Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy
Abstract
Simple Summary
Abstract
1. Introduction
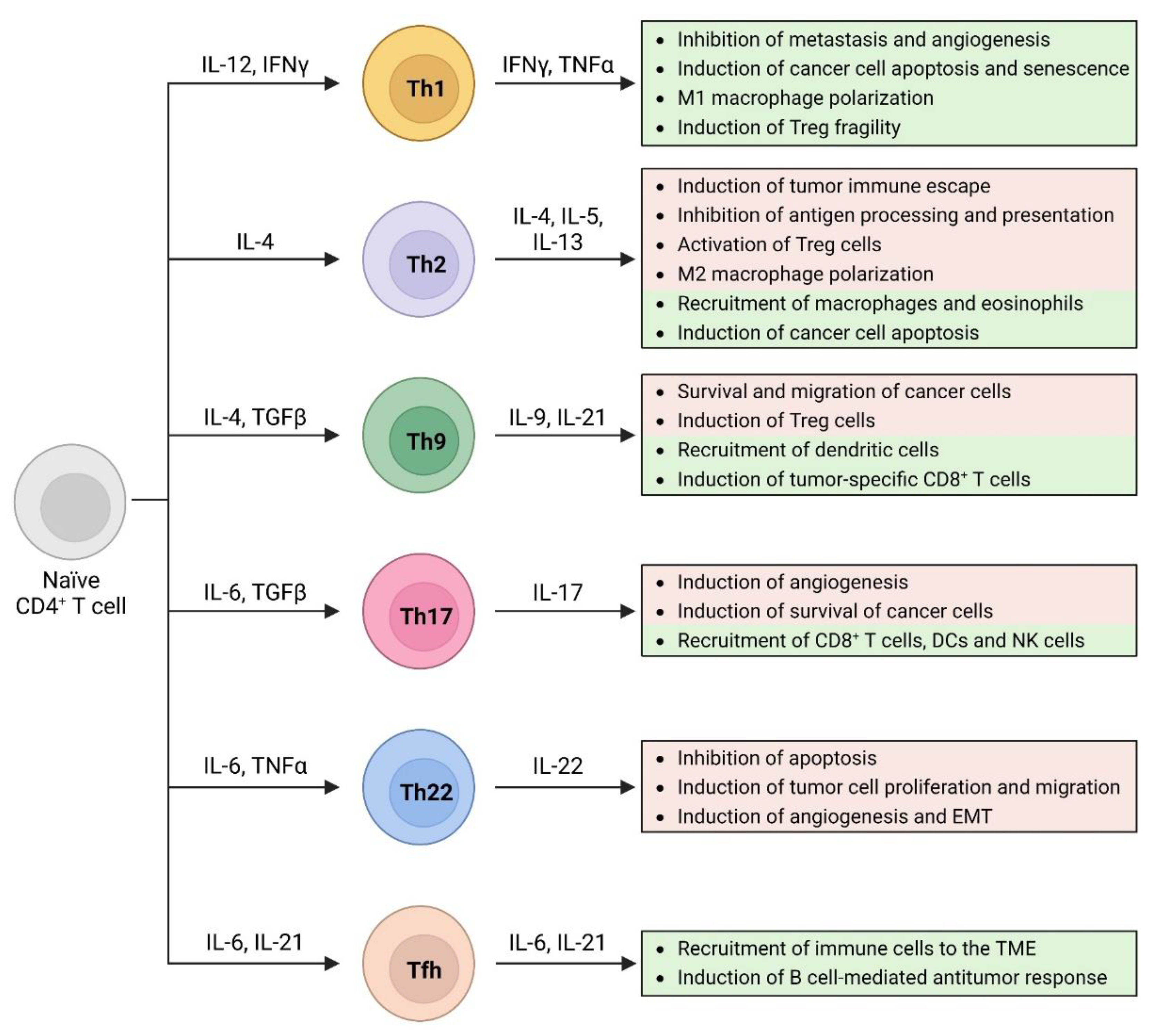
2. Th1 Cells
2.1. Th1 Cells and Cancer
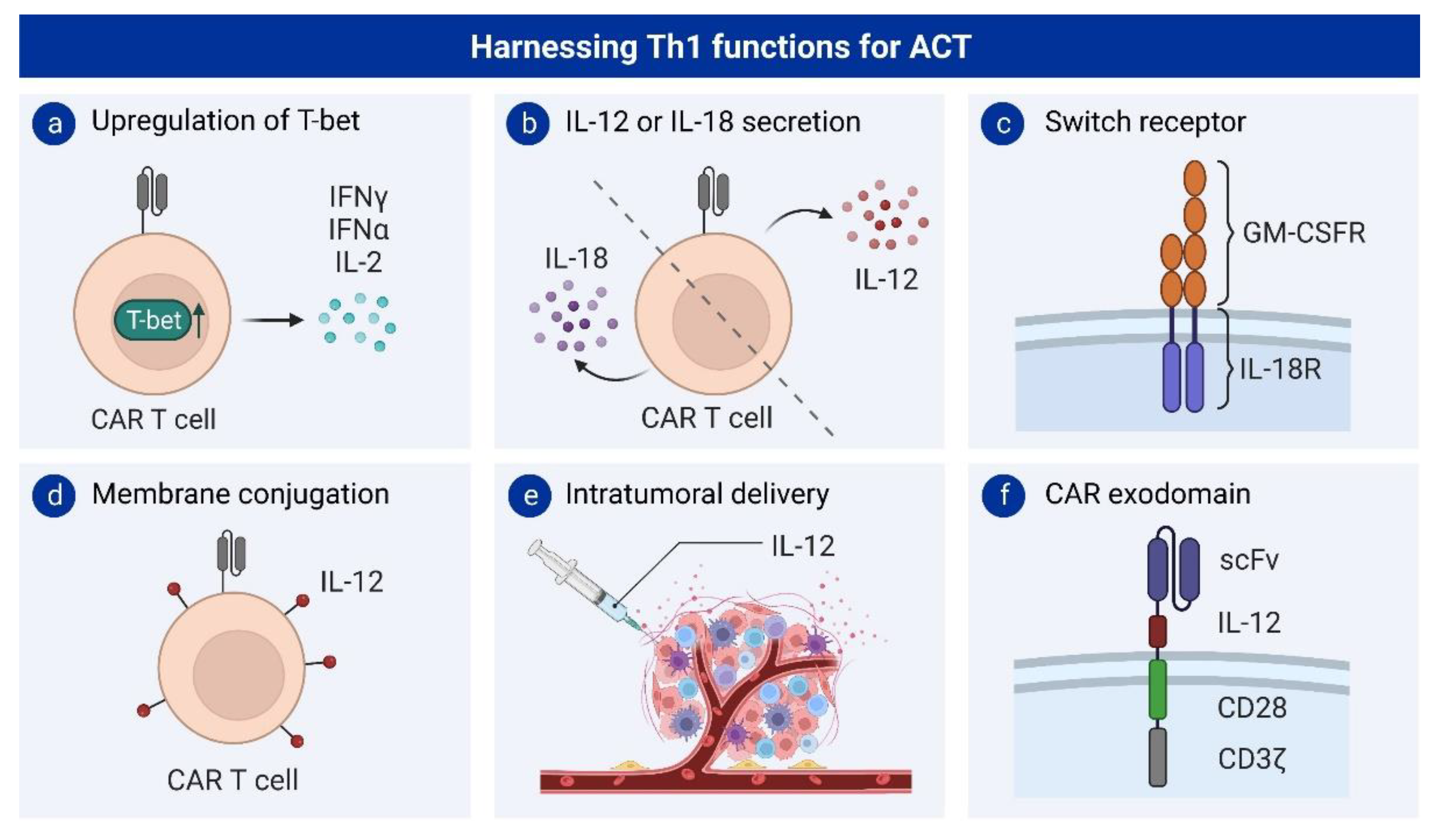
2.2. Th1 Cells and Adoptive T Cell Therapy
2.2.1. Interleukin 12 Engineering to Induce Th1 Differentiation
2.2.2. Interleukin 18 Engineering to Induce Th1 Differentiation
3. Th2 Cells
3.1. Th2 Cells and Cancer
3.2. Th2 Cells and Adoptive T Cell Therapy
4. Th9 Cells
4.1. Th9 Cells and Cancer
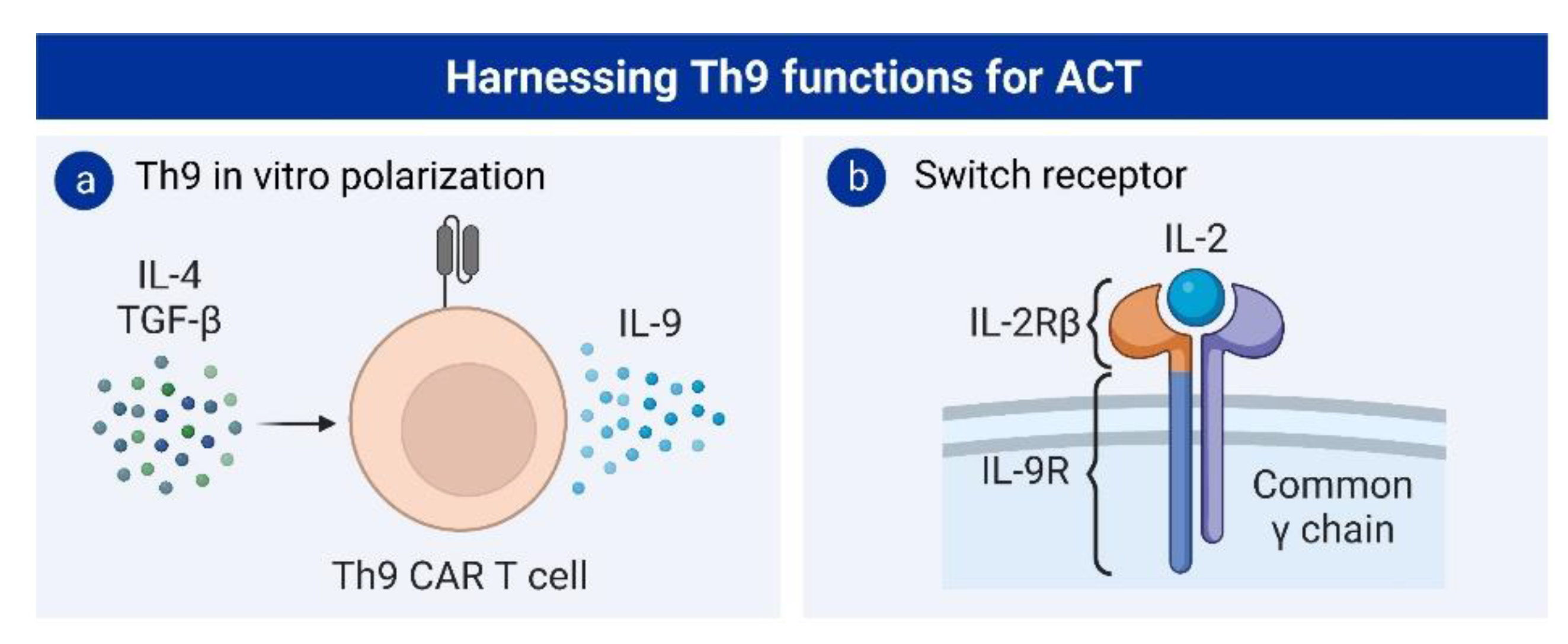
4.2. Th9 Cells and Adoptive T-Cell Therapy
5. Th17 Cells
5.1. Th17 Cells and Cancer
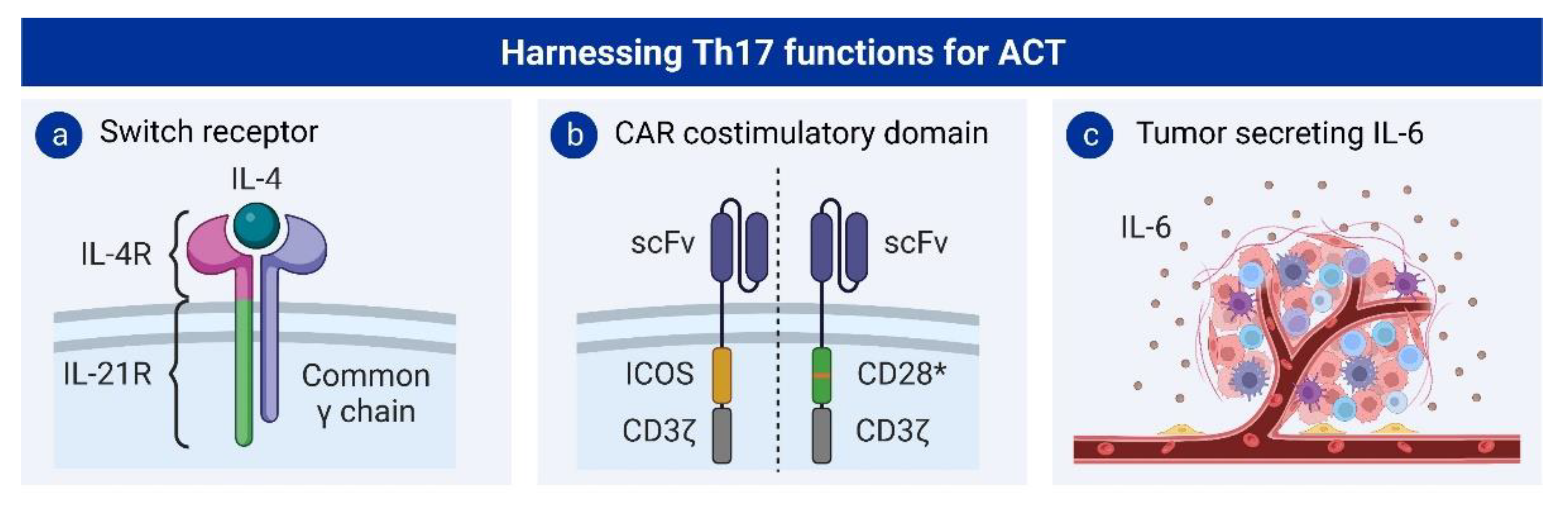
5.2. Th17 Cells and Adoptive T Cell Therapy
6. Th22 Cells
Th22 Cells and Cancer
7. T Follicular Helper Cells
Tfh Cells and Cancer
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive cellular therapy in solid tumor malignancies: Review of the literature and challenges ahead. J. Immunother. Cancer 2021, 9, e002723. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [PubMed]
- Alnefaie, A.; Albogami, S.; Asiri, Y.; Ahmad, T.; Alotaibi, S.S.; Al-Sanea, M.M.; Althobaiti, H. Chimeric Antigen Receptor T-Cells: An Overview of Concepts, Applications, Limitations, and Proposed Solutions. Front. Bioeng. Biotechnol. 2022, 10, 797440. [Google Scholar] [CrossRef]
- Shafer, P.; Kelly, L.M.; Hoyos, V. Cancer Therapy With TCR-Engineered T Cells: Current Strategies, Challenges, and Prospects. Front. Immunol. 2022, 13, 835762. [Google Scholar] [CrossRef]
- Ostroumov, D.; Fekete-Drimusz, N.; Saborowski, M.; Kuhnel, F.; Woller, N. CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 2018, 75, 689–713. [Google Scholar] [CrossRef]
- Kennedy, R.; Celis, E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 2008, 222, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Babala, N.; Melief, C.J.M.; Kastenmuller, W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Schattner, E.J.; Mascarenhas, J.; Bishop, J.; Yoo, D.H.; Chadburn, A.; Crow, M.K.; Friedman, S.M. CD4+ T-cell induction of Fas-mediated apoptosis in Burkitt’s lymphoma B cells. Blood 1996, 88, 1375–1382. [Google Scholar] [CrossRef]
- Thomas, W.D.; Hersey, P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J. Immunol. 1998, 161, 2195–2200. [Google Scholar] [CrossRef]
- Schoenberger, S.P.; Toes, R.E.; van der Voort, E.I.; Offringa, R.; Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998, 393, 480–483. [Google Scholar] [CrossRef]
- Kennedy, R.; Celis, E. T helper lymphocytes rescue CTL from activation-induced cell death. J. Immunol. 2006, 177, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- DuPage, M.; Bluestone, J.A. Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease. Nat. Rev. Immunol. 2016, 16, 149–163. [Google Scholar] [CrossRef]
- Scott, P. IL-12: Initiation cytokine for cell-mediated immunity. Science 1993, 260, 496–497. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef]
- Nakanishi, K. Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front. Immunol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Tsutsui, H.; Yoshimoto, T.; Adachi, O.; Yoshida, N.; Kishimoto, T.; Okamura, H.; Nakanishi, K.; Akira, S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 1998, 8, 383–390. [Google Scholar] [CrossRef]
- Magram, J.; Connaughton, S.E.; Warrier, R.R.; Carvajal, D.M.; Wu, C.Y.; Ferrante, J.; Stewart, C.; Sarmiento, U.; Faherty, D.A.; Gately, M.K. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 1996, 4, 471–481. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a Heterodimeric Cytokine Composed of EBI3 and p28 Protein, Induces Proliferation of Naive CD4+ T Cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010, 238, 247–262. [Google Scholar] [CrossRef]
- Bradley, L.M.; Dalton, D.K.; Croft, M. A direct role for IFN-gamma in regulation of Th1 cell development. J. Immunol. 1996, 157, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pages, F.; Sautes-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-gamma in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Karachaliou, N.; Gonzalez-Cao, M.; Crespo, G.; Drozdowskyj, A.; Aldeguer, E.; Gimenez-Capitan, A.; Teixido, C.; Molina-Vila, M.A.; Viteri, S.; De Los Llanos Gil, M.; et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther. Adv. Med. Oncol. 2018, 10, 1758834017749748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, C.; Zhang, Q.; Ye, J.; Wang, F.; Zhang, Y.; Hunborg, P.; Varvares, M.A.; Hoft, D.F.; Hsueh, E.C.; et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015, 6, 17462–17478. [Google Scholar] [CrossRef]
- Hoepner, S.; Loh, J.M.; Riccadonna, C.; Derouazi, M.; Maroun, C.Y.; Dietrich, P.Y.; Walker, P.R. Synergy between CD8 T cells and Th1 or Th2 polarised CD4 T cells for adoptive immunotherapy of brain tumours. PLoS ONE 2013, 8, e63933. [Google Scholar] [CrossRef]
- Slattery, M.L.; Lundgreen, A.; Bondurant, K.L.; Wolff, R.K. Interferon-signaling pathway: Associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis 2011, 32, 1660–1667. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Su, Q.; Huang, H.; Zhou, P.; Luan, J.; Liu, J.; Wang, J.; Chen, X. Expression of Th1- Th2- and Th17-associated cytokines in laryngeal carcinoma. Oncol. Lett. 2016, 12, 1941–1948. [Google Scholar] [CrossRef]
- Wang, D.; Aguilar, B.; Starr, R.; Alizadeh, D.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; Forman, S.J.; Brown, C.E. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight 2018, 3, e99048. [Google Scholar] [CrossRef]
- Dillard, P.; Koksal, H.; Maggadottir, S.M.; Winge-Main, A.; Pollmann, S.; Menard, M.; Myhre, M.R.; Maelandsmo, G.M.; Florenes, V.A.; Gaudernack, G.; et al. Targeting Telomerase with an HLA Class II-Restricted TCR for Cancer Immunotherapy. Mol. Ther. 2021, 29, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Gacerez, A.T.; Sentman, C.L. T-bet promotes potent antitumor activity of CD4(+) CAR T cells. Cancer Gene Ther. 2018, 25, 117–128. [Google Scholar] [CrossRef]
- Kueberuwa, G.; Kalaitsidou, M.; Cheadle, E.; Hawkins, R.E.; Gilham, D.E. CD19 CAR T Cells Expressing IL-12 Eradicate Lymphoma in Fully Lymphoreplete Mice through Induction of Host Immunity. Mol. Ther. Oncolytics 2018, 8, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Adu-Berchie, K.; Brockman, J.M.; Pezone, M.; Zhang, D.K.Y.; Zhou, J.; Pyrdol, J.W.; Wang, H.; Wucherpfennig, K.W.; Mooney, D.J. Cytokine conjugation to enhance T cell therapy. Proc. Natl. Acad. Sci. USA 2023, 120, e2213222120. [Google Scholar] [CrossRef]
- Agliardi, G.; Liuzzi, A.R.; Hotblack, A.; De Feo, D.; Nunez, N.; Stowe, C.L.; Friebel, E.; Nannini, F.; Rindlisbacher, L.; Roberts, T.A.; et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat. Commun. 2021, 12, 444. [Google Scholar] [CrossRef]
- Koneru, M.; Purdon, T.J.; Spriggs, D.; Koneru, S.; Brentjens, R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumor in vivo. OncoImmunology 2015, 4, e994446. [Google Scholar] [CrossRef]
- You, F.; Jiang, L.; Zhang, B.; Lu, Q.; Zhou, Q.; Liao, X.; Wu, H.; Du, K.; Zhu, Y.; Meng, H.; et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified Anti-MUC1 chimeric antigen receptor transduced T cells. Sci. China Life Sci. 2016, 59, 386–397. [Google Scholar] [CrossRef]
- Koneru, M.; O’Cearbhaill, R.; Pendharkar, S.; Spriggs, D.R.; Brentjens, R.J. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J. Transl. Med. 2015, 13, 102. [Google Scholar] [CrossRef]
- Chinnasamy, D.; Yu, Z.; Kerkar, S.P.; Zhang, L.; Morgan, R.A.; Restifo, N.P.; Rosenberg, S.A. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 2012, 18, 1672–1683. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Grote, D.M.; Ziesmer, S.C.; Niki, T.; Hirashima, M.; Novak, A.J.; Witzig, T.E.; Ansell, S.M. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J. Clin. Investig. 2012, 122, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Di, S.; Shi, B.; Zhang, H.; Wang, Y.; Wu, X.; Luo, H.; Wang, H.; Li, Z.; Jiang, H. Armored Inducible Expression of IL-12 Enhances Antitumor Activity of Glypican-3-Targeted Chimeric Antigen Receptor-Engineered T Cells in Hepatocellular Carcinoma. J. Immunol. 2019, 203, 198–207. [Google Scholar] [CrossRef]
- Zhang, L.; Kerkar, S.P.; Yu, Z.; Zheng, Z.; Yang, S.; Restifo, N.P.; Rosenberg, S.A.; Morgan, R.A. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol. Ther. 2011, 19, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morgan, R.A.; Beane, J.D.; Zheng, Z.; Dudley, M.E.; Kassim, S.H.; Nahvi, A.V.; Ngo, L.T.; Sherry, R.M.; Phan, G.Q.; et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 2015, 21, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Davies, J.S.; Serna, C.; Yu, Z.; Restifo, N.P.; Rosenberg, S.A.; Morgan, R.A.; Hinrichs, C.S. Enhanced efficacy and limited systemic cytokine exposure with membrane-anchored interleukin-12 T-cell therapy in murine tumor models. J. Immunother. Cancer 2020, 8, e000210. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Q.; Zhang, W.; Du, H.; Chen, Y.; Zhao, Q.; Dao, L.; Xia, X.; Natalie Wall, F.; Zhang, Z.; et al. Cell membrane-anchored and tumor-targeted IL-12 (attIL12)-T cell therapy for eliminating large and heterogeneous solid tumors. J. Immunother. Cancer 2022, 10, e003633. [Google Scholar] [CrossRef] [PubMed]
- Hombach, A.; Barden, M.; Hannappel, L.; Chmielewski, M.; Rappl, G.; Sachinidis, A.; Abken, H. IL12 integrated into the CAR exodomain converts CD8(+) T cells to poly-functional NK-like cells with superior killing of antigen-loss tumors. Mol. Ther. 2022, 30, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, Z.; Sun, M.; Li, B.; Pan, F.; Ma, A.; Liao, J.; Yin, T.; Tang, X.; Huang, G.; et al. IL-12 nanochaperone-engineered CAR T cell for robust tumor-immunotherapy. Biomaterials 2022, 281, 121341. [Google Scholar] [CrossRef]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep. 2017, 20, 3025–3033. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 2017, 21, 3205–3219. [Google Scholar] [CrossRef]
- Avanzi, M.P.; Yeku, O.; Li, X.; Wijewarnasuriya, D.P.; van Leeuwen, D.G.; Cheung, K.; Park, H.; Purdon, T.J.; Daniyan, A.F.; Spitzer, M.H.; et al. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep. 2018, 23, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.; Kuehle, J.; Dragon, A.C.; Galla, M.; Kloth, C.; Rudek, L.S.; Sandalcioglu, I.E.; Neyazi, B.; Moritz, T.; Meyer, J.; et al. Design and Characterization of an “All-in-One” Lentiviral Vector System Combining Constitutive Anti-G(D2) CAR Expression and Inducible Cytokines. Cancers 2020, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Sand, L.G.L.; Bell, M.; Patil, S.L.; Langfitt, D.; Gottschalk, S. A Chimeric GM-CSF/IL18 Receptor to Sustain CAR T-cell Function. Cancer Discov. 2021, 11, 1661–1671. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Millward, M.; Mainwaring, P.; Kefford, R.; Logan, T.; Pavlick, A.; Kathman, S.J.; Laubscher, K.H.; Dar, M.M.; Kirkwood, J.M. A phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer 2009, 115, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Damsky, W.; Weizman, O.-E.; Mcgeary, M.K.; Hartmann, K.P.; Rosen, C.E.; Fischer, S.; Jackson, R.; Flavell, R.A.; Wang, J.; et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 2020, 583, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Detry, S.; Andries, J.; Bloch, Y.; Gabay, C.; Clancy, D.M.; Savvides, S.N. Structural basis of human IL-18 sequestration by the decoy receptor IL-18 binding protein in inflammation and tumor immunity. J. Biol. Chem. 2022, 298, 101908. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E.; Zhu, J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]
- Maier, E.; Duschl, A.; Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 2012, 42, 2827–2833. [Google Scholar] [CrossRef]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef]
- Zhu, J. Transcriptional regulation of Th2 cell differentiation. Immunol. Cell Biol. 2010, 88, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ellyard, J.I.; Simson, L.; Parish, C.R. Th2-mediated anti-tumour immunity: Friend or foe? Tissue Antigens 2007, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ubukata, H.; Motohashi, G.; Tabuchi, T.; Nagata, H.; Konishi, S.; Tabuchi, T. Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J. Surg. Oncol. 2010, 102, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, T.; Shigemasa, K.; Arihiro, K.; Fujii, T.; Nagai, N.; Ohama, K. Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncol. Rep. 2005, 13, 1153–1158. [Google Scholar] [CrossRef]
- Sheu, B.C.; Lin, R.H.; Lien, H.C.; Ho, H.N.; Hsu, S.M.; Huang, S.C. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J. Immunol. 2001, 167, 2972–2978. [Google Scholar] [CrossRef] [PubMed]
- Nevala, W.K.; Vachon, C.M.; Leontovich, A.A.; Scott, C.G.; Thompson, M.A.; Markovic, S.N.; Melanoma Study Group of the Mayo Clinic Cancer, C. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin. Cancer Res. 2009, 15, 1931–1939. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Schreck, S.; Friebel, D.; Buettner, M.; Distel, L.; Grabenbauer, G.; Young, L.S.; Niedobitek, G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol. Oncol. 2009, 27, 31–39. [Google Scholar] [CrossRef]
- Palma, M.; Gentilcore, G.; Heimersson, K.; Mozaffari, F.; Nasman-Glaser, B.; Young, E.; Rosenquist, R.; Hansson, L.; Osterborg, A.; Mellstedt, H. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica 2017, 102, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.K.; Maresh, E.L.; Shen, D.; Elshimali, Y.; Apple, S.; Horvath, S.; Mah, V.; Bose, S.; Chia, D.; Chang, H.R.; et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum. Pathol. 2010, 41, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Tepper, R.I.; Coffman, R.L.; Leder, P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 1992, 257, 548–551. [Google Scholar] [CrossRef]
- Gooch, J.L.; Christy, B.; Yee, D. STAT6 mediates interleukin-4 growth inhibition in human breast cancer cells. Neoplasia 2002, 4, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Toi, M. Interleukin-4 and breast cancer. Breast Cancer 2000, 7, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Iwakabe, K.; Sekimoto, M.; Ohmi, Y.; Yahata, T.; Nakui, M.; Sato, T.; Habu, S.; Tashiro, H.; Sato, M.; et al. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J. Exp. Med. 1999, 190, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [CrossRef]
- Lorvik, K.B.; Hammarstrom, C.; Fauskanger, M.; Haabeth, O.A.; Zangani, M.; Haraldsen, G.; Bogen, B.; Corthay, A. Adoptive Transfer of Tumor-Specific Th2 Cells Eradicates Tumors by Triggering an In Situ Inflammatory Immune Response. Cancer Res. 2016, 76, 6864–6876. [Google Scholar] [CrossRef]
- Jacenik, D.; Karagiannidis, I.; Beswick, E.J. Th2 cells inhibit growth of colon and pancreas cancers by promoting anti-tumorigenic responses from macrophages and eosinophils. Br. J. Cancer 2022, 128, 387–397. [Google Scholar] [CrossRef]
- Golumbek, P.T.; Lazenby, A.J.; Levitsky, H.I.; Jaffee, L.M.; Karasuyama, H.; Baker, M.; Pardoll, D.M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science 1991, 254, 713–716. [Google Scholar] [CrossRef]
- Pippin, B.A.; Rosenstein, M.; Jacob, W.F.; Chiang, Y.; Lotze, M.T. Local IL-4 delivery enhances immune reactivity to murine tumors: Gene therapy in combination with IL-2. Cancer Gene Ther. 1994, 1, 35–42. [Google Scholar]
- Pericle, F.; Giovarelli, M.; Colombo, M.P.; Ferrari, G.; Musiani, P.; Modesti, A.; Cavallo, F.; Di Pierro, F.; Novelli, F.; Forni, G. An efficient Th2-type memory follows CD8+ lymphocyte-driven and eosinophil-mediated rejection of a spontaneous mouse mammary adenocarcinoma engineered to release IL-4. J. Immunol. 1994, 153, 5659–5673. [Google Scholar] [CrossRef] [PubMed]
- Stoppacciaro, A.; Paglia, P.; Lombardi, L.; Parmiani, G.; Baroni, C.; Colombo, M.P. Genetic modification of a carcinoma with the IL-4 gene increases the influx of dendritic cells relative to other cytokines. Eur. J. Immunol. 1997, 27, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Rodolfo, M.; Zilocchi, C.; Accornero, P.; Cappetti, B.; Arioli, I.; Colombo, M.P. IL-4-transduced tumor cell vaccine induces immunoregulatory type 2 CD8 T lymphocytes that cure lung metastases upon adoptive transfer. J. Immunol. 1999, 163, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Noffz, G.; Qin, Z.; Kopf, M.; Blankenstein, T. Neutrophils but not eosinophils are involved in growth suppression of IL-4-secreting tumors. J. Immunol. 1998, 160, 345–350. [Google Scholar] [CrossRef]
- Eguchi, J.; Kuwashima, N.; Hatano, M.; Nishimura, F.; Dusak, J.E.; Storkus, W.J.; Okada, H. IL-4-transfected tumor cell vaccines activate tumor-infiltrating dendritic cells and promote type-1 immunity. J. Immunol. 2005, 174, 7194–7201. [Google Scholar] [CrossRef]
- Modesti, A.; Masuelli, L.; Modica, A.; D’Orazi, G.; Scarpa, S.; Bosco, M.C.; Forni, G. Ultrastructural evidence of the mechanisms responsible for interleukin-4-activated rejection of a spontaneous murine adenocarcinoma. Int. J. Cancer 1993, 53, 988–993. [Google Scholar] [CrossRef]
- Atkins, M.B.; Vachino, G.; Tilg, H.J.; Karp, D.D.; Robert, N.J.; Kappler, K.; Mier, J.W. Phase I evaluation of thrice-daily intravenous bolus interleukin-4 in patients with refractory malignancy. J. Clin. Oncol. 1992, 10, 1802–1809. [Google Scholar] [CrossRef]
- Gilleece, M.H.; Scarffe, J.H.; Ghosh, A.; Heyworth, C.M.; Bonnem, E.; Testa, N.; Stern, P.; Dexter, T.M. Recombinant human interleukin 4 (IL-4) given as daily subcutaneous injections--a phase I dose toxicity trial. Br. J. Cancer 1992, 66, 204–210. [Google Scholar] [CrossRef]
- Stadler, W.M.; Rybak, M.E.; Vogelzang, N.J. A phase II study of subcutaneous recombinant human interleukin-4 in metastatic renal cell carcinoma. Cancer 1995, 76, 1629–1633. [Google Scholar] [CrossRef]
- Lebel-Binay, S.; Laguerre, B.; Quintin-Colonna, F.; Conjeaud, H.; Magazin, M.; Miloux, B.; Pecceu, F.; Caput, D.; Ferrara, P.; Fradelizi, D. Experimental gene therapy of cancer using tumor cells engineered to secrete interleukin-13. Eur. J. Immunol. 1995, 25, 2340–2348. [Google Scholar] [CrossRef]
- Ma, H.L.; Whitters, M.J.; Jacobson, B.A.; Donaldson, D.D.; Collins, M.; Dunussi-Joannopoulos, K. Tumor cells secreting IL-13 but not IL-13Ralpha2 fusion protein have reduced tumorigenicity in vivo. Int. Immunol. 2004, 16, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Matsui, S.; Noben-Trauth, N.; Chen, H.; Watson, C.; Donaldson, D.D.; Carbone, D.P.; Paul, W.E.; Berzofsky, J.A. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 2000, 1, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Q.; Zhang, Z.; Wang, J.; Li, S.; Zhang, J.; Liu, G. TH9 cell differentiation, transcriptional control and function in inflammation, autoimmune diseases and cancer. Oncotarget 2016, 7, 71001–71012. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Hufford, M.M.; Olson, M.R. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 2015, 15, 295–307. [Google Scholar] [CrossRef]
- Neurath, M.F.; Kaplan, M.H. Th9 cells in immunity and immunopathological diseases. Semin. Immunopathol. 2017, 39, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, J.; Cai, Z.; Li, B.; Sun, L.; Shen, Y.; Wang, S.; Wang, Z.; Wang, Z.; Wang, Y.; et al. Th9 Cell Differentiation and Its Dual Effects in Tumor Development. Front. Immunol. 2020, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Dardalhon, V.; Awasthi, A.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9 + IL-10 + Foxp3(-) effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef]
- Jash, A.; Sahoo, A.; Kim, G.C.; Chae, C.S.; Hwang, J.S.; Kim, J.E.; Im, S.H. Nuclear factor of activated T cells 1 (NFAT1)-induced permissive chromatin modification facilitates nuclear factor-kappaB (NF-kappaB)-mediated interleukin-9 (IL-9) transactivation. J. Biol. Chem. 2012, 287, 15445–15457. [Google Scholar] [CrossRef]
- Chang, H.C.; Sehra, S.; Goswami, R.; Yao, W.; Yu, Q.; Stritesky, G.L.; Jabeen, R.; McKinley, C.; Ahyi, A.N.; Han, L.; et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 2010, 11, 527–534. [Google Scholar] [CrossRef]
- Yashiro, T.; Kubo, M.; Ogawa, H.; Okumura, K.; Nishiyama, C. PU. 1 Suppresses Th2 Cytokine Expression via Silencing of GATA3 Transcription in Dendritic Cells. PLoS ONE 2015, 10, e0137699. [Google Scholar] [CrossRef]
- Wang, A.; Pan, D.; Lee, Y.H.; Martinez, G.J.; Feng, X.H.; Dong, C. Cutting edge: Smad2 and Smad4 regulate TGF-beta-mediated Il9 gene expression via EZH2 displacement. J. Immunol. 2013, 191, 4908–4912. [Google Scholar] [CrossRef] [PubMed]
- Staudt, V.; Bothur, E.; Klein, M.; Lingnau, K.; Reuter, S.; Grebe, N.; Gerlitzki, B.; Hoffmann, M.; Ulges, A.; Taube, C.; et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 2010, 33, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lu, K.; Li, P.; Lv, X.; Wang, X. Overexpression of IL-9 induced by STAT6 activation promotes the pathogenesis of chronic lymphocytic leukemia. Int. J. Clin. Exp. Pathol. 2014, 7, 2319–2323. [Google Scholar]
- Vink, A.; Renauld, J.C.; Warnier, G.; Van Snick, J. Interleukin-9 stimulates in vitro growth of mouse thymic lymphomas. Eur. J. Immunol. 1993, 23, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Lai, R.; Lin, Q.; Lau, E.; Thomazy, D.M.; Calame, D.; Ford, R.J.; Kwak, L.W.; Kirken, R.A.; Amin, H.M. Autocrine release of interleukin-9 promotes Jak3-dependent survival of ALK+ anaplastic large-cell lymphoma cells. Blood 2006, 108, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dhamija, B.; Marathe, S.; Ghosh, S.; Dwivedi, A.; Karulkar, A.; Sharma, N.; Sengar, M.; Sridhar, E.; Bonda, A.; et al. The Th9 Axis Reduces the Oxidative Stress and Promotes the Survival of Malignant T Cells in Cutaneous T-Cell Lymphoma Patients. Mol. Cancer Res. 2020, 18, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.L.; Gao, J.M.; Li, P.P.; Wang, X. IL-9 contributes to immunosuppression mediated by regulatory T cells and mast cells in B-cell non-hodgkin’s lymphoma. J. Clin. Immunol. 2011, 31, 1084–1094. [Google Scholar] [CrossRef]
- Eller, K.; Wolf, D.; Huber, J.M.; Metz, M.; Mayer, G.; McKenzie, A.N.; Maurer, M.; Rosenkranz, A.R.; Wolf, A.M. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J. Immunol. 2011, 186, 83–91. [Google Scholar] [CrossRef]
- Merz, H.; Houssiau, F.A.; Orscheschek, K.; Renauld, J.C.; Fliedner, A.; Herin, M.; Noel, H.; Kadin, M.; Mueller-Hermelink, H.K.; Van Snick, J.; et al. Interleukin-9 expression in human malignant lymphomas: Unique association with Hodgkin’s disease and large cell anaplastic lymphoma. Blood 1991, 78, 1311–1317. [Google Scholar] [CrossRef]
- Nagato, T.; Kobayashi, H.; Kishibe, K.; Takahara, M.; Ogino, T.; Ishii, H.; Oikawa, K.; Aoki, N.; Sato, K.; Kimura, S.; et al. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin. Cancer Res. 2005, 11, 8250–8257. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.; Xue, G.; Bi, E.; Ma, X.; Wang, A.; Qian, J.; Dong, C.; Yi, Q. Th9 Cells Represent a Unique Subset of CD4+ T Cells Endowed with the Ability to Eradicate Advanced Tumors. Cancer Cell 2018, 33, 1048–1060.e1047. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Hong, S.; Li, H.; Park, J.; Hong, B.; Wang, L.; Zheng, Y.; Liu, Z.; Xu, J.; He, J.; et al. Th9 cells promote antitumor immune responses in vivo. J. Clin. Investig. 2012, 122, 4160–4171. [Google Scholar] [CrossRef] [PubMed]
- Purwar, R.; Schlapbach, C.; Xiao, S.; Kang, H.S.; Elyaman, W.; Jiang, X.; Jetten, A.M.; Khoury, S.J.; Fuhlbrigge, R.C.; Kuchroo, V.K.; et al. Robust tumor immunity to melanoma mediated by interleukin-9–producing T cells. Nat. Med. 2012, 18, 1248–1253. [Google Scholar] [CrossRef]
- Vegran, F.; Berger, H.; Boidot, R.; Mignot, G.; Bruchard, M.; Dosset, M.; Chalmin, F.; Rebe, C.; Derangere, V.; Ryffel, B.; et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol. 2014, 15, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahid, A.; Cydzik, M.; Prodeus, A.; Alwash, M.; Stanojcic, M.; Thompson, M.; Huang, E.H.; Shively, J.E.; Gray-Owen, S.D.; Gariepy, J. Induction of antigen-specific TH 9 immunity accompanied by mast cell activation blocks tumor cell engraftment. Int. J. Cancer 2016, 139, 841–853. [Google Scholar] [CrossRef]
- Park, J.; Li, H.; Zhang, M.; Lu, Y.; Hong, B.; Zheng, Y.; He, J.; Yang, J.; Qian, J.; Yi, Q. Murine Th9 cells promote the survival of myeloid dendritic cells in cancer immunotherapy. Cancer Immunol. Immunother. 2014, 63, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Jin, G.; Fang, J.; Lu, Y. IL-4 together with IL-1beta induces antitumor Th9 cell differentiation in the absence of TGF-beta signaling. Nat. Commun. 2019, 10, 1376. [Google Scholar] [CrossRef]
- You, F.P.; Zhang, J.; Cui, T.; Zhu, R.; Lv, C.Q.; Tang, H.T.; Sun, D.W. Th9 cells promote antitumor immunity via IL-9 and IL-21 and demonstrate atypical cytokine expression in breast cancer. Int. Immunopharmacol. 2017, 52, 163–167. [Google Scholar] [CrossRef]
- Tan, H.; Wang, S.; Zhao, L. A tumour-promoting role of Th9 cells in hepatocellular carcinoma through CCL20 and STAT3 pathways. Clin. Exp. Pharmacol. Physiol. 2017, 44, 213–221. [Google Scholar] [CrossRef]
- Ye, Z.J.; Zhou, Q.; Yin, W.; Yuan, M.L.; Yang, W.B.; Xiong, X.Z.; Zhang, J.C.; Shi, H.Z. Differentiation and immune regulation of IL-9-producing CD4+ T cells in malignant pleural effusion. Am. J. Respir. Crit. Care Med. 2012, 186, 1168–1179. [Google Scholar] [CrossRef]
- Liu, L.; Bi, E.; Ma, X.; Xiong, W.; Qian, J.; Ye, L.; Su, P.; Wang, Q.; Xiao, L.; Yang, M.; et al. Enhanced CAR-T activity against established tumors by polarizing human T cells to secrete interleukin-9. Nat. Commun. 2020, 11, 5902. [Google Scholar] [CrossRef]
- Kalbasi, A.; Siurala, M.; Su, L.L.; Tariveranmoshabad, M.; Picton, L.K.; Ravikumar, P.; Li, P.; Lin, J.-X.; Escuin-Ordinas, H.; Da, T.; et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 2022, 607, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Reisser, T.; Halbgebauer, D.; Scheurer, J.; Wolf, L.; Leithauser, F.; Beyersdorf, N.; Fischer-Posovszky, P.; Debatin, K.M.; Strauss, G. In vitro-generated alloantigen-specific Th9 cells mediate antileukemia cytotoxicity in the absence of graft-versus-host disease. Leukemia 2020, 34, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkhurst, C.N.; Muratet, M.; et al. A validated regulatory network for Th17 cell specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef]
- Stadhouders, R.; Lubberts, E.; Hendriks, R.W. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J. Autoimmun. 2018, 87, 1–15. [Google Scholar] [CrossRef]
- Yang, X.O.; Nurieva, R.; Martinez, G.J.; Kang, H.S.; Chung, Y.; Pappu, B.P.; Shah, B.; Chang, S.H.; Schluns, K.S.; Watowich, S.S.; et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008, 29, 44–56. [Google Scholar] [CrossRef]
- Maruyama, T.; Kono, K.; Mizukami, Y.; Kawaguchi, Y.; Mimura, K.; Watanabe, M.; Izawa, S.; Fujii, H. Distribution of Th17 cells and FoxP3+ regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010, 101, 1947–1954. [Google Scholar] [CrossRef]
- Fu, L.Q.; Yang, X.; Cai, M.H.; Yao, J.Y.; Jin, W.W.; Mou, Y.P.; Ma, Y.Y. Role of Treg/Th17 Imbalance, Microbiota and miRNAs in Pancreatic Cancer: Therapeutic Options. Crit. Rev. Immunol. 2020, 40, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Kagamu, H.; Miura, S.; Hiura, T.; Miyabayashi, T.; Itoh, R.; Kuriyama, H.; Tanaka, H.; Tanaka, J.; Yoshizawa, H.; et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin. Cancer Res. 2008, 14, 6770–6779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X.; Wu, X.; Zhang, T.; Zhu, Q.; Wang, X.; Wang, H.; Wang, K.; Lin, Y.; Wang, X. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 2018, 6, 1578–1592. [Google Scholar] [CrossRef]
- Qianmei, Y.; Zehong, S.; Guang, W.; Hui, L.; Lian, G. Recent advances in the role of Th17/Treg cells in tumor immunity and tumor therapy. Immunol. Res. 2021, 69, 398–414. [Google Scholar] [CrossRef]
- Ye, J.; Livergood, R.S.; Peng, G. The role and regulation of human Th17 cells in tumor immunity. Am. J. Pathol. 2013, 182, 10–20. [Google Scholar] [CrossRef]
- Muranski, P.; Boni, A.; Antony, P.A.; Cassard, L.; Irvine, K.R.; Kaiser, A.; Paulos, C.M.; Palmer, D.C.; Touloukian, C.E.; Ptak, K.; et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008, 112, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Muranski, P.; Borman, Z.A.; Kerkar, S.P.; Klebanoff, C.A.; Ji, Y.; Sanchez-Perez, L.; Sukumar, M.; Reger, R.N.; Yu, Z.; Kern, S.J.; et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011, 35, 972–985. [Google Scholar] [CrossRef]
- Paulos, C.M.; Carpenito, C.; Plesa, G.; Suhoski, M.M.; Varela-Rohena, A.; Golovina, T.N.; Carroll, R.G.; Riley, J.L.; June, C.H. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci. Transl. Med. 2010, 2, 55ra78. [Google Scholar] [CrossRef]
- Kryczek, I.; Zhao, E.; Liu, Y.; Wang, Y.; Vatan, L.; Szeliga, W.; Moyer, J.; Klimczak, A.; Lange, A.; Zou, W. Human TH17 cells are long-lived effector memory cells. Sci. Transl. Med. 2011, 3, 104ra100. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Luo, H.; Sun, Y.; Shi, B.; Sun, R.; Li, Z. An IL-4/21 Inverted Cytokine Receptor Improving CAR-T Cell Potency in Immunosuppressive Solid-Tumor Microenvironment. Front. Immunol. 2019, 10, 1691. [Google Scholar] [CrossRef]
- Guedan, S.; Chen, X.; Madar, A.; Carpenito, C.; McGettigan, S.E.; Frigault, M.J.; Lee, J.; Posey, A.D., Jr.; Scholler, J.; Scholler, N.; et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 2014, 124, 1070–1080. [Google Scholar] [CrossRef]
- Guedan, S.; Madar, A.; Casado-Medrano, V.; Shaw, C.; Wing, A.; Liu, F.; Young, R.M.; June, C.H.; Posey, A.D., Jr. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J. Clin. Investig. 2020, 130, 3087–3097. [Google Scholar] [CrossRef]
- Gnerlich, J.L.; Mitchem, J.B.; Weir, J.S.; Sankpal, N.V.; Kashiwagi, H.; Belt, B.A.; Porembka, M.R.; Herndon, J.M.; Eberlein, T.J.; Goedegebuure, P.; et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J. Immunol. 2010, 185, 4063–4071. [Google Scholar] [CrossRef]
- Azizi, G.; Yazdani, R.; Mirshafiey, A. Th22 cells in autoimmunity: A review of current knowledge. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 108–117. [Google Scholar]
- Baba, N.; Rubio, M.; Kenins, L.; Regairaz, C.; Woisetschlager, M.; Carballido, J.M.; Sarfati, M. The aryl hydrocarbon receptor (AhR) ligand VAF347 selectively acts on monocytes and naive CD4+ Th cells to promote the development of IL-22-secreting Th cells. Hum. Immunol. 2012, 73, 795–800. [Google Scholar] [CrossRef]
- Eyerich, K.; Eyerich, S. Th22 cells in allergic disease. Allergo. J. Int. 2015, 24, 1–7. [Google Scholar] [CrossRef]
- Jiang, R.; Tan, Z.; Deng, L.; Chen, Y.; Xia, Y.; Gao, Y.; Wang, X.; Sun, B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology 2011, 54, 900–909. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, H.; Deng, L.; Hou, J.; Shi, R.; Yao, M.; Gao, Y.; Yao, A.; Wang, X.; Yu, L.; et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013, 13, 59. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, G.; Lu, G.; Ye, J.; Cui, L.; Zeng, Z.; Chen, J.; Zhou, J. Th22 Cells/IL-22 Serves as a Protumor Regulator to Drive Poor Prognosis through the JAK-STAT3/MAPK/AKT Signaling Pathway in Non-Small-Cell Lung Cancer. J. Immunol. Res. 2022, 2022, 8071234. [Google Scholar] [CrossRef]
- Kobold, S.; Volk, S.; Clauditz, T.; Kupper, N.J.; Minner, S.; Tufman, A.; Duwell, P.; Lindner, M.; Koch, I.; Heidegger, S.; et al. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J. Thorac. Oncol. 2013, 8, 1032–1042. [Google Scholar] [CrossRef]
- Zhuang, Y.; Peng, L.S.; Zhao, Y.L.; Shi, Y.; Mao, X.H.; Guo, G.; Chen, W.; Liu, X.F.; Zhang, J.Y.; Liu, T.; et al. Increased intratumoral IL-22-producing CD4+ T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol. Immunother. 2012, 61, 1965–1975. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Xing, H.; Wang, L.; Zhang, G.; Yu, N.; Wang, J.; Guo, W.; Jiang, J. Elevated Th22 cells and related cytokines in patients with epithelial ovarian cancer. Medicine 2017, 96, e8359. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Suarez-Gosalvez, J.; Voigt, C.; Markota, A.; Giannou, A.D.; Schubel, M.; Jobst, J.; Zhang, T.; Dorr, J.; Markl, F.; et al. T cell-derived interleukin-22 drives the expression of CD155 by cancer cells to suppress NK cell function and promote metastasis. Immunity 2023, 56, 143–161.e111. [Google Scholar] [CrossRef]
- Rui, J.; Chunming, Z.; Binbin, G.; Na, S.; Shengxi, W.; Wei, S. IL-22 promotes the progression of breast cancer through regulating HOXB-AS5. Oncotarget 2017, 8, 103601–103612. [Google Scholar] [CrossRef]
- Kryczek, I.; Lin, Y.; Nagarsheth, N.; Peng, D.; Zhao, L.; Zhao, E.; Vatan, L.; Szeliga, W.; Dou, Y.; Owens, S.; et al. IL-22+CD4+ T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 2014, 40, 772–784. [Google Scholar] [CrossRef]
- Hernandez, P.; Gronke, K.; Diefenbach, A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 2018, 48, 15–31. [Google Scholar] [CrossRef]
- Lim, C.; Savan, R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014, 25, 257–271. [Google Scholar] [CrossRef]
- Gurney, A.L. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int. Immunopharmacol. 2004, 4, 669–677. [Google Scholar] [CrossRef]
- Zheng, Y.; Danilenko, D.M.; Valdez, P.; Kasman, I.; Eastham-Anderson, J.; Wu, J.; Ouyang, W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 2007, 445, 648–651. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, K.; Lam, A.K.; Huang, J.; Qiu, F.; Qiao, B.; Zhang, Y. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2020, 9, 640–652. [Google Scholar] [CrossRef]
- Qi, H. T follicular helper cells in space-time. Nat. Rev. Immunol. 2016, 16, 612–625. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Forster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.H.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef]
- Ahearne, M.J.; Willimott, S.; Pinon, L.; Kennedy, D.B.; Miall, F.; Dyer, M.J.; Wagner, S.D. Enhancement of CD154/IL4 proliferation by the T follicular helper (Tfh) cytokine, IL21 and increased numbers of circulating cells resembling Tfh cells in chronic lymphocytic leukaemia. Br. J. Haematol. 2013, 162, 360–370. [Google Scholar] [CrossRef]
- Cha, Z.; Zang, Y.; Guo, H.; Rechlic, J.R.; Olasnova, L.M.; Gu, H.; Tu, X.; Song, H.; Qian, B. Association of peripheral CD4+ CXCR5+ T cells with chronic lymphocytic leukemia. Tumour. Biol. 2013, 34, 3579–3585. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; de Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G.; et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Ma, Q.-Y.; Huang, D.-Y.; Zhang, H.-J.; Chen, J.; Miller, W.; Chen, X.-F. Function of follicular helper T cell is impaired and correlates with survival time in non-small cell lung cancer. Int. Immunopharmacol. 2016, 41, 1–7. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Niogret, J.; Berger, H.; Rebe, C.; Mary, R.; Ballot, E.; Truntzer, C.; Thibaudin, M.; Derangere, V.; Hibos, C.; Hampe, L.; et al. Follicular helper-T cells restore CD8+-dependent antitumor immunity and anti-PD-L1/PD-1 efficacy. J. Immunother. Cancer 2021, 9, e002157. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu-Sanz, D.; Kobold, S. Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy. Cancers 2023, 15, 1650. https://doi.org/10.3390/cancers15061650
Andreu-Sanz D, Kobold S. Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy. Cancers. 2023; 15(6):1650. https://doi.org/10.3390/cancers15061650
Chicago/Turabian StyleAndreu-Sanz, David, and Sebastian Kobold. 2023. "Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy" Cancers 15, no. 6: 1650. https://doi.org/10.3390/cancers15061650
APA StyleAndreu-Sanz, D., & Kobold, S. (2023). Role and Potential of Different T Helper Cell Subsets in Adoptive Cell Therapy. Cancers, 15(6), 1650. https://doi.org/10.3390/cancers15061650




