Phase 1 Study to Evaluate the Safety of Reducing the Prophylactic Dose of Dexamethasone around Docetaxel Infusion in Patients with Prostate and Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
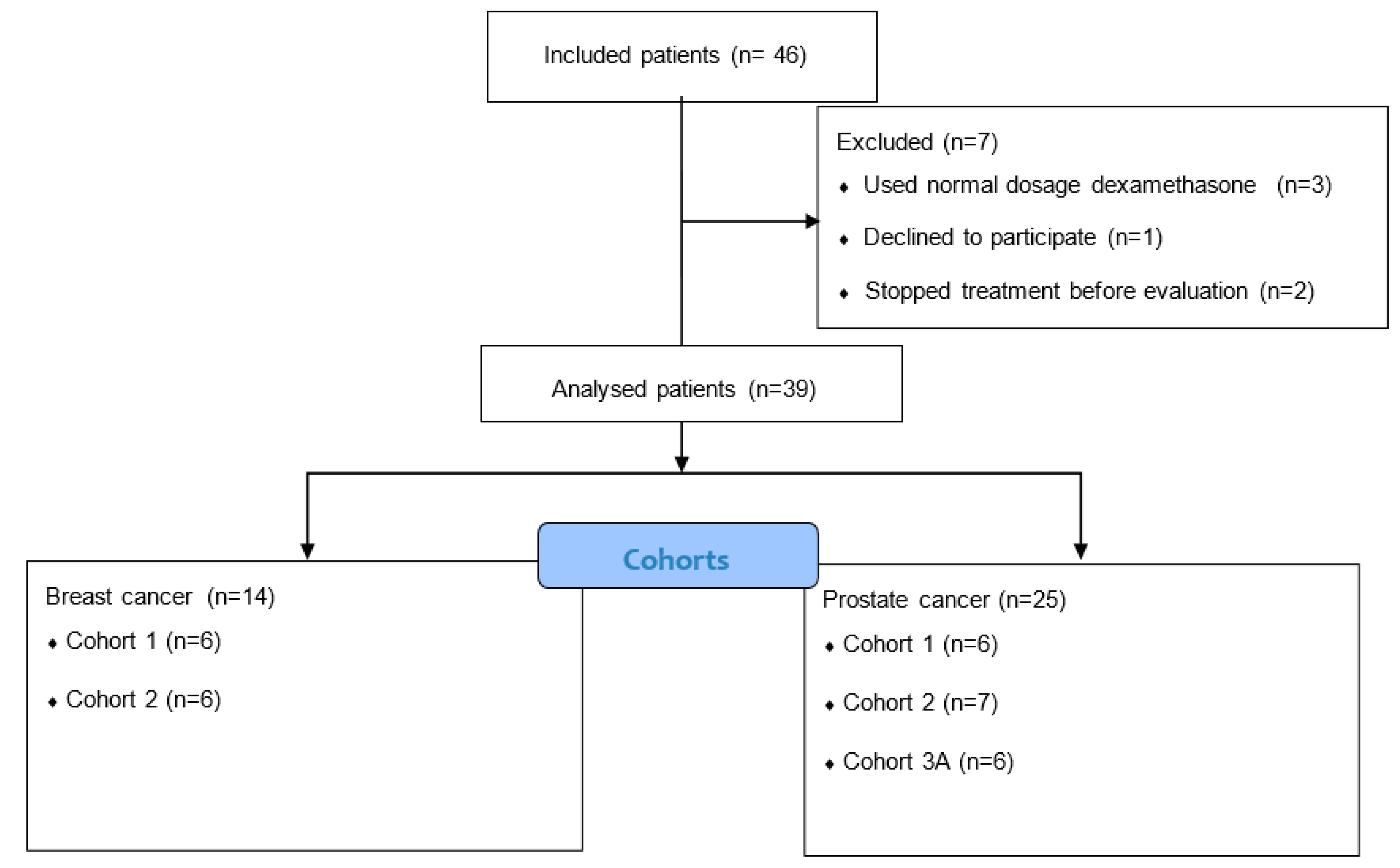
2.1. Study Design
2.2. Patient
2.3. Endpoint
2.4. Quality of Life
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Toxicity
3.3. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Pazdur, R. Docetaxel. J. Clin. Oncol. 1995, 13, 2643–2655. [Google Scholar] [CrossRef]
- Schrijvers, D.; Wanders, J.; Dirix, L.; Prove, A.; Vonck, I.; Van, O.A.; Kaye, S. Coping with toxicities of docetaxel (Taxotere). Ann. Oncol. 1993, 4, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, V.; Giavina-Bianchi, P.; Picard, M.; Caiado, J.; Castells, M. Drug desensitization in the management of hypersensitivity reactions to monoclonal antibodies and chemotherapy. BioDrugs 2014, 28, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Dieras, V.; Chevallier, B.; Kerbrat, P.; Krakowski, I.; Roche, H.; Misset, J.L.; Lentz, M.A.; Azli, N.; Murawsky, M.; Riva, A.; et al. A multicentre phase II study of docetaxel 75 mg m-2 as first-line chemotherapy for patients with advanced breast cancer: Report of the Clinical Screening Group of the EORTC. European Organization for Research and Treatment of Cancer. Br. J. Cancer 1996, 74, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A.; Seidman, A.D.; Crown, J.P.; Balmaceda, C.; Freilich, R.; Gilewski, T.A.; Hakes, T.B.; Currie, V.; Lebwohl, D.E.; Baselga, J.; et al. Phase II and pharmacologic study of docetaxel as initial chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996, 14, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Ravdin, P.M.; Burris, H.A., III; Cook, G.; Eisenberg, P.; Kane, M.; Bierman, W.A.; Mortimer, J.; Genevois, E.; Bellet, R.E. Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J. Clin. Oncol. 1995, 13, 2879–2885. [Google Scholar] [CrossRef]
- Ten Bokkel Huinink, W.W.; Prove, A.M.; Piccart, M.; Steward, W.; Tursz, T.; Wanders, J.; Franklin, H.; Clavel, M.; Verweij, J.; Alakl, M.; et al. A phase II trial with docetaxel (Taxotere) in second line treatment with chemotherapy for advanced breast cancer. A study of the EORTC Early Clinical Trials Group. Ann. Oncol. 1994, 5, 527–532. [Google Scholar] [CrossRef]
- Chevallier, B.; Fumoleau, P.; Kerbrat, P.; Dieras, V.; Roche, H.; Krakowski, I.; Azli, N.; Bayssas, M.; Lentz, M.A.; Van, G.M. Docetaxel is a major cytotoxic drug for the treatment of advanced breast cancer: A phase II trial of the Clinical Screening Cooperative Group of the European Organization for Research and Treatment of Cancer. J. Clin. Oncol. 1995, 13, 314–322. [Google Scholar] [CrossRef]
- Fumoleau, P.; Chevallier, B.; Kerbrat, P.; Krakowski, Y.; Misset, J.L.; Maugard-Louboutin, C.; Dieras, V.; Azli, N.; Bougon, N.; Riva, A.; et al. A multicentre phase II study of the efficacy and safety of docetaxel as first-line treatment of advanced breast cancer: Report of the Clinical Screening Group of the EORTC. Ann. Oncol. 1996, 7, 165–171. [Google Scholar] [CrossRef]
- Valero, V.; Jones, S.E.; Von Hoff, D.D.; Booser, D.J.; Mennel, R.G.; Ravdin, P.M.; Holmes, F.A.; Rahman, Z.; Schottstaedt, M.W.; Erban, J.K.; et al. A phase II study of docetaxel in patients with paclitaxel-resistant metastatic breast cancer. J. Clin. Oncol. 1998, 16, 3362–3368. [Google Scholar] [CrossRef]
- EMA Label Docetaxel. Available online: https://www.ema.europa.eu/en/documents/overview/taxotere-epar-summary-public_en.pdf (accessed on 12 February 2023).
- Riva, A.; Fumoleau, P.; Roché, H. Efficacy and safety of different corticosteroid premedications in breast cancer patients treated with Taxotere. Proc. Am. Soc Clin Oncol. 1997. [Google Scholar]
- Hickish, T.; Astras, G.; Thomas, P.; Penfold, S.; Purandare, L.; Hickish, T.F.; Kerr, D. Glucose intolerance during adjuvant chemotherapy for breast cancer. J. Natl. Cancer Inst. 2009, 101, 537. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.; Chiew, K.S.; Galica, J.; Pond, G.R.; Tannock, I.F. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br. J. Cancer 2006, 94, 1011–1015. [Google Scholar] [CrossRef]
- Yoo, K.E.; Kang, R.Y.; Lee, J.Y.; Lee, Y.J.; Suh, S.Y.; Kim, K.S.; Kim, H.S.; Lee, S.H.; Lee, B.K. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer 2015, 23, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Kim, E.S.; Dong, W.; Feng, L.; Hortobagyi, G.N.; Giordano, S.H. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann. Oncol. 2013, 24, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Marrone, M.T.; Selvin, E.; Barber, J.R.; Platz, E.A.; Joshu, C.E. Hyperglycemia, classified with multiple biomarkers simultaneously in men without diabetes, and risk of fatal prostate cancer. Cancer Prev. Res. 2019, 12, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Pfitzenmaier, J. Glucocorticoid use in prostate cancer and other solid tumours: Implications for effectiveness of cytotoxic treatment and metastases. Lancet Oncol. 2006, 7, 425–430. [Google Scholar] [CrossRef]
- Kumar, R. Emerging role of glucocorticoid receptor in castration resistant prostate cancer: A potential therapeutic target. J. Cancer 2020, 11, 696–701. [Google Scholar] [CrossRef]
- Zhou, F.; Shi, Y.; Zhao, G.; Aufderklamm, S.; Murray, K.S.; Jin, B. A narrative review of the role of glucocorticoid receptors in prostate cancer: Developments in last 5 years. Transl. Androl. Urol. 2022, 11, 1189–1199. [Google Scholar] [CrossRef]
- Sakellakis, M.; Flores, L.J. Is the glucocorticoid receptor a key player in prostate cancer?: A literature review. Medicine 2022, 101, e29716. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Q. The role of glucocorticoid receptor in prostate cancer progression: From bench to bedside. Int. Urol. Nephrol. 2017, 49, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Puhr, M.; Hoefer, J.; Eigentler, A.; Ploner, C.; Handle, F.; Schaefer, G.; Kroon, J.; Leo, A.; Heidegger, I.; Eder, I.; et al. The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clin. Cancer Res. 2018, 24, 927–938. [Google Scholar] [CrossRef]
- McCune, J.S.; Hawke, R.L.; LeCluyse, E.L.; Gillenwater, H.H.; Hamilton, G.; Ritchie, J.; Lindley, C. In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin. Pharmacol. Ther. 2000, 68, 356–366. [Google Scholar] [CrossRef]
- Teo, Y.L.; Saetaew, M.; Chanthawong, S.; Yap, Y.S.; Chan, E.C.; Ho, H.K.; Chan, A. Effect of CYP3A4 inducer dexamethasone on hepatotoxicity of lapatinib: Clinical and in vitro evidence. Breast Cancer Res. Treat. 2012, 133, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). 2011. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 12 February 2023).
- Chan, S.; Friedrichs, K.; Noel, D.; Pinter, T.; Van Belle, S.; Vorobiof, D.; Duarte, R.; Gil Gil, M.; Bodrogi, I.; Murray, E.; et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J. Clin. Oncol. 1999, 17, 2341–2354. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Erban, J.; Overmoyer, B.; Budd, G.T.; Hutchins, L.; Lower, E.; Laufman, L.; Sundaram, S.; Urba, W.J.; Pritchard, K.I.; et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J. Clin. Oncol. 2005, 23, 5542–5551. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Kang, R.Y.; Yoo, K.S.; Han, H.J.; Lee, J.Y.; Lee, S.H.; Kim, D.W.; Lee, Y.J. Evaluation of the effects and adverse drug reactions of low-dose dexamethasone premedication with weekly docetaxel. Support Care Cancer 2017, 25, 429–437. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Yang, Y.; Cheng, H.; Li, C.; He, Q. Pretreatment with different doses of dexamethasone in the prevention of docetaxel-induced hypersensitivity. Pak. J. Pharm. Sci. 2017, 30, 61–65. [Google Scholar]
- Chen, N.X.; Zhao, F.F.; Yan, F.; Zhang, X.X. Safe dose reduction of steroid pre-medication for docetaxel in head and neck neoplasm treatment. Acta Otolaryngol. 2016, 136, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, J.D.; Herrington, J.D. Single premedication dose of dexamethasone 20 mg IV before docetaxel administration. J. Oncol. Pharm. Pract. 2011, 17, 155–159. [Google Scholar] [CrossRef]
- Jacobs, C.; Hutton, B.; Mazzarello, S.; Smith, S.; Joy, A.; Amir, E.; Ibrahim, M.F.; Gregario, N.; Daigle, K.; Eggert, L.; et al. Optimisation of steroid prophylaxis schedules in breast cancer patients receiving docetaxel chemotherapy-a survey of health care providers and patients. Support Care Cancer 2015, 23, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Gjafa, E.; Ng, K.; Grunewald, T.; Galazi, M.; Skyllberg, E.; Wilson, P.; Alifrangis, C.; Shamash, J. Neutropenic sepsis rates in patients receiving BEP chemotherapy using olanzapine and reduced doses of dexamethasone compared to a standard antiemetic regimen. BJU Int. 2021, 127, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.J.; Klijn, J.; Paridaens, R.; Nooij, M.; Mauriac, L.; Coleman, R.; Bontenbal, M.; Awada, A.; Selleslags, J.; Van, V.A.; et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: Final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J. Clin. Oncol. 1997, 15, 3149–3155. [Google Scholar] [CrossRef] [PubMed]
- Syrigou, E.; Dannos, I.; Kotteas, E.; Makrilia, N.; Tourkantonis, I.; Dilana, K.; Gkiozos, I.; Saif, M.W.; Syrigos, K.N. Hypersensitivity reactions to docetaxel: Retrospective evaluation and development of a desensitization protocol. Int. Arch. Allergy Immunol. 2011, 156, 320–324. [Google Scholar] [CrossRef]
- Parinyanitikul, N.; Tanpipattanakul, W.; Poovorawan, N.; Rattananupong, T.; Laoitthi, P.; Sithidetphaiboon, P.; Thanasanvimon, S.; Sriuranpong, V. Incidence of infusion hypersensitivity reaction after withholding dexamethasone premedication in early breast cancer patients not experiencing two previous cycles of infusion hypersensitivity reaction for weekly paclitaxel chemotherapy. Support Care Cancer 2018, 26, 2471–2477. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Vaz-Luis, I.; Di Meglio, A.; Hu, J.; Li, T.; Rees, R.; Sinclair, N.; Milisits, L.; Leone, J.P.; Constantine, M.; et al. Prospective Study Testing a Simplified Paclitaxel Premedication Regimen in Patients with Early Breast Cancer. Oncologist 2021, 26, 927–933. [Google Scholar] [CrossRef]
- Ferroni, P.; Riondino, S.; Laudisi, A.; Portarena, I.; Formica, V.; Alessandroni, J.; D’Alessandro, R.; Orlandi, A.; Costarelli, L.; Cavaliere, F.; et al. Pretreatment Insulin Levels as a Prognostic Factor for Breast Cancer Progression. Oncologist 2016, 21, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Wang, C.Y.; Neuhouser, M.L.; Xiao, L.; Smith, A.W.; Reding, K.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R.; et al. Associations of insulin-like growth factor and insulin-like growth factor binding protein-3 with mortality in women with breast cancer. Int. J. Cancer 2013, 132, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 2009, 27, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Teply, B.A.; Luber, B.; Denmeade, S.R.; Antonarakis, E.S. The influence of prednisone on the efficacy of docetaxel in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 72–78. [Google Scholar] [CrossRef]
- Tanaka, N.; Nishimura, K.; Okajima, E.; Ina, K.; Ogawa, O.; Nagata, H.; Akakura, K.; Fujimoto, K.; Gotoh, M.; Teramukai, S.; et al. Docetaxel-based chemotherapy combined with dexamethasone 1 mg daily oral administration for castration-resistant prostate cancer: Long-term outcomes. Int. J. Urol. 2019, 26, 797–803. [Google Scholar] [CrossRef]
- Tanaka, N.; Nishimura, K.; Okajima, E.; Ina, K.; Ogawa, O.; Nagata, H.; Akakura, K.; Fujimoto, K.; Gotoh, M.; Teramukai, S.; et al. The efficacy and safety of docetaxel-based chemotherapy combined with dexamethasone 1 mg daily oral administration: JMTO Pca 10-01 phase II trial. Jpn. J. Clin. Oncol. 2017, 47, 247–251. [Google Scholar] [CrossRef]
- Yang, Z.; Ni, Y.; Zhao, D.; Zhang, Y.; Wang, J.; Jiang, L.; Chen, D.; Wu, Z.; Wang, Y.; He, L.; et al. Corticosteroid switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients with biochemical progression on abiraterone acetate plus prednisone. BMC Cancer 2021, 21, 919. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Sobhani, N.; Corona, S.P.; D’Angelo, A. Corticosteroid switch after progression on abiraterone acetate plus prednisone. Int. J. Clin. Oncol. 2020, 25, 240–246. [Google Scholar] [CrossRef]
| Breast Cancer (Docetaxel Dosage 75–100 mg/m2) | ||||||
|---|---|---|---|---|---|---|
| Day −1 | Day 0 | Day 1 | ||||
| Cohort 1 | 8 mg | 4 mg | 8 mg | 4 mg | 8 mg | 4 mg |
| Cohort 2 | 8 mg | 8 mg | 8 mg | |||
| Cohort 3 | 4 mg | 8 mg | 4 mg | |||
| Prostate cancer (Docetaxel dosage 75 mg/m2) | ||||||
| Day −1 | Day 0 | |||||
| Cohort 1 | 8 mg | 8 mg | ||||
| Cohort 2 | - | 8 mg | ||||
| Cohort 3A | - | 4 mg, with 2dd 5 mg prednisone continuously | ||||
| Cohort 3B | - | 4 mg, without 2dd 5 mg prednisone continuously | ||||
| Prostate Cancer (N = 28) | Breast Cancer (N = 18) | |
|---|---|---|
| Median Age (range), Years | 69.5 (55–80) | 54 (34–67) |
| Median Body Mass Index (range), kg/m2 | 26.9 (21.3–32.7) | 26.4 (18.6–40.4) |
| WHO Status | ||
| Grade 0 | 15 (54%) | 12 (67%) |
| Grade 1 | 6 (21%) | 3 (17%) |
| Grade 2 | 1 (4%) | 0 (0%) |
| Missing | 6 (21%) | 3 (17%) |
| Stage | ||
| I | 0 (0%) | 3 (17%) |
| II | 0 (0%) | 12 (67%) |
| III | 0 (0%) | 1 (6%) |
| IV | 28 (100%) | 2 (11%) |
| Chemotherapy Regimen | ||
| (Neo)adjuvant | 0 (0%) | 16 (89%) |
| Palliative | 28 (100%) | 2 (11%) |
| Docetaxel Dosage | ||
| 75 mg/m2 | 28 (100%) | 2 (11%) |
| 100 mg/m2 | 0 (0%) | 16 (89%) |
| Treatment | ||
| Monotherapy Docetaxel | 28 (100%) | 14 (78%) |
| Combination Chemotherapy | 0 (0%) | 4 (22%) |
| Breast Cancer | Number of Patients Evaluated | Median Cycles of Docetaxel (Range) | Mean Cumulative Dose of Docetaxel (Range) | HSR (Any Grade) | HSR (Grade III/IV) | Fluid Retention * (Any Grade) | Fluid Retention (Grade III/IV) |
| Cohort 1 | 6 | 4 (4) | 351 mg/m2 (325–400) | 0 | 0 | 1/6 (grade I) | 0 |
| Cohort 2 | 6 | 4 (1–4) | 307 mg/m2 (100–400) | 0 | 0 | 1/6 (grade II) | 0 |
| Cohort 3 | 2 | 6 (6) | 525 mg/m2 (450–600) | 0 | 0 | 0 | 0 |
| Prostate Cancer | Number of Patients Evaluated | Median Cycles of Docetaxel (Range) | Mean Cumulative Dose of Docetaxel (Range) | HSR (Any Grade) | HSR (Grade III/IV) | Fluid Retention * (Any Grade) | Fluid Retention (Grade III/IV) |
| Cohort 1 | 6 | 6 (1–9) | 445 mg/m2 (75–645) | 0 | 0 | 1/6 (grade II) | 0 |
| Cohort 2 | 7 | 6 (5–6) | 407 mg/m2 (225–450) | 1/6 (grade I) ** | 0 | 0 | 0 |
| Cohort 3A | 6 | 6 (2–6) | 372 mg/m2 (150–450) | 0 | 0 | 1/6 (grade I/II) | 0 |
| Cohort 3B | 6 | 6 (5–6) | 434 mg/m2 (356–450) | 0 | 0 | 2/6 (grade I/II) | 0 |
| Febrile Neutropenia | Nausea | Hyperglycaemia | |
|---|---|---|---|
| Cohorts 1 | 3/12 (grade IV) * | 5/12 (grade I–II) | 4/12 (grade I–II) |
| Cohorts 2 | 1/13 (grade IV) * | 5/13 (grade I) | 3/13 (grade I) |
| Cohorts 3 | 0/14 | 2/14 (grade I–II) | 1/14 (grade I) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugtenberg, R.T.; de Groot, S.; Houtsma, D.; Dezentjé, V.O.; Vulink, A.J.E.; Fischer, M.J.; Portielje, J.E.A.; van der Hoeven, J.J.M.; Gelderblom, H.; Pijl, H.; et al. Phase 1 Study to Evaluate the Safety of Reducing the Prophylactic Dose of Dexamethasone around Docetaxel Infusion in Patients with Prostate and Breast Cancer. Cancers 2023, 15, 1691. https://doi.org/10.3390/cancers15061691
Lugtenberg RT, de Groot S, Houtsma D, Dezentjé VO, Vulink AJE, Fischer MJ, Portielje JEA, van der Hoeven JJM, Gelderblom H, Pijl H, et al. Phase 1 Study to Evaluate the Safety of Reducing the Prophylactic Dose of Dexamethasone around Docetaxel Infusion in Patients with Prostate and Breast Cancer. Cancers. 2023; 15(6):1691. https://doi.org/10.3390/cancers15061691
Chicago/Turabian StyleLugtenberg, Rieneke T., Stefanie de Groot, Danny Houtsma, Vincent O. Dezentjé, Annelie J. E. Vulink, Maarten J. Fischer, Johanneke E. A. Portielje, Jacobus J. M. van der Hoeven, Hans Gelderblom, Hanno Pijl, and et al. 2023. "Phase 1 Study to Evaluate the Safety of Reducing the Prophylactic Dose of Dexamethasone around Docetaxel Infusion in Patients with Prostate and Breast Cancer" Cancers 15, no. 6: 1691. https://doi.org/10.3390/cancers15061691
APA StyleLugtenberg, R. T., de Groot, S., Houtsma, D., Dezentjé, V. O., Vulink, A. J. E., Fischer, M. J., Portielje, J. E. A., van der Hoeven, J. J. M., Gelderblom, H., Pijl, H., & Kroep, J. R. (2023). Phase 1 Study to Evaluate the Safety of Reducing the Prophylactic Dose of Dexamethasone around Docetaxel Infusion in Patients with Prostate and Breast Cancer. Cancers, 15(6), 1691. https://doi.org/10.3390/cancers15061691







