Therapy Resistance of Glioblastoma in Relation to the Subventricular Zone: What Is the Role of Radiotherapy?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment and Data Collection
2.3. SVZ MRI Characteristics, Delineation and Dosimetric Data
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Prognostic Factors for PFS
3.3. Prognostic Factors for OS
3.4. Impact of SVZ Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Barani, I.J.; Cuttino, L.W.; Benedict, S.H.; Todor, D.; Bump, E.A.; Wu, Y.; Chung, T.D.; Broaddus, W.C.; Lin, P.-S. Neural Stem Cell-Preserving External-Beam Radiotherapy of Central Nervous System Malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 978–985. [Google Scholar] [CrossRef]
- Sundar, S.J.; Hsieh, J.K.; Manjila, S.; Lathia, J.D.; Sloan, A. The Role of Cancer Stem Cells in Glioblastoma. Neurosurg. Focus 2014, 37, E6. [Google Scholar] [CrossRef] [PubMed]
- Temple, S. The Development of Neural Stem Cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef]
- Benmelouka, A.Y.; Munir, M.; Sayed, A.; Attia, M.S.; Ali, M.M.; Negida, A.; Alghamdi, B.S.; Kamal, M.A.; Barreto, G.E.; Ashraf, G.M.; et al. Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2258. [Google Scholar] [CrossRef]
- Goffart, N.; Kroonen, J.; Rogister, B. Glioblastoma-Initiating Cells: Relationship with Neural Stem Cells and the Micro-Environment. Cancers 2013, 5, 1049–1071. [Google Scholar] [CrossRef]
- Matarredona, E.R.; Pastor, A.M. Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications. Front. Oncol. 2019, 9, 779. [Google Scholar] [CrossRef]
- Stiles, C.D.; Rowitch, D.H. Glioma Stem Cells: A Midterm Exam. Neuron 2008, 58, 832–846. [Google Scholar] [CrossRef]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The Role of SVZ Stem Cells in Glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human Hippocampal Neurogenesis Drops Sharply in Children to Undetectable Levels in Adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Lim, D.A.; Cha, S.; Mayo, M.C.; Chen, M.-H.; Keles, E.; VandenBerg, S.; Berger, M.S. Relationship of Glioblastoma Multiforme to Neural Stem Cell Regions Predicts Invasive and Multifocal Tumor Phenotype. Neuro Oncol. 2007, 9, 424–429. [Google Scholar] [CrossRef]
- Mistry, A.M.; Dewan, M.C.; White-Dzuro, G.A.; Brinson, P.R.; Weaver, K.D.; Thompson, R.C.; Ihrie, R.A.; Chambless, L.B. Decreased Survival in Glioblastomas Is Specific to Contact with the Ventricular-Subventricular Zone, Not Subgranular Zone or Corpus Callosum. J. Neurooncol. 2017, 132, 341–349. [Google Scholar] [CrossRef]
- Berger, F.; Gay, E.; Pelletier, L.; Tropel, P.; Wion, D. Development of Gliomas: Potential Role of Asymmetrical Cell Division of Neural Stem Cells. Lancet Oncol. 2004, 5, 511–514. [Google Scholar] [CrossRef]
- Elicin, O.; Inac, E.; Uzel, E.K.; Karacam, S.; Uzel, O.E. Relationship between Survival and Increased Radiation Dose to Subventricular Zone in Glioblastoma Is Controversial. J. Neurooncol. 2014, 118, 413–419. [Google Scholar] [CrossRef]
- Darázs, B.; Ruskó, L.; Végváry, Z.; Ferenczi, L.; Dobi, Á.; Paczona, V.; Varga, Z.; Fodor, E.; Hideghéty, K. Subventricular Zone Volumetric and Dosimetric Changes during Postoperative Brain Tumor Irradiation and Its Impact on Overall Survival. Phys. Med. 2019, 68, 35–40. [Google Scholar] [CrossRef]
- Foro Arnalot, P.; Pera, O.; Rodriguez, N.; Sanz, X.; Reig, A.; Membrive, I.; Ortiz, A.; Granados, R.; Algara, M. Influence of Incidental Radiation Dose in the Subventricular Zone on Survival in Patients with Glioblastoma Multiforme Treated with Surgery, Radiotherapy, and Temozolomide. Clin. Transl. Oncol. 2017, 19, 1225–1231. [Google Scholar] [CrossRef]
- Bender, K.; Träger, M.; Wahner, H.; Onken, J.; Scheel, M.; Beck, M.; Ehret, F.; Budach, V.; Kaul, D. What Is the Role of the Subventricular Zone in Radiotherapy of Glioblastoma Patients? Radiother. Oncol. 2021, 158, 138–145. [Google Scholar] [CrossRef]
- Murchison, S.C.; Wiksyk, B.; Gossman, S.; Jensen, B.; Sayers, D.; Lesperance, M.; Truong, P.T.; Alexander, A. Subventricular Zone Radiation Dose and Outcome for Glioblastoma Treated Between 2006 and 2012. Cureus 2018, 10, e3618. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Bonsu, J.M.; Redmond, K.J.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. Implications of Irradiating the Subventricular Zone Stem Cell Niche. Stem Cell Res. 2016, 16, 387–396. [Google Scholar] [CrossRef]
- Klein, M.; Postma, T.J.; Taphoorn, M.J.B.; Aaronson, N.K.; Vandertop, W.P.; Muller, M.; van der Ploeg, H.M.; Heimans, J.J. The Prognostic Value of Cognitive Functioning in the Survival of Patients with High-Grade Glioma. Neurology 2003, 61, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Taphoorn, M.J.; Klein, M. Cognitive Deficits in Adult Patients with Brain Tumours. Lancet Neurol. 2004, 3, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Nair, V.; Paul, S.N.; Kannan, S.; Moiyadi, A.; Epari, S.; Jalali, R. Can Irradiation of Potential Cancer Stem-Cell Niche in the Subventricular Zone Influence Survival in Patients with Newly Diagnosed Glioblastoma? J. Neurooncol. 2012, 109, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hallaert, G.; Pinson, H.; van den Broecke, C.; Sweldens, C.; van Roost, D.; Kalala, J.-P.; Boterberg, T. Survival Impact of Incidental Subventricular Zone Irradiation in IDH-Wildtype Glioblastoma. Acta Oncol. 2021, 60, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Barendsen, G.W. Dose Fractionation, Dose Rate and Iso-Effect Relationships for Normal Tissue Responses. Int. J. Radiat. Oncol.*Biol.*Phys. 1982, 8, 1981–1997. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Lavell, E.; Quiñones-Hinojosa, A.; Guerrero-Cazares, H. Regulation of Subventricular Zone-Derived Cells Migration in the Adult Brain. In Stem Cell Biology in Neoplasms of the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–21. [Google Scholar]
- Hellström, N.A.K.; Björk-Eriksson, T.; Blomgren, K.; Kuhn, H.G. Differential Recovery of Neural Stem Cells in the Subventricular Zone and Dentate Gyrus After Ionizing Radiation. Stem Cells 2009, 27, 634–641. [Google Scholar] [CrossRef]
- Achanta, P.; Capilla-Gonzalez, V.; Purger, D.; Reyes, J.; Sailor, K.; Song, H.; Garcia-Verdugo, J.M.; Gonzalez-Perez, O.; Ford, E.; Quinones-Hinojosa, A. Subventricular Zone Localized Irradiation Affects the Generation of Proliferating Neural Precursor Cells and the Migration of Neuroblasts. Stem Cells 2012, 30, 2548–2560. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Guerrero-Cazares, H.; Bonsu, J.M.; Gonzalez-Perez, O.; Achanta, P.; Wong, J.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. The Subventricular Zone Is Able to Respond to a Demyelinating Lesion After Localized Radiation. Stem Cells 2014, 32, 59–69. [Google Scholar] [CrossRef]
- Haskins, W.E.; Zablotsky, B.L.; Foret, M.R.; Ihrie, R.A.; Alvarez-Buylla, A.; Eisenman, R.N.; Berger, M.S.; Lin, C.-H.A. Molecular Characteristics in MRI-Classified Group 1 Glioblastoma Multiforme. Front. Oncol. 2013, 3, 182. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, T.N.; Kukekov, V.G.; Laywell, E.D.; Suslov, O.N.; Vrionis, F.D.; Steindler, D.A. Human Cortical Glial Tumors Contain Neural Stem-Like Cells Expressing Astroglial and Neuronal Markers in Vitro. Glia 2002, 39, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Harrabi, S.B.; Bougatf, N.; Bernhardt, D.; Mohr, A.; Rieber, J.; Koelsche, C.; Rieken, S.; Debus, J. Do Increased Doses to Stem-Cell Niches during Radiation Therapy Improve Glioblastoma Survival? Stem Cells Int. 2016, 2016, 8793462. [Google Scholar] [CrossRef]
- Evers, P.; Lee, P.P.; DeMarco, J.; Agazaryan, N.; Sayre, J.W.; Selch, M.; Pajonk, F. Irradiation of the Potential Cancer Stem Cell Niches in the Adult Brain Improves Progression-Free Survival of Patients with Malignant Glioma. BMC Cancer 2010, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Eppinga, W.; Lagerwaard, F.; Cloughesy, T.; Slotman, B.; Nghiemphu, P.L.; Wang, P.-C.; Kupelian, P.; Agazaryan, N.; Demarco, J.; et al. Evaluation of High Ipsilateral Subventricular Zone Radiation Therapy Dose in Glioblastoma: A Pooled Analysis. Int. J. Radiat. Oncol.*Biol.*Phys. 2013, 86, 609–615. [Google Scholar] [CrossRef]
- Chen, L.; Guerrero-Cazares, H.; Ye, X.; Ford, E.; McNutt, T.; Kleinberg, L.; Lim, M.; Chaichana, K.; Quinones-Hinojosa, A.; Redmond, K. Increased Subventricular Zone Radiation Dose Correlates with Survival in Glioblastoma Patients after Gross Total Resection. Int. J. Radiat. Oncol.*Biol.*Phys. 2013, 86, 616–622. [Google Scholar] [CrossRef]
- Bruil, D.E.; David, S.; Nagtegaal, S.H.J.; de Sonnaville, S.F.A.M.; Verhoeff, J.J.C. Irradiation of the Subventricular Zone and Subgranular Zone in High- and Low-Grade Glioma Patients: An Atlas-Based Analysis on Overall Survival. Neurooncol. Adv. 2022, 4, vdab193. [Google Scholar] [CrossRef]
- Muracciole, X.; El-amine, W.; Tabouret, E.; Boucekine, M.; Barlier, A.; Petrirena, G.; Harivony, T.; Solignac, L.; Chinot, O.L.; Macagno, N.; et al. Negative Survival Impact of High Radiation Doses to Neural Stem Cells Niches in an IDH-Wild-Type Glioblastoma Population. Front. Oncol. 2018, 8, 426. [Google Scholar] [CrossRef]
- Achari, R.; Arunsingh, M.; Badgami, R.K.; Saha, A.; Chatterjee, S.; Shrimali, R.K.; Mallick, I.; Arun, B. High-Dose Neural Stem Cell Radiation May Not Improve Survival in Glioblastoma. Clin. Oncol. 2017, 29, 335–343. [Google Scholar] [CrossRef]
- Şuşman, S.; Leucuţa, D.-C.; Kacso, G.; Florian, Ş. loan High Dose vs Low Dose Irradiation of the Subventricular Zone in Patients with Glioblastoma—A Systematic Review and Meta-Analysis. Cancer Manag. Res. 2019, 11, 6741–6753. [Google Scholar] [CrossRef]
- Hallaert, G.; Pinson, H.; van den Broecke, C.; Vanhauwaert, D.; van Roost, D.; Boterberg, T.; Kalala, J.P. Subventricular Zone Contacting Glioblastoma: Tumor Size, Molecular Biological Factors and Patient Survival. Acta Oncol. 2020, 59, 1474–1479. [Google Scholar] [CrossRef]
- Berendsen, S.; van Bodegraven, E.; Seute, T.; Spliet, W.G.M.; Geurts, M.; Hendrikse, J.; Schoysman, L.; Huiszoon, W.B.; Varkila, M.; Rouss, S.; et al. Adverse Prognosis of Glioblastoma Contacting the Subventricular Zone: Biological Correlates. PLoS ONE 2019, 14, e0222717. [Google Scholar] [CrossRef]
- Pope, W.B.; Sayre, J.; Perlina, A.; Villablanca, J.P.; Mischel, P.S.; Cloughesy, T.F. MR Imaging Correlates of Survival in Patients with High-Grade Gliomas. AJNR Am. J. Neuroradiol. 2005, 26, 2466–2474. [Google Scholar]
- Beiriger, J.; Habib, A.; Jovanovich, N.; Kodavali, C.V.; Edwards, L.; Amankulor, N.; Zinn, P.O. The Subventricular Zone in Glioblastoma: Genesis, Maintenance, and Modeling. Front. Oncol. 2022, 12, 790976. [Google Scholar] [CrossRef]
- Kappadakunnel, M.; Eskin, A.; Dong, J.; Nelson, S.F.; Mischel, P.S.; Liau, L.M.; Ngheimphu, P.; Lai, A.; Cloughesy, T.F.; Goldin, J.; et al. Stem Cell Associated Gene Expression in Glioblastoma Multiforme: Relationship to Survival and the Subventricular Zone. J. Neurooncol. 2010, 96, 359–367. [Google Scholar] [CrossRef]
- de Luca, C.; Virtuoso, A.; Papa, M.; Certo, F.; Barbagallo, G.M.V.; Altieri, R. Regional Development of Glioblastoma: The Anatomical Conundrum of Cancer Biology and Its Surgical Implication. Cells 2022, 11, 1349. [Google Scholar] [CrossRef]
- Fyllingen, E.H.; Bø, L.E.; Reinertsen, I.; Jakola, A.S.; Sagberg, L.M.; Berntsen, E.M.; Salvesen, Ø.; Solheim, O. Survival of Glioblastoma in Relation to Tumor Location: A Statistical Tumor Atlas of a Population-Based Cohort. Acta Neurochir. 2021, 163, 1895–1905. [Google Scholar] [CrossRef]
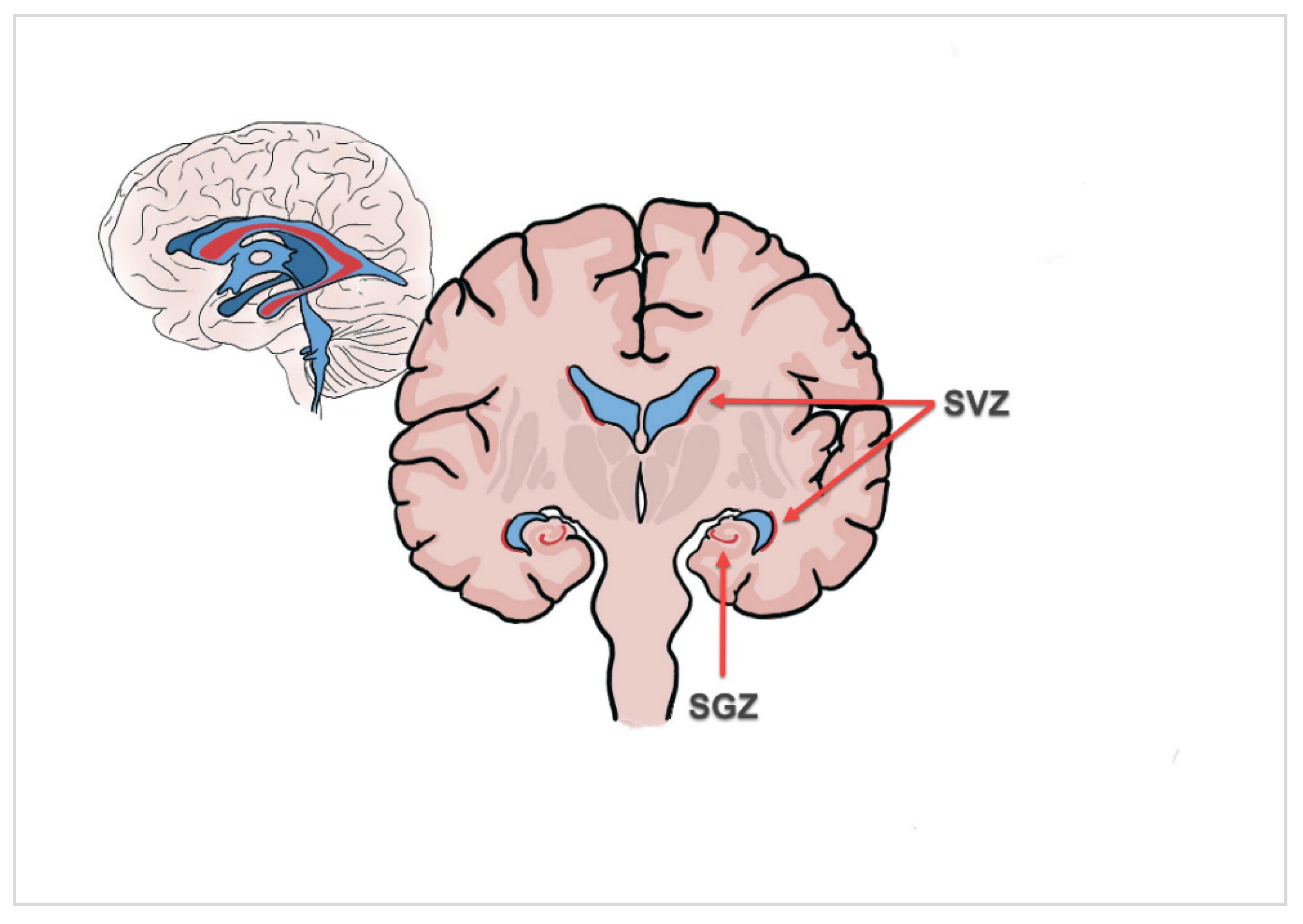

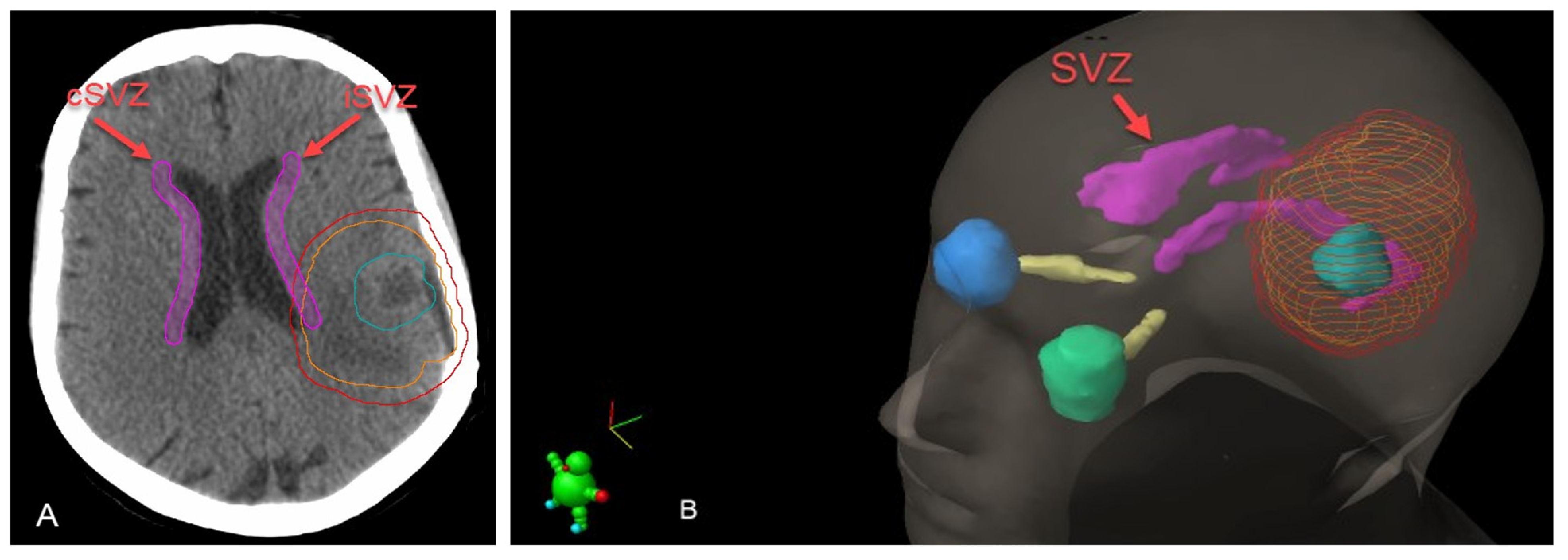

| Characteristics | Median (Range)/n (%) |
|---|---|
| n = 147 | |
| Age, years | 64 (15–89) |
| Sex | |
| Female | 48 (32.7) |
| Male | 99 (67.3) |
| Karnofsky Performance Score | |
| 90–100% | 89 (60.6) |
| 80% | 34 (23.1) |
| ≤70% | 24 (16.3) |
| Multiple glioblastoma | |
| Multifocal | 7 (4.8) |
| Multicentric | 31 (21.1) |
| Glioblastoma classification | |
| Type I | 100 (68) |
| Type II | 4 (2.7) |
| Type III | 41 (27.9) |
| Type IV | 2 (1.4) |
| O6-methylguanine methyltransferase | |
| Methylated | 67 (45.6) |
| Non-methylated | 79 (53.7) |
| NA | 1 (0.7) |
| Isocitrate dehydrogenase-1 gene | |
| Wild-type | 133 (90.5) |
| Mutant | 11 (7.5) |
| NA | 3 (2) |
| Extent of resection | |
| Gross total resection | 67 (45.6) |
| Subtotal resection | 46 (31.3) |
| Biopsy | 34 (23.1) |
| Radiotherapy | |
| Definitive | 33 (22.4) |
| Adjuvant | 114 (77.6) |
| Radiotherapy dose/fractionation | |
| Conventional (2 Gy) | 95 (64.6) |
| Hypo-fractionation (2.67 Gy) | 52 (34.7) |
| Steroid usage | 43 (29.3) |
| Dmean iSVZ, Gy BED2 Gy 80 Gy = EQD2 40 Gy | 85.8 (2.10–123.40) |
| ≥80 Gy | 92 (62.6) |
| <80 Gy | 55 (37.4) |
| Dmax iSVZ, Gy BED2 Gy 100 Gy = EQD2 50 Gy | 122.4 (46.2–143.7) |
| ≥100 Gy | 96 (65.3) |
| <100 Gy | 51 (34.7) |
| Dmean cSVZ, Gy BED2 Gy 64 Gy = EQD2 32 Gy | 47.2 (1.63–114) |
| <64 Gy | 111 (75.5) |
| ≥64 Gy | 36 (24.5) |
| Dmax cSVZ, Gy BED2 100 Gy = EQD2 50 Gy | 82.2 (12.4–124.8) |
| ≥100 Gy | 42 (28.6) |
| <100 Gy | 105 (71.4) |
| Chemotherapy | |
| Concomitant + adjuvant | 109 (74.1) |
| Only concomitant | 15 (10.2) |
| Only adjuvant | 5 (3.4) |
| Chemotherapy regimen | |
| Temozolomid | 126 (85.7) |
| Bevacizumab | 3 (2) |
| NA | 18 (12.2) |
| Recurrence | |
| In field | 100 (68) |
| Out of field | 21 (14.3) |
| NA | 12 (8.2) |
| Salvage therapy | |
| Radiotherapy | 8 (5.4) |
| Surgery | 31 (21.1) |
| Chemotherapy | 58 (39.5) |
| Best supportive care | 36 (24.5) |
| NA | 14 (9.5) |
| Response assessment in neuro-oncology (RANO) | |
| Complete response | 1 (0.7) |
| Partial response | 9 (6.1) |
| Stable disease | 43 (29.3) |
| Progression | 81 (55.1) |
| NA | 13 (8.8) |
| Final outcome | |
| Alive | 8 (5.4) |
| Dead | 135 (91.8) |
| NA | 4 (2.7) |
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Factors | p Value | Hazards Ratio (HR) | 95% Confidence Interval | p Value | Hazards Ratio (HR) | 95% Confidence Interval |
| Karnofsky Performance Score (ref. = >70%) | <0.001 | 2.834 | 1.624–4.945 | 0.028 | 1.953 | 1.074–3.551 |
| O6-methylguanine methyltransferase (ref. = non-methylated) | 0.008 | 0.599 | 0.410–0.874 | 0.012 | 0.616 | 0.413–0.899 |
| Extent of resection (ref. = biopsy) | 0.010 | 1.832 | 1.154–2.909 | |||
| Chemotherapy (ref. = yes) | 0.024 | 1.925 | 1.090–3.400 | |||
| Dmean iSVZ BED2 Gy (ref. = ≥80 Gy) | 0.092 | 1.380 | 0.949–2.007 | |||
| Dmax iSVZ BED2 Gy (ref. = ≥100 Gy) | 0.001 | 2.000 | 1.337–2.992 | 0.004 | 1.904 | 1.223–2.965 |
| Dmean cSVZ BED2 Gy (ref. <64 Gy) | 0.042 | 1.580 | 1.016–2.457 | 0.034 | 1.662 | 1.039–2.659 |
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| p Value | Hazards Ratio (HR) | 95% Confidence Interval | p Value | Hazards Ratio (HR) | 95% Confidence Interval | |
| Karnofsky Performance Score (ref. = >70%) | <0.001 | 2.426 | 1.543–3.814 | 0.034 | 1.679 | 1.039–2.713 |
| Age (ref. = <70 years) | 0.032 | 1.513 | 1.037–2.207 | |||
| O6-methylguanine methyl-transferase (ref. = non-methylated) | 0.005 | 0.609 | 0.430–0.863 | 0.010 | 0.632 | 0.446–0.897 |
| Chemotherapy (ref. = yes) | 0.005 | 2.079 | 1.255–3.445 | |||
| Dmean iSVZ BED2 Gy (ref. = ≥80 Gy) | 0.020 | 1.515 | 1.067–2.153 | |||
| Dmax iSVZ BED2 Gy (ref. = ≥100 Gy) | 0.001 | 1.877 | 1.314–2.683 | <0.001 | 2.254 | 1.476–3.442 |
| Dmean cSVZ BED2 Gy (ref. <64 Gy) | 0.001 | 1.957 | 1.303–2.940 | |||
| Dmax cSVZ BED2 Gy (ref. = <100 Gy) | 0.008 | 1.688 | 1.143–2.492 | 0.002 | 2.070 | 1.296–3.307 |
| SVZ Dose | Subgroup | Median PFS (mo) | 95% Confidence Interval | p Value | Median OS (mo) | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|---|
| Dmean iSVZ | ≥80 Gy (BED2 Gy) ≥40 Gy (EQD2) | 8 | 6.846–9.154 | 0.072 | 15 | 12.702–17.298 | 0.016 |
| <80 Gy (BED2 Gy) <40 Gy (EQD2) | 6 | 4.788–7.212 | 13 | 11.052–14.948 | |||
| Dmax iSVZ | ≥100 Gy (BED2 Gy) ≥50 Gy (EQD2) | 8 | 6.978–9.022 | <0.001 | 16 | 13.658–18.342 | <0.001 |
| <100 Gy (BED2 Gy) <50 Gy | 6 | 5.036–6.964 | 11 | 6.885–15.115 | |||
| Dmean cSVZ | ≥64 Gy (BED2 Gy) ≥32 Gy (EQD2) | 6 | 4.907–7.093 | 0.030 | 11 | 6.780–15.220 | 0.001 |
| <64Gy (BED2 Gy) <32 Gy (EQD2) | 8 | 6.983–9.017 | 15 | 12.744–17.256 | |||
| Dmax cSVZ | ≥100 Gy (BED2 Gy) ≥50 Gy (EQD2) | 6 | 5.224–6.776 | 0.090 | 12 | 9.352–14.648 | 0.006 |
| <100Gy (BED2 Gy) <50 Gy (EQD2) | 8 | 7.013–8.987 | 15 | 12.509–17.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermiş, E.; Althaus, A.; Blatti, M.; Uysal, E.; Leiser, D.; Norouzi, S.; Riggenbach, E.; Hemmatazad, H.; Ahmadli, U.; Wagner, F. Therapy Resistance of Glioblastoma in Relation to the Subventricular Zone: What Is the Role of Radiotherapy? Cancers 2023, 15, 1677. https://doi.org/10.3390/cancers15061677
Ermiş E, Althaus A, Blatti M, Uysal E, Leiser D, Norouzi S, Riggenbach E, Hemmatazad H, Ahmadli U, Wagner F. Therapy Resistance of Glioblastoma in Relation to the Subventricular Zone: What Is the Role of Radiotherapy? Cancers. 2023; 15(6):1677. https://doi.org/10.3390/cancers15061677
Chicago/Turabian StyleErmiş, Ekin, Alexander Althaus, Marcela Blatti, Emre Uysal, Dominic Leiser, Shokoufe Norouzi, Elena Riggenbach, Hossein Hemmatazad, Uzeyir Ahmadli, and Franca Wagner. 2023. "Therapy Resistance of Glioblastoma in Relation to the Subventricular Zone: What Is the Role of Radiotherapy?" Cancers 15, no. 6: 1677. https://doi.org/10.3390/cancers15061677
APA StyleErmiş, E., Althaus, A., Blatti, M., Uysal, E., Leiser, D., Norouzi, S., Riggenbach, E., Hemmatazad, H., Ahmadli, U., & Wagner, F. (2023). Therapy Resistance of Glioblastoma in Relation to the Subventricular Zone: What Is the Role of Radiotherapy? Cancers, 15(6), 1677. https://doi.org/10.3390/cancers15061677





