Radiotherapy Alone Versus Concurrent or Adjuvant Chemoradiotherapy for Nasopharyngeal Carcinoma Patients with Negative Epstein–Barr Virus DNA after Induction Chemotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
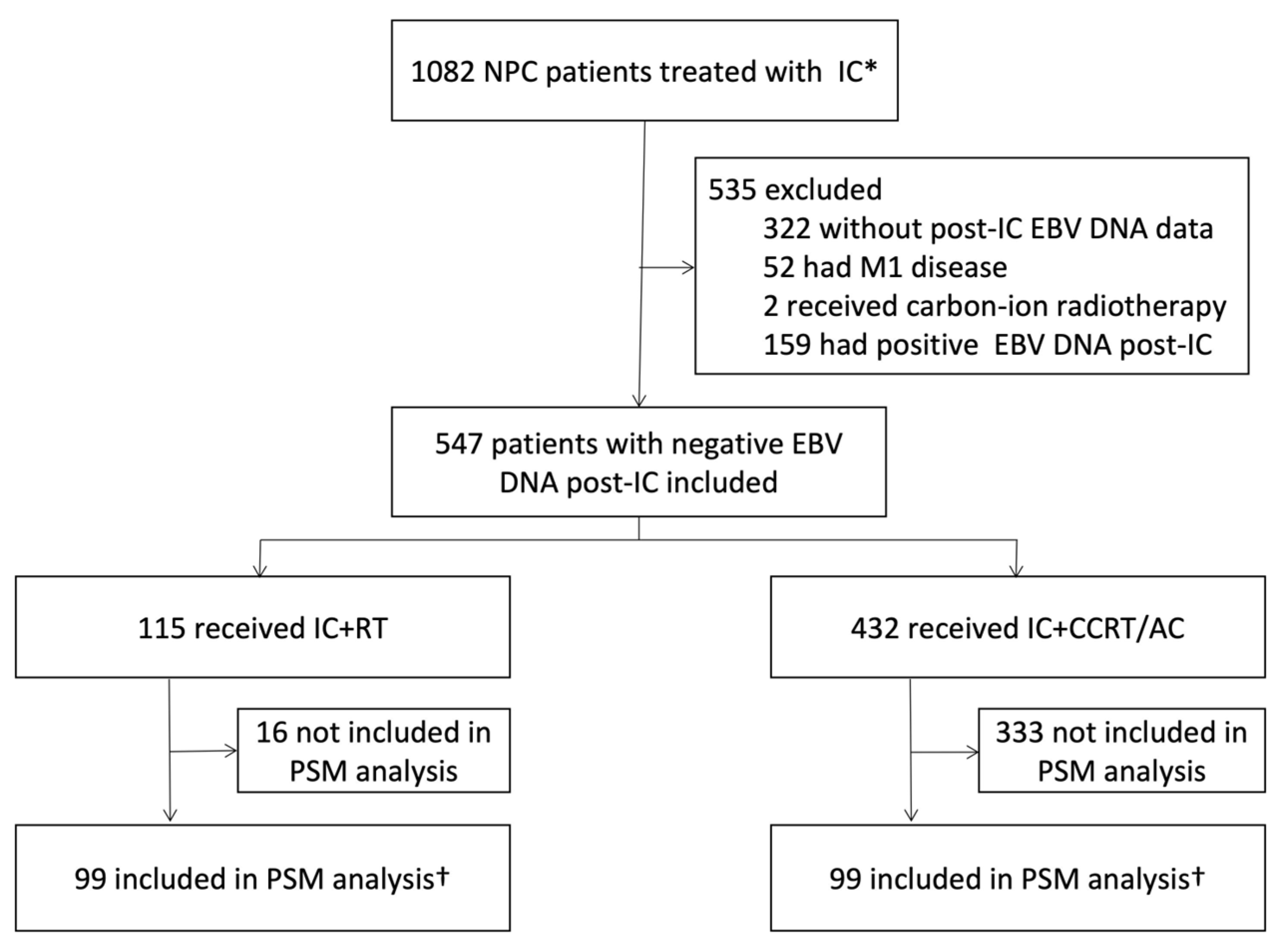
2.1. Patients
2.2. Plasma EBV DNA Detection
2.3. Treatments
2.4. Follow-Up and Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
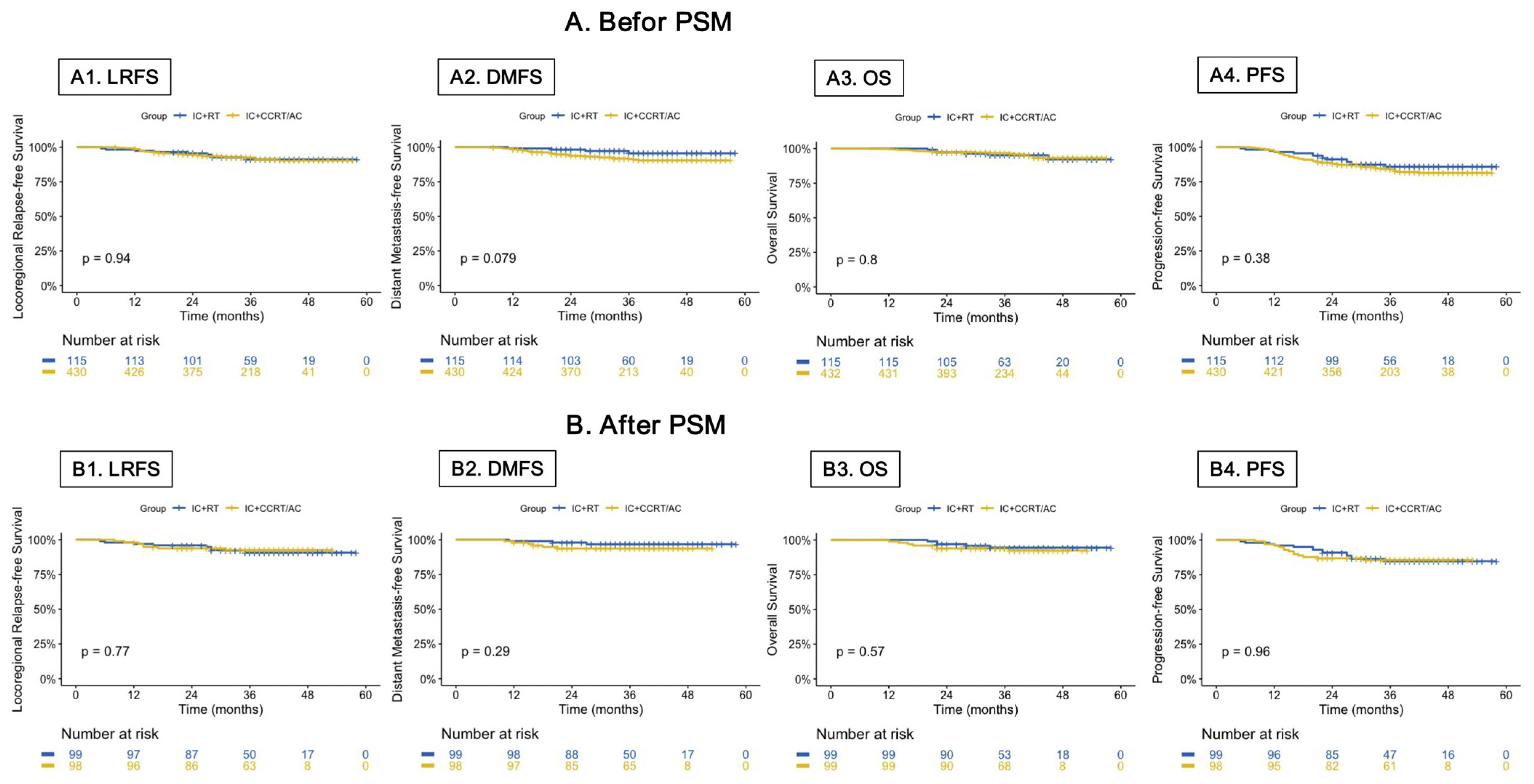
3.2. Survival Outcomes
3.3. Prognostic Analysis
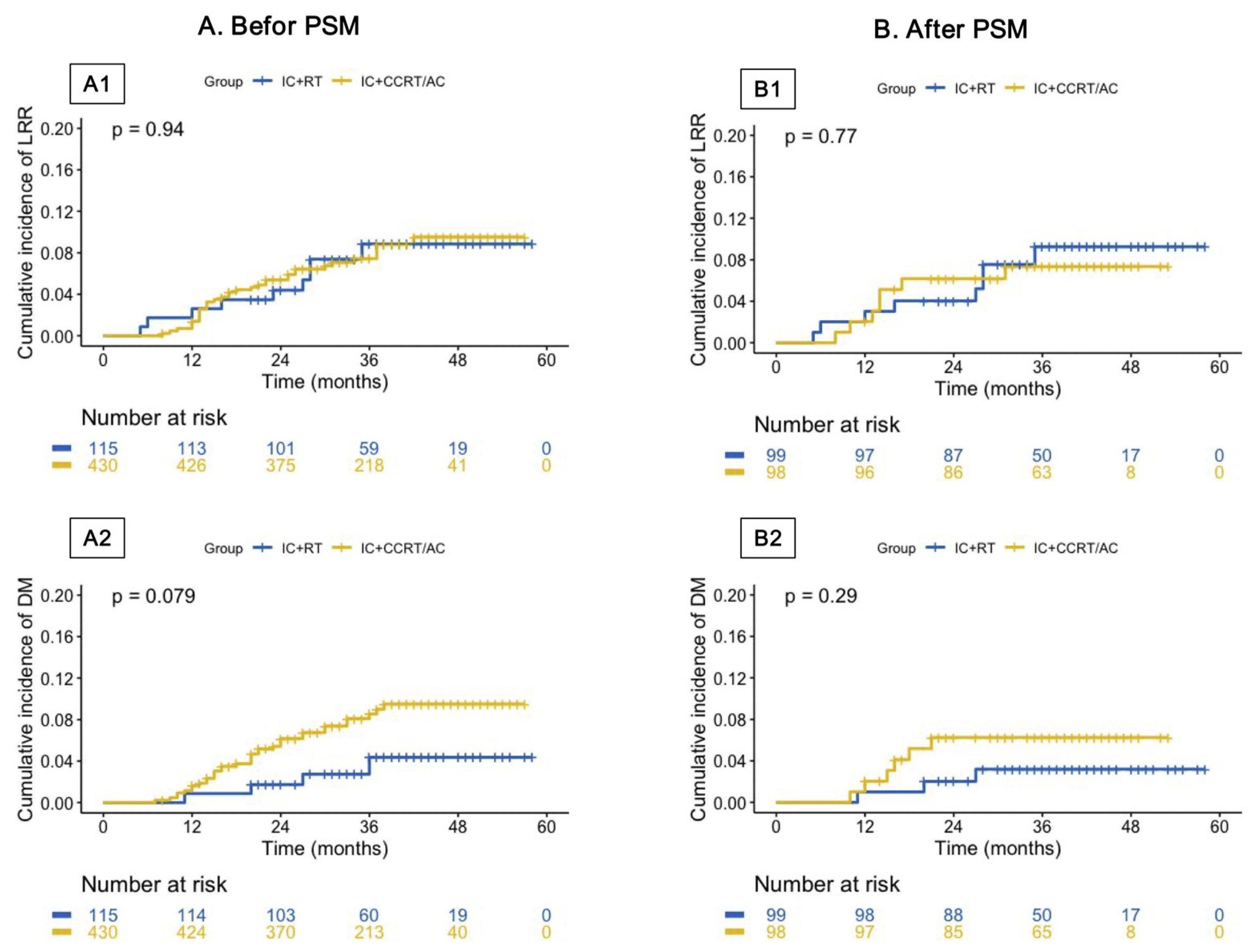
3.4. Treatment Failures
3.5. Toxicities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.W.; Ma, B.B.; Ng, W.T.; Chan, A.T. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J. Clin. Oncol. 2015, 33, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology: Head and Neck Cancers; Version 2; National Comprehensive Cancer Network (NCCN): Fort Washington, PA, USA, 2022. [Google Scholar]
- Tang, L.L.; Chen, Y.P.; Chen, C.B.; Chen, M.Y.; Chen, N.Y.; Chen, X.Z.; Du, X.J.; Fang, W.F.; Feng, M.; Gao, J.; et al. The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. 2021, 41, 1195–1227. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; LeBlanc, M.; Giri, P.G.; Fu, K.K.; Cooper, J.; Vuong, T.; Forastiere, A.A.; Adams, G.; Sakr, W.A.; Schuller, D.E.; et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J. Clin. Oncol. 1998, 16, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.M.; Fan, Y.; Wang, X.X.; Xie, Q.C.; Sun, J.G.; Chen, Z.T.; Zhu, B. Increased treatment-related mortality with additional cisplatin-based chemotherapy in patients with nasopharyngeal carcinoma treated with standard radiotherapy. Radiother. Oncol. 2012, 104, 279–285. [Google Scholar] [CrossRef]
- Lee, A.W.; Lau, W.H.; Tung, S.Y.; Chua, D.T.; Chappell, R.; Xu, L.; Siu, L.; Sze, W.M.; Leung, T.W.; Sham, J.S.; et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J. Clin. Oncol. 2005, 23, 6966–6975. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wen, Y.F.; Guo, L.; Liu, H.; Huang, P.Y.; Mo, H.Y.; Li, N.W.; Xiang, Y.Q.; Luo, D.H.; Qiu, F.; et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: Phase III randomized trial. J. Natl. Cancer Inst. 2011, 103, 1761–1770. [Google Scholar] [CrossRef]
- Lee, A.W.; Tung, S.Y.; Chua, D.T.; Ngan, R.K.; Chappell, R.; Tung, R.; Siu, L.; Ng, W.T.; Sze, W.K.; Au, G.K.; et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J. Natl. Cancer Inst. 2010, 102, 1188–1198. [Google Scholar] [CrossRef]
- Wee, J.; Tan, E.H.; Tai, B.C.; Wong, H.B.; Leong, S.S.; Tan, T.; Chua, E.T.; Yang, E.; Lee, K.M.; Fong, K.W.; et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J. Clin. Oncol. 2005, 23, 6730–6738. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, C.; Wang, L.; Yan, F.; Sun, Q.; Ye, Z.; Liu, T.; Fu, Z.; Jiang, Y. Influence of concurrent chemotherapy on locoregionally advanced nasopharyngeal carcinoma treated with neoadjuvant chemotherapy plus intensity-modulated radiotherapy: A retrospective matched analysis. Sci. Rep. 2020, 10, 2489. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Z.; Wang, Y.; He, J.; Chai, J.; Wei, Z.; Guan, H.; Wang, J.; Liu, Z.; Li, R.; et al. Induction chemotherapy followed by intensity-modulated radiotherapy versus concurrent chemoradiotherapy in nasopharyngeal carcinoma: A retrospective analysis. Clin. Otolaryngol. 2021, 46, 976–982. [Google Scholar] [CrossRef]
- Liu, L.T.; Liang, Y.J.; Guo, S.S.; Mo, H.Y.; Guo, L.; Wen, Y.F.; Xie, H.J.; Tang, Q.N.; Sun, X.S.; Liu, S.L.; et al. Induction chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in the treatment of different risk locoregionally advanced nasopharyngeal carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Sun, X.S.; Lu, Z.J.; Chen, Q.Y.; Lin, H.X.; Tang, L.Q.; Bei, J.X.; Guo, L.; Mai, H.Q. Nomogram Predicting the Benefits of Adding Concurrent Chemotherapy to Intensity-Modulated Radiotherapy After Induction Chemotherapy in Stages II-IVb Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10, 539321. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.M.; Lee, V.H.F.; Ng, W.T.; Strojan, P.; Saba, N.F.; Rinaldo, A.; Willems, S.M.; Rodrigo, J.P.; Forastiere, A.A.; Ferlito, A. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur. J. Cancer 2021, 153, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Chen, Y.; Zhou, G.; Qi, Z.; Tan, K.R.L.; Wang, H.; Lin, L.; Chen, F.; Zhang, L.; Huang, X.; et al. Liquid biopsy tracking during sequential chemo-radiotherapy identifies distinct prognostic phenotypes in nasopharyngeal carcinoma. Nat. Commun. 2019, 10, 3941. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ouyang, T.; Xiong, Y.; Ba, L.; Li, Q.; Qiu, M.; Zou, Z.; Peng, G. Prognostic Value of Plasma Epstein-Barr Virus DNA Levels Pre- and Post-Neoadjuvant Chemotherapy in Patients With Nasopharyngeal Carcinoma. Front. Oncol. 2021, 11, 714433. [Google Scholar] [CrossRef]
- Huang, C.L.; Sun, Z.Q.; Guo, R.; Liu, X.; Mao, Y.P.; Peng, H.; Tian, L.; Lin, A.H.; Li, L.; Shao, J.Y.; et al. Plasma Epstein-Barr Virus DNA Load After Induction Chemotherapy Predicts Outcome in Locoregionally Advanced Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 355–361. [Google Scholar] [CrossRef]
- Chen, F.P.; Luo, Y.S.; Chen, K.; Li, J.Y.; Huo, L.Q.; Shi, L.; Ou-Yang, Y.; Cao, X.P. Circulating Epstein-Barr virus DNA level post induction chemotherapy contributes to prognostication in advanced-stage nasopharyngeal carcinoma. Eur. J. Cancer 2021, 151, 63–71. [Google Scholar] [CrossRef]
- ICRU. Prescribing, Recording, and Reporting Photon Beam Therapy; International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1999; Volume 62. [Google Scholar]
- Ou, X.; Zhou, X.; Shi, Q.; Xing, X.; Yang, Y.; Xu, T.; Shen, C.; Wang, X.; He, X.; Kong, L.; et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: New insight into the value of total dose of cisplatin and radiation boost. Oncotarget 2015, 6, 38381–38397. [Google Scholar] [CrossRef]
- Li, W.F.; Chen, N.Y.; Zhang, N.; Hu, G.Q.; Xie, F.Y.; Sun, Y.; Chen, X.Z.; Li, J.G.; Zhu, X.D.; Hu, C.S.; et al. Concurrent chemoradiotherapy with/without induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma: Long-term results of phase 3 randomized controlled trial. Int. J. Cancer 2019, 145, 295–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hu, G.Q.; Zhang, N.; Zhu, X.D.; Yang, K.Y.; Jin, F.; Shi, M.; Chen, Y.P.; Hu, W.H.; et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019, 381, 1124–1135. [Google Scholar] [CrossRef]
- Liu, L.T.; Tang, L.Q.; Chen, Q.Y.; Zhang, L.; Guo, S.S.; Guo, L.; Mo, H.Y.; Zhao, C.; Guo, X.; Cao, K.J.; et al. The Prognostic Value of Plasma Epstein-Barr Viral DNA and Tumor Response to Neoadjuvant Chemotherapy in Advanced-Stage Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, C.S.; Chen, X.Z.; Hu, G.Q.; Cheng, Z.B.; Sun, Y.; Li, W.X.; Chen, Y.Y.; Xie, F.Y.; Liang, S.B.; et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W.F.; Chen, N.Y.; Zhang, N.; Hu, G.Q.; Xie, F.Y.; Sun, Y.; Chen, X.Z.; Li, J.G.; Zhu, X.D.; et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016, 17, 1509–1520. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, X.; Zhou, Q.; Yang, K.Y.; Jin, F.; Zhu, X.D.; Shi, M.; Hu, G.Q.; Hu, W.H.; Sun, Y.; et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: A multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet 2021, 398, 303–313. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Before PSM (n = 547) | After PSM (n = 198) | |||||
|---|---|---|---|---|---|---|---|
| IC + CCRT/AC (n = 432) n (%) | IC + RT (n = 115) n (%) | p Value | IC + CCRT/AC (n = 99) n (%) | IC + RT (n = 99) n (%) | p Value | ||
| Age (years) | <45 | 168 (38.9) | 26 (22.6) | 0.002 | 30 (30.3) | 26 (26.3) | 0.636 |
| ≥45 | 264 (61.1) | 89 (77.4) | 69 (69.7) | 73 (73.7) | |||
| Gender | Female | 118 (27.3) | 36 (31.3) | 0.466 | 27 (27.3) | 28 (28.3) | 1.000 |
| Male | 314 (72.7) | 79 (68.7) | 72 (72.7) | 71 (71.7) | |||
| T category a | T1–2 | 129 (29.9) | 34 (29.6) | 1.000 | 30 (30.3) | 32 (32.3) | 0.878 |
| T3–4 | 303 (70.1) | 81 (70.4) | 69 (69.7) | 67 (67.7) | |||
| N category a | N0–1 | 116 (26.9) | 44 (38.3) | 0.023 | 28 (28.3) | 35 (35.4) | 0.360 |
| N2–3 | 316 (73.1) | 71 (61.7) | 71 (71.7) | 64 (64.6) | |||
| Clinical stage a | II-III | 215 (49.8) | 74 (64.3) | 0.007 | 57 (57.6) | 58 (58.6) | 1.000 |
| IVa | 217 (50.2) | 41 (35.7) | 42 (42.4) | 41 (41.4) | |||
| Pre-IC EBV DNA (copies/mL) | <500 | 120 (27.8) | 39 (33.9) | 0.241 | 31 (31.3) | 33 (33.3) | 0.879 |
| ≥500 | 312 (72.2) | 76 (66.1) | 68 (68.7) | 66 (66.7) | |||
| IC regimen | TP | 196 (45.4) | 91 (79.1) | <0.001 | 77 (77.8) | 75 (75.8) | 0.127 |
| GP | 232 (53.7) | 20 (17.4) | 22 (22.2) | 20 (20.2) | |||
| PF | 4 (0.9) | 4 (3.5) | 0 (0.0) | 4 (4.0) | |||
| IC cycle | 1 | 5 (1.2) | 3 (2.6) | <0.001 | 1 (1.0) | 3 (3.0) | 0.551 |
| 2 | 317 (73.4) | 58 (50.4) | 57 (57.6) | 53 (53.5) | |||
| ≥3 | 110 (25.5) | 54 (47.0) | 41 (41.4) | 43 (43.4) | |||
| Targeted therapy b | no | 371 (85.9) | 100 (87.0) | 0.885 | 84 (84.8) | 84 (84.8) | 1.000 |
| yes | 61 (14.1) | 15 (13.0) | 15 (15.2) | 15 (15.2) | |||
| Site | IC + CCRT/AC (n = 432) n (%) | IC + RT (n = 115) n (%) |
|---|---|---|
| Locoregional recurrence | ||
| Local only | 20 (4.6) | 3 (2.6) |
| Regional only | 4 (0.9) | 4 (3.5) |
| Local and regional | 4 (0.9) | 1 (0.9) |
| Distant metastases | ||
| Distant only | 29 (6.7) | 3 (2.6) |
| Local and distant | 1 (0.2) | 1 (0.9) |
| Regional and distant | 3 (0.7) | 0 |
| Local, regional, and distant | 2 (0.5) | 0 |
| Total locoregional failure | 34 (7.9) | 9 (7.8) |
| Total distant failure | 35 (8.1) | 4 (3.5) |
| Death (any cause) | 19 (4.4) | 6 (5.2) |
| NPC cause | 14 (3.2) | 2 (1.7) |
| Non-NPC cause | 0 | 1 (0.9) |
| Unknow cause | 5 (1.2) | 3 (2.6) |
| Toxicity | IC + CCRT (n = 44) n (%) | IC + RT (n = 99) n (%) | p Value |
|---|---|---|---|
| Leukopenia | 6 (13.6) | 2 (2) | 0.017 |
| Neutropenia | 0 | 1 (1) | 1.000 |
| Anemia | 3 (6.8) | 0 | 0.046 |
| Thrombocytopenia | 1 (2.3) | 0 | 0.676 |
| Mucositis | 17 (38.6) | 37 (37.4) | 0.886 |
| Dermatitis | 5 (11.4) | 9 (9.1) | 0.907 |
| AST/ALT increase | 0 | 0 | - |
| BUN increase | 0 | 0 | - |
| Creatinine increase | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Pan, G.; Du, C.; Hu, C.; Ying, H. Radiotherapy Alone Versus Concurrent or Adjuvant Chemoradiotherapy for Nasopharyngeal Carcinoma Patients with Negative Epstein–Barr Virus DNA after Induction Chemotherapy. Cancers 2023, 15, 1689. https://doi.org/10.3390/cancers15061689
Kong F, Pan G, Du C, Hu C, Ying H. Radiotherapy Alone Versus Concurrent or Adjuvant Chemoradiotherapy for Nasopharyngeal Carcinoma Patients with Negative Epstein–Barr Virus DNA after Induction Chemotherapy. Cancers. 2023; 15(6):1689. https://doi.org/10.3390/cancers15061689
Chicago/Turabian StyleKong, Fangfang, Guangsen Pan, Chengrun Du, Chaosu Hu, and Hongmei Ying. 2023. "Radiotherapy Alone Versus Concurrent or Adjuvant Chemoradiotherapy for Nasopharyngeal Carcinoma Patients with Negative Epstein–Barr Virus DNA after Induction Chemotherapy" Cancers 15, no. 6: 1689. https://doi.org/10.3390/cancers15061689
APA StyleKong, F., Pan, G., Du, C., Hu, C., & Ying, H. (2023). Radiotherapy Alone Versus Concurrent or Adjuvant Chemoradiotherapy for Nasopharyngeal Carcinoma Patients with Negative Epstein–Barr Virus DNA after Induction Chemotherapy. Cancers, 15(6), 1689. https://doi.org/10.3390/cancers15061689





