.R. Phospholipase A2 Drives Tumorigenesis and Cancer Aggressiveness through Its Interaction with Annexin A1. Cells 2021, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.V.G.K.; Chintala, R.; Duddukuri, G.R. Targeting Arachidonic Acid Pathway by Natural Products for Cancer Prevention and Therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.-M.; Kim, K.-T. G Protein-Coupled Receptor Signalling and Cross-Talk. Cell. Signal. 2002, 14, 397–405. [Google Scholar] [CrossRef]
- Tuluc, F.; Lai, J.P.; Kilpatrick, L.E.; Evans, D.L.; Douglas, S.D. Neurokinin 1 Receptor Isoforms and the Control of Innate Immunity. Trends Immunol. 2009, 30, 271–276. [Google Scholar] [CrossRef]
- Garcia-Recio, S.; Gascón, P. Biological and Pharmacological Aspects of the NK1-Receptor. Biomed Res. Int. 2015, 2015, 495704. [Google Scholar] [CrossRef]
- HPA. The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 31 October 2022).
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A Genome-Wide Transcriptomic Analysis of Protein-Coding Genes in Human Blood Cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Blasco, V.; Pinto, F.M.; Fernández-Atucha, A.; Prados, N.; Tena-Sempere, M.; Fernández-Sánchez, M.; Candenas, L. Altered Expression of the Kisspeptin/KISS1R and Neurokinin B/NK3R Systems in Mural Granulosa and Cumulus Cells of Patients with Polycystic Ovarian Syndrome. J. Assist. Reprod. Genet. 2019, 36, 113–120. [Google Scholar] [CrossRef]
- Cañete, H.; Dorta, I.; Hernández, M.; Cejudo Roman, A.; Candenas, L.; Pinto, F.M.; Valladares, F.; Báez, D.; Montes de Oca, F.; Bello, A.R.; et al. Differentially Regulated Expression of Neurokinin B (NKB)/NK3 Receptor System in Uterine Leiomyomata. Hum. Reprod. 2013, 28, 1799–1808. [Google Scholar] [CrossRef]
- Singh, D.; Joshi, D.D.; Hameed, M.; Qian, J.; Gascón, P.; Maloof, P.B.; Mosenthal, A.; Rameshwar, P. Increased Expression of Preprotachykinin-I and Neurokinin Receptors in Human Breast Cancer Cells: Implications for Bone Marrow Metastasis. Proc. Natl. Acad. Sci. USA 2000, 97, 388–393. [Google Scholar] [CrossRef]
- King, R.; Brain, S.D. CGRP. In Handbook of Biologically Active Peptides; Academic Press: Amsterdam, The Netherlands, 2013; pp. 1394–1466. [Google Scholar]
- Montuenga, L.M.; Martínez, A.; Miller, M.J.; Unsworth, E.J.; Cuttitta, F. Expression of Adrenomedullin and Its Receptor during Embryogenesis Suggests Autocrine or Paracrine Modes of Action. Endocrinology 1997, 138, 440–451. [Google Scholar] [CrossRef]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a Multifunctional Regulatory Peptide. Endocr. Rev. 2000, 21, 138–167. [Google Scholar] [CrossRef]
- Kim, W.; Moon, S.-O.; Sung, M.J.; Kim, S.H.; Lee, S.; So, J.-N.; Park, S.K. Angiogenic Role of Adrenomedullin through Activation of Akt, Mitogen-activated Protein Kinase, and Focal Adhesion Kinase in Endothelial Cells. FASEB J. 2003, 17, 1–19. [Google Scholar] [CrossRef]
- Iwase, T.; Nagaya, N.; Fujii, T.; Itoh, T.; Ishibashi-Ueda, H.; Yamagishi, M.; Miyatake, K.; Matsumoto, T.; Kitamura, S.; Kangawa, K. Adrenomedullin Enhances Angiogenic Potency of Bone Marrow Transplantation in a Rat Model of Hindlimb Ischemia. Circulation 2005, 111, 356–362. [Google Scholar] [CrossRef]
- Kato, H.; Shichiri, M.; Marumo, F.; Hirata, Y. Adrenomedullin as an Autocrine/Paracrine Apoptosis Survival Factor for Rat Endothelial Cells. Endocrinology 1997, 138, 2615–2620. [Google Scholar] [CrossRef]
- Martinez, A. The Effects of Adrenomedullin Overexpression in Breast Tumor Cells. Cancer Spectr. Knowl. Environ. 2002, 94, 1226–1237. [Google Scholar] [CrossRef]
- Nikitenko, L.L. Adrenomedullin Is an Autocrine Regulator of Endothelial Growth in Human Endometrium. Mol. Hum. Reprod. 2000, 6, 811–819. [Google Scholar] [CrossRef]
- Oehler, M.K.; Hague, S.; Rees, M.C.; Bicknell, R. Adrenomedullin Promotes Formation of Xenografted Endometrial Tumors by Stimulation of Autocrine Growth and Angiogenesis. Oncogene 2002, 21, 2815–2821. [Google Scholar] [CrossRef]
- Hippenstiel, S.; Witzenrath, M.; Schmeck, B.; Hocke, A.; Krisp, M.; Krüll, M.; Seybold, J.; Seeger, W.; Rascher, W.; Schütte, H.; et al. Adrenomedullin Reduces Endothelial Hyperpermeability. Circ. Res. 2002, 91, 618–625. [Google Scholar] [CrossRef]
- Zhao, Y.; Hague, S.; Manek, S.; Zhang, L.; Bicknell, R.; Rees, M.C. PCR Display Identifies Tamoxifen Induction of the Novel Angiogenic Factor Adrenomedullin by a Non Estrogenic Mechanism in the Human Endometrium. Oncogene 1998, 16, 409–415. [Google Scholar] [CrossRef]
- Nakamura, M.; Han, B.; Nunobiki, O.; Kakudo, K. Adrenomedullin: A Tumor Progression Factor via Angiogenic Control. Curr. Cancer Drug Targets 2006, 6, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ewers, M.; Mielke, M.M.; Hampel, H. Blood-Based Biomarkers of Microvascular Pathology in Alzheimer’s Disease. Exp. Gerontol. 2010, 45, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kucukosmanoglu, E.; Keskin, O.; Karcin, M.; Cekmen, M.; Balat, A. Plasma Adrenomedullin Levels in Children with Asthma: Any Relation with Atopic Dermatitis? Allergol. Immunopathol. 2012, 40, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.L.; Brooke, G.S.; DiMarchi, R.D.; Gold, G. Human and Rat Amylin Have No Effects on Insulin Secretion in Isolated Rat Pancreatic Islets. Biochem. Biophys. Res. Commun. 1991, 177, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Mather, K.J.; Paradisi, G.; Leaming, R.; Hook, G.; Steinberg, H.O.; Fineberg, N.; Hanley, R.; Baron, A.D. Role of Amylin in Insulin Secretion and Action in Humans: Antagonist Studies across the Spectrum of Insulin Sensitivity. Diabetes Metab. Res. Rev. 2002, 18, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Chen, S.; Lutz, T.A.; Parkes, D.G.; Roth, J.D. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol. Rev. 2015, 67, 564–600. [Google Scholar] [CrossRef]
- Gong, J.; Kong, L.; Yang, L.; Nie, Y.Z.; Liang, Y.; Teng, C.B. Toxicity Reduction of Human Islet Amyloid Polypeptide by Trim-Away Technique in Insulinoma Cells. Yi Chuan 2020, 42, 586–598. (In Chinese) [Google Scholar]
- Muff, R.; Born, W.; Lutz, T.A.; Fischer, J.A. Biological Importance of the Peptides of the Calcitonin Family as Revealed by Disruption and Transfer of Corresponding Genes. Peptides 2004, 25, 2027–2038. [Google Scholar] [CrossRef]
- Garelja, M.L.; Hay, D.L. A Narrative Review of the Calcitonin Peptide Family and Associated Receptors as Migraine Targets: Calcitonin Gene-Related Peptide and Beyond. Headache J. Head Face Pain 2022, 62, 1093–1104. [Google Scholar] [CrossRef]
- Naot, D.; Musson, D.S.; Cornish, J. The Activity of Peptides of the Calcitonin Family in Bone. Physiol. Rev. 2019, 99, 781–805. [Google Scholar] [CrossRef]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the Pharmacology of Calcitonin/CGRP Family of Peptides: IUPHAR Review 25: Pharmacology of the Calcitonin/CGRP Family. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef]
- Andreotti, G.; Vitale, R.M.; Avidan-Shpalter, C.; Amodeo, P.; Gazit, E.; Motta, A. Converting the Highly Amyloidogenic Human Calcitonin into a Powerful Fibril Inhibitor by Three-Dimensional Structure Homology with a Non-Amyloidogenic Analogue. J. Biol. Chem. 2011, 286, 2707–2718. [Google Scholar] [CrossRef]
- Rosenfeld, M.G.; Mermod, J.-J.; Amara, S.G.; Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W.; Evans, R.M. Production of a Novel Neuropeptide Encoded by the Calcitonin Gene via Tissue-Specific RNA Processing. Nature 1983, 304, 129–135. [Google Scholar] [CrossRef]
- Amara, S.G.; Arriza, J.L.; Leff, S.E.; Swanson, L.W.; Evans, R.M.; Rosenfeld, M.G. Expression in Brain of a Messenger RNA Encoding a Novel Neuropeptide Homologous to Calcitonin Gene-Related Peptide. Science 1985, 229, 1094–1097. [Google Scholar] [CrossRef]
- Hillyard, C.J.; Abeyasekera, G.; Craig, R.; Myers, C.; Stevenson, J.; Macintyre, I. Katacalcin: A New Plasma Calcium-Lowering Hormone. Lancet 1983, 321, 846–848. [Google Scholar] [CrossRef]
- Josephs, T.M.; Belousoff, M.J.; Liang, Y.-L.; Piper, S.J.; Cao, J.; Garama, D.J.; Leach, K.; Gregory, K.J.; Christopoulos, A.; Hay, D.L.; et al. Structure and Dynamics of the CGRP Receptor in Apo and Peptide-Bound Forms. Science 2021, 372, eabf7258. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs Regulate the Transport and Ligand Specificity of the Calcitonin-Receptor-like Receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Simms, J.; Routledge, S.; Uddin, R.; Poyner, D. The Structure of the CGRP and Related Receptors. Calcitonin Gene-Relat. Pept. CGRP Mech. 2018, 255, 23–36. [Google Scholar] [CrossRef]
- Cong, Z.; Liang, Y.-L.; Zhou, Q.; Darbalaei, S.; Zhao, F.; Feng, W.; Zhao, L.; Xu, H.E.; Yang, D.; Wang, M.-W. Structural Perspective of Class B1 GPCR Signaling. Trends Pharmacol. Sci. 2022, 43, 321–334. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Khoshouei, M.; Deganutti, G.; Glukhova, A.; Koole, C.; Peat, T.S.; Radjainia, M.; Plitzko, J.M.; Baumeister, W.; Miller, L.J.; et al. Cryo-EM Structure of the Active, Gs-Protein Complexed, Human CGRP Receptor. Nature 2018, 561, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Egea, S.C.; Dickerson, I.M. Direct Interactions between Calcitonin-Like Receptor (CLR) and CGRP-Receptor Component Protein (RCP) Regulate CGRP Receptor Signaling. Endocrinology 2012, 153, 1850–1860. [Google Scholar] [CrossRef]
- Dickerson, I. Role of CGRP-Receptor Component Protein (RCP) in CLR/RAMP Function. Curr. Protein Pept. Sci. 2013, 14, 407–415. [Google Scholar] [CrossRef]
- Evans, B.N.; Rosenblatt, M.I.; Mnayer, L.O.; Oliver, K.R.; Dickerson, I.M. CGRP-RCP, a Novel Protein Required for Signal Transduction at Calcitonin Gene-Related Peptide and Adrenomedullin Receptors. J. Biol. Chem. 2000, 275, 31438–31443. [Google Scholar] [CrossRef] [PubMed]
- ter Haar, E.; Koth, C.M.; Abdul-Manan, N.; Swenson, L.; Coll, J.T.; Lippke, J.A.; Lepre, C.A.; Garcia-Guzman, M.; Moore, J.M. Crystal Structure of the Ectodomain Complex of the CGRP Receptor, a Class-B GPCR, Reveals the Site of Drug Antagonism. Structure 2010, 18, 1083–1093. [Google Scholar] [CrossRef]
- Shi, L.; Lehto, S.G.; Zhu, D.X.D.; Sun, H.; Zhang, J.; Smith, B.P.; Immke, D.C.; Wild, K.D.; Xu, C. Pharmacologic Characterization of AMG 334, a Potent and Selective Human Monoclonal Antibody against the Calcitonin Gene-Related Peptide Receptor. J. Pharmacol. Exp. Ther. 2015, 356, 223–231. [Google Scholar] [CrossRef]
- Garces, F.; Mohr, C.; Zhang, L.; Huang, C.-S.; Chen, Q.; King, C.; Xu, C.; Wang, Z. Molecular Insight into Recognition of the CGRPR Complex by Migraine Prevention Therapy Aimovig (Erenumab). Cell Rep. 2020, 30, 1714–1723.e6. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, M.; Vuong, T.; Taura, T.; Wilson, D.S.; Stratton, J.R.; Mackenzie, K.D. Migraine Therapeutics Differentially Modulate the CGRP Pathway. Cephalalgia 2021, 41, 499–514. [Google Scholar] [CrossRef]
- Klein, K.R.; Matson, B.C.; Caron, K.M. The Expanding Repertoire of Receptor Activity Modifying Protein (RAMP) Function. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 65–71. [Google Scholar] [CrossRef]
- Cottrell, G.S. CGRP Receptor Signalling Pathways. Calcitonin Gene-Relat. Pept. CGRP Mech. 2018, 255, 37–64. [Google Scholar] [CrossRef]
- Routledge, S.J.; Simms, J.; Clark, A.; Yeung, H.Y.; Wigglesworth, M.J.; Dickerson, I.M.; Kitchen, P.; Ladds, G.; Poyner, D.R. Receptor Component Protein, an Endogenous Allosteric Modulator of Family B G Protein Coupled Receptors. Biochim. Biophys. Acta BBA—Biomembr. 2020, 1862, 183174. [Google Scholar] [CrossRef] [PubMed]
- Conner, A.C.; Simms, J.; Howitt, S.G.; Wheatley, M.; Poyner, D.R. The Second Intracellular Loop of the Calcitonin Gene-Related Peptide Receptor Provides Molecular Determinants for Signal Transduction and Cell Surface Expression. J. Biol. Chem. 2006, 281, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.S.; Raddant, A.C.; Woolley, M.J.; Russo, A.F.; Hay, D.L. CGRP Receptor Antagonist Activity of Olcegepant Depends on the Signalling Pathway Measured. Cephalalgia 2018, 38, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Suekane, A.; Ichikawa, T.; Saito, Y.; Nakahata, S.; Morishita, K. The CGRP Receptor Antagonist MK0974 Induces EVI1 high AML Cell Apoptosis by Disrupting ERK Signaling. Anticancer Res. 2022, 42, 4743–4752. [Google Scholar] [CrossRef]
- Mancinelli, R.; Ceci, L.; Kennedy, L.; Francis, H.; Meadows, V.; Chen, L.; Carpino, G.; Kyritsi, K.; Wu, N.; Zhou, T.; et al. The Effects of Taurocholic Acid on Biliary Damage and Liver Fibrosis Are Mediated by Calcitonin-Gene-Related Peptide Signaling. Cells 2022, 11, 1591. [Google Scholar] [CrossRef]
- Yarwood, R.E.; Imlach, W.L.; Lieu, T.; Veldhuis, N.A.; Jensen, D.D.; Klein Herenbrink, C.; Aurelio, L.; Cai, Z.; Christie, M.J.; Poole, D.P.; et al. Endosomal Signaling of the Receptor for Calcitonin Gene-Related Peptide Mediates Pain Transmission. Proc. Natl. Acad. Sci. USA 2017, 114, 12309–12314. [Google Scholar] [CrossRef]
- Bigioni, M.; Benzo, A.; Irrissuto, C.; Maggi, C.A.; Goso, C. Role of NK-1 and NK-2 Tachykinin Receptor Antagonism on the Growth of Human Breast Carcinoma Cell Line MDA-MB-231. Anticancer Drugs 2005, 16, 1083–1089. [Google Scholar] [CrossRef]
- Nizam, E.; Erin, N. Differential Consequences of Neurokinin Receptor 1 and 2 Antagonists in Metastatic Breast Carcinoma Cells; Effects Independent of Substance P. Biomed. Pharmacother. 2018, 108, 263–270. [Google Scholar] [CrossRef]
- Gutierrez, S.; Boada, M.D. Neuropeptide-Induced Modulation of Carcinogenesis in a Metastatic Breast Cancer Cell Line (MDA-MB-231LUC+). Cancer Cell Int. 2018, 18, 216. [Google Scholar] [CrossRef]
- Fang, W.; Fu, C.; Chen, X.; Mou, X.; Liu, F.; Qian, J.; Zhao, P.; Zheng, Y.; Zheng, Y.; Deng, J.; et al. Neurokinin-2 Receptor Polymorphism Predicts Lymph Node Metastasis in Colorectal Cancer Patients. Oncol. Lett. 2015, 9, 2003–2006. [Google Scholar] [CrossRef]
- Xiang, H.; Toyoshima, Y.; Shen, W.; Wang, X.; Okada, N.; Kii, S.; Nagato, T. IFN-α/β-Mediated NK2R Expression Is Related to the Malignancy of Colon Cancer Cells. Cancer Sci. 2021, 113, 2513–2525. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.; Nardelli, F.; Manzini, S.; Maggi, C.A. Substance P Activates Responses Correlated with Tumour Growth in Human Glioma Cell Lines Bearing Tachykinin NK1 Receptors. Br. J. Cancer 1999, 79, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Christofides, K.; Jones, C.E. Endocytic Recycling Prevents Copper Accumulation in Astrocytoma Cells Stimulated with Copper-Bound Neurokinin B. Biochem. Biophys. Res. Commun. 2020, 523, 739–744. [Google Scholar] [CrossRef]
- McGregor, G.P.; Fehmann, C.; Hartel, R.; Voigt, K.H.; Göke, B.; Göke, R. Investigations of the Expression and Post-Translational Processing of the Preprotachykinin-I (PPT-I) Gene by Rat Pancreatic, Insulin-Producing Tumor Cell-Lines. Regul. Pept. 1993, 46, 444–446. [Google Scholar] [CrossRef] [PubMed]
- McGregor, G.P.; Hartel, R.; Fehmann, H.-C.; Lankat-Buttgereit, B.; Göke, B.; Göke, R.; Voigt, K. Characterisation of the Expression and Post-Translational Processing of the Preprotachykinin-I Gene and the Regulated Release of Tachykinins by the RINm5F Cell-Line. FEBS Lett. 1992, 312, 187–191. [Google Scholar] [CrossRef]
- Bishop, A.E.; Hamid, Q.A.; Adams, C.; Bretherton-Watt, D.; Jones, P.M.; Denny, P.; Stamp, G.W.H.; Hurt, R.L.; Grimelius, L.; Harmar, A.J.; et al. Expression of Tachykinins by Ileal and Lung Carcinoid Tumors Assessed by Combined in Situ Hybridization, Immunocytochemistry, and Radioimmunoassay. Cancer 1989, 63, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Norheim, I.; Wilander, E.; öberg, K.; Theodorsson-Norheim, E.; Lundqvist, M.-L.; Lindgren, P.; Bergh, J. Tachykinin Production by Carcinoid Tumours in Culture. Eur. J. Cancer Clin. Oncol. 1987, 23, 689–695. [Google Scholar] [CrossRef]
- Turner, G.B.; Johnston, B.T.; McCance, D.R.; McGinty, A.; Watson, R.G.P.; Patterson, C.C.; Ardill, J.E.S. Circulating Markers of Prognosis and Response to Treatment in Patients with Midgut Carcinoid Tumours. Gut 2006, 55, 1586–1591. [Google Scholar] [CrossRef]
- Makridis, C.; Theodorsson, E.; Åkerström, G.; Öberg, K.; Knutson, L. Increased Intestinal Non-Substance P Tachykinin Concentrations in Malignant Midgut Carcinoid Disease: Tachykinins in Carcinoid Disease. J. Gastroenterol. Hepatol. 2002, 14, 500–507. [Google Scholar] [CrossRef]
- McGregor, G.P.; Gaedicke, G.; Voigt, K. Neurokinin-Immunoreactivity in Human Neuroblastomas: Evidence for Selective Expression of the Preprotachykinin (PPT) II Gene. FEBS Lett. 1990, 277, 83–87. [Google Scholar] [CrossRef]
- Mukerji, I.; Ramkissoon, S.H.; Reddy, K.K.R.; Rameshwar, P. Autocrine Proliferation of Neuroblastoma Cells Is Partly Mediated through Neurokinin Receptors: Relevance to Bone Marrow Metastasis. J. Neurooncol. 2005, 71, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Mukai, H.; Kako, K.; Nakayama, K.; Munekata, E. Further Identification of Neurokinin Receptor Types and Mechanisms of Calcium Signaling Evoked by Neurokinins in the Murine Neuroblastoma C1300 Cell Line. J. Neurochem. 2002, 67, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Advenier, C.; Naline, E.; Toty, L.; Bakdach, H.; Emonds-Alt, X.; Vilain, P.; Brelière, J.-C.; Fur, G.L. Effects on the Isolated Human Bronchus of SR 48968, a Potent and Selective Nonpeptide Antagonist of the Neurokinin A (NK2) Receptors. Am. Rev. Respir. Dis. 1992, 146, 1177–1181. [Google Scholar] [CrossRef]
- Obata, K.; Shimo, T.; Okui, T.; Matsumoto, K.; Takada, H.; Takabatake, K.; Kunisada, Y.; Ibaragi, S.; Yoshioka, N.; Kishimoto, K.; et al. Role of Neurokinin 3 Receptor Signaling in Oral Squamous Cell Carcinoma. Anticancer Res. 2017, 11, 6119–6123. [Google Scholar] [CrossRef]
- Kage, R.; Conlon, J.M. Neurokinin B in a Human Pheochromocytoma Measured with a Specific Radioimmunoassay. Peptides 1989, 10, 713–716. [Google Scholar] [CrossRef]
- Demitsu, T.; Murata, S.; Kakurai, M.; Kiyosawa, T.; Yaoita, H. Immunocytochemical Characterization of Malignant Schwannoma-Derived Cells in Culture. J. Dermatol. 1997, 24, 1–6. [Google Scholar] [CrossRef]
- Woltering, E.A.; Voros, B.A.; Thiagarajan, R.; Beyer, D.T.; Ramirez, R.A.; Wang, Y.-Z.; Mamikunian, G.; Boudreaux, J.P. Plasma Neurokinin A Levels Predict Survival in Well-Differentiated Neuroendocrine Tumors of the Small Bowel. Pancreas 2018, 47, 843–848. [Google Scholar] [CrossRef]
- Ardill, J.E.; Johnston, B.T.; McCance, D.R.; Eatock, M. Neurokinin A Monitoring of Response to Interferon Alpha in a Patient with an Advanced Small Bowel Neuroendocrine Tumour Uncontrolled by Somatostatin Analogue Therapy. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 297–301. [Google Scholar] [CrossRef]
- Ardill, J.E.; McCance, D.R.; Stronge, W.V.; Johnston, B.T. Raised Circulating Neurokinin A Predicts Prognosis in Metastatic Small Bowel Neuroendocrine Tumours. Lowering Neurokinin A Indicates Improved Prognosis. Ann. Clin. Biochem. Int. J. Lab. Med. 2016, 53, 259–264. [Google Scholar] [CrossRef]
- Ardill, J.E.S.; Armstrong, L.; Smye, M.; Doherty, R.; McCance, D.R.; Johnston, B.T. Neuroendocrine Tumours of the Small Bowel: Interpretation of Raised Circulating Chromogranin A, Urinary 5 Hydroxy Indole Acetic Acid and Circulating Neurokinin A. QJM 2016, 109, 111–115. [Google Scholar] [CrossRef]
- Diebold, A.E.; Boudreaux, J.P.; Wang, Y.-Z.; Anthony, L.B.; Uhlhorn, A.P.; Ryan, P.; Mamikunian, P.; Mamikunian, G.; Woltering, E.A. Neurokinin A Levels Predict Survival in Patients with Stage IV Well Differentiated Small Bowel Neuroendocrine Neoplasms. Surgery 2012, 152, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Balks, H.J.; Conlon, J.M.; Creutzfeldt, W.; Stöckmann, F. Effect of a Long-Acting Somatostatin Analogue (Octreotide) on Circulating Tachykinins and the Pentagastrin-Induced Carcinoid Flush. Eur. J. Clin. Pharmacol. 1989, 36, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Theodorsson-Norheim, E.; Andersson, M.; Jornvall, H.; Norheim, I.; Oberg, K.; Jacobsson, G. Isolation and Characterization of Neurokinin A, Neurokinin A(3–10) and Neurokinin A(4–10) from a Neutral Water Extract of a Metastatic Ileal Carcinoid Tumour. Eur. J. Biochem. 1987, 166, 693–698. [Google Scholar] [CrossRef] [PubMed]
- González-Santana, A.; Marrero-Hernández, S.; Dorta, I.; Hernández, M.; Pinto, F.M.; Báez, D.; Bello, A.R.; Candenas, L.; Almeida, T.A. Altered Expression of the Tachykinins Substance P/Neurokinin A/Hemokinin-1 and Their Preferred Neurokinin 1/Neurokinin 2 Receptors in Uterine Leiomyomata. Fertil. Steril. 2016, 106, 1521–1529. [Google Scholar] [CrossRef]
- Ogasawara, M.; Murata, J.; Ayukawa, K.; Saiki, I. Differential Effect of Intestinal Neuropeptides on Invasion and Migration of Colon Carcinoma Cells in Vitro. Cancer Lett. 1997, 119, 125–130. [Google Scholar] [CrossRef]
- Björk, J.; Nilsson, J.; Hultcrantz, R.; Johansson, C. Growth-Regulatory Effects of Sensory Neuropeptides, Epidermal Growth Factor, Insulin, and Somatostatin on the Non-Transformed Intestinal Epithelial Cell Line IEC-6 and the Colon Cancer Cell Line HT 29. Scand. J. Gastroenterol. 1993, 28, 879–884. [Google Scholar] [CrossRef]
- Heuillet, E.; Ménager, J.; Fardin, V.; Flamand, O.; Bock, M.; Garret, C.; Crespo, A.; Fallourd, A.M.; Doble, A. Characterization of a Human NK 1 Tachykinin Receptor in the Astrocytoma Cell Line U 373 MG. J. Neurochem. 1993, 60, 868–876. [Google Scholar] [CrossRef]
- Tung, W.-L.; Lee, C.-M. Effects of Tachykinins on [3H] Taurine Release from Human Astrocytoma Cells (U-373 MG). Brain Res. 1991, 549, 171–173. [Google Scholar] [CrossRef]
- Conlon, J.M.; Deacon, C.F.; Bailey, C.J.; Flatt, P.R. Effects of a Transplantable Insulinoma upon Regulatory Peptide Concentrations in the Gastrointestinal Tract of the Rat. Diabetologia 1986, 29, 334–338. [Google Scholar] [CrossRef]
- Hanna, F.W.F.; Ardill, J.E.S.; Johnston, C.F.; Cunningham, R.T.; Curry, W.J.; Russell, C.F.J.; Buchanan, K.D. Regulatory Peptides and Other Neuroendocrine Markers in Medullary Carcinoma of the Thyroid. J. Endocrinol. 1997, 152, 275–281. [Google Scholar] [CrossRef]
- Takahashi, K.; Satoh, F.; Hara, E.; Murakami, O.; Kumabe, T.; Tominaga, T.; Kayama, T.; Yoshimoto, T.; Shibahara, S. Production and Secretion of Adrenomedullin by Cultured Choroid Plexus Carcinoma Cells. J. Neurochem. 2002, 68, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Karpinich, N.O.; Kechele, D.O.; Espenschied, S.T.; Willcockson, H.H.; Fedoriw, Y.; Caron, K.M. Adrenomedullin Gene Dosage Correlates with Tumor and Lymph Node Lymphangiogenesis. FASEB J. 2013, 27, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Hay, D.L.; Walker, C.S.; Poyner, D.R. Adrenomedullin and Calcitonin Gene-Related Peptide Receptors in Endocrine-Related Cancers: Opportunities and Challenges. Endocr. Relat. Cancer 2010, 18, C1–C14. [Google Scholar] [CrossRef] [PubMed]
- Zudaire, E.; Martínez, A.; Cuttitta, F. Adrenomedullin and Cancer. Regul. Pept. 2003, 112, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Nikitenko, L.L.; Fox, S.B.; Kehoe, S.; Rees, M.C.P.; Bicknell, R. Adrenomedullin and Tumour Angiogenesis. Br. J. Cancer 2006, 94, 1–7. [Google Scholar] [CrossRef]
- Cabarcas, S.M.; Mathews, L.A.; Farrar, W.L. The Cancer Stem Cell Niche-There Goes the Neighborhood? Int. J. Cancer 2011, 129, 2315–2327. [Google Scholar] [CrossRef]
- Talero, E.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, A.C.; Illanes, M.; Motilva, V. Role of Different Inflammatory and Tumor Biomarkers in the Development of Ulcerative Colitis-Associated Carcinogenesis. Inflamm. Bowel Dis. 2011, 17, 696–710. [Google Scholar] [CrossRef]
- Warrington, J.I.; Richards, G.O.; Wang, N. The Role of the Calcitonin Peptide Family in Prostate Cancer and Bone Metastasis. Curr. Mol. Biol. Rep. 2017, 3, 197–203. [Google Scholar] [CrossRef]
- Valiya Veettil, M.; Krishna, G.; Roy, A.; Ghosh, A.; Dutta, D.; Kumar, B.; Chakraborty, S.; Anju, T.R.; Sharma-Walia, N.; Chandran, B. Kaposi’s Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells. J. Virol. 2020, 94, e01692-19. [Google Scholar] [CrossRef]
- Gluexam, T.; Grandits, A.M.; Schlerka, A.; Nguyen, C.H.; Etzler, J.; Finkes, T.; Fuchs, M.; Scheid, C.; Heller, G.; Hackl, H.; et al. CGRP Signaling via CALCRL Increases Chemotherapy Resistance and Stem Cell Properties in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2019, 20, 5826. [Google Scholar] [CrossRef]
- Larrue, C.; Guiraud, N.; Mouchel, P.-L.; Dubois, M.; Farge, T.; Gotanègre, M.; Bosc, C.; Saland, E.; Nicolau-Travers, M.-L.; Sabatier, M.; et al. Adrenomedullin-CALCRL Axis Controls Relapse-Initiating Drug Tolerant Acute Myeloid Leukemia Cells. Nat. Commun. 2021, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Di Liddo, R.; Bridi, D.; Gottardi, M.; De Angeli, S.; Grandi, C.; Tasso, A.; Bertalot, T.; Martinelli, G.; Gherlinzoni, F.; Conconi, M.T. Adrenomedullin in the Growth Modulation and Differentiation of Acute Myeloid Leukemia Cells. Int. J. Oncol. 2016, 48, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Angeli, D.; Petracci, E.; Fonzi, E.; Vedovato, S.; Sperotto, A.; Padella, A.; Ghetti, M.; Ferrari, A.; Robustelli, V.; et al. Adrenomedullin Expression Characterizes Leukemia Stem Cells and Associates With an Inflammatory Signature in Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 684396. [Google Scholar] [CrossRef]
- Ismail, E.A.R.; El-Mogy, M.I.; Mohamed, D.S.; El-Farrash, R.A.H. Methylation Pattern of Calcitonin (CALCA) Gene in Pediatric Acute Leukemia. J. Pediatr. Hematol. Oncol. 2011, 33, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Morimoto, R.; Hirose, T.; Satoh, F.; Totsune, K. Adrenomedullin 2/Intermedin in the Hypothalamo–Pituitary–Adrenal Axis. J. Mol. Neurosci. 2011, 43, 182–192. [Google Scholar] [CrossRef]
- Mouri, T.; Takahashi, K.; Sone, M.; Murakami, O.; Ohneda, M.; Itoi, K.; Imai, Y.; Yoshinaga, K.; Sasano, N. Calcitonin Gene-Related Peptide-like Immunoreactivities in Pheochromocytomas. Peptides 1989, 10, 201–204. [Google Scholar] [CrossRef]
- Takahashi, K.; Satoh, F.; Sone, M.; Totsune, K.; Arihara, Z.; Noshiro, T.; Mouri, T.; Murakami, O. Expression of Adrenomedullin MRNA in Adrenocortical Tumors and Secretion of Adrenomedullin by Cultured Adrenocortical Carcinoma Cells. Peptides 1998, 19, 1719–1724. [Google Scholar] [CrossRef]
- Zeng, Z.-P.; Liu, D.-M.; Li, H.-Z.; Fan, X.-R.; Liu, G.-Q.; Yan, W.-G.; Tong, A.-L.; Zheng, X. Expression and Effect of Adrenomedullin in Pheochromocytoma. Ann. N. Y. Acad. Sci. 2006, 1073, 270–276. [Google Scholar] [CrossRef]
- Liu, D.-M.; Zeng, Z.-P.; Li, H.-Z.; Fan, X.-R.; Liu, G.-Q.; Yan, W.-G.; Tong, A.-L.; Zheng, X. Expression of Adrenomedullin and Its Receptor MRNA in the Tissues of Normal Adrenal Medulla and Pheochromocytoma. Zhonggou Yi Xue Ke Yuan Xue Bao Acta Acad. Med. Sin. 2005, 4, 452–456. [Google Scholar]
- Morimoto, R.; Satoh, F.; Murakami, O.; Hirose, T.; Totsune, K.; Imai, Y.; Arai, Y.; Suzuki, T.; Sasano, H.; Ito, S.; et al. Expression of Adrenomedullin 2/Intermedin in Human Adrenal Tumors and Attached Non-Neoplastic Adrenal Tissues. J. Endocrinol. 2008, 198, 175–183. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, X.; Li, F.; Zhao, Y.; Guo, Y.; Yang, R. RNA Interference Targeting Adrenomedullin Induces Apoptosis and Reduces the Growth of Human Bladder Urothelial Cell Carcinoma. Med. Oncol. 2013, 30, 616. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.K.; Fischer, D.C.; Orlowska-Volk, M.; Herrle, F.; Kieback, D.G.; Rees, M.C.P.; Bicknell, R. Tissue and Plasma Expression of the Angiogenic Peptide Adrenomedullin in Breast Cancer. Br. J. Cancer 2003, 89, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Siclari, V.A.; Mohammad, K.S.; Tompkins, D.R.; Davis, H.; McKenna, C.R.; Peng, X.; Wessner, L.L.; Niewolna, M.; Guise, T.A.; Suvannasankha, A.; et al. Tumor-Expressed Adrenomedullin Accelerates Breast Cancer Bone Metastasis. Breast Cancer Res. 2014, 16, 458. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-L.; Chen, S.-L.; Huang, Y.-H.; Yang, X.; Wang, C.-H.; He, J.-H.; Yun, J.-P.; Luo, R.-Z. Adrenomedullin Inhibits Tumor Metastasis and Is Associated with Good Prognosis in Triple-Negative Breast Cancer Patients. Am. J. Transl. Res. 2020, 12, 773–786. [Google Scholar] [PubMed]
- Benyahia, Z.; Dussault, N.; Cayol, M.; Sigaud, R.; Berenguer-Daizé, C.; Delfino, C.; Tounsi, A.; Garcia, S.; Martin, P.-M.; Mabrouk, K.; et al. Stromal Fibroblasts Present in Breast Carcinomas Promote Tumor Growth and Angiogenesis through Adrenomedullin Secretion. Oncotarget 2017, 8, 15744–15762. [Google Scholar] [CrossRef]
- Lu, Y.-M.; Zhong, J.-B.; Wang, H.-Y.; Yu, X.-F.; Li, Z.-Q. The Prognostic Value of Intermedin in Patients with Breast Cancer. Dis. Mark. 2015, 2015, 862158. [Google Scholar] [CrossRef]
- Kong, L.; Xiong, Y.; Wang, D.; Huang, L.; Li, M.; Feng, Z.; Zhou, Y.; Zhang, H.; Liu, F.; Xiao, F.; et al. Intermedin (Adrenomedullin 2) Promotes Breast Cancer Metastasis via Src/c-Myc-Mediated Ribosome Production and Protein Translation. Breast Cancer Res. Treat. 2022, 195, 91–103. [Google Scholar] [CrossRef]
- Papantoniou, V.; Tsiouris, S.; Sotiropoulou, M.; Valsamaki, P.; Koutsikos, J.; Ptohis, N.; Dimitrakakis, C.; Sotiropoulou, E.; Melissinou, M.; Nakopoulou, L.; et al. The Potential Role of Calcitonin Gene-Related Peptide (CGRP) in Breast Carcinogenesis and Its Correlation With 99mTc-(V)DMSA Scintimammography. Am. J. Clin. Oncol. 2007, 30, 420–427. [Google Scholar] [CrossRef]
- Zhao, H.; Ning, L.; Wang, Z.; Li, H.; Qiao, D.; Yao, Y.; Qin, H. Calcitonin Gene-Related Peptide Inhibits Osteolytic Factors Induced by Osteoblast In Co-Culture System with Breast Cancer. Cell Biochem. Biophys. 2014, 70, 1097–1104. [Google Scholar] [CrossRef]
- Moriyama, T.; Otani, T.; Maruo, T. Expression of Adrenomedullin by Human Placental Cytotrophoblasts and Choriocarcinoma JAr Cells. J. Clin. Endocrinol. Metab. 2001, 86, 3958–3961. [Google Scholar] [CrossRef]
- Uemura, M.; Yamamoto, H.; Takemasa, I.; Mimori, K.; Mizushima, T.; Ikeda, M.; Sekimoto, M.; Doki, Y.; Mori, M. Hypoxia-Inducible Adrenomedullin in Colorectal Cancer. Anticancer Res. 2011, 31, 507–514. [Google Scholar] [PubMed]
- Nouguerède, E.; Berenguer, C.; Garcia, S.; Bennani, B.; Delfino, C.; Nanni, I.; Dahan, L.; Gasmi, M.; Seitz, J.; Martin, P.; et al. Expression of Adrenomedullin in Human Colorectal Tumors and Its Role in Cell Growth and Invasion in Vitro and in Xenograft Growth in Vivo. Cancer Med. 2013, 2, 196–207. [Google Scholar] [CrossRef]
- Hikosaka, T.; Tsuruda, T.; Nagata, S.; Kuwasako, K.; Tsuchiya, K.; Hoshiko, S.; Inatsu, H.; Chijiiwa, K.; Kitamura, K. Adrenomedullin Production Is Increased in Colorectal Adenocarcinomas; Its Relation to Matrix Metalloproteinase-9. Peptides 2011, 32, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, W.-D.; Lee, S.; Park, J.-H. Adrenomedullin Is Involved in the Progression of Colonic Adenocarcinoma. Mol. Med. Rep. 2012, 6, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gala, M.; Yamamoto, M.; Pino, M.S.; Kikuchi, H.; Shue, D.S.; Shirasawa, S.; Austin, T.R.; Lynch, M.P.; Rueda, B.R.; et al. Adrenomedullin Is a Therapeutic Target in Colorectal Cancer: K-Ras Regulates Adrenomedullin in Hypoxia. Int. J. Cancer 2014, 134, 2041–2050. [Google Scholar] [CrossRef]
- Ochoa-Callejero, L.; García-Sanmartín, J.; Martínez-Herrero, S.; Rubio-Mediavilla, S.; Narro-Íñiguez, J.; Martínez, A. Small Molecules Related to Adrenomedullin Reduce Tumor Burden in a Mouse Model of Colitis-Associated Colon Cancer. Sci. Rep. 2017, 7, 17488. [Google Scholar] [CrossRef] [PubMed]
- White, D.M.; Leah, J.D.; Zimmermann, M. The Localization and Release of Substance P and Calcitonin Gene-Related Peptide at Nerve Fibre Endings in Rat Cutaneous Nerve Neuroma. Brain Res. 1989, 503, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.J.; Chitcholtan, K.; Dann, J.M.; Guilford, P.; Harris, G.; Lewis, L.K.; Nagase, J.; Welkamp, A.A.W.; Zwerus, R.; Sykes, P.H. Adrenomedullin Interacts with VEGF in Endometrial Cancer and Has Varied Modulation in Tumours of Different Grades. Gynecol. Oncol. 2012, 125, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.K.; Norbury, C.; Hague, S.; Rees, M.C.; Bicknell, R. Adrenomedullin Inhibits Hypoxic Cell Death by Upregulation of Bcl-2 in Endometrial Cancer Cells: A Possible Promotion Mechanism for Tumour Growth. Oncogene 2001, 20, 2937–2945. [Google Scholar] [CrossRef]
- Nunobiki, O.; Nakamura, M.; Taniguchi, E.; Utsunomiya, H.; Mori, I.; Tsubota, Y.; Mabuchi, Y.; Kakudo, K. Adrenomedullin, Bcl-2 and Microvessel Density in Normal, Hyperplastic and Neoplastic Endometrium. Pathol. Int. 2009, 59, 530–536. [Google Scholar] [CrossRef]
- Dallmayer, M.; Li, J.; Ohmura, S.; Alba Rubio, R.; Baldauf, M.C.; Hölting, T.L.B.; Musa, J.; Knott, M.M.L.; Stein, S.; Cidre-Aranaz, F.; et al. Targeting the CALCB/RAMP1 Axis Inhibits Growth of Ewing Sarcoma. Cell Death Dis. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Peng, L.; Wang, Q.; Chen, N.; Teng, Y.; Wang, T.; Mao, F.; Zhang, J.; Cheng, P.; Liu, Y.; et al. Degranulation of Mast Cells Induced by Gastric Cancer-Derived Adrenomedullin Prompts Gastric Cancer Progression. Cell Death Dis. 2018, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.P.; Serrano, J.; Amorim, M.A.; Pozo-Rodrigalvarez, A.; Martinez-Murillo, R. Adrenomedullin and Nitric Oxide: Implications for the Etiology and Treatment of Primary Brain Tumors. CNS Neurol. Disord. Drug Targets 2011, 10, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Metellus, P.; Voutsinos-Porche, B.; Nanni-Metellus, I.; Colin, C.; Fina, F.; Berenguer, C.; Dussault, N.; Boudouresque, F.; Loundou, A.; Intagliata, D.; et al. Adrenomedullin Expression and Regulation in Human Glioblastoma, Cultured Human Glioblastoma Cell Lines and Pilocytic Astrocytoma. Eur. J. Cancer 2011, 47, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Ouafik, L.; Sauze, S.; Boudouresque, F.; Chinot, O.; Delfino, C.; Fina, F.; Vuaroqueaux, V.; Dussert, C.; Palmari, J.; Dufour, H.; et al. Neutralization of Adrenomedullin Inhibits the Growth of Human Glioblastoma Cell Lines in Vitro and Suppresses Tumor Xenograft Growth in Vivo. Am. J. Pathol. 2002, 160, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Boudouresque, F.; Berthois, Y.; Martin, P.-M.; Figarella-Branger, D.; Chinot, O.; Ouafik, L. Role of Adrenomedullin in Glioblastomas Growth. Bull Cancer 2005, 4, 317–326. [Google Scholar]
- Ouafik, L.; Berenguer-Daize, C.; Berthois, Y. Adrenomedullin Promotes Cell Cycle Transit and Up-Regulates Cyclin D1 Protein Level in Human Glioblastoma Cells through the Activation of c-Jun/JNK/AP-1 Signal Transduction Pathway. Cell. Signal. 2009, 21, 597–608. [Google Scholar] [CrossRef]
- Lim, S.Y.; Ahn, S.-H.; Park, H.; Lee, J.; Choi, K.; Choi, C.; Choi, J.H.; Park, E.-M.; Choi, Y.-H. Transcriptional Regulation of Adrenomedullin by Oncostatin M in Human Astroglioma Cells: Implications for Tumor Invasion and Migration. Sci. Rep. 2014, 4, 6444. [Google Scholar] [CrossRef]
- He, Z.; Cheng, M.; Hu, J.; Liu, L.; Liu, P.; Chen, L.; Cao, D.; Tang, J. MiR-1297 Sensitizes Glioma Cells to Temozolomide (TMZ) Treatment through Targeting Adrenomedullin (ADM). J. Transl. Med. 2022, 20, 443. [Google Scholar] [CrossRef]
- Huang, L.; Wang, D.; Feng, Z.; Zhao, H.; Xiao, F.; Wei, Y.; Zhang, H.; Li, H.; Kong, L.; Li, M.; et al. Inhibition of Intermedin (Adrenomedullin 2) Suppresses the Growth of Glioblastoma and Increases the Antitumor Activity of Temozolomide. Mol. Cancer Ther. 2021, 20, 284–295. [Google Scholar] [CrossRef]
- Gitter, B.D.; Cox, L.M.; Carlson, C.D.; May, P.C. Human Amylin Stimulates Inflammatory Cytokine Secretion from Human Glioma Cells. Neuroimmunomodulation 2000, 7, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.; Liu, Z.; Wang, X.; Ji, T. The Neuropeptide Calcitonin Gene-Related Peptide Links Perineural Invasion with Lymph Node Metastasis in Oral Squamous Cell Carcinoma. BMC Cancer 2021, 21, 1254. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.; Wang, X.; Ji, T. Calcitonin Gene-related Peptide: A Promising Bridge between Cancer Development and Cancer-associated Pain in Oral Squamous Cell Carcinoma (Review). Oncol. Lett. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Yoon, J.-H.; Lee, J.-H.; Yu, S.J.; Myung, S.J.; Kim, W.; Gwak, G.-Y.; Lee, S.-H.; Lee, S.-M.; Jang, J.J.; et al. Hypoxia-Inducible Adrenomedullin Accelerates Hepatocellular Carcinoma Cell Growth. Cancer Lett. 2008, 271, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T.; Seki, N.; Miwa, S.; Kobayashi, A.; Soeda, J.; Nimura, Y.; Kawasaki, S.; Miyagawa, S. Identification of Genes Associated with Multiple Nodules in Hepatocellular Carcinoma Using CDNA Microarray: Multicentric Occurrence or Intrahepatic Metastasis? Hepatogastroenterology 2008, 55, 865–872. [Google Scholar]
- Zhou, C.; Zheng, Y.; Li, L.; Zhai, W.; Li, R.; Liang, Z.; Zhao, L. Adrenomedullin Promotes Intrahepatic Cholangiocellular Carcinoma Metastasis and Invasion by Inducing Epithelial-Mesenchymal Transition. Oncol. Rep. 2015, 34, 610–616. [Google Scholar] [CrossRef]
- Li, F.; Yang, R.; Zhang, X.; Liu, A.; Zhao, Y.; Guo, Y. Silencing of Hypoxia-inducible Adrenomedullin Using RNA Interference Attenuates Hepatocellular Carcinoma Cell Growth in Vivo. Mol. Med. Rep. 2014, 10, 1295–1302. [Google Scholar] [CrossRef]
- Qu, Z.; Jiang, Y.; Xu, M.; Lu, M.Z.; Zhou, B.; Ding, Y. Correlation of Adrenomedullin with the Erythropoietin Receptor and Microvessel Density in Hepatocellular Carcinoma. Arch. Med. Sci. 2015, 5, 978–981. [Google Scholar] [CrossRef]
- Guo, X.; Schmitz, J.C.; Kenney, B.C.; Uchio, E.M.; Kulkarni, S.; Cha, C.H. Intermedin Is Overexpressed in Hepatocellular Carcinoma and Regulates Cell Proliferation and Survival. Cancer Sci. 2012, 103, 1474–1480. [Google Scholar] [CrossRef]
- Shang, H.; Hao, Z.; Fu, X.; Hua, X.; Ma, Z.; Ai, F.; Feng, Z.; Wang, K.; Li, W.; Li, B. Intermedin Promotes Hepatocellular Carcinoma Cell Proliferation through the Classical Wnt Signaling Pathway. Oncol. Lett. 2018, 15, 5966–5970. [Google Scholar] [CrossRef]
- Xiao, F.; Li, H.; Feng, Z.; Huang, L.; Kong, L.; Li, M.; Wang, D.; Liu, F.; Zhu, Z.; Wei, Y.; et al. Intermedin Facilitates Hepatocellular Carcinoma Cell Survival and Invasion via ERK1/2-EGR1/DDIT3 Signaling Cascade. Sci. Rep. 2021, 11, 488. [Google Scholar] [CrossRef]
- Sheriff, S.; Fischer, J.E.; Balasubramaniam, A. Characterization of Amylin Binding Sites in a Human Hepatoblastoma Cell Line. Peptides 1992, 13, 1193–1199. [Google Scholar] [CrossRef]
- Buyukberber, S.; Sari, I.; Camci, C.; Buyukberber, N.M.; Sevinc, A.; Turk, H.M. Adrenomedullin Expression Does Not Correlate with Survival in Lung Cancer. Med. Oncol. 2007, 24, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Portal-Nuñez, S.; Shankavaram, U.T.; Rao, M.; Datrice, N.; Atay, S.; Aparicio, M.; Camphausen, K.A.; Fernández-Salguero, P.M.; Chang, H.; Lin, P.; et al. Aryl Hydrocarbon Receptor-Induced Adrenomedullin Mediates Cigarette Smoke Carcinogenicity in Humans and Mice. Cancer Res. 2012, 72, 5790–5800. [Google Scholar] [CrossRef] [PubMed]
- Edbrooke, M.R.; Parker, D.; McVey, J.H.; Riley, J.H.; Sorenson, G.D.; Pettengill, O.S.; Craig, R.K. Expression of the Human Calcitonin/CGRP Gene in Lung and Thyroid Carcinoma. EMBO J. 1985, 4, 715–724. [Google Scholar] [CrossRef]
- Schifter, S.; Johannsen, L.; Bunker, C.; Brickell, P.; Bork, E.; Lindeberg, H.; Faber, J. Calcitonin Gene-Related Peptide in Small Cell Lung Carcinomas. Clin. Endocrinol. 1993, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, Y.; Bong, R.; Ding, Y.; Song, N.; Wang, X.; Song, X.; Luo, Y. Tumor-Associated Macrophages Promote Angiogenesis and Melanoma Growth via Adrenomedullin in a Paracrine and Autocrine Manner. Clin. Cancer Res. 2011, 17, 7230–7239. [Google Scholar] [CrossRef]
- Zhang, Z.-L.; Huang, S.-X.; Lin, S.; Chai, L. Plasma Adrenomedullin Levels and Nasopharyngeal Carcinoma Prognosis. Clin. Chim. Acta 2015, 440, 172–176. [Google Scholar] [CrossRef]
- Dötsch, J.; Harmjanz, A.; Christiansen, H.; Hänze, J.; Lampert, F.; Rascher, W. Gene Expression of Neuronal Nitric Oxide Synthase and Adrenomedullin in Human Neuroblastoma Using Real-Time PCR. Int. J. Cancer 2000, 88, 172–175. [Google Scholar] [CrossRef]
- Zimmermann, U.; Fischer, J.A.; Muff, R. Adrenomedullin and Calcitonin Gene-Related Peptide Interact with the Same Receptor in Cultured Human Neuroblastoma SK-N-MC Cells. Peptides 1995, 16, 421–424. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hoppe, S.; Papadopoulos, T.; Linder, V.; Mohr, B.; Hahn, E.G.; Lohmann, T.; Schuppan, D. Adrenomedullin Is a Novel Marker of Tumor Progression in Neuroendocrine Carcinomas. Horm. Metab. Res. 2006, 38, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.d.L.; Peterle, G.T.; dos Santos, M.; Trivilin, L.O.; Mendes, S.O.; de Oliveira, M.M.; dos Santos, J.G.; Stur, E.; Agostini, L.P.; Couto, C.V.M.d.S.; et al. JMJD1A, H3K9me1, H3K9me2 and ADM Expression as Prognostic Markers in Oral and Oropharyngeal Squamous Cell Carcinoma. PLoS ONE 2018, 13, e0194884. [Google Scholar] [CrossRef]
- Dai, X.; Ma, W.; He, X.J.; Jha, R.K. Elevated Expression of Adrenomedullin Is Correlated with Prognosis and Disease Severity in Osteosarcoma. Med. Oncol. 2013, 30, 347. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Y.; Hao, C.-P.; Ling, M.; Guo, C.-H.; Ma, W. Hypoxia-Induced Apoptosis Is Blocked by Adrenomedullin via Upregulation of Bcl-2 in Human Osteosarcoma Cells. Oncol. Rep. 2015, 34, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, P.-L.; Vuaroqueaux, V.; Daurés, J.-P.; Houafic, L.; Martin, P.-M.; Laffargue, F.; Maudelonde, T. Expression of Adrenomedullin in Human Ovaries, Ovarian Cysts and Cancers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhang, S.; Miao, Y.; Han, Z.; Zhang, X.; Wen, F.; Zhang, Y. Adrenomedullin Expression in Epithelial Ovarian Cancers and Promotes HO8910 Cell Migration Associated with Upregulating Integrin A5β1 and Phosphorylating FAK and Paxillin. J. Exp. Clin. Cancer Res. 2012, 31, 19. [Google Scholar] [CrossRef]
- Li, M.; Hong, L.; Liao, M.; Guo, G. Expression and Clinical Significance of Focal Adhesion Kinase and Adrenomedullin in Epithelial Ovarian Cancer. Oncol. Lett. 2015, 10, 1003–1007. [Google Scholar] [CrossRef]
- Zhang, Y. Effect of Inhibition of the Adrenomedullin Gene on the Growth and Chemosensitivity of Ovarian Cancer Cells. Oncol. Rep. 2012, 27, 1461–1466. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Ma, J.; Pang, X.; Dong, M. Adrenomedullin Promotes Angiogenesis in Epithelial Ovarian Cancer through Upregulating Hypoxiainducible Factor-1α and Vascular Endothelial Growth Factor. Sci. Rep. 2017, 16, 40524. [Google Scholar] [CrossRef]
- Hata, K. Expression of the Adrenomedullin Gene in Epithelial Ovarian Cancer. Mol. Hum. Reprod. 2000, 6, 867–872. [Google Scholar] [CrossRef]
- Keleg, S.; Kayed, H.; Jiang, X.; Penzel, R.; Giese, T.; Büchler, M.W.; Friess, H.; Kleeff, J. Adrenomedullin Is Induced by Hypoxia and Enhances Pancreatic Cancer Cell Invasion. Int. J. Cancer 2007, 121, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Chen, J.; Wang, J.; Okada, F.; Sugiyama, T.; Kobayashi, T.; Shindo, M.; Higashino, F.; Katoh, H.; Asaka, M.; et al. Adrenomedullin Antagonist Suppresses in Vivo Growth of Human Pancreatic Cancer Cells in SCID Mice by Suppressing Angiogenesis. Oncogene 2003, 22, 1238–1242. [Google Scholar] [CrossRef]
- Aggarwai, G.; Ramachandran, V.; Javeed, N.; Arumugam, T.; Dutta, S. Adrenomedullin Is Up-Regulated in Patients With Pancreatic Cancer and Causes Insulin Resistance in β Cells and Mice. Gastroenterology 2012, 6, 1510–1517. [Google Scholar] [CrossRef]
- Letizia, C.; Tamburrano, G.; Alo, P.; Paoloni, A.; Caliumi, C.; Marinoni, E.; di Iorio, R.; d’Erasmo, E. Adrenomedullin, a New Peptide, in Patients with Insulinoma. Eur. J. Endocrinol. 2001, 144, 517–520. [Google Scholar] [CrossRef]
- Ramachandran, V.; Arumugam, T.; Hwang, R.F.; Greenson, J.K.; Simeone, D.M.; Logsdon, C.D. Adrenomedullin Is Expressed in Pancreatic Cancer and Stimulates Cell Proliferation and Invasion in an Autocrine Manner via the Adrenomedullin Receptor, ADMR. Cancer Res. 2007, 67, 2666–2675. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Tanaka, M.; Kamiyoshi, A.; Sakurai, T.; Ichikawa-Shindo, Y.; Kawate, H.; Cui, N.; Wei, Y.; Tanaka, M.; Kakihara, S.; et al. Deficiency of the Adrenomedullin-RAMP3 System Suppresses Metastasis through the Modification of Cancer-Associated Fibroblasts. Oncogene 2020, 39, 1914–1930. [Google Scholar] [CrossRef]
- Xu, M.; Qi, F.; Zhang, S.; Ma, X.; Wang, S.; Wang, C.; Fu, Y.; Luo, Y. Adrenomedullin Promotes the Growth of Pancreatic Ductal Adenocarcinoma through Recruitment of Myelomonocytic Cells. Oncotarget 2016, 7, 55043–55056. [Google Scholar] [CrossRef] [PubMed]
- Hollander, L.L.; Guo, X.; Salem, R.R.; Cha, C.H. The Novel Tumor Angiogenic Factor, Adrenomedullin-2, Predicts Survival in Pancreatic Adenocarcinoma. J. Surg. Res. 2015, 197, 219–224. [Google Scholar] [CrossRef]
- Mäkimattila, S.; Hietaniemi, K.; Kiviluoto, T.; Timonen, T.; Järvien, H. In Vivo Glucose-Stimulated Amylin Secretion Is Increased in Nondiabetic Patients with Pancreatic Cancer. Metabolism 2001, 50, 1036–1042. [Google Scholar] [CrossRef]
- Gao, F.; Liu, G.; Wang, J.; Huang, S.; Ding, F.; Lian, W.; Lv, X.; Guo, Y.; Fan, X.; Zhang, S.; et al. Methylation of CALCA and CALCB in Pancreatic Ductal Adenocarcinoma. Oxid. Med. Cell. Longev. 2021, 2021, 2088345. [Google Scholar] [CrossRef]
- Ramachandran, V.; Arumugam, T.; Langley, R.; Hwang, R.F.; Vivas-Mejia, P.; Sood, A.K.; Lopez-Berestein, G.; Logsdon, C.D. The ADMR Receptor Mediates the Effects of Adrenomedullin on Pancreatic Cancer Cells and on Cells of the Tumor Microenvironment. PLoS ONE 2009, 4, e7502. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T. Immunocytochemical Staining for Islet Amyloid Polypeptide in Pancreatic Endocrine Tumors. Islets 2011, 3, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.; Kovacs, K.; Scheithauer, B.; Rotondo, F.; Salehi, F.; Lloyd, R. Adrenomedullin Expression in Pituitary Adenomas and Nontumoral Adenohypophyses. Histol. Histopathol. 2007, 23, 11–17. [Google Scholar] [CrossRef]
- Rocchi, P.; Boudouresque, F.; Zamora, A.J.; Muracciole, X.; Lechevallier, E.; Martin, P.M.; Ouafik, L. Expression of Adrenomedullin and Peptide Amidation Activity in Human Prostate Cancer and in Human Prostate Cancer Cell Lines. Cancer Res. 2001, 3, 1196–1206. [Google Scholar]
- Berenguer-Daizé, C.; Boudouresque, F.; Bastide, C.; Tounsi, A.; Benyahia, Z.; Acunzo, J.; Dussault, N.; Delfino, C.; Baeza, N.; Daniel, L.; et al. Adrenomedullin Blockade Suppresses Growth of Human Hormone–Independent Prostate Tumor Xenograft in Mice. Clin. Cancer Res. 2013, 19, 6138–6150. [Google Scholar] [CrossRef]
- Calvo, A.; Abasolo, I.; Jiménez, N.; Wang, Z.; Montuenga, L. Adrenomedullin and Proadrenomedullin N-Terminal 20 Peptide in the Normal Prostate and in Prostate Carcinoma: AM and PAMP in the Prostate. Microsc. Res. Tech. 2002, 57, 98–104. [Google Scholar] [CrossRef]
- Abasolo, I.; Wang, Z.; Montuenga, L.M.; Calvo, A. Adrenomedullin Inhibits Prostate Cancer Cell Proliferation through a CAMP-Independent Autocrine Mechanism. Biochem. Biophys. Res. Commun. 2004, 322, 878–886. [Google Scholar] [CrossRef]
- Abasolo, I.; Montuenga, L.M.; Calvo, A. Adrenomedullin Prevents Apoptosis in Prostate Cancer Cells. Regul. Pept. 2006, 133, 115–122. [Google Scholar] [CrossRef]
- Oulidi, A.; Bokhobza, A.; Gkika, D.; Vanden Abeele, F.; Lehen’kyi, V.; Ouafik, L.; Mauroy, B.; Prevarskaya, N. TRPV2 Mediates Adrenomedullin Stimulation of Prostate and Urothelial Cancer Cell Adhesion, Migration and Invasion. PLoS ONE 2013, 8, e64885. [Google Scholar] [CrossRef]
- Wang, X.-L.; Wang, Y.-Y.; He, H.-D.; Xie, X.; Yu, Z.-J.; Pan, Y.-M. Association of Plasma Intermedin Levels with Progression and Metastasis in Men after Radical Prostatectomy for Localized Prostatic Cancer. Cancer Biomark. 2015, 15, 799–805. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, Y.; Morita, T. Serum Calcitonin Gene-Related Peptide Levels in Untreated Prostate Cancer Patients: CGRP Level in Untreated Prostate Cancer. Int. J. Urol. 2006, 13, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kobayashi, Y.; Morita, T. Significance of Serum Calcitonin Gene-Related Peptide Levels in Prostate Cancer Patients Receiving Hormonal Therapy. Urol. Int. 2009, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Sheng, D.; Shao, Y.; Zhang, Q.; Peng, Y. Neuronal Calcitonin Gene-Related Peptide Promotes Prostate Tumor Growth in the Bone Microenvironment. Peptides 2021, 135, 170423. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, J.; Thiesson, H.; Walter, S.; Ottosen, P.D.; Skøtt, O.; Jensen, B.L. Tissue Expression and Plasma Levels of Adrenomedullin in Renal Cancer Patients. Clin. Sci. 2006, 111, 61–70. [Google Scholar] [CrossRef]
- Nikitenko, L.L.; Leek, R.; Henderson, S.; Pillay, N.; Turley, H.; Generali, D.; Gunningham, S.; Morrin, H.R.; Pellagatti, A.; Rees, M.C.P.; et al. The G-Protein–Coupled Receptor CLR Is Upregulated in an Autocrine Loop with Adrenomedullin in Clear Cell Renal Cell Carcinoma and Associated with Poor Prognosis. Clin. Cancer Res. 2013, 19, 5740–5748. [Google Scholar] [CrossRef]
- Fujita, Y.; Mimata, H.; Nasu, N.; Nomura, T.; Nomura, Y.; Nakagawa, M. Involvement of Adrenomedullin Induced by Hypoxia in Angiogenesis in Human Renal Cell Carcinoma. Int. J. Urol. 2002, 9, 285–295. [Google Scholar] [CrossRef]
- Venkatanarayan, A.; Raulji, P.; Norton, W.; Chakravarti, D.; Coarfa, C.; Su, X.; Sandur, S.K.; Ramirez, M.S.; Lee, J.; Kingsley, C.V.; et al. IAPP-Driven Metabolic Reprogramming Induces Regression of P53-Deficient Tumours in Vivo. Nature 2015, 517, 626–630. [Google Scholar] [CrossRef]
- Kim, J.T.; Lim, M.A.; Lee, S.E.; Kim, H.J.; Koh, H.Y.; Lee, J.H.; Jun, S.M.; Kim, J.M.; Kim, K.H.; Shin, H.S.; et al. Adrenomedullin2 Stimulates Progression of Thyroid Cancer in Mice and Humans under Nutrient Excess Conditions. J. Pathol. 2022, 258, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Alevizaki, M.; Grigorakis, S.; Tseleni-Balafouta, S.; Alevizaki, C.; Philippou, G.; Anastasiou, E.; Souvatzoglou, A. High Plasma Amylin/Islet Amyloid Polypeptide Levels in Patients with Residual Medullary Thyroid Carcinoma after Total Thyroidectomy. Eur. J. Endocrinol. 2001, 145, 585–589. [Google Scholar] [CrossRef]
- Haller-Brem, S.; Muff, R.; Petermann, J.B.; Born, W.; Roos, B.A.; Fischer, J.A. Role of Cytosolic Free Calcium Concentration in the Secretion of Calcitonin Gene-Related Peptide and Calcitonin from Medullary Thyroid Carcinoma Cells. Endocrinology 1987, 121, 1272–1277. [Google Scholar] [CrossRef]
- Pacini, F.; Fugazzola, L.; Basolo, F.; Elisei, R.; Pinchera, A. Expression of Calcitonin Gene-Related Peptide in Medullary Thyroid Cancer. J. Endocrinol. Investig. 1992, 15, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Takeuchi, S.; Otani, T.; Maruo, T. Implications of Adrenomedullin Expression in the Invasion of Squamous Cell Carcinoma of the Uterine Cervix. Int. J. Clin. Oncol. 2001, 6, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Takeuchi, S.; Ohara, N.; Maruo, T. Paradoxically Abundant Expression of Bcl-2 and Adrenomedullin in Invasive Cervical Squamous Carcinoma. Int. J. Clin. Oncol. 2003, 8, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yoshinoya, A.; Murakami, O.; Totsune, K.; Shibahara, S. Production and Secretion of Two Vasoactive Peptides, Adrenomedullin and Endothelin-1, by Cultured Human Adrenocortical Carcinoma Cells. Peptides 2000, 21, 251–256. [Google Scholar] [CrossRef]
- Thouënnon, E.; Pierre, A.; Tanguy, Y.; Guillemot, J.; Manecka, D.-L.; Guérin, M.; Ouafik, L.; Muresan, M.; Klein, M.; Bertherat, J.; et al. Expression of Trophic Amidated Peptides and Their Receptors in Benign and Malignant Pheochromocytomas: High Expression of Adrenomedullin RDC1 Receptor and Implication in Tumoral Cell Survival. Endocr. Relat. Cancer 2010, 17, 637–651. [Google Scholar] [CrossRef]
- Letizia, C.; De Toma, G.; Caliumi, C.; Cerci, S.; Massa, R.; Di Iorio, R.; Alo, P.; Marinoni, E.; Diacinti, D.; D’Erasmo, E. Plasma Adrenomedullin Concentrations in Patients with Adrenal Pheochromocytoma. Horm. Metab. Res. 2001, 33, 290–294. [Google Scholar] [CrossRef]
- Cotesta, D.; Caliumi, C.; Alò, P.; Petramala, L.; Reale, M.G.; Masciangelo, R.; Signore, A.; Cianci, R.; D’Erasmo, E.; Letizia, C. High Plasma Levels of Human Chromogranin a and Adrenomedullin in Patients with Pheochromocytoma. Tumori J. 2005, 91, 53–58. [Google Scholar] [CrossRef]
- Kobayashi, H.; Itoh, S.; Yanagita, T.; Yokoo, H.; Sugano, T.; Wada, A. Expression of Adrenomedullin and Proadrenomedullin N-Terminal 20 Peptide in PC12 Cells after Exposure to Nerve Growth Factor. Neuroscience 2004, 125, 973–980. [Google Scholar] [CrossRef]
- Brekhman, V.; Lugassie, J.; Zaffryar-Eilot, S.; Sabo, E.; Kessler, O.; Smith, V.; Golding, H.; Neufeld, G. Receptor Activity Modifying Protein-3 Mediates the Protumorigenic Activity of Lysyl Oxidase-like Protein-2. FASEB J. 2011, 25, 55–65. [Google Scholar] [CrossRef]
- Paré, M.; Darini, C.Y.; Yao, X.; Chignon-Sicard, B.; Rekima, S.; Lachambre, S.; Virolle, V.; Aguilar-Mahecha, A.; Basik, M.; Dani, C.; et al. Breast Cancer Mammospheres Secrete Adrenomedullin to Induce Lipolysis and Browning of Adjacent Adipocytes. BMC Cancer 2020, 20, 784. [Google Scholar] [CrossRef]
- Nakayama, M.; Takahashi, K.; Murakami, O.; Shirato, K.; Shibahara, S. Induction of Adrenomedullin by Hypoxia and Cobalt Chloride in Human Colorectal Carcinoma Cells. Biochem. Biophys. Res. Commun. 1998, 243, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Eissele, R.; Neuhaus, C.; Trautmann, M.E.; Funk, A.; Arnold, R.; Höfler, H. Immunoreactivity and Expression of Amylin in Gastroenteropancreatic Endocrine Tumors. Am. J. Phatologie 1993, 1, 283–291. [Google Scholar]
- Godlewski, J.; Kaleczyc, J. Somatostatin, Substance P and Calcitonin Gene-Related Peptide-Positive Intramural Nerve Structures of the Human Large Intestine Affected by Carcinoma. Folia Histochem. Cytobiol. 2010, 48, 475–483. [Google Scholar] [CrossRef]
- Bozkurt, K.K.; Yalçın, Y.; Erdemoğlu, E.; Tatar, B.; Erdemoğlu, E.; Çerçi, S.S.; Çiriş, İ.M.; Başpınar, Ş.; Uğuz, A.; Kapucuoğlu, N. The Role of Immunohistochemical Adrenomedullin and Bcl-2 Expression in Development of Type-1 Endometrial Adenocarcinoma. Pathol.—Res. Pract. 2016, 212, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Cerci, S.S.; Yalçın, Y.; Bozkurt, K.K.; Erdemoğlu, E.; Tatar, B. Hypoxia-Inducible Factor-1α, Adrenomedullin and Bcl-2 Although Expected Are Not Related to Increased Uptake of Fluorine-18-Fluorodeoxyglucose in Endometrial Cancer. Hell. J. Nuc. Med. 2015, 18, 228–232. [Google Scholar]
- Höppener, J.W.M.; Steenbergh, P.H.; Slebos, R.J.C.; Visser, A.; Lips, C.J.M.; Jansz, H.S.; Bechet, J.M.; Lenoir, G.M.; Born, W.; Haller-Brem, S.; et al. Expression of the Second Calcitonin/Calcitonin Gene-Related Peptide Gene in Ewing Sarcoma Cell Lines. J. Clin. Endocrinol. Metab. 1987, 64, 809–817. [Google Scholar] [CrossRef]
- Qiao, F.; Fang, J.; Xu, J.; Zhao, W.; Ni, Y.; Akuo, B.A.; Zhang, W.; Liu, Y.; Ding, F.; Li, G.; et al. The Role of Adrenomedullin in the Pathogenesis of Gastric Cancer. Oncotarget 2017, 8, 88464–88474. [Google Scholar] [CrossRef]
- Benes, L. The Immunohistochemical Expression of Calcitonin Receptor-like Receptor (CRLR) in Human Gliomas. J. Clin. Pathol. 2004, 57, 172–176. [Google Scholar] [CrossRef]
- Kitamuro, T.; Takahashi, K.; Nakayama, M.; Murakami, O.; Hida, W.; Shirato, K.; Shibahara, S. Induction of Adrenomedullin During Hypoxia in Cultured Human Glioblastoma Cells. J. Neurochem. 2002, 75, 1826–1833. [Google Scholar] [CrossRef]
- Takahashi, K.; Satoh, F.; Hara, E.; Sone, M.; Murakami, O.; Kayama, T.; Yoshimoto, T.; Shibahara, S. Production and Secretion of Adrenomedullin From Glial Cell Tumors and Its Effects on CAMP Production. Peptides 1997, 18, 1117–1124. [Google Scholar] [CrossRef]
- Takahashi, K.; Udono-Fujimori, R.; Totsune, K.; Murakami, O.; Shibahara, S. Suppression of Cytokine-Induced Expression of Adrenomedullin and Endothelin-1 by Dexamethasone in T98G Human Glioblastoma Cells. Peptides 2003, 24, 1053–1062. [Google Scholar] [CrossRef]
- McIlvried, L.A.; Atherton, M.A.; Horan, N.L.; Goch, T.N.; Scheff, N.N. Sensory Neurotransmitter Calcitonin Gene-Related Peptide Modulates Tumor Growth and Lymphocyte Infiltration in Oral Squamous Cell Carcinoma. Adv. Biol. 2022, 6, e2200019. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.-J.; Xiao, F.; Wei, Y.; Ke, W.; Xin, H.-B. Intermedin: A Novel Regulator for Vascular Remodeling and Tumor Vessel Normalization by Regulating Vascular Endothelial-Cadherin and Extracellular Signal–Regulated Kinase. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2721–2732. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Hao, Z.Q.; Fu, X.B.; Hua, X.D.; Ma, Z.H.; Ai, F.L.; Feng, Z.Q.; Wang, K.; Li, W.X.; Li, B. Intermedin Promotes Hepatic Carcinoma Cell Proliferation through Upregulation of miR-155. Int. J. Clin. Exp. Pahotlogy 2018, 11, 3961–3968. [Google Scholar]
- Greillier, L.; Tounsi, A.; Berenguer-Daizé, C.; Dussault, N.; Delfino, C.; Benyahia, Z.; Cayol, M.; Mabrouk, K.; Garcia, S.; Martin, P.-M.; et al. Functional Analysis of the Adrenomedullin Pathway in Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2016, 11, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Miller, M.J.; Unsworth, E.J.; Siegfried, J.M.; Cuttitta, F. Expression of Adrenomedullin in Normal Human Lung and in Pulmonary Tumors. Endocrinology 1995, 136, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y. Immunohistochemical Analysis of Calcitonin and Calcitonin Gene-Related Peptide in Human Lung. Hum. Pathol. 1989, 20, 896–902. [Google Scholar] [CrossRef]
- MartÍnez, A.; Elsasser, T.H.; Muro-Cacho, C.; Moody, T.W.; Miller, M.J.; Macri, C.J.; Cuttitta, F. Expression of Adrenomedullin and Its Receptor in Normal and Malignant Human Skin: A Potential Pluripotent Role in the Integument. Endocrinology 1997, 138, 5597–5604. [Google Scholar] [CrossRef]
- Kitamuro, T.; Takahashi, K.; Totsune, K.; Nakayama, M.; Murakami, O.; Hida, W.; Shirato, K.; Shibahara, S. Differential Expression of Adrenomedullin and Its Receptor Component, Receptor Activity Modifying Protein (RAMP) 2 during Hypoxia in Cultured Human Neuroblastoma Cells. Peptides 2001, 22, 1795–1801. [Google Scholar] [CrossRef]
- Xu, Y.; Krukoff, T.L. Adrenomedullin Stimulates Nitric Oxide Release from SK-N-SH Human Neuroblastoma Cells by Modulating Intracellular Calcium Mobilization. Endocrinology 2005, 146, 2295–2305. [Google Scholar] [CrossRef]
- Deville, J.-L.; Bartoli, C.; Berenguer, C.; Fernandez-Sauze, S.; Kaafarani, I.; Delfino, C.; Fina, F.; Salas, S.; Muracciole, X.; Mancini, J.; et al. Expression and Role of Adrenomedullin in Renal Tumors and Value of Its MRNA Levels as Prognostic Factor in Clear-Cell Renal Carcinoma. Int. J. Cancer 2009, 125, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; Ma, W.; Han, X.; He, X.; Dai, X. Downregulation of Adrenomedullin Leads to the Inhibition of the Tumorigenesis via VEGF Pathway in Human and Nude Mice Osteosarcoma Models. Arch. Med. Res. 2019, 50, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, P.-L.; Daurés, J.-P.; Ouafik, L.; Martin, P.-M.; Laffargue, F.; Maudelonde, T. Steroids and Adrenomedullin Growth Patterns in Human Ovarian Cancer Cells: Estrogenic-Regulation Assay. Gynecol. Oncol. 2003, 91, 651–656. [Google Scholar] [CrossRef]
- Liu, J.; Bützow, R.; Hydén-Granskog, C.; Voutilainen, R. Expression of Adrenomedullin in Human Ovaries, Ovarian Sex Cord–Stromal Tumors and Cultured Granulosa–Luteal Cells. Gynecol. Endocrinol. 2009, 25, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Shang, H.; Pang, X.; Zhao, Y. Basic Fibroblast Growth Factor Upregulates Adrenomedullin Expression in Ovarian Epithelial Carcinoma Cells via JNK-AP-1 Pathway. Regul. Pept. 2009, 157, 44–50. [Google Scholar] [CrossRef]
- Pang, X.; Shang, H.; Deng, B.; Wen, F.; Zhang, Y. The Interaction of Adrenomedullin and Macrophages Induces Ovarian Cancer Cell Migration via Activation of RhoA Signaling Pathway. Int. J. Mol. Sci. 2013, 14, 2774–2787. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, P.; Pang, X.; Hu, Y.; Zhang, Y. Adrenomedullin Up-Regulates the Expression of Vascular Endothelial Growth Factor in Epithelial Ovarian Carcinoma Cells via JNK/AP-1 Pathway. Int. J. Gynecol. Cancer 2015, 25, 953–960. [Google Scholar] [CrossRef]
- Javeed, N.; Sagar, G.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Bhattacharya, S.; Truty, M.; Petersen, G.M.; Kaufman, R.J.; Chari, S.T.; et al. Pancreatic Cancer–Derived Exosomes Cause Paraneoplastic β-Cell Dysfunction. Clin. Cancer Res. 2015, 21, 1722–1733. [Google Scholar] [CrossRef]
- Sagar, G.; Sah, R.P.; Javeed, N.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Giorgadze, N.; Tchkonia, T.; Kirkland, J.L.; Chari, S.T.; et al. Pathogenesis of Pancreatic Cancer Exosome-Induced Lipolysis in Adipose Tissue. Gut 2016, 65, 1165–1174. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Srivastava, S.K.; Singh, S.; Tyagi, N.; Arora, S.; Carter, J.E.; Khushman, M.; Singh, A.P. MYB Promotes Desmoplasia in Pancreatic Cancer through Direct Transcriptional Up-Regulation and Cooperative Action of Sonic Hedgehog and Adrenomedullin. J. Biol. Chem. 2016, 291, 16263–16270. [Google Scholar] [CrossRef]
- Ding, X.; Flatt, P.R.; Permert, J.; Adrian, T.E. Pancreatic Cancer Cells Selectively Stimulate Islet β Cells to Secrete Amylin. Gastroenterology 1998, 114, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Stridsberg, M.; Eriksson, B.; Lundqvist, G.; Skogseid, B.; Wilander, E.; Öberg, K. Islet Amyloid Polypeptide (IAPP) in Patients with Neuroendocrine Tumours. Regul. Pept. 1995, 55, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, F.; Vidal, S.; Bell, D.; Horvath, E.; Kovacs, K.; Scheithauer, B.W.; Lloyd, R.V. Immunohistochemical Localization of Amylin in Human Pancreas, Thyroid, Pituitary and Their Tumors. Acta Histochem. 2003, 105, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.E.; Ding, X.-Z.; Young, C.M.; Adrian, T.E. The Specificity of Amylin for the Diagnosis of Pancreatic Adenocarcinoma. Int. J. Gastrointest. Cancer 2002, 31, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chari, S.T.; Klee, G.G.; Miller, L.J.; Raimondo, M.; DiMagno, E.P. Islet Amyloid Polypeptide Is Not a Satisfactory Marker for Detecting Pancreatic Cancer. Gastroenterology 2001, 121, 640–645. [Google Scholar] [CrossRef]
- Garnett, S.; de Bruyns, A.; Provencher-Tom, V.; Dutchak, K.; Shu, R.; Dankort, D. Metabolic Regulator IAPP (Amylin) Is Required for BRAF and RAS Oncogene-Induced Senescence. Mol. Cancer Res. 2021, 19, 874–885. [Google Scholar] [CrossRef]
- kim, H.S.; Joo, S.S.; Yoo, J.-M. The Correlation between Amylin and Insulin by Treatment with 2-Deoxy-D-Glucose and/or Mannose in Rat Insulinoma INS-1E Cells. J. Physiol. Pharmacol. 2022, 72, 102–123. [Google Scholar] [CrossRef]
- Williams, A.J.K.; Coates, P.J.; Lowe, D.G.; McLEAN, C.; Gale, E.A.M. Immunochemical Investigation of Insulinomas for Islet Amyloid Polypeptide and Insulin: Evidence for Differential Synthesis and Storage. Histopathology 1992, 21, 215–223. [Google Scholar] [CrossRef]
- Oskarsson, M.E.; Singh, K.; Wang, J.; Vlodavsky, I.; Li, J.; Westermark, G.T. Heparan Sulfate Proteoglycans Are Important for Islet Amyloid Formation and Islet Amyloid Polypeptide-Induced Apoptosis. J. Biol. Chem. 2015, 290, 15121–15132. [Google Scholar] [CrossRef]
- Magruder, J.T.; Elahi, D.; Andersen, D.K. Diabetes and Pancreatic Cancer: Chicken or Egg? Pancreas 2011, 40, 339–351. [Google Scholar] [CrossRef]
- Bretherton-Watt, D.; Ghatei, M.A.; Bloom, S.E.; Williams, S.; Bloom, S.R. Islet Amyloid Polypeptide-like Immunoreactivity in Human Tissue and Endocrine Tumors. J. Clin. Endocrinol. Metab. 1993, 76, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, Y.; Liu, W.; Mai, Y. Silibinin Ameliorates Amylin-Induced Pancreatic β-Cell Apoptosis Partly via Upregulation of GLP-1R/PKA Pathway. Mol. Cell Biochem. 2019, 1–2, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, J.; MacGibbon, G.; Dragunow, M.; Cooper, G.J.S. Increased Expression and Activation of C-Jun Contributes to Human Amylin-Induced Apoptosis in Pancreatic Islet β-Cells. J. Mol. Biol. 2002, 324, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Abasolo, I.; Yang, L.; Haleem, R.; Xiao, W.; Pio, R.; Cuttitta, F.; Montuenga, L.M.; Kozlowski, J.M.; Calvo, A.; Wang, Z. Overexpression of Adrenomedullin Gene Markedly Inhibits Proliferation of PC3 Prostate Cancer Cells in Vitro and in Vivo. Mol. Cell. Endocrinol. 2003, 199, 179–187. [Google Scholar] [CrossRef]
- Gonzalez-Moreno, O.; Calvo, A.; Joshi, B.H.; Abasolo, I.; Leland, P.; Wang, Z.; Montuenga, L.; Puri, R.K.; Green, J.E. Gene Expression Profiling Identifies IL-13 Receptor ?2 Chain as a Therapeutic Target in Prostate Tumor Cells Overexpressing Adrenomedullin. Int. J. Cancer 2005, 114, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.H.; Leland, P.; Calvo, A.; Green, J.E.; Puri, R.K. Human Adrenomedullin Up-Regulates Interleukin-13 Receptor A2 Chain in Prostate Cancer In Vitro and In Vivo: A Novel Approach to Sensitize Prostate Cancer to Anticancer Therapy. Cancer Res. 2008, 68, 9311–9317. [Google Scholar] [CrossRef]
- Jiménez, N.; Abasolo, I.; Jongsma, J.; Calvo, A.; Garayoa, M.; van der Kwast, T.H.; van Steenbrugge, G.J.; Montuenga, L.M. Androgen-Independent Expression of Adrenomedullin and Peptidylglycine Alphaamidating Monooxygenase in Human Prostatic Carcinoma. Mol. Carcinog. 2003, 38, 14–24. [Google Scholar] [CrossRef]
- Berenguer, C.; Boudouresque, F.; Dussert, C.; Daniel, L.; Muracciole, X.; Grino, M.; Rossi, D.; Mabrouk, K.; Figarella-Branger, D.; Martin, P.-M.; et al. Adrenomedullin, an Autocrine/Paracrine Factor Induced by Androgen Withdrawal, Stimulates ‘Neuroendocrine Phenotype’ in LNCaP Prostate Tumor Cells. Oncogene 2008, 27, 506–518. [Google Scholar] [CrossRef]
- Smith, R.S.; Gao, L.; Bledsoe, G.; Chao, L.; Chao, J. Intermedin Is a New Angiogenic Growth Factor. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1040–H1047. [Google Scholar] [CrossRef]
- Nagakawa, O.; Ogasawara, M.; Fujii, H.; Murakami, K.; Murata, J.; Fuse, H.; Saiki, I. Effect of Prostatic Neuropeptides on Invasion and Migration of PC-3 Prostate Cancer Cells. Cancer Lett. 1998, 133, 27–33. [Google Scholar] [CrossRef]
- Di Sant’Agnese, P.A. Neuroendocrine Differentiation in Carcinoma of the Prostate. Diagnostic, Prognostic, and Therapeutic Implications. Cancer 1992, 70, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Hagner, S.; Stahl, U.; Grimm, T.; Sturzl, M.; Lang, R.E. Expression of Calcitonin Receptor-like Receptor in Human Vascular Tumours. J. Clin. Pathol. 2006, 59, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Stables, J.M.; Beresford, I.J.M.; Arkinstall, S.; Ireland, S.J.; Walsh, D.M.; Seale, P.W.; Ward, P.; Hagan, R.M. GR138676, a Novel Peptidic Tachykinin Antagonist Which Is Potent at NK3 Receptors. Neuropeptides 1994, 27, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, S.; Wang, S.; Shi, L.; Wang, C.; Zhang, J.; Gao, Y.; Li, G.; Qi, Y.; An, X.; et al. Targeting Neurokinin-3 Receptor: A Novel Anti-Angiogenesis Strategy for Cancer Treatment. Oncotarget 2017, 8, 40713–40723. [Google Scholar] [CrossRef]
- Kitani, M.; Sakata, J.; Asada, Y.; Kitamura, K.; Eto, T. Distribution and Expression of Adrenomedullin in Human Gastrointestinal Tissue. Ann. Clin. Biochem. Int. J. Lab. Med. 1998, 35, 643–648. [Google Scholar] [CrossRef]
- Marutsuka, K.; Nawa, Y.; Asada, Y.; Hara, S.; Kitamura, K.; Eto, T.; Sumiyoshi, A. Adrenomedullin and Proadrenomudullin N-Terminal 20 Peptide (PAMP) Are Present in Human Colonic Epithelia and Exert an Antimicrobial Effect. Exp. Physiol. 2001, 86, 543–545. [Google Scholar] [CrossRef]
- Miller, M.J.; Martínez, A.; Unsworth, E.J.; Thiele, C.J.; Moody, T.W.; Elsasser, T.; Cuttitta, F. Androgen-Independent Expression of Adrenomedullin and Peptidylglycine Alphaamidating Monooxygenase in Human Prostatic Carcinoma. J. Biol. Chem. 1996, 271, 23345–23351. [Google Scholar] [CrossRef]
- Kaafarani, I.; Fernandez-Sauze, S.; Berenguer, C.; Chinot, O.; Delfino, C.; Dussert, C.; Metellus, P.; Boudouresque, F.; Mabrouk, K.; Grisoli, F.; et al. Targeting Adrenomedullin Receptors with Systemic Delivery of Neutralizing Antibodies Inhibits Tumor Angiogenesis and Suppresses Growth of Human Tumor Xenografts in Mice. FASEB J. 2009, 23, 3424–3435. [Google Scholar] [CrossRef]
- Martínez, A.; Weaver, C.; López, J.; Bhathena, S.J.; Elsasser, T.H.; Miller, M.J.; Moody, T.W.; Unsworth, E.J.; Cuttitta, F. Regulation of Insulin Secretion and Blood Glucose Metabolism by Adrenomedullin. Endocrinology 1996, 137, 2626–2632. [Google Scholar] [CrossRef]
- Vázquez, R.; Riveiro, M.E.; Berenguer-Daizé, C.; O’Kane, A.; Gormley, J.; Touzelet, O.; Rezai, K.; Bekradda, M.; Ouafik, L. Targeting Adrenomedullin in Oncology: A Feasible Strategy With Potential as Much More Than an Alternative Anti-Angiogenic Therapy. Front. Oncol. 2021, 10, 589218. [Google Scholar] [CrossRef]
- Larráyoz, I.M.; Martínez-Herrero, S.; García-Sanmartín, J.; Ochoa-Callejero, L.; Martínez, A. Adrenomedullin and Tumour Microenvironment. J. Transl. Med. 2014, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Hirata, Y.; Iwasaki, H.; Sato, K.; Watanabe, T.X.; Inui, T.; Nakajima, K.; Sakakibara, S.; Marumo, F. Structure-Activity Relationship of Adrenomedullin, a Novel Vasodilatory Peptide, in Cultured Rat Vascular Smooth Muscle Cells. Endocrinology 1994, 135, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Avgoustou, P.; Jailani, A.B.A.; Zirimwabagabo, J.-O.; Tozer, M.J.; Gibson, K.R.; Glossop, P.A.; Mills, J.E.J.; Porter, R.A.; Blaney, P.; Bungay, P.J.; et al. Discovery of a First-in-Class Potent Small Molecule Antagonist against the Adrenomedullin-2 Receptor. ACS Pharmacol. Transl. Sci. 2020, 3, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Zirimwabagabo, J.-O.; Jailani, A.B.A.; Avgoustou, P.; Tozer, M.J.; Gibson, K.R.; Glossop, P.A.; Mills, J.E.J.; Porter, R.A.; Blaney, P.; Wang, N.; et al. Discovery of a First-In-Class Small Molecule Antagonist against the Adrenomedullin-2 Receptor: Structure–Activity Relationships and Optimization. J. Med. Chem. 2021, 64, 3299–3319. [Google Scholar] [CrossRef]
- Jailani, A.B.A.; Bigos, K.J.A.; Avgoustou, P.; Egan, J.L.; Hathway, R.A.; Skerry, T.M.; Richards, G.O. Targeting the Adrenomedullin-2 Receptor for the Discovery and Development of Novel Anti-Cancer Agents. Expert Opin. Drug Discov. 2022, 17, 839–848. [Google Scholar] [CrossRef]
- Miseki, T.; Kawakami, H.; Natsuizaka, M.; Darmanin, S.; Cui, H.Y.; Chen, J.; Fu, Q.; Okada, F.; Shindo, M.; Higashino, F.; et al. Suppression of Tumor Growth by Intra-Muscular Transfer of Naked DNA Encoding Adrenomedullin Antagonist. Cancer Gene Ther. 2007, 14, 39–44. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Hida, K.; Hida, Y.; Muraki, C.; Ohga, N. Adrenomedullin Antagonist Suppresses Tumor Formation in Renal Cell Carcinoma through Inhibitory Effects on Tumor Endothelial Cells and Endothelial Progenitor Mobilization. Int. J. Oncol. 2010, 36, 1379–1386. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Qiao, N.; Meng, Q.; Zhang, M.; Wang, X.; Jia, J.; Yang, S.; Qu, C.; Li, W.; et al. Adrenomedullin Blockade Suppresses Sunitinib-Resistant Renal Cell Carcinoma Growth by Targeting the ERK/MAPK Pathway. Oncotarget 2016, 7, 63374–63387. [Google Scholar] [CrossRef]
- Tanaka, M.; Koyama, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Liu, T.; Xian, X.; Imai, A.; Zhai, L.; et al. The Endothelial Adrenomedullin-RAMP2 System Regulates Vascular Integrity and Suppresses Tumour Metastasis. Cardiovasc. Res. 2016, 111, 398–409. [Google Scholar] [CrossRef]
- Zudaire, E.; Martínez, A.; Garayoa, M.; Pío, R.; Kaur, G.; Woolhiser, M.R.; Metcalfe, D.D.; Hook, W.A.; Siraganian, R.P.; Guise, T.A.; et al. Adrenomedullin Is a Cross-Talk Molecule That Regulates Tumor and Mast Cell Function during Human Carcinogenesis. Am. J. Pathol. 2006, 168, 280–291. [Google Scholar] [CrossRef]
- Martínez-Herrero, S.; Martínez, A. Cancer Protection Elicited by a Single Nucleotide Polymorphism Close to the Adrenomedullin Gene. J. Clin. Endocrinol. Metab. 2013, 98, E807–E810. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Hsu, S.Y.T. Development of Chimeric and Bifunctional Antagonists for CLR/RAMP Receptors. PLoS ONE 2019, 14, e0216996. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, F.; Kong, L.; Wang, D.; Li, H.; Wei, Y.; Tan, C.; Zhao, H.; Zhang, T.; Cao, G.; et al. Intermedin Enlarges the Vascular Lumen by Inducing the Quiescent Endothelial Cell Proliferation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Al-Keilani, M.; Alsmadi, D.; Darweesh, R.; Alzoubi, K. Pramlintide, an Antidiabetic, Is Antineoplastic in Colorectal Cancer and Synergizes with Conventional Chemotherapy. Clin. Pharmacol. Adv. Appl. 2018, 10, 23–29. [Google Scholar] [CrossRef]
- Chiba, T.; Yamaguchi, A.; Yamatani, T.; Nakamura, A.; Morishita, T.; Inui, T.; Fukase, M.; Noda, T.; Fujita, T. Calcitonin Gene-Related Peptide Receptor Antagonist Human CGRP-(8-37). Am. J. Physiol.-Endocrinol. Metab. 1989, 256, E331–E335. [Google Scholar] [CrossRef]
- Balood, M.; Ahmadi, M.; Eichwald, T.; Ahmadi, A.; Majdoubi, A.; Roversi, K.; Roversi, K.; Lucido, C.T.; Restaino, A.C.; Huang, S.; et al. Nociceptor Neurons Affect Cancer Immunosurveillance. Nature 2022, 611, 405–412. [Google Scholar] [CrossRef]
- Pío, R.; Martínez, A.; Unsworth, E.J.; Kowalak, J.A.; Bengoechea, J.A.; Zipfel, P.F.; Elsasser, T.H.; Cuttitta, F. Complement Factor H Is a Serum-Binding Protein for Adrenomedullin, and the Resulting Complex Modulates the Bioactivities of Both Partners. J. Biol. Chem. 2001, 276, 12292–12300. [Google Scholar] [CrossRef]
- Chirgwin, J.M.; Mohammad, K.S.; Guise, T.A. Tumor-Bone Cellular Interactions in Skeletal Metastases. J. Musculoeskelet Neuronal Interact. 2004, 4, 308–318. [Google Scholar]
- Majkowska-Pilip, A.; Halik, P.K.; Gniazdowska, E. The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy. Pharmaceutics 2019, 11, 443. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme Corp. Radiolabeled CGRP Antagonists. WO2011014383A1 (2011), 3 February 2011. [Google Scholar]
- Tang, B.; Yong, X.; Xie, R.; Li, Q.-W.; Yang, S.-M. Vasoactive Intestinal Peptide Receptor-Based Imaging and Treatment of Tumors. Int. J. Oncol. 2014, 44, 1023–1031. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).