Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
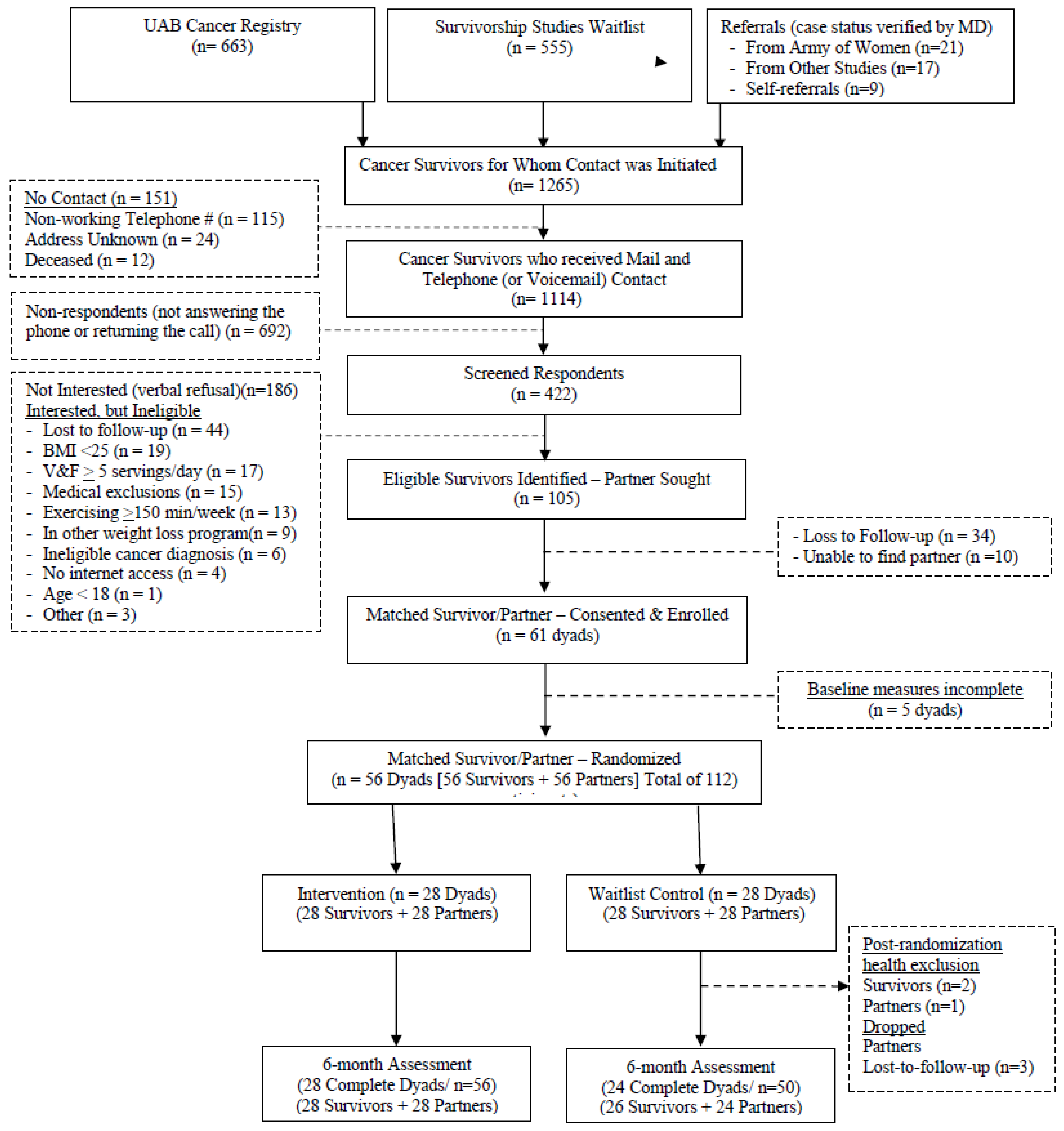
2.2. Participants: Recruitment, Screening, Consent, and Randomization
2.3. DUET Intervention
2.4. Waitlist Control
2.5. Measures
2.5.1. Anthropometric Measures (Captured at Baseline and 6 Months)
- Body Weight (Primary Outcome): The participant stepped on the scale without shoes in light clothing while the process was captured via Zoom (focusing on the scale display to make sure the scale was “zeroed-out” prior to weighing). Weight was measured twice, with both values averaged for analyses.
- Waist circumference: This was measured with unmarked ribbons to reduce bias [22]; two sets of ribbons were mailed to each dyad for assessments. Participants bared their midriffs on Zoom and were instructed to place one end of the ribbon on the umbilicus. Partners then wrapped the ribbon evenly around the waist. Participants rotated in front of the camera, and assessors ensured the ribbon was parallel to the floor and snug against the skin. Upon exhale, partners used a felt-tip marker to mark the point of overlap. The process was repeated with the 2nd ribbon. Both ribbons were returned to the study office, where they were measured by staff, and the average was used for analyses.
2.5.2. Dietary Intake (Captured at Baseline and 6 Months)
2.5.3. Physical Activity and Sleep (Captured at Baseline and 6 Months)
2.5.4. Physical Performance (Captured at Baseline and 6 Months)
2.5.5. Circulating Biomarkers (Captured at Baseline and 6 Months)
2.5.6. Online Surveys
- Comorbidity/Symptoms: The Older Americans Resources and Services (OARS) Comorbidity Index (modified version) assessed the number of medical conditions and functional impact by ascertaining either an affirmative or negative response to 21 unique medical conditions and 22 symptoms [27].
- Quality of Life (QOL): QOL was measured by the PROMIS global QOL to assess physical, mental, and social health domains, as well as usual activities, fatigue, and pain [28].
- Social Support: Validated 5-point scales by Sallis et al. [31] assessed social support for exercise and dietary change.
- Barriers: Thirty-one common barriers were assessed to a diet low in fat and sugar, increased V and Fs and whole grains, and exercise (e.g., “low calorie foods don’t taste good,” “I don’t know how to cook or prepare low calorie foods,” “I am too busy to (…follow a low calorie diet…exercise),” ”I don’t enjoy exercise, etc.) [32,33,34].
- Demographics: Self-reported data were collected on height, race/ethnicity, age, educational and marital status, current smoking status, income range, and relationship with the dyadic partner (i.e., spouse, child, parent, sibling, friend, or other). Because eHealth literacy also could serve as a potential moderator, three items from the eHEALS eHealth Literacy scale, [35], i.e., “I know how to use the Internet to answer my health questions; ”I know how to use the health information I find on the Internet to help me,” and “I have the skills I need to evaluate the health resources I find on the Internet” (5-item Likert scale ranging from strongly agree to disagree) were included in the baseline online survey. In addition, given substantial data showing that depression is a strong moderator of weight loss interventions [36], the Center for Epidemiologic Studies of Depression (CES-D; Boston short form; 20 items; yes-no format), was administered at baseline [37].
2.5.7. Safety
2.6. Statistical Considerations
3. Results
3.1. Study Sample, Retention, and Safety
3.2. Changes in Adiposity
3.3. Changes in Dietary Intake and Physical Activity
3.4. Changes in Physical Performance
3.5. Changes in Circulating Biomarkers
3.6. Changes in Patient-Reported Outcomes
3.7. Model Dyadic Terms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 16 February 2023).
- Mitka, M. IOM report: Aging US population, rising costs, and complexity of cases add up to crisis in cancer care. JAMA 2013, 310, 1549–1550. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the IARC working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Ligibel, J.A.; Alfano, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Burger, R.A.; Chlebowski, R.T.; Fabian, C.J.; Gucalp, A.; Hershman, D.L.; Hudson, M.M.; et al. American Society of Clinical Oncology Position Statement on Obesity and Cancer. J. Clin. Oncol. 2014, 32, 3568–3574. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Social Foundations of Thought and Action: A social Cognitive Theory; Prentice-Hall, Inc: Hoboken, NJ, USA, 1986. [Google Scholar]
- Black, D.R.; Gleser, L.J.; Kooyers, K.J. A meta-analytic evaluation of couples weight-loss programs. Health Psychol. 1990, 9, 330–347. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Jones, L.W.; Snyder, D.C.; Sloane, R.J.; Kimmick, G.G.; Hughes, D.C.; Badr, H.J.; Miller, P.E.; Burke, L.E.; Lipkus, I.M. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer 2014, 120, 2522–2534. [Google Scholar] [CrossRef]
- Carmack, C.L.; Parker, N.H.; Demark-Wahnefried, W.; Shely, L.; Baum, G.; Yuan, Y.; Giordano, S.H.; Rodriguez-Bigas, M.; Pettaway, C.; Basen-Engquist, K. Healthy Moves to improve lifestyle behaviors of cancer survivors and their spouses: Feasibility and preliminary results of intervention efficacy. Nutrients 2021, 13, 4460. [Google Scholar] [CrossRef]
- Pekmezi, D.W.; Crane, T.E.; Oster, R.A.; Rogers, L.Q.; Hoenemeyer, T.; Farrell, D.; Cole, W.W.; Wolin, K.; Badr, H.; Demark-Wahnefried, W. Rationale and methods for a randomized controlled trial of a dyadic, web-based, weight loss intervention among cancer survivors and partners: The DUET study. Nutrients 2021, 13, 3472. [Google Scholar] [CrossRef]
- Gray, M.S.; Judd, S.E.; Sloane, R.; Snyder, D.C.; Miller, P.E.; Demark-Wahnefried, W. Rural-urban differences in health behaviors and outcomes among older, overweight, long-term cancer survivors in the RENEW randomized control trial. Cancer Causes Control 2019, 30, 301–309. [Google Scholar] [CrossRef]
- Kaur, H.; Fernández, J.R.; Locher, J.L.; Demark-Wahnefried, W. Rural and urban differences in vegetable and fruit consumption among older cancer survivors in the deep south: An exploratory cross-sectional study. J. Acad. Nutr. Diet. 2022, 122, 1717–1724. [Google Scholar] [CrossRef]
- Williams, V.A.; Brown, N.I.; Johnson, R.; Ainsworth, M.C.; Farrell, D.; Barnes, M.; Perumean-Chaney, S.; Fontaine, K.; Martin, M.Y.; Pekmezi, D.; et al. A web-based lifestyle intervention for cancer survivors: Feasibility and acceptability of SurvivorSHINE. J. Cancer Ed. 2022, 37, 1773–1781. [Google Scholar] [CrossRef]
- Williams, V.; Brown, N.; Moore, J.X.; Farrell, D.; Perumean-Chaney, S.; Schleicher, E.; Fontaine, K.; Demark-Wahnefried, W.; Pekmezi, D. Web-based lifestyle interventions for survivors of cancer: Usability study. JMIR Form. Res. 2022, 6, e30974. [Google Scholar] [CrossRef] [PubMed]
- Kelley, H.H. The “stimulus field” for interpersonal phenomena: The source of language and thought about interpersonal events. Pers. Social Psychol. Rev. 1997, 1, 140–169. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; McBride, C.M.; Pollak, K.I.; Puleo, E.; Butterfield, R.M.; Emmons, K.M. Understanding health behavior change among couples: An interdependence and communal coping approach. Social Sci. Med. 2006, 62, 1369–1380. [Google Scholar] [CrossRef]
- World Cancere Research Fund. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. 2018. Available online: https://www.wcrf.org/dietandcancer (accessed on 16 February 2023).
- Greer, S.; Watson, M. Mental adjustment to cancer: Its measurement and prognostic importance. Cancer Surv. 1987, 6, 439–453. [Google Scholar]
- Frankenfield, D.C.; Rowe, W.A.; Smith, J.S.; Cooney, R.N. Validation of several established equations for resting metabolic rate in obese and nonobese people. J. Am. Diet. Assoc. 2003, 103, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Hoenemeyer, T.W.; Cole, W.W.; Oster, R.A.; Pekmezi, D.W.; Pye, A.; Demark-Wahnefried, W. Test/retest reliability and validity of remote vs. in-person anthropometric and physical performance assessments in cancer survivors and supportive partners. Cancers (Basel) 2022, 14, 1075. [Google Scholar] [CrossRef]
- Freudenheim, J.L.; Darrow, S.L. Accuracy of self-measurement of body fat distribution by waist, hip, and thigh circumferences. Nutr. Cancer 1991, 15, 179–186. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef]
- Rogers, L.Q.; McAuley, E.; Anton, P.M.; Courneya, K.S.; Vicari, S.; Hopkins-Price, P.; Verhulst, S.; Mocharnuk, R.; Hoelzer, K. Better exercise adherence after treatment for cancer (BEAT Cancer) study: Rationale, design, and methods. Contemp. Clin. Trials 2012, 33, 124–137. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J. Cancer Surv. 2015, 9, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Borsch-Supan, A.; Weiss, L.M.; Borsch-Supan, M.; Potter, A.J.; Cofferen, J.; Kerschner, E. Dried blood spot collection, sample quality, and fieldwork conditions: Structural validations for conversion into standard values. Am. J. Hum. Biol. 2021, 33, e23517. [Google Scholar] [CrossRef] [PubMed]
- Fillenbaum, G. Multidimensional Functional Assessment of Older Adults; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Cella, D.; Riley, W.; Stone, A.; Rothrock, N.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.M.; Abrams, D.B.; Niaura, R.S.; Eaton, C.A.; Rossi, J.S. Self-efficacy in weight management. J. Consult. Clin. Psychol. 1991, 59, 739–744. [Google Scholar] [CrossRef]
- McAuley, E.; Blissmer, B. Self-efficacy determinants and consequences of physical activity. Exerc. Sport Sci. Rev. 2000, 28, 85–88. [Google Scholar]
- Sallis, J.F.; Grossman, R.M.; Pinski, R.B.; Patterson, T.L.; Nader, P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987, 16, 825–836. [Google Scholar] [CrossRef]
- Arroyave, W.D.; Clipp, E.C.; Miller, P.E.; Jones, L.W.; Ward, D.S.; Bonner, M.J.; Rosoff, P.M.; Snyder, D.C.; Demark-Wahnefried, W. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol. Nurs. Forum. 2008, 35, 121–130. [Google Scholar] [CrossRef]
- Vijan, S.; Stuart, N.S.; Fitzgerald, J.T.; Ronis, D.L.; Hayward, R.A.; Slater, S.; Hofer, T.P. Barriers to following dietary recommendations in Type 2 diabetes. Diabet. Med. 2005, 22, 32–38. [Google Scholar] [CrossRef]
- Rogers, L.Q.; Courneya, K.S.; Shah, P.; Dunnington, G.; Hopkins-Price, P. Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: A pilot study. Eur. J. Cancer Care 2007, 16, 55–66. [Google Scholar] [CrossRef]
- Norman, C.D.; Skinner, H.A. eHEALS: The eHealth Literacy Scale. JMIR 2006, 8, e27. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Kohout, F.J.; Berkman, L.F.; Evans, D.A.; Cornoni-Huntley, J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J. Aging. Health 1993, 5, 179–193. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Lyons, K.S.; Dieckmann, N.F.; Lee, C.S.; Mitrim, Z.; Beer, T.M. Study protocol for the Exercising Together© trial: A randomized, controlled trial of partnered exercise for couples coping with cancer. Trials 2021, 22, 579. [Google Scholar] [CrossRef]
- Cho, D.; Milbury, K.; Liao, Y.; Pettaway, C.A.; Gregg, J.R.; Li, Y.; McNeill, L.H. Study protocol: One plus one can be greater than two-Ecological momentary assessment for Black prostate cancer survivors and partners. PLoS ONE 2021, 16, e0255614. [Google Scholar] [CrossRef]
- Hallward, L.; Chemtob, K.; Lambert, S.D.; Duncan, L.R. Prostate cancer survivors’ and caregivers’ experiences using behavior change techniques during a web-based self-management and physical activity program: A qualitative study. J. Clin. Med. 2020, 9, 3244. [Google Scholar] [CrossRef] [PubMed]
- Bantum, E.O.; Albright, C.L.; White, K.K.; Berenberg, J.L.; Layi, G.; Ritter, P.L.; Laurent, D.; Plant, K.; Lorig, K. Surviving and thriving with cancer using a Web-based health behavior change intervention: Randomized controlled trial. JMIR 2014, 16, e54. [Google Scholar]
- Kanera, I.M.; Bolman, C.A.; Willems, R.A.; Mesters, I.; Lechner, L. Lifestyle-related effects of the web-based Kanker Nazorg Wijzer (Cancer Aftercare Guide) intervention for cancer survivors: A randomized controlled trial. J. Cancer Surv. 2016, 10, 883–897. [Google Scholar] [CrossRef]
- Sturgeon, K.M.; Brown, J.C.; Sears, D.D.; Sarwer, D.B.; Schmitz, K.H. WISER survivor trial: Combined effect of exercise and weight loss interventions on inflammation in breast cancer survivors. Med. Sci. Sport. Exerc. 2023, 55, 209–215. [Google Scholar] [CrossRef]
- Reeves, M.M.; Terranova, C.O.; Winkler, E.A.H.; McCarthy, N.; Hickman, I.J.; Ware, R.S.; Lawler, S.P.; Eakin, E.G.; Demark-Wahnefried, W. Effect of a remotely delivered weight loss intervention in early-stage breast ancer: Randomized controlled trial. Nutrients 2021, 13, 4091. [Google Scholar] [CrossRef]
- Befort, C.A.; Kimler, B.F.; Bantis, L.E.; Phillips, T.A.; Fabian, C.J. Effects of weight loss and weight regain on circulating biomarkers in overweight/obese breast cancer survivors enrolled in a weight loss trial in the rural midwest. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, T.J.; Dyer, A.M.; Rogers, C.J.; Schmitz, K.H.; Sturgeon, K.M. Effects of diet and exercise-induced weight loss on biomarkers of inflammation in breast cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N. Biological variation in serum lipid concentrations. Scand. J. Clin. Lab. Invest. Suppl. 1990, 198, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Morey, M.C.; Snyder, D.C.; Sloane, R.; Cohen, H.J.; Peterson, B.; Hartman, T.J.; Miller, P.; Mitchell, D.C.; Demark-Wahnefried, W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: A randomized controlled trial. JAMA 2009, 301, 1883–1891. [Google Scholar] [CrossRef]
- Kaiser Family Foundation. Loneliness and Social Isolation in the United States, the United Kingdom, and Japan: An International Survey; Kaiser Family Foundation: San Francisco, CA, USA, 2018. [Google Scholar]
- U.S. Bureau of Census. Quick Facts: United States Census. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045221 (accessed on 16 February 2023).
- Lyles, C.M.; Kay, L.S.; Crepaz, N.; Herbst, J.H.; Passin, W.F.; Kim, A.S.; Rama, S.M.; Thadiparthi, S.; DeLuca, J.B.; Mullins, M.M. Best-evidence interventions: Findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Am. J. Public Health 2007, 97, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, P.B.; Rowland, J.H.; Paskett, E.D.; Van Leeuwen, F.; Moskowitz, C.; Katta, S.; Wollins, D.; Robison, L.L. Identification of key gaps in cancer survivorship research: Findings from the American Society of Clinical Oncology survey. J. Oncol. Pract. 2016, 12, 190–193. [Google Scholar] [CrossRef]
- Adams, D.V.; Long, S.; Fleury, M.E. Association of remote technology use and other decentralization tools with patient likelihood to enroll in cancer clinical trials. JAMA Netw. Open 2022, 5, e2220053. [Google Scholar] [CrossRef]
| Characteristic | Overall (n = 112) | Waitlist (n = 56) | Intervention (n = 56) |
|---|---|---|---|
Cancer Diagnoses among Survivors (n/%)
| 45/56 (80.3%) 11/56 (19.7%) | 22/28 (78.6%) 6/28 (21.4%) | 23/28 (82.1%) 5/28 (17.9%) |
Cancer Stage among Survivors (n/%)
| 23/56 (41.1%) 6/56 (10.7%) 1/56 (1.8%) 26/56 (46.4%) | 11/28 (39.3%) 3/28 (10.7%) 0/28 (0%) 14/28 (50%) | 12/28 (42.9%) 3/28 (10.7%) 1/28 (3.6%) 12/28 (42.9%) |
| Months elapsed since diagnosis: Mean (sd)/range | 67.3 (72.0)/0–303 | 63.1 (62.7)/12–206 | 71.0 (80.4)/10–303 |
Partner’s relationship to survivor, miles between them
| 23/56 (41.1%) 7/56 (12.5%) 6/56 (10.7%) 17/56 (30.4%) 3/56 (5.3%) 7.1 (12.4)/0–56 | 12/28 (42.9%) 5/28 (17.8%) 3/28 (10.7%) 8/28 (28.6%) 0/28 (0%) 5.7 (7.5)/0–31 | 11/28 (39.3%) 2/28 (7.2%) 3/28 (10.7%) 9/28 (32.1%) 3/28 (10.7%) 8.5 (16.0)/0–56 |
| Female gender (n/%) | 86 (76.8%) | 42/56 (75.0%) | 44/56 (78.6%) |
| Age (years): Mean (sd)/range | 58.4 (12.7)/23–81 | 58.7 (13.0)/24–81 | 58.1 (12.5)/23–78 |
Race (n/%)
| 70 (62.5%) 41 (36.6%) 1 (0.9%) | 34 (60.7%) 22 (39.3%) 0 (0%) | 36 (64.3%) 19 (33.9%) 1 (1.8%) |
Educational Status (n/%)
| 16 (14.3%) 34 (30.4%) 59 (53.5%) 2 (1.8%) | 8 (14.3%) 14 (25.0%) 32 (57.1%) 2 (3.6%) | 8 (14.3%) 20 (35.7%) 28 (50.0%) 0 (0%) |
| eHealth Literacy: Mean (sd)/range ¶ | 3.93 (0.77)/1.63–5.0 | 4.06 (0.78) 1.63–5.0 | 3.93 (0.77)/1.63–5.0 |
Income (n/%)
| 18 (16.1%) 48 (42.8%) 46 (41.1%) | 7 (12.5%) 28 (50.0%) 21 (37.5%) | 11 (19.6%) 20 (35.7%) 25 (44.6%) |
Employment (n/%)
| 62 (55.4%) 36 (32.1%) 14 (12.5%) | 32 (57.1%) 17 (30.4%) 7 (12.5%) | 30 (53.6%) 19 (33.9%) 7 (12.5%) |
| Rural Residence (n/%) | 9 (8.0%) | 6 (10.7%) | 3 (5.4%) |
| BMI: Mean (sd)/range | 32.4 (2.75) 25–51 | 33.3 (6.8) 25–51 | 31.4 (4.9) 25–45 |
| Daily servings of Vand Fs: Mean (sd)/range | 1.8 (1.1) 0.2–6.3 | 1.9 (1.2) 0.2–6.3 | 1.7 (1.0) 0.2–3.8 |
| Weekly minutes of MVPA: Mean (sd)/range | 43.8 (54.7) 0–280 | 43.7 (59.1) 0–225 | 43.8 (60.5) 0–280 |
| Current Smoker (n/%) | 5 (4.5%) | 1 (1.8%) | 4 (7.1%) |
| Number of Comorbidities: Mean (sd)/range | 3.0 (2.6) 0–11 | 3.5 (2.7) 0–11 | 2.6 (2.4) 0–10 |
| Depressive Symptoms (CESD): Mean (sd)/range § | 3.2 (6.3) 0–24 | 3.9 (6.1) 0–24 | 3.9 (6.1) 0–24 |
| Waitlist Control (WL) | DUET Intervention | Significance (p-Values) | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | 6M Mean (SD) | Baseline Mean (SD) | 6M Mean (SD) | Between Arm | Within Arm | Time × Arm | |
| Weight (kg) (Primary Outcome) | |||||||
| Survivors Partners Dyads | 88.0 (17.6) 96.7 (22.6) 92.4 (20.6) | 86.9 (16.1) 95.3 (22.9) 91.3 (20.3) | 86.8 (18.2) 87.6 (14.0) 87.2 (16.1) | 83.8 (18.4) * 84.9 (15.6) * 84.4 (16.9) * | 0.656 0.069 0.044 | 0.001 0.001 <0.001 | 0.090 0.280 0.033 |
| Waist Circumference (cm) | |||||||
| Survivors Partners Dyads | 107.1 (17.7) 111.3 (15.3) 109.2 (16.7) | 104.6 (14.3) 108.4 (16.4) * 106.6 (15.6) * | 106.0 (11.7) 106.5 (9.5) 106.2 (10.6) | 102.5 (13.4) * 103.3 (9.5) * 102.9 (11.5) * | 0.667 0.144 0.128 | 0.003 <0.001 <0.001 | 0.579 0.594 0.339 |
| Calorie Intake/day Survivors Partners Dyads | 1645.9 (535.0) 1553.5 (483.7) 1596.9 (515.6) | 1532.0 (568.9) 1467.2 (501.3) 1497.7 (542.5) | 1400.0 (439.0) 1570.2 (498.3) 1485.1 (473.1) | 1265.6 (305.1) 1378.5 (408.3) * 1321.0 (360.6) * | 0.027 0.716 0.053 | 0.046 0.012 0.001 | 0.836 0.286 0.365 |
| Healthy Eating Index Survivors Partners Dyads | 51.8 (10.9) 55.5 (10.5) 54.0 (10.6) | 50.8 (12.2) 54.6 (12.2) 52.6 (12.5) | 53.9 (13.7) 52.2 (12.0) 53.1 (12.8) | 58.8 (15.2) 53.9 (13.4) 56.4 (14.4) | 0.092 0.416 0.396 | 0.360 0.874 0.589 | 0.163 0.587 0.130 |
| MVPA Self-Report Survivors Partners Dyad | 51.9 (61.6) 43.3 (61.8) 45.6 (61.4) | 57.3 (61.9) 58.1 (75.4) 57.9 (68.6) | 48.5 (67.8) 39.1 (52.9) 43.1 (60.4) | 103.9(104.7) * 81.6(113.5) 92.7(108.8) * | 0.448 0.299 0.099 | 0.011 0.025 <0.001 | 0.189 0.356 0.229 |
| MVPA Accelerometry Survivors Partners Dyads | 162.6 (172.4) 148.0 (169.0) 157.7 (171.3) | 122.2 (190.2) 146.0 (278.4) 139.0 (236.8) † | 108.5 (122.6) 146.4 (121.8) 127.5 (122.4) | 150.3 (199.0) * 132.0 (271.4) 141.0 (236.4) | 0.726 0.655 0.633 | 0.047 <0.001 <0.001 | 0.265 0.819 0.600 |
| Waitlist Control (WL) | DUET Intervention | Significance (p-Values) | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | 6M Mean (SD) | Baseline Mean (SD) | 6M Mean (SD) | Between Arm | Within Arm | Time × Arm | |
| 30-sec Chair Stand (reps) Survivors Partners Dyads | 10.5 (2.7) 11.5 (3.0) 11.0 (2.9) * | 12.2 (3.9) 14.0 (5.8) 13.1 (5.0) | 10.3 (3.1) 11.0 (3.4) 10.7 (3.2) | 13.1 (3.6) * 13.5 (3.3) * 13.3 (3.4) * | 0.645 0.624 0.871 | <0.001 <0.001 <0.001 | 0.301 0.995 0.380 |
| 8′ Get-up-and-Go (sec) Survivors Partners Dyads | 8.1 (2.7) 7.4 (1.5) 7.8 (2.3) | 7.6 (2.8) 7.3 (1.8) 7.6 (2.3) | 7.7 (1.7) 7.6 (1.6) 7.6 (1.6) | 6.9 (1.5) * 7.1 (2.0) 7.0 (1.8) * | 0.290 0.980 0.107 | 0.004 0.084 0.001 | 0.470 0.304 0.211 |
| Sit-and-Reach (cm) Survivors Partners Dyads | −0.2 (4.6) 0.3 (3.6) 0.1 (4.0) | −0.3 (4.7) 0.8 (3.6) 0.4 (4.1) | −0.8 (3.0) −0.5 (3.8) −0.6 (3.4) | 0.6 (2.0) * 0.5 (2.8) 0.6 (2.4) * | 0.889 0.583 0.573 | 0.036 0.025 0.002 | 0.015 0.520 0.043 |
| Back Scratch (cm) Survivors Partners Dyads | −3.7 (3.5) −2.5 (3.4) −3.0 (3.4) | −3.7 (3.2) −2.3 (3.3) −2.9 (3.3) | −3.3 (3.5) −3.7 (3.6) −3.5 (3.6) | −2.9 (3.9) −3.3 (3.6) −3.1 (3.7) | 0.627 0.260 0.511 | 0.187 0.237 0.104 | 0.217 0.699 0.147 |
| 2-min Step Test (reps) Survivors Partners Dyads | 81.0 (22.9) 79.7 (20.8) 80.3 (22.0) | 80.4 (23.0) 83.0 (22.5) 80.9 (22.6) | 79.9 (23.1) 79.1 (24.2) 79.4 (23.4) | 84.4 (23.5) * 87.3 (23.6) 85.8 (23.4) | 0.806 0.766 0.442 | 0.396 0.016 0.037 | 0.258 0.298 0.077 |
| Waitlist (WL) Control | DUET Intervention | Significance (p-Values) | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | 6M Mean (SD) | Baseline Mean (SD) | 6M Mean (SD) | Between Arm | Within Arm | Time × Arm | |
| Glucose (mg/dL) Survivors Partners Dyads | 87.8 (25.6) 88.2 (17.8) 88.3 (22.6) | 62.6 (34.2) * 65.8 (44.3) 61.2 (38.0) | 89.0 (23.1) 82.7 (21.6) 85.9 (22.4) | 71.4 (23.1) 67.4 (34.5) * 69.4 (29.2) * | 0.175 0.599 0.185 | 0.002 0.002 <0.001 | 0.188 0.393 0.148 |
| Total Chol. (mg/dL) Survivors Partners Dyads | 269.5 (69.6) 280.3 (58.2) 276.9 (64.4) | 252.1 (34.0) 238.1 (42.1) 245.6 (39.2) | 268.2 (50.7) 269.5 (59.4) 268.8 (54.6) | 255.5 (44.8) 249.3 (38.4) 252.3 (41.3) | 0.713 0.969 0.868 | 0.139 0.005 0.002 | 0.791 0.303 0.377 |
| HDL Chol. (mg/dL) Survivors Partners Dyads | 74.2 (11.7) 72.3 (10.8) 73.5 (11.4) | 68.1 (14.4) † 67.6 (12.7) 68.0 (13.8) † | 68.4 (10.9) 69.0 (11.9) 68.7 (11.3) | 63.2 (10.6) 61.2 (10.5) † 62.1 (10.5)† | 0.130 0.119 0.017 | <0.001 0.002 <0.001 | 0.593 0.405 0.977 |
| CRP (mg/dL) Survivors Partners Dyads | 2.0 (1.4) 3.2 (2.6) 2.7 (2.1) | 1.7 (1.5) 2.7 (2.2) 2.3 (1.9) | 4.5 (7.4) 2.7 (4.0) 3.6 (6.0) | 3.0 (3.5) 2.3 (4.5) 2.6 (4.0) * | 0.182 0.062 0.403 | 0.041 0.142 0.025 | 0.824 0.328 0.183 |
| Leptin Survivors Partners Dyads | 102.2 (71.6) 113.0 (76.7) 108.1 (75.0) | 91.6 (50.2) 116.0 (79.3) 103.7 (68.6) | 101.8 (65.1) 74.0 (51.0) 88.2 (59.7) | 100.9 (86.5) 83.8 (74.8) 92.1 (80.1) | 0.767 0.017 0.161 | 0.689 0.945 0.705 | 0.735 0.523 0.448 |
| Adiponectin Survivors Partners Dyads | 34.6 (23.1) 26.3 (9.9) 31.1 (18.8) | 31.9 (15.2) 24.7 (9.4) 28.4 (13.4) | 26.8 (20.8) 25.2 (13.6) 26.0 (17.5) | 23.8 (11.1) 23.3 (12.6) 23.5 (11.7) | 0.283 0.380 0.113 | 0.343 0.174 0.043 | 0.137 0.247 0.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demark-Wahnefried, W.; Oster, R.A.; Crane, T.E.; Rogers, L.Q.; Cole, W.W.; Kaur, H.; Farrell, D.; Parrish, K.B.; Badr, H.J.; Wolin, K.Y.; et al. Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners. Cancers 2023, 15, 1577. https://doi.org/10.3390/cancers15051577
Demark-Wahnefried W, Oster RA, Crane TE, Rogers LQ, Cole WW, Kaur H, Farrell D, Parrish KB, Badr HJ, Wolin KY, et al. Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners. Cancers. 2023; 15(5):1577. https://doi.org/10.3390/cancers15051577
Chicago/Turabian StyleDemark-Wahnefried, Wendy, Robert A. Oster, Tracy E. Crane, Laura Q. Rogers, W. Walker Cole, Harleen Kaur, David Farrell, Kelsey B. Parrish, Hoda J. Badr, Kathleen Y. Wolin, and et al. 2023. "Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners" Cancers 15, no. 5: 1577. https://doi.org/10.3390/cancers15051577
APA StyleDemark-Wahnefried, W., Oster, R. A., Crane, T. E., Rogers, L. Q., Cole, W. W., Kaur, H., Farrell, D., Parrish, K. B., Badr, H. J., Wolin, K. Y., & Pekmezi, D. W. (2023). Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners. Cancers, 15(5), 1577. https://doi.org/10.3390/cancers15051577







