Survival in People Living with HIV with or without Recurrence of Hepatocellular Carcinoma after Invasive Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics of HCC/PLWH with a Fatal Outcome or Not
3.2. Biochemistry at Invasive Therapy
3.3. Biochemistry at Last Evaluation
3.4. Characteristics of HCC/PLWH and Recurrence
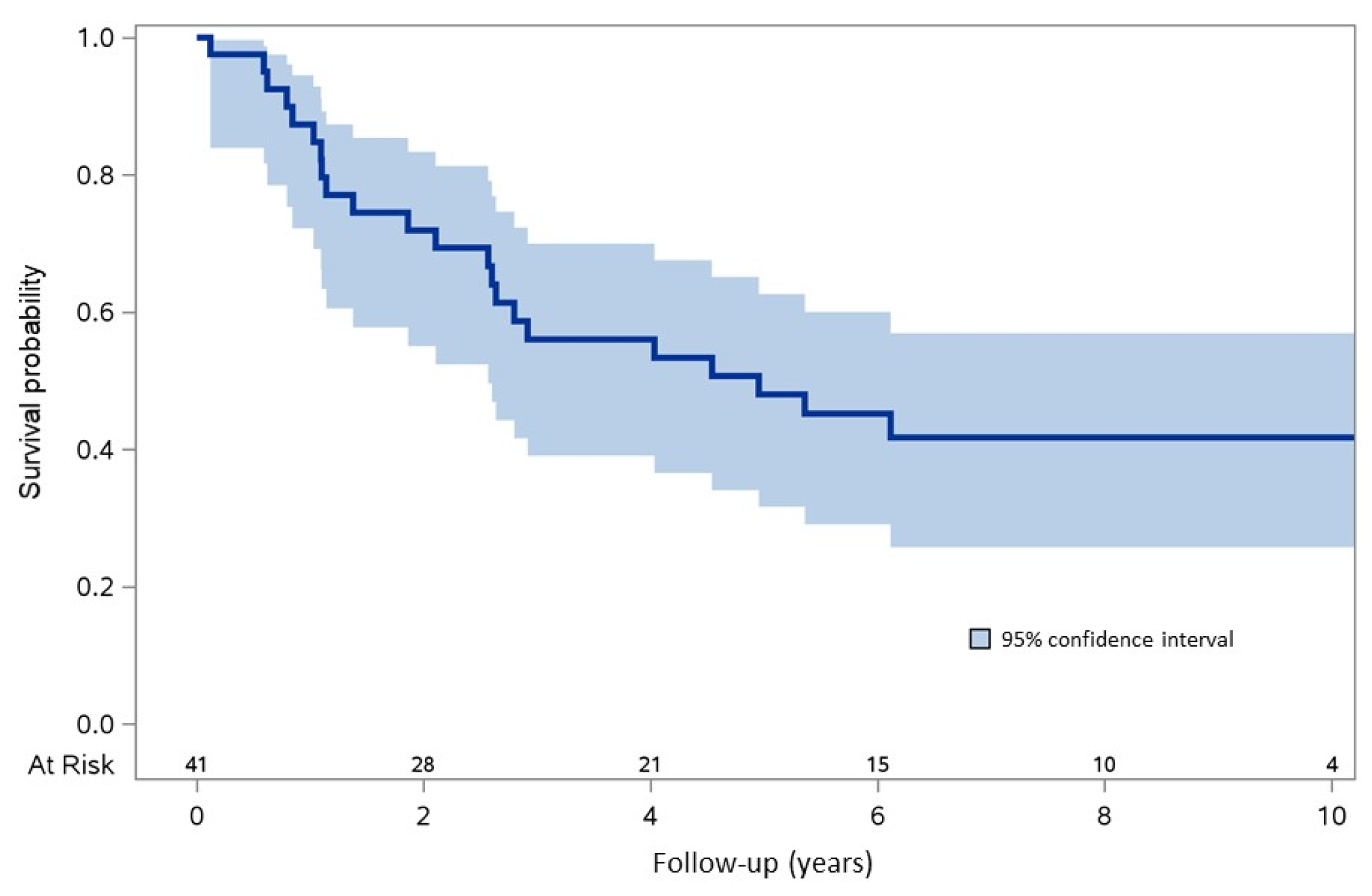
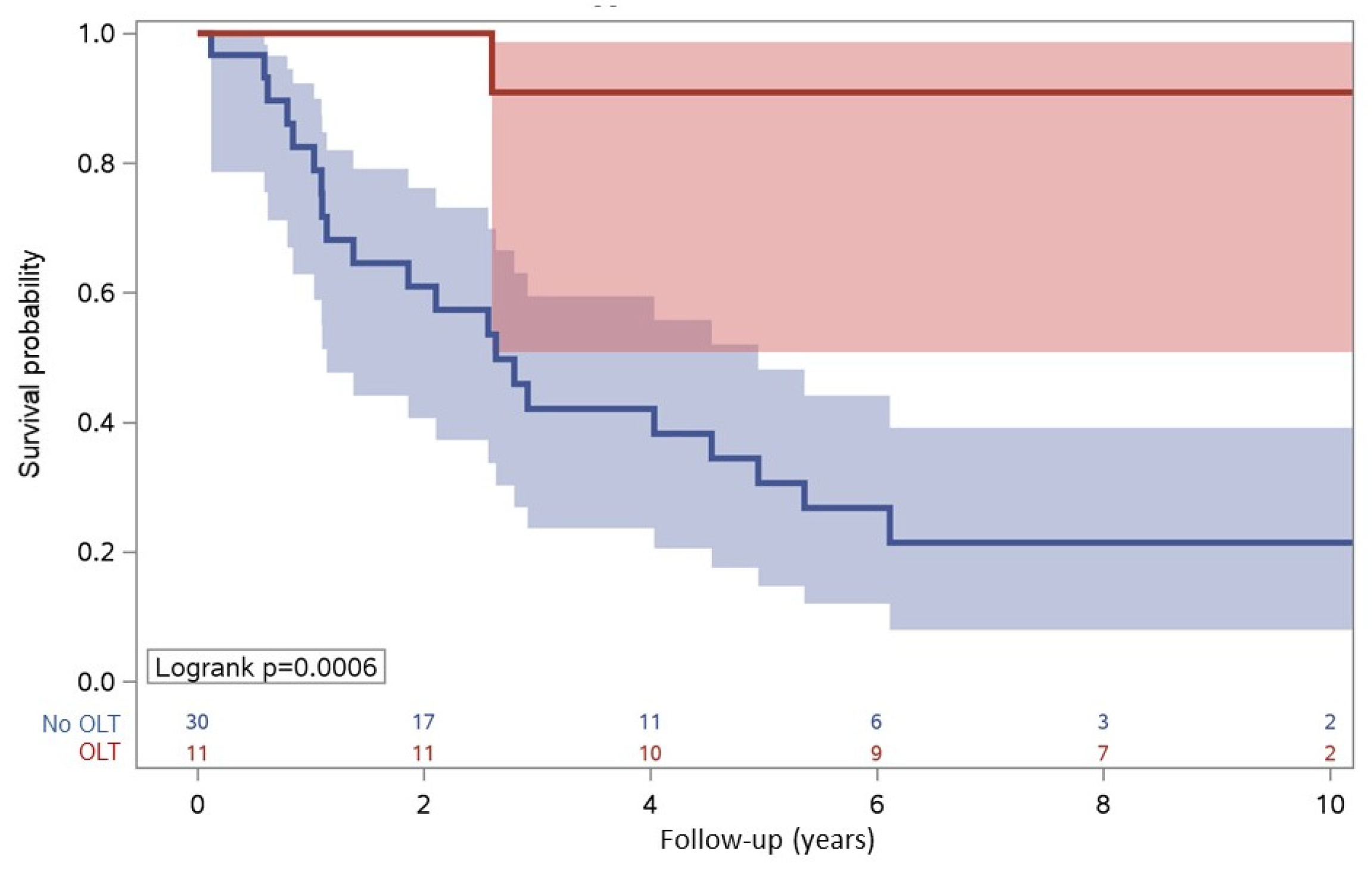
3.5. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mocroft, A.; Phillips, A.N.; Gatell, J.; Ledergerber, B.; Fisher, M.; Clumeck, N.; Losso, M.; Lazzarin, A.; Fatkenheuer, G.; Lundgren, J.D.; et al. Normalisation of CD4 counts in participants with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: An observational cohort study. Lancet 2007, 370, 407–413. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Hamouda, O.; Sannes, M.; Boufassa, F.; Johnson, A.M.; Lambert, P.; Porter, K.; for the CASCADE Collaboration. Changes in the Risk of Death After HIV Seroconversion Compared with Mortality in the General Population. JAMA 2008, 300, 51–59. [Google Scholar] [CrossRef]
- Rosenthal, E.; Roussillon, C.; Salmon-Céron, D.; Georget, A.; Hénard, S.; Huleux, T.; Gueit, I.; Mortier, E.; Costagliola, D.; Morlat, P.; et al. Liver-related deaths in HIV-infected participants between 1995 and 2010 in France: The Mortavic 2010 study in collaboration with the Agence Nationale de Recherche sur le SIDA (ANRS) EN 20 Mortalité 2010 survey. HIV Med. 2015, 16, 230–239. [Google Scholar] [CrossRef]
- Joshi, D.; O’Grady, J.; Dieterich, D.; Gazzard, B.; Agarwal, K. Increasing burden of liver disease in participants with HIV infection. Lancet 2011, 377, 1198–1209. [Google Scholar] [CrossRef]
- Clifford, G.M.; Rickenbach, M.; Polesel, J.; Maso, L.D.; Steffen, I.; Ledergerber, B.; Rauch, A.; Probst-Hensch, N.M.; Bouchardy, C.; Levi, F.; et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS 2008, 22, 2135–2141. [Google Scholar] [CrossRef]
- Berretta, M.; Martellotta, F.; Di Francia, R.; Spina, M.; Vaccher, E.; Balestreri, L.; Borsatti, E.; Bearz, A.; De Paoli, P.; Tirelli, U. Clinical presentation and outcome of non-AIDS defining cancers, in HIV-infected participants in the ART-era: The Italian Cooperative Group on AIDS and tumors activity. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3619–3634. [Google Scholar]
- Yopp, A.C.; Subramanian, M.; Jain, M.K.; Mansour, J.C.; Schwarz, R.E.; Balch, G.C.; Singal, A.G. Presentation, treatment, and clinical outcomes of participants with hepatocellular carcinoma, with and without human immunodeficiency virus infection. Clin. Gastroenterol. Hepatol. 2012, 10, 1284–1290. [Google Scholar] [CrossRef]
- Bräu, N.; Fox, R.K.; Xiao, P.; Marks, K.; Naqvi, Z.; Taylor, L.E.; Trikha, A.; Sherman, M.; Sulkowski, M.S.; Dieterich, D.T.; et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected participants: A U.S.-Canadian multicenter study. J. Hepatol. 2007, 47, 527–537. [Google Scholar] [CrossRef]
- Pinato, D.J.; Allara, E.; Chen, T.-Y.; Trevisani, F.; Minguez, B.; Zoli, M.; Harris, M.; Pria, A.D.; Merchante, N.; Platt, H.; et al. Influence of HIV Infection on the Natural History of Hepatocellular Carcinoma: Results From a Global Multicohort Study. J. Clin. Oncol. 2019, 37, 296–304. [Google Scholar] [CrossRef]
- Lim, C.; Goutte, N.; Gervais, A.; Vullierme, M.-P.; Valla, D.C.; Degos, F.; Farges, O. Standardized care management ensures similar survival rates in HIV-positive and HIV-negative participants with hepatocellular carcinoma. J. Acquir. Immune Defic. Syndr. 2012, 61, 581–587. [Google Scholar] [CrossRef]
- Berretta, M.; Garlassi, E.; Cacopardo, B.; Cappellani, A.; Guaraldi, G.; Cocchi, S.; De Paoli, P.; Lleshi, A.; Izzi, I.; Torresin, A.; et al. Hepatocellular Carcinoma in HIV-Infected Patients: Check Early, Treat Hard. Oncologist 2011, 16, 1258–1269. [Google Scholar] [CrossRef]
- Giordano, T.P.; Kramer, J.R.; Souchek, J.; Richardson, P.; El-Serag, H.B. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: A cohort study, 1992–2001. Arch. Intern. Med. 2004, 164, 2349–2354. [Google Scholar] [CrossRef]
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Wong, J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in participants with preserved liver function: Implications for a strategy of salvage transplantation. Ann. Surg. 2002, 235, 373–382. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2021, 76, 681–693. [Google Scholar] [CrossRef]
- Motomura, T.; Shirabe, K.; Mano, Y.; Muto, J.; Toshima, T.; Umemoto, Y.; Fukuhara, T.; Uchiyama, H.; Ikegami, T.; Yoshizumi, T.; et al. Neutrophil–lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J. Hepatol. 2013, 58, 58–64. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Wang, Y.; Yao, X.; Yang, J.; Li, J. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembo-ization. PLoS ONE 2015, 10, e0119312. [Google Scholar] [CrossRef]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Tanaka, K.; Fushiya, N.; Koike, K.; Nishino, H.; Matsushima, M. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2014, 22, 803–810. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef]
- Fan, F.; Dong, G.; Han, C.; Ding, W.; Li, X.; Dong, X.; Wang, Z.; Liang, P.; Yu, J. Peripheral immune factors aiding clinical parameter for better early recurrence prediction of hepatocellular carcinoma after thermal ablation. Int. J. Hyperth. 2023, 40, 2172219. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Z.; Zhou, L.; Qi, Z.; Xing, S.; Lv, J.; Shi, J.; Fu, B.; Liu, Z.; Zhang, J.-Y.; et al. Impairment of CD4+ cyto- toxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2013, 58, 139–149. [Google Scholar] [CrossRef]
- Morsica, G.; Galli, L.; Messina, E.; Cibarelli, A.; Bagaglio, S.; Poli, A.; Ranzenigo, M.; Castagna, A.; Hasson, H.; Uberti-Foppa, C. Levels of Alpha-Fetoprotein and Association with Mortality in Hepatocellular Carcinoma of HIV-1-Infected Patients. J. Oncol. 2022, 10, e3586064. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef]
- Vittinghoff, E.; McCulloch, C.E. Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. Am. J. Epidemiol. 2007, 165, 710–718. [Google Scholar] [CrossRef]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef]
- Taborelli, M.; Suligoi, B.; Toffolutti, F.; Frova, L.; Grande, E.; Grippo, F.; Pappagallo, M.; Pugliese, L.; Regine, V.; Serraino, D.; et al. Excess liver-related mortality among people with AIDS compared to the general population: An Italian nationwide cohort study using multiple causes of death. HIV Med. 2020, 21, 642–649. [Google Scholar] [CrossRef]
- Pinato, D.J.; Sharma, R.; Citti, C.; Platt, H.; Ventura-Cots, M.; Allara, E.; Chen, T.-Y.; Pria, A.D.; Jain, M.; Minguez, B.; et al. The albumin-bilirubin grade uncovers the prognostic relationship between hepatic reserve and immune dysfunction in HIV-associated hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 47, 95–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, L.X.; Feng, F.S.; Yang, J.; Wei, G. A simple CD4+ T cells to FIB-4 ratio for evaluating prognosis of BCLC-B hepatocellular carcinoma: A retrospective cohort study. BMC Cancer 2022, 22, 311. [Google Scholar] [CrossRef]
- Debruyne, E.N.; Delanghe, J.R. Diagnosing and monitoring hepatocellular carcinoma with alpha-fetoprotein: New aspects and applications. Clin. Chim. Acta 2008, 395, 19–26. [Google Scholar] [CrossRef]
- Tyson, G.L.; Duan, Z.; Kramer, J.R.; Davila, J.A.; Richardson, P.A.; El-Serag, H.B. Level of fetoprotein predicts mortality among participants with hepatitis C–related hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 989–994. [Google Scholar] [CrossRef]
- Chan, M.Y.; She, W.H.; Dai, W.C.; Tsang, S.H.Y.; Chok, K.S.H.; Chan, A.C.Y.; Fung, J.; Lo, C.M.; Cheung, T.T. Prognostic value of preoperative alpha-fetoprotein (AFP) level in participants receiving curative hepatectomy-an analysis of 1182 participants in Hong Kong. Transl. Gastroenterol. Hepatol. 2019, 4, 52. [Google Scholar] [CrossRef]
- Bai, D.-S.; Zhang, C.; Chen, P.; Jin, S.-J.; Jiang, G.-Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017, 7, 12870. [Google Scholar] [CrossRef]
- op den Winkel, M.; Nagel, D.; Sappl, J.; op den Winkel, P.; Lamerz, R.; Zech, C.J.; Straub, G.; Nickel, T.; Rentsch, M.; Stieber, P.; et al. Prognosis of participants with hepatocellular carcinoma. Validation and ranking of established staging-systems in a large western HCC-cohort. PLoS ONE 2012, 7, e45066. [Google Scholar] [CrossRef]
- Farinati, F.; Sergio, A.; Giacomin, A.; Di Nolfo, M.A.; Del Poggio, P.; Benvegnù, L.; Rapaccini, G.; Zoli, M.; Borzio, F.; Giannini, E.; et al. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur. J. Gastroenterol. Hepatol. 2009, 21, 1212–1218. [Google Scholar] [CrossRef]
- Ruggieri, A.; Barbati, C.; Malorni, W. Cellular and molecular mechanisms involved in hepatocellular carcinoma gender disparity. Int. J. Cancer 2010, 127, 499–504. [Google Scholar] [CrossRef]
- Kanda, T.; Takahashi, K.; Nakamura, M.; Nakamoto, S.; Wu, S.; Haga, Y.; Sasaki, R.; Jiang, X.; Yokosuka, O. Androgen Receptor Could Be a Potential Therapeutic Target in Patients with Advanced Hepatocellular Carcinoma. Cancers 2017, 9, 43. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O. The androgen receptor as an emerging target in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2015, 2, 91–99. [Google Scholar] [CrossRef]
- Tian, Y.E.; Xie, X.U.; Lin, Y.; Tan, G.; Zhong, W.U. Androgen receptor in hepatocarcinogenesis: Recent developments and perspectives. Oncol. Lett. 2015, 9, 1983–1988. [Google Scholar] [CrossRef]
- Zheng, B.; Zhu, Y.-J.; Wang, H.-Y.; Chen, L. Gender disparity in hepatocellular carcinoma (HCC): Multiple underlying mechanisms. Sci. China Life Sci. 2017, 60, 575–584. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Kim, S.-H.; Moon, D.-B.; Kim, W.-J.; Kang, W.-H.; Kwon, J.H.; Jwa, E.K.; Cho, H.-D.; Ha, S.-M.; Chung, Y.-K.; Lee, S.-G. Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation. HepatoBiliary Surg. Nutr. 2016, 5, 461–469. [Google Scholar] [CrossRef]
- Montalvá, E.; Cantos, M.; Boscà, A.; Rubín, A.; Vinaixa, C.; Granero, P.; Maupoey, J.; López-Andújar, R. Prognostic Value of Pre-transplantation Serum Alpha-Fetoprotein Levels in Hepatocellular Carcinoma Recurrence. Transplant. Proc. 2016, 48, 2966–2968. [Google Scholar] [CrossRef]
- Li, B.; Liu, A.; Wen, Y.; Yang, G.; Zhao, J.; Li, X.; Mao, Y.; Li, B. The prognostic values of serum markers in hepatocellular carcinoma after invasive therapies based on real-world data. J. Clin. Lab. Anal. 2021, 35, e23932. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Liang, B.Y.; Xiong, M.; Zhan, D.Q.; Wei, S.; Wang, G.P.; Chen, Y.-F.; Chen, X.-P. Long-term outcomes of repeat hepatic resection in participants with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: A single-center experience in China. Ann. Surg. Oncol. 2012, 19, 2515–2525. [Google Scholar] [CrossRef]
- Zhou, Y.; Sui, C.; Li, B.; Yin, Z.; Tan, Y.; Yang, J. Repeat hepatectomy for recurrent hepatocellular carcinoma: A local experience and a systematic review. World J. Surg. Oncol. 2010, 8, 55. [Google Scholar] [CrossRef]
- Merchante, N.; Rodríguez-Fernández, M.; Figueruela, B.; Rodríguez-Arrondo, F.; Revollo, B.; Ibarra, S.; Téllez, F.; Merino, E.; Montero-Alonso, M.; Galindo, M.; et al. Impact of HIV on the survival of hepatocellular carcinoma in hepatitis C virus-infected participants. AIDS 2020, 34, 1497–1507. [Google Scholar] [CrossRef]
- Puoti, M.; Bruno, R.; Soriano, V.; Donato, F.; Gaeta, G.B.; Quinzan, G.P.; Precone, D.; Gelatti, U.; Asensi, V.; Vaccher, E.; et al. Hepatocellular carcinoma in HIV-infected participants: Epidemiological features, clinical presentation and outcome. AIDS 2004, 18, 2285–2293. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Li, S.; Wang, Y.; Zhou, J.; Liu, L.; Wang, C. Comparative analysis of presentation and outcome after liver resection of participants with hepatocellular carcinoma with and without HIV. J. Acquir. Immune Defic. Syndr. 2021, 86, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Agüero, F.; Forner, A.; Manzardo, C.; Valdivieso, A.; Blanes, M.; Barcena, R.; Rafecas, A.; Castells, L.; Abradelo, M.; Torre-Cisneros, J.; et al. Human immunodeficiency virus infection does not worsen prognosis of liver transplantation for hepatocellular carcinoma. Hepatology 2015, 63, 488–498. [Google Scholar] [CrossRef]
- Eman, P.; Chacon, E.; Gupta, M.; Berger, J.C.; Shah, M.B.; El Haddad, H.E.; El-Husseini, A.; Cruz, A.D.; Grigorian, A.; Mei, X.; et al. Long term outcomes of participants transplanted for hepatocellular carcinoma with human immunodeficiency virus infection. HPB 2019, 21, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.G.; Kottilil, S.; Terrault, N.; Amara, D.; Husson, J.; Huprikar, S.; Florman, S.; Sulkowski, M.S.; Durand, C.M.; Luetkemeyer, A.F.; et al. Retrospective-prospective study of safety and efficacy of sofosbuvir-based direct-acting antivirals in HIV/HCV-coinfected participants with decompensated liver disease pre– or post–liver transplant. Am. J. Transplant. 2021, 21, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, G.P.; Berretta, M.; Guaraldi, G.; Magistri, P.; Esposito, G.; Ballarin, R.; Serra, V.; Di Sandro, S.; Di Benedetto, F. Liver transplantation for HCC in HIV-infected participants: Long-term single-center experience. Cancers 2021, 13, 4727. [Google Scholar] [CrossRef] [PubMed]

| Variable | Overall (Number = 41) | Died (Number = 22) | Alive (Number = 19) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 53 (49–56) | 53 (49–56) | 54 (50–58) | 0.267 | |
| Sex, male | 34 (83) | 16 (72) | 18 (95) | 0.010 | |
| Years of HIV infection | 22.75 (12.67–26.41) | 21.02 (10.11–25.34) | 22.75 (16.22–27.08) | 0.278 | |
| Years since first ART | 11.95 (7.29–16.84) | 10.64 (5.11–15.9) | 14.95 (8.78–19.39) | 0.206 | |
| Number of nodules > 3 | 3 (7) | 3 (14) | 0 (0) | 0.010 | |
| Cancer embolus in portal vein | No | 29 (70.7) | 15 (68.2) | 14 (73.7) | 0.744 |
| Yes | 12 (29.3) | 7 (31.8) | 5 (26.3) | ||
| Extra-hepatic spread | No | 37 (90.2) | 19 (86.4) | 18 (94.7) | 0.610 |
| Yes | 4 (9.8) | 3 (13.6) | 1 (5.3) | ||
| Child Pugh Turcotte | A | 33 (80.5) | 17 (77.3) | 16 (84.2) | 0.563 |
| B | 7 (17.1) | 4 (18.2) | 3 (15.8) | ||
| C | 1 (2.4) | 1 (4.5) | 0 | ||
| BCLC | 0/A | 20 (48.8) | 7 (31.8) | 13 (68.4) | 0.063 |
| B | 8 (19.5) | 6 (27.3) | 2 (10.5) | ||
| C/D | 13 (31.7) | 9 (40.9) | 4 (21.1) | ||
| Treatment | 0.0038 | ||||
| OLT | 11 (26.8) | 1 (4.5) | 10 (52.6) | ||
| LR | 6 (14.6) | 4 (18.2) | 2 (10.5) | ||
| RFTA | 6 (14.6) | 3 (13.6) | 3 (15.8) | ||
| CRE | 18 (43.9) | 14 (63.6) | 4 (21.0) | ||
| AFP, ng/mL | 27.8 (8.7–135.2) | 41.4 (14.6–347.7) | 14.4 (7.3–23.95) | 0.015 | |
| AFP, ng/mL | <28.8 | 19 (51.4) | 6 (28.6) | 13 (81.3) | 0.002 |
| ≥28.8 | 18 (48.6) | 15 (71.4) | 3 (18.8) | ||
| AST, U/L | 70 (34–100) | 71 (38–100) | 45 (31–158) | 0.855 | |
| ALT, U/L | 67 (33–99) | 64 (32–99) | 68 (35–104) | 0.466 | |
| Bilirubin, mg/dL | 1 (0.78–1.75) | 1 (0.78–1.52) | 1 (0.78–1.75) | 0.824 | |
| Albumin (g/L) | 39.9 (37.26–41.11) | 39.9 (37.26–41.11) | 40.31 (33.78–43.03) | 1.000 | |
| CD4 cells count, mmc | 433 (262–722) | 333.5 (246–722) | 530 (345–764) | 0.218 | |
| CD8 cells count, mmc | 732 (445–1209) | 589 (307–757) | 1021 (621–1392) | 0.02 | |
| CD4/CD8 ratio | 0.68 (0.34–0.98) | 0.71 (0.35–1.11) | 0.42 (0.31–0.94) | 0.264 | |
| Neutrophils, cells 109/L | 2.85 (2.4–3.4) | 2.6 (1.8–3.2) | 3.3 (2.4–4.4) | 0.082 | |
| Lymphocytes, cells 109/L | 1.7 (1.2–2.6) | 1.5 (1–2.2) | 2.3 (1.6–2.6) | 0.05 | |
| Neutrophils/lymphocytes, ratio | 1.58 (1.12–2.14) | 1.36 (1.06–2.36) | 1.59 (1.28–2.11) | 0.521 | |
| Platelets count, 109/L | 107 (64–141) | 100 (64–123) | 110.5 (63–154) | 0.325 | |
| PTs/INR | 1.06 (1.03–1.28) | 1.06 (0.99–1.37) | 1.08 (1.05–1.21) | 0.953 | |
| Creatinine, mg/dL | 0.74 (0.68–0.86) | 0.72 (0.66–0.88) | 0.75 (0.72–0.86) | 0.444 | |
| Anti-HCV positive | 32 (78) | 17 (77.3) | 15 (78.9) | 0.976 | |
| HBsAg positive | 8 (19.5) | 4 (18.2) | 4 (21.1) | 0.883 | |
| HIV-RNA > 50 copies/mL | 4 (9.8) | 2 (9.1) | 2 (10.5) | 0.560 | |
| Unknown | 14 (34.1) | 6 (27.3) | 8 (42.1) |
| Variable | Overall (Number = 41) | Died (Number = 22) | Alive (Number = 19) | p-Value | |
|---|---|---|---|---|---|
| AFP, ng/mL | 22.6 (7.7–216.2) | 46.2 (8.9–889.9) | 13.7 (6.9–27.9) | 0.060 | |
| AFP, ng/mL | <28.8 | 20 (57.1) | 8 (40) | 12 (80) | 0.036 |
| ≥28.8 | 15 (42.9) | 12 (60) | 3 (20) | ||
| AST, U/L | 58.5 (32.5–130.5) | 80 (41–170) | 37.5 (27–71) | 0.030 | |
| ALT, U/L | 43 (28–96) | 49 (31–96) | 32 (22–83) | 0.167 | |
| Bilirubin, mg/dL | 0.9 (0.57–2.07) | 1.74 (0.8–2.97) | 0.74 (0.47–0.9) | 0.005 | |
| Albumin, g/L | 40.85 (36.55–44.85) | 38.6 (35.8–45) | 42.55 (40.5–44.7) | 0.175 | |
| CD4 cells count, mmc | 368 (250–561) | 305 (136–491) | 400 (348–623) | 0.035 | |
| CD4+/CD8 ratio | 0.65 (0.38–1.01) | 0.7 (0.38–1.15) | 0.57 (0.37–0.96) | 0.392 | |
| CD8 cells count, mmc | 552 (379.5–915) | 384.5 (272–562) | 873 (513–1313) | 0.0007 | |
| Neutrophils, 109/L | 3.8 (2.6–4.7) | 3.3 (2.3–5.4) | 3.9 (2.9–4.6) | 0.570 | |
| Lymphocytes, 109/L | 1.35 (0.9–2.1) | 0.95 (0.7–1.6) | 1.95 (1.2–2.5) | 0.005 | |
| Neutrophils/lymphocytes | 2.46 (1.43–4.53) | 3.5 (1.43–8.67) | 2.06 (1.48–2.7) | 0.068 | |
| Platelets, 109/L | 129.5 (83–189.5) | 102.5 (66–165) | 151.5 (124–204) | 0.073 | |
| Creatinine, mg/dL | 0.95 (0.78–1.2) | 0.82 (0.76–0.98) | 1.13 (0.94–1.44) | 0.005 | |
| PTs/INR | 1.07 (1–1.15) | 1.15 (1.05–1.19) | 1.02 (0.99–1.08) | 0.008 | |
| HIV-RNA, copies/mL | 377 (126–19621) | 377 (140–12170) | 13591 (111–27071) | 0.999 | |
| HIV-RNA, ≥50 copies/mL | 31 (75.6) | 15 (68.2) | 16 (84.2) | 0.402 | |
| Unknown | 2 (4.9) | 1 (4.5) | 1 (5.3) |
| Variable | Overall (Number = 41) | Died (Number = 22) | Alive (Number = 19) | p-Value | |
|---|---|---|---|---|---|
| CHILD Pugh Turcotte | 0.563 | ||||
| A | 33 (80.5) | 17 (77.3) | 16 (84.2) | ||
| B | 7 (17.1) | 4 (18.2) | 3 (15.8) | ||
| C | 1 (2.4) | 1 (4.5) | 0 (0) | ||
| BCLC | 0.063 | ||||
| 0/A | 20 (48.8) | 7 (31.8) | 13 (68.4) | ||
| B | 8 (19.5) | 6 (27.3) | 2 (10.5) | ||
| C/D | 13 (31.7) | 9 (40.9) | 4 (21.1) | ||
| AFP, ng/mL | 13.2 (2.7–333.2) | 211 (20–3066′) | 2.55 (2–6.0) | <0.0001 | |
| AFP, ng/mL | 0.0002 | ||||
| <28.8 | 24 (61.5%) | 7 (33.3) | 17 (94.4) | ||
| ≥28.8 | 15 (38.5%) | 14 (66.7) | 1 (5.6) | ||
| Albumin, g/L | 36.69 (31.51–40.26) | 33.1 (28.52–38.52) | 40.27 (35.54–42.2) | 0.002 | |
| AST, IU/L | 58 (32–158) | 126 (90–208) | 34 (25–48) | 0.0001 | |
| ALT, IU/L | 43 (25–80) | 63 (35–99) | 28 (20–47) | 0.008 | |
| Bilirubin, mg/dL | 1.05 (0.67–1.94) | 1.04 (0.53–1.78) | 1.28 (0.7–2.09) | 0.480 | |
| CD4 cell count, mmc | 343 (215–497) | 217.5 (143–341) | 433 (348–614) | 0.0004 | |
| CD4/CD8 | 0.7 (0.41–0.89) | 0.64 (0.42–0.89) | 0.72 (0.37–0.96) | 0.855 | |
| CD8 cell count, mmc | 546 (329–852) | 383 (236–582) | 848 (538–1139) | 0.0005 | |
| Lymphocytes, 109/L | 1.5 (0.9–2) | 0.9 (0.8–1.6) | 1.9 (1.2–2.2) | 0.002 | |
| Neutrophils, 109/L | 3.9 (2.9–5.4) | 4.2 (2.3–6.8) | 3.75 (3.1–4.1) | 0.468 | |
| Neutrophils/lymphocytes | 2.67 (2–4.2) | 4.2 (2.67–5.53) | 2.03 (1.59–2.91) | 0.001 | |
| Platelets, 109/L | 151 (100–248) | 110.5 (72–259) | 170 (142–240) | 0.187 | |
| Creatinine, mg/dL | 1.02 (0.83–1.38) | 0.93 (0.78–1.08) | 1.14 (1.02–1.57) | 0.023 | |
| PTs/INR | 1.1 (1.03–1.19) | 1.1 (1–1.22) | 1.1 (1.03–1.18) | 0.915 | |
| HIV-RNA, copies/mL | 88 (64–12170) | 65 (63–6118) | 13591 (111–27071) | 0.247 | |
| HIV-RNA, ≥50 copies/mL | 6 (14.6%) | 4 (18.2) | 2 (10.5%) | 0.489 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoni, C.; Galli, L.; Lolatto, R.; Hasson, H.; Siribelli, A.; Messina, E.; Castagna, A.; Uberti Foppa, C.; Morsica, G. Survival in People Living with HIV with or without Recurrence of Hepatocellular Carcinoma after Invasive Therapy. Cancers 2023, 15, 1653. https://doi.org/10.3390/cancers15061653
Bertoni C, Galli L, Lolatto R, Hasson H, Siribelli A, Messina E, Castagna A, Uberti Foppa C, Morsica G. Survival in People Living with HIV with or without Recurrence of Hepatocellular Carcinoma after Invasive Therapy. Cancers. 2023; 15(6):1653. https://doi.org/10.3390/cancers15061653
Chicago/Turabian StyleBertoni, Costanza, Laura Galli, Riccardo Lolatto, Hamid Hasson, Alessia Siribelli, Emanuela Messina, Antonella Castagna, Caterina Uberti Foppa, and Giulia Morsica. 2023. "Survival in People Living with HIV with or without Recurrence of Hepatocellular Carcinoma after Invasive Therapy" Cancers 15, no. 6: 1653. https://doi.org/10.3390/cancers15061653
APA StyleBertoni, C., Galli, L., Lolatto, R., Hasson, H., Siribelli, A., Messina, E., Castagna, A., Uberti Foppa, C., & Morsica, G. (2023). Survival in People Living with HIV with or without Recurrence of Hepatocellular Carcinoma after Invasive Therapy. Cancers, 15(6), 1653. https://doi.org/10.3390/cancers15061653






