LL-37 Might Promote Local Invasion of Melanoma by Activating Melanoma Cells and Tumor-Associated Macrophages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Tumor Inoculation and Treatment
2.3. Cell Lines and Cell Culture
2.4. RNA Extraction and Quantitative Real-Time PCR Experiments
2.5. Western Blotting
2.6. In Vitro Angiogenesis Assay
2.7. Culture of M2 Macrophages from Human Peripheral Blood Monocytes
2.8. Cytokine Enzyme-Linked Immunosorbent Assays (ELISAs)
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabriek, B.O.; Dijkstra, C.D.; Berg, T.K.V.D. The macrophage scavenger receptor CD163. Immunobiology 2005, 210, 153–160. [Google Scholar] [CrossRef]
- Chamilos, G.; Gregorio, J.; Meller, S.; Lande, R.; Kontoyiannis, D.P.; Modlin, R.L.; Gilliet, M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood 2012, 120, 3699–3707. [Google Scholar] [CrossRef]
- Takahashi, T.; Kulkarni, N.N.; Lee, E.Y.; Zhang, L.-J.; Wong, G.C.L.; Gallo, R.L. Cathelicidin promotes inflammation by enabling binding of self-RNA to cell surface scavenger receptors. Sci. Rep. 2018, 8, 4032. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, H.; Delputte, P.L.; Nauwynck, H.J. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol. Immunol. 2010, 47, 1650–1660. [Google Scholar] [CrossRef]
- Lau, S.K.; Chu, P.G.; Weiss, L.M. CD163: A Specific Marker of Macrophages in Paraffin-Embedded Tissue Samples. Am. J. Clin. Pathol. 2004, 122, 794–801. [Google Scholar] [CrossRef]
- Fuentes-Duculan, J.; Bonifacio, K.M.; Hawkes, J.E.; Kunjravia, N.; Cueto, I.; Li, X.; Gonzalez, J.; Garcet, S.; Krueger, J.G. Autoantigens ADAMTSL5 and LL37 are significantly upregulated in active Psoriasis and localized with keratinocytes, dendritic cells and other leukocytes. Exp. Dermatol. 2017, 26, 1075–1082. [Google Scholar] [CrossRef]
- Fang, H.; Hou, Y.; Zhuang, H.; Wang, C. The effects of Malassezia in the activation of Interleukin (IL)-23/IL-17 axis in Psoriasis. New Microbiol. 2022, 45, 130–137. [Google Scholar] [PubMed]
- Ohuchi, K.; Amagai, R.; Ikawa, T.; Muto, Y.; Roh, Y.; Endo, J.; Maekawa, T.; Kambayashi, Y.; Asano, Y.; Fujimura, T. Plasminogen activating inhibitor-1 promotes angiogenesis in cutaneous angiosarcomas. Exp. Dermatol. 2023, 32, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Subhadarshani, S.; Yusuf, N.; Elmets, C.A. IL-23 and the Tumor Microenvironment. Adv. Exp. Med. Biol. 2021, 1290, 89–98. [Google Scholar]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.J.; Choi, J.M.; Lee, K.H.; Kim, T.Y.; Cho, B.K.; Jung, J.Y.; Chung, K.Y.; Cho, D.; Park, H.J. The antimicrobial peptide human cationic antimicrobial protein-18/cathelicidin LL-37 as a putative growth factor for malignant melanoma. Br. J. Dermatol. 2010, 163, 959–967. [Google Scholar] [CrossRef]
- Jia, J.; Zheng, Y.; Wang, W.; Shao, Y.; Li, Z.; Wang, Q.; Wang, Y.; Yan, H. Antimicrobial peptide LL-37 promotes YB-1 expression, and the viability, migration and invasion of malignant elanoma cells. Mol. Med. Rep. 2016, 15, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Manarang, J.C.; Otteson, D.C.; McDermott, A.M. Expression of Antimicrobial Peptides by Uveal and Cutaneous Melanoma Cells and Investigation of Their Role in Tumor Cell Migration and Vasculogenic Mimicry. Curr. Eye Res. 2017, 42, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Okabe, T.; Tanita, K.; Sato, Y.; Lyu, C.; Kambayashi, Y.; Maruyama, S.; Aiba, S.; Chunbing, L. A novel technique to diagnose non-melanoma skin cancer by thermal conductivity measurements: Correlations with cancer stromal factors. Exp. Dermatol. 2019, 28, 1029–1035. [Google Scholar] [CrossRef]
- Lyu, C.; Fujimura, T.; Amagai, R.; Ohuchi, K.; Sato, Y.; Tanita, K.; Matsushita, S.; Fujisawa, Y.; Otsuka, A.; Yamamoto, Y.; et al. Increased expression of dermal LL37 may trigger migration of CCR7+ regulatory T cells in extramammary Paget’s disease. J. Dermatol. Sci. 2020, 99, 65–68. [Google Scholar] [CrossRef]
- Lai, Y.P.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. The human cathelicidin hCAP18/LL-37: A multifunctional peptide involved in mycobacterial infections. Peptides 2010, 31, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Amagai, R.; Takahashi, T.; Terui, H.; Fujimura, T.; Yamasaki, K.; Aiba, S.; Asano, Y. The Antimicrobial Peptide Cathelicidin Exerts Immunomodulatory Effects via Scavenger Receptors. Int. J. Mol. Sci. 2023, 24, 875. [Google Scholar] [CrossRef]
- Zeth, K.; Sancho-Vaello, E. The Human Antimicrobial Peptides Dermcidin and LL-37 Show Novel Distinct Pathways in Membrane Interactions. Front. Chem. 2017, 5, 86. [Google Scholar] [CrossRef]
- Choi, M.; Jo, J.; Lee, M.; Park, J.; Yao, T.; Park, Y. Cathelicidin-related antimicrobial peptide mediates skeletal muscle degeneration caused by injury and Duchenne muscular dystrophy in mice. J. Cachex Sarcopenia Muscle 2022, 13, 3091–3105. [Google Scholar] [CrossRef]
- Mihailovic, P.M.; Lio, W.M.; Yano, J.; Zhao, X.; Zhou, J.; Chyu, K.; Shah, P.K.; Cercek, B.; Dimayuga, P.C. The cathelicidin pro-tein CRAMP is a potential atherosclerosis self-antigen in ApoE(-/-) mice. PLoS ONE 2017, 12, e0187432. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, A.; Fujimura, T.; Furudate, S.; Kambayashi, Y.; Yamauchi, T.; Yagita, H.; Aiba, S. Immunomodulatory effect of peritumorally administered interferon-beta on melanoma through tumor-associated macrophages. Oncoimmunology 2015, 4, e1047584. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Miyagawa, T.; Fukui, Y.; Toyama, S.; Omatsu, J.; Awaji, K.; Norimatsu, Y.; Watanabe, Y.; Yoshizaki, A.; Sato, S.; et al. Endothelial CCR6 expression due to FLI1 deficiency contributes to vasculopathy associated with systemic sclerosis. Arthritis Res. Ther. 2021, 23, 283. [Google Scholar] [CrossRef]
- Ohuchi, K.; Kambayashi, Y.; Hidaka, T.; Fujimura, T. Plasminogen Activating Inhibitor-1 Might Predict the Efficacy of Anti-PD1 Antibody in Advanced Melanoma Patients. Front. Oncol. 2021, 11, 798385. [Google Scholar] [CrossRef]
- Fujimura, T.; Kakizaki, A.; Furudate, S.; Aiba, S. A possible interaction between periostin and CD163+ skin-resident macrophages in pemphigus vulgaris and bullous pemphigoid. Exp. Dermatol. 2017, 26, 1193–1198. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshikawa, S.; Minagawa, A.; Takenouchi, T.; Yokota, K.; Uchi, H.; Noma, N.; Nakamura, Y.; Asai, J.; Kato, J.; et al. Classification of 3097 patients from the Japanese melanoma study database using the American joint committee on cancer eighth edition cancer staging system. J. Dermatol. Sci. 2019, 94, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Yu, T.; Sang, Y.; Gao, X. Tumor-promoting effect of IL-23 in mammary cancer mediated by infiltration of M2 macro-phages and neutrophils in tumor microenvironment. Biochem. Biophys. Res. Commun. 2017, 482, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Li, Y.; He, H.; Ding, H.; Chen, K.; Du, J.; Chen, T.; Wu, Z.; Liu, H.; Wang, D.; et al. IL-23 production of liver inflammatory macrophages to damaged hepatocytes promotes hepatocellular carcinoma development after chronic hepatitis B virus infection. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 3759–3770. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, R.; Meng, E.; Wu, H. CXCL5: A coachman to drive cancer progression. Front. Oncol. 2022, 12, 944494. [Google Scholar] [CrossRef]
- Fujimura, T.; Aiba, S. Significance of Immunosuppressive Cells as a Target for Immunotherapies in Melanoma and Non-Melanoma Skin Cancers. Biomolecules 2020, 10, 1087. [Google Scholar] [CrossRef]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Damme, J.V.; Walz, A.; Marriott, D.; et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, M.; Chen, G.; Wang, W.; Zhang, P.; Yue, Y.; Guan, Z.; Wang, X.; Fan, J. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Int. J. Oncol. 2019, 54, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, Z.Q.; Zong, Y.P.; Ou, B.C.; Shen, X.H.; Feng, H.; Zheng, M.H.; Zhao, J.K.; Lu, A.G. CXCL5 induces tumor angiogenesis via enhancing the expression of FOXD1 mediated by the AKT/NFkB pathway in colorectal cancer. Cell Death Dis 2019, 10, 178. [Google Scholar] [CrossRef]
- Soler-Cardona, A.; Forsthuber, A.; Lipp, K.; Ebersberger, S.; Heinz, M.; Schossleitner, K.; Buchberger, E.; Gröger, M.; Petzelbauer, P.; Hoeller, C.; et al. CXCL5 Facilitates Melanoma Cell–Neutrophil Interaction and Lymph Node Metastasis. J. Investig. Dermatol. 2018, 138, 1627–1635. [Google Scholar] [CrossRef]
- Liu, L.; Sun, H.; Wu, S.; Tan, H.; Sun, Y.; Liu, X.; Si, S.; Xu, L.; Huang, J.; Zhou, W.; et al. IL-17A promotes CXCR2-dependent angiogenesis in a mouse model of liver cancer. Mol. Med. Rep. 2019, 20, 1065–1074. [Google Scholar] [CrossRef]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef]
- Qian, X.; Gu, L.; Ning, H.; Zhang, Y.; Hsueh, E.C.; Fu, M.; Hu, X.; Wei, L.; Hoft, D.F.; Liu, J. Increased Th17 Cells in the Tumor Microenvironment Is Mediated by IL-23 via Tumor-Secreted Prostaglandin E2. J. Immunol. 2013, 190, 5894–5902. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Bai, J.; Xue, Y.; Peng, Q. MMP1 and MMP9 are potential prognostic biomarkers and targets for uveal melanoma. BMC Cancer 2021, 21, 1068. [Google Scholar] [CrossRef]
- Pandita, A.; Ekstrand, M.; Bjursten, S.; Zhao, Z.; Fogelstrand, P.; Le Gal, K.; Ny, L.; Bergo, M.O.; Karlsson, J.; Nilsson, J.A.; et al. Intussusceptive Angiogenesis in Human Metastatic Malignant Melanoma. Am. J. Pathol. 2021, 191, 2023–2038. [Google Scholar] [CrossRef]
- Fujimura, T. Stromal Factors as a Target for Immunotherapy in Melanoma and Non-Melanoma Skin Cancers. Int. J. Mol. Sci. 2022, 23, 4044. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.P.; Raja, R.; Thorat, D.; Soundararajan, G.; Patil, T.V.; Kundu, G.C. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene 2013, 33, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
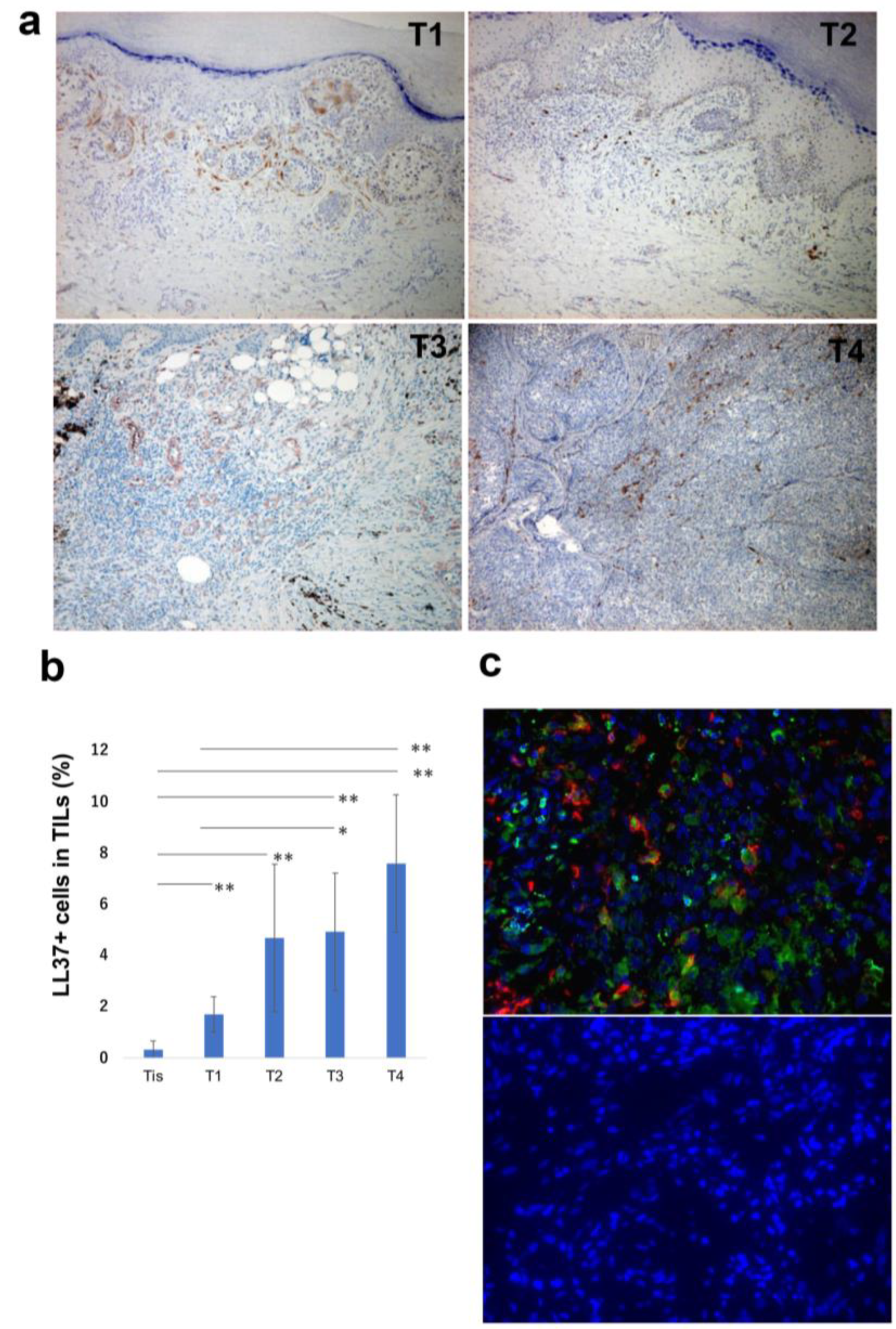
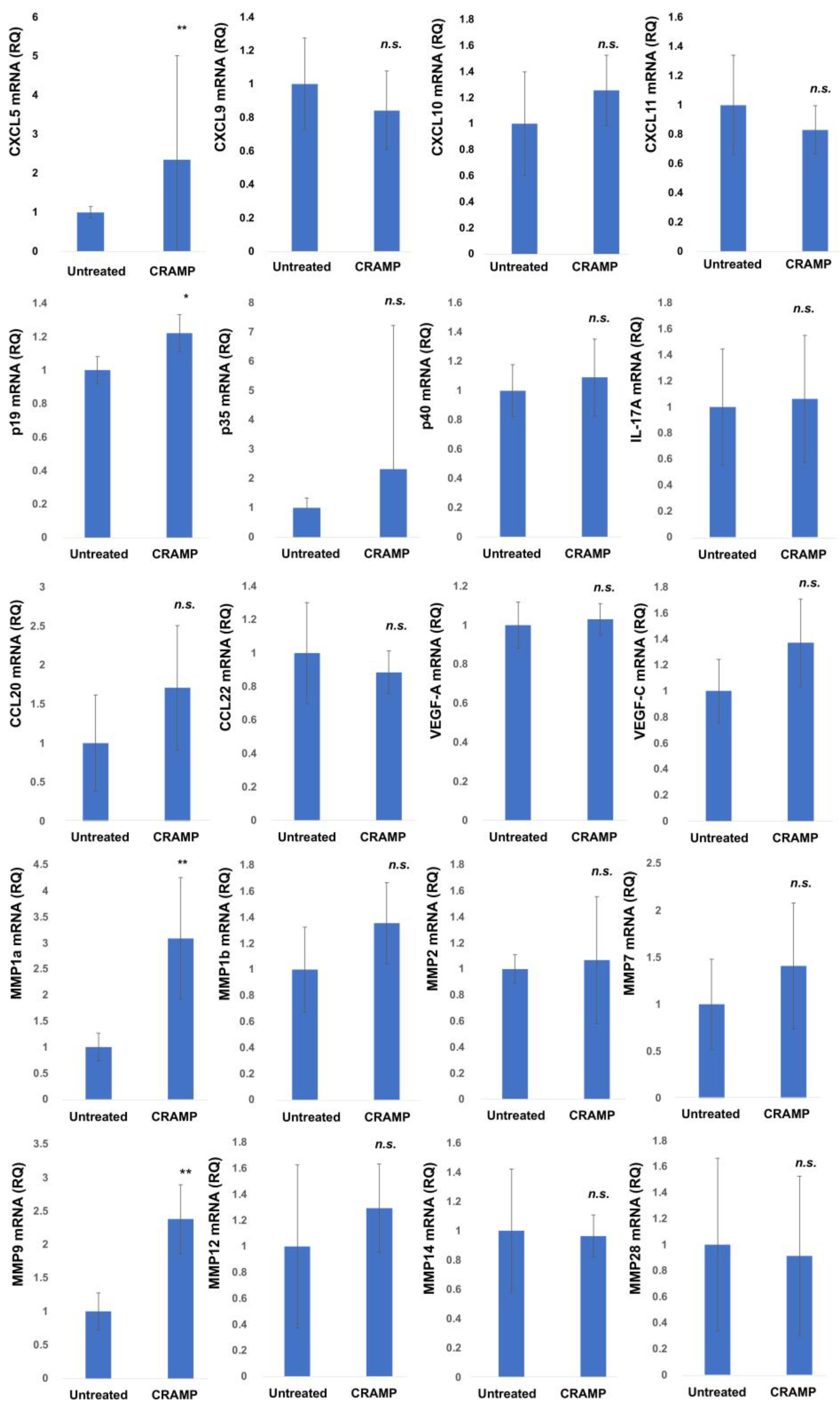

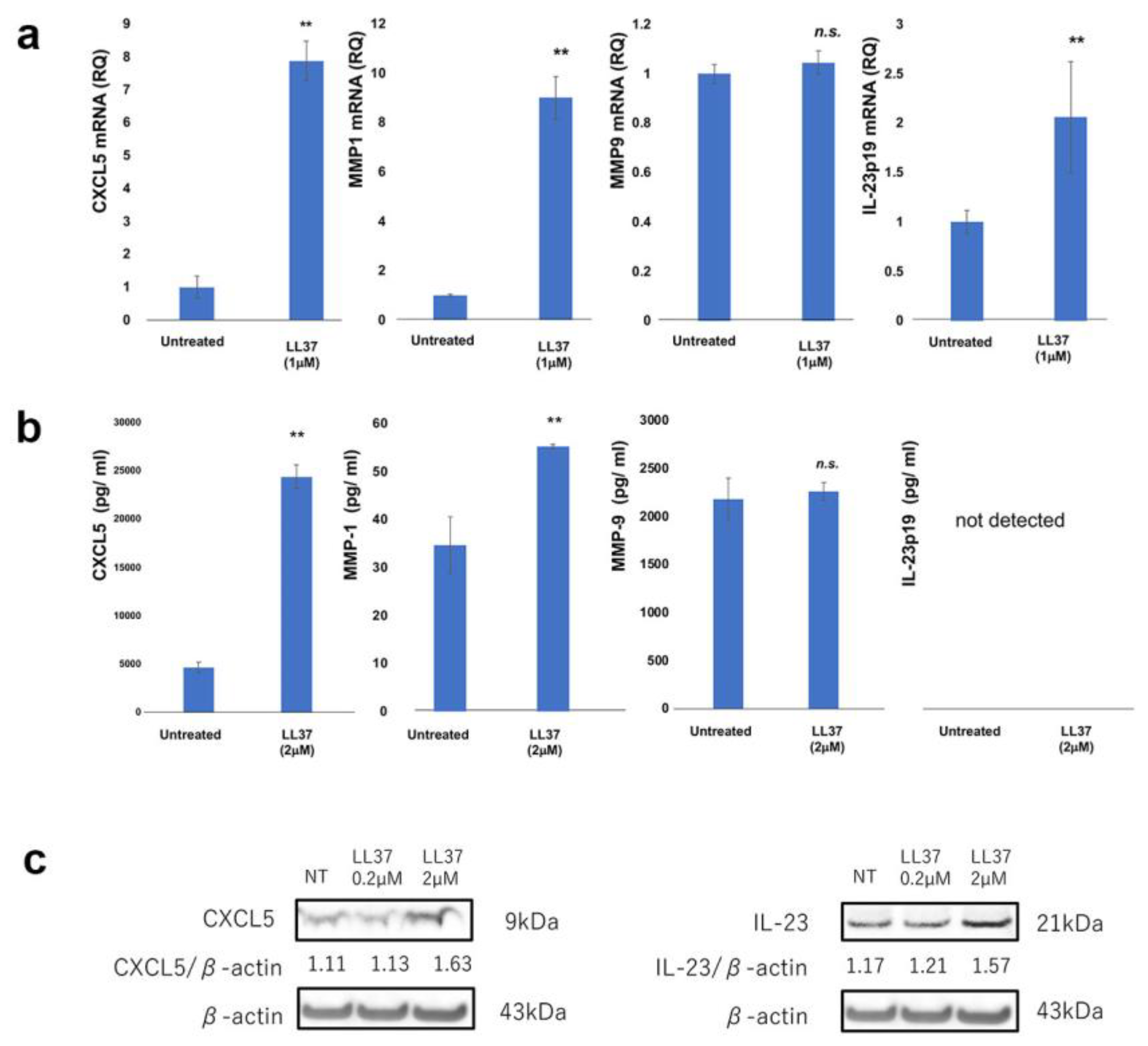
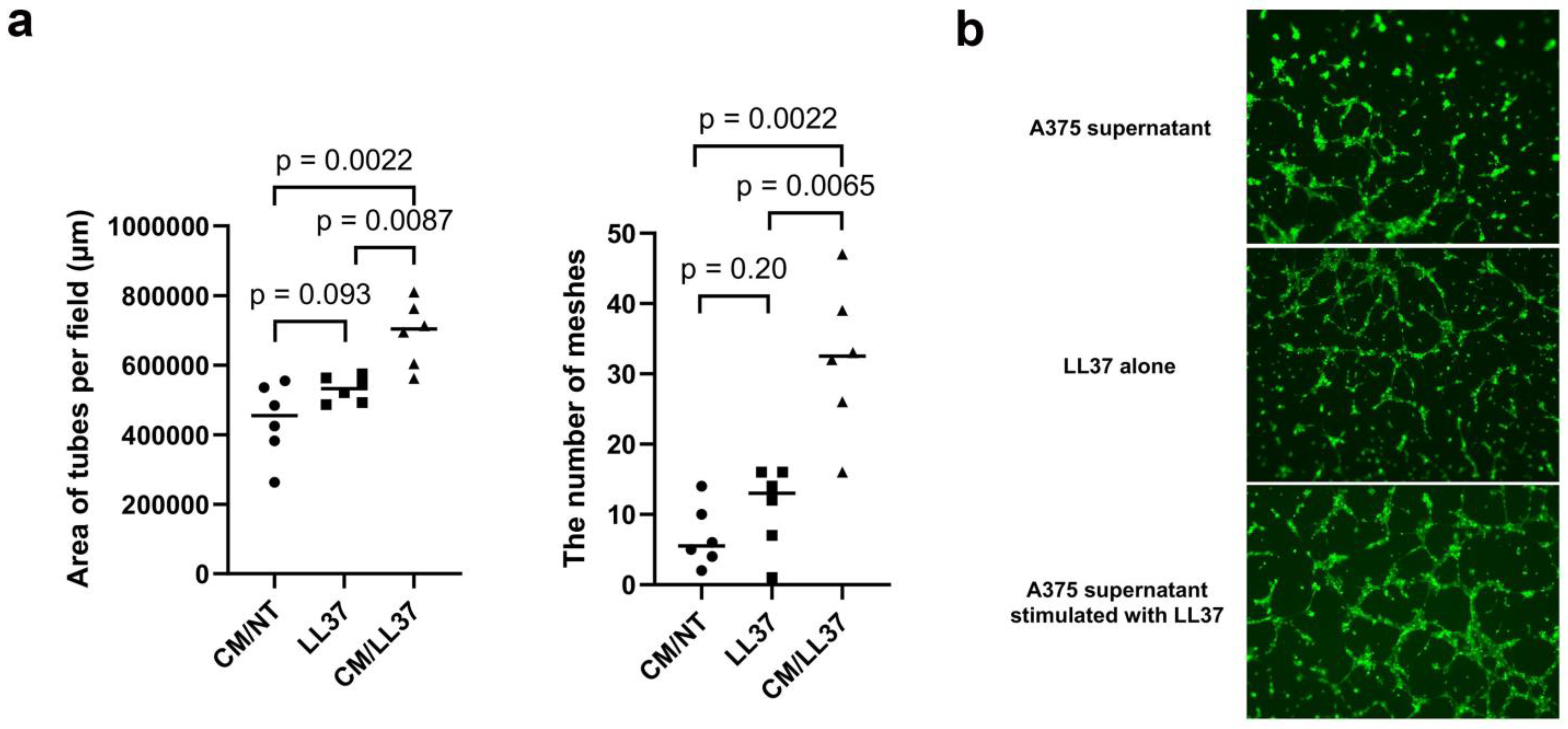

| Age | Sex | Subtype | Tumor Thickness (mm) | TNM | Stage | ||
|---|---|---|---|---|---|---|---|
| case 1 | 69 | M | acral | 0 | TisN0M0 | 0 | |
| case 2 | 78 | F | acral | 0 | TisN0M0 | 0 | |
| case 3 | 72 | F | acral | 0 | TisN0M0 | 0 | |
| case 4 | 67 | M | acral | 0 | TisN0M0 | 0 | |
| case 5 | 75 | F | acral | 0 | TisN0M0 | 0 | |
| Tis | case 6 | 49 | F | acral | 0 | TisN0M0 | 0 |
| case 7 | 86 | F | acral | 0 | TisN0M0 | 0 | |
| case 8 | 69 | F | acral | 0 | TisN0M0 | 0 | |
| case 9 | 85 | M | acral | 0 | TisN0M0 | 0 | |
| case 10 | 21 | F | non-CSD | 0 | TisN0M0 | 0 | |
| case 11 | 78 | F | CSD | 0.5 | T1aN0M0 | IA | |
| case 12 | 79 | F | CSD | 0.5 | T1aN0M0 | IA | |
| case 13 | 62 | M | acral | 0.35 | T1aN0M0 | IA | |
| case 14 | 65 | M | acral | 0.90 | T1aN0M0 | IA | |
| case 15 | 48 | F | acral | 0.90 | T1bN0M0 | IB | |
| T1 | case 16 | 36 | F | acral | 0.60 | T1aN0M0 | IA |
| case 17 | 43 | F | acral | 0.80 | T1aN0M0 | IA | |
| case 18 | 72 | M | acral | 0.42 | T1aN0M0 | IA | |
| case 19 | 75 | M | acral | 0.70 | T1bN0M0 | IB | |
| case 20 | 65 | M | CSD | 0.05 | T1bN0M0 | IA | |
| case 21 | 64 | F | acral | 1.02 | T2bN0M0 | IIA | |
| case 22 | 46 | F | acral | 1.05 | T2aN0M0 | IB | |
| case 23 | 81 | F | CSD | 1.20 | T2aN0M0 | IB | |
| case 24 | 88 | F | CSD | 1.50 | T2bN0M0 | IIA | |
| case 25 | 72 | M | acral | 1.80 | T2bN0M0 | IIA | |
| T2 | case 26 | 65 | M | non-CSD | 1.50 | T2aN0M0 | IB |
| case 27 | 42 | F | acral | 1.80 | T2aN0M0 | IB | |
| case 28 | 69 | F | non-CSD | 1.03 | T2aN0M0 | IB | |
| case 29 | 21 | F | acral | 1.30 | T2aN0M0 | IB | |
| case 30 | 21 | F | non-CSD | 1.3 | T2aN0M0 | IB | |
| case 31 | 47 | M | acral | 3.5 | T3aN3cM0 | IIIC | |
| case 32 | 63 | F | acral | 3 | T3aN0M0 | IIA | |
| case 33 | 83 | F | CSD | 2 | T3aN0M0 | IIA | |
| case 34 | 62 | F | CSD | 3 | T3aN1aM0 | IIIB | |
| case 35 | 79 | M | acral | 3.5 | T3aN1bM0 | IIIB | |
| T3 | case 36 | 79 | F | acral | 3.2 | T3aN0M0 | IIA |
| case 37 | 64 | F | acral | 2 | T3bN1aM0 | IIIC | |
| case 38 | 84 | F | acral | 2 | T3aN0M0 | IIA | |
| case 39 | 81 | F | acral | 3 | T3aN0M0 | IIA | |
| case 40 | 71 | M | non-CSD | 3 | T3aN0M0 | IIA | |
| case 41 | 74 | F | acral | 6 | T4bN0M0 | IIC | |
| case 42 | 82 | F | acral | 6 | T4aN0M0 | IIB | |
| case 43 | 77 | M | acral | 6 | T4aN0M0 | IIB | |
| case 44 | 90 | F | CSD | 4.4 | T4aN0M0 | IIB | |
| case 45 | 69 | F | acral | 4.5 | T4bN1aM0 | IIIC | |
| T4 | case 46 | 39 | M | CSD | 9 | T4bN2aM0 | IIIC |
| case 47 | 70 | M | acral | 14 | T4bN0M0 | IIC | |
| case 48 | 35 | M | non-CSD | 20 | T4bN1aM1b | IV | |
| case 49 | 84 | M | acral | 6 | T4bN0M0 | IIC | |
| case 50 | 47 | M | CSD | 8 | T4bN2bM0 | IIIC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohuchi, K.; Ikawa, T.; Amagai, R.; Takahashi, T.; Roh, Y.; Endo, J.; Kambayashi, Y.; Asano, Y.; Fujimura, T. LL-37 Might Promote Local Invasion of Melanoma by Activating Melanoma Cells and Tumor-Associated Macrophages. Cancers 2023, 15, 1678. https://doi.org/10.3390/cancers15061678
Ohuchi K, Ikawa T, Amagai R, Takahashi T, Roh Y, Endo J, Kambayashi Y, Asano Y, Fujimura T. LL-37 Might Promote Local Invasion of Melanoma by Activating Melanoma Cells and Tumor-Associated Macrophages. Cancers. 2023; 15(6):1678. https://doi.org/10.3390/cancers15061678
Chicago/Turabian StyleOhuchi, Kentaro, Tetsuya Ikawa, Ryo Amagai, Toshiya Takahashi, Yuna Roh, Junko Endo, Yumi Kambayashi, Yoshihide Asano, and Taku Fujimura. 2023. "LL-37 Might Promote Local Invasion of Melanoma by Activating Melanoma Cells and Tumor-Associated Macrophages" Cancers 15, no. 6: 1678. https://doi.org/10.3390/cancers15061678
APA StyleOhuchi, K., Ikawa, T., Amagai, R., Takahashi, T., Roh, Y., Endo, J., Kambayashi, Y., Asano, Y., & Fujimura, T. (2023). LL-37 Might Promote Local Invasion of Melanoma by Activating Melanoma Cells and Tumor-Associated Macrophages. Cancers, 15(6), 1678. https://doi.org/10.3390/cancers15061678







