Simple Summary
In the past 20 years, the development of targeted therapies that can be matched to a tumor’s molecular and immune abnormalities has resulted in the improvement of outcomes for patients suffering from advanced aggressive malignancies. Remarkably, non-small cell lung cancer (NSCLC) has become the poster child for a lethal malignancy in which numerous molecular aberrations have become druggable. Similar to NSCLC, there are limited responses in cholangiocarcinoma (CCA) to conventional chemotherapy. Next-generation sequencing has identified novel genomic alterations in CCA that vary between patients. Gene- and immune-targeted therapies are leading to a new era of precision/personalized medicine for patients with CCA. Herein, we review the current status of molecularly matched precision-targeted therapy for CCA.
Abstract
In the past two decades, molecular targeted therapy has revolutionized the treatment landscape of several malignancies. Lethal malignancies such as non-small cell lung cancer (NSCLC) have become a model for precision-matched immune- and gene-targeted therapies. Multiple small subgroups of NSCLC defined by their genomic aberrations are now recognized; remarkably, taken together, almost 70% of NSCLCs now have a druggable anomaly. Cholangiocarcinoma (CCA) is a rare tumor with a poor prognosis. Novel molecular alterations have been recently identified in patients with CCA, and the potential for targeted therapy is being realized. In 2019, a fibroblast growth factor receptor 2 (FGFR2) inhibitor, pemigatinib, was the first approved targeted therapy for patients with locally advanced or metastatic intrahepatic CCA who had FGFR2 gene fusions or rearrangement. More regulatory approvals for matched targeted therapies as second-line or subsequent treatments in advanced CCA followed, including additional drugs that target FGFR2 gene fusion/rearrangement. Recent tumor-agnostic approvals include (but are not limited to) drugs that target mutations/rearrangements in the following genes and are hence applicable to CCA: isocitrate dehydrogenase 1 (IDH1); neurotrophic tropomyosin-receptor kinase (NTRK); the V600E mutation of the BRAF gene (BRAFV600E); and high tumor mutational burden, high microsatellite instability, and gene mismatch repair-deficient (TMB-H/MSI-H/dMMR) tumors. Ongoing trials investigate HER2, RET, and non-BRAFV600E mutations in CCA and improvements in the efficacy and safety of new targeted treatments. This review aims to present the current status of molecularly matched targeted therapy for advanced CCA.
1. Introduction
In the past two decades, molecular targeted and immune therapy has revolutionized the treatment landscape of several malignancies. Recent findings highlighted that matched targeted therapy improved the response rate and prolonged the survival of patients with advanced cancers [1,2]. Some of the most notable achievements are in non-small cell lung cancer (NSCLC), a disease for which the number of actionable biomarkers has increased rapidly. Indeed, NSCLC is now almost a poster child for the benefits of precision medicine, with almost 70% of NSCLCs having a biomarker-based therapy (including but not limited to EGFR and ERBB2 alterations, mismatch repair gene defects, high tumor mutational burden, BRAF V600E, NTRK fusions, ALK fusions, ROS1 fusions, and RET alterations) [3,4,5,6,7,8,9,10]. Moreover, molecular diagnostics have supported the development of drugs and therapeutic antibodies targeting specific receptors, antigens, or molecular pathways crucial for tumor cell proliferation and invasion, tumor growth, immunity, and metastases across various malignancies [11].
Cholangiocarcinoma (CCA) is a promising candidate for targeted therapy due to its diverse molecular features [12]. The incidence of CCA is low in the Western world, with between 0.35 and 2 cases per a 100,000 population per year [13]. The global incidence of CCA has steadily increased over the last 30 years, from 0.1 to 0.6 cases per a 100,000 population [13]. CCA is a highly aggressive malignancy, with a 5-year OS for locally advanced or metastatic disease of less than 10% [14]. CCA arises from the intrahepatic and extrahepatic biliary epithelium [15]. Anatomically, 60–70% of cases are classified as perihilar CCA (pCCA); 20–30% of cases are distal CCA (dCCA); and 5–10% of cases are intrahepatic CCA (iCAA) [15]. Current population statistics show an increased prevalence of CCA [15]. In early-stage CCA, the primary treatment includes surgical resection and adjuvant chemotherapy, while systemic chemotherapy is the standard treatment for advanced-stage CCA [16]. Patients with CCA often present with late-stage disease, and the prognosis is poor [16].
Molecular techniques, including next-generation sequencing (NGS), have identified novel mutations in tumors from patients with CCA (Figure 1) [17]. Patients with CCA show substantial variations in their molecular profiling and genetic aberrations according to their anatomic locations (Table 1). Promising therapeutic molecular targets for CCA have been identified and include isocitrate dehydrogenases 1 and 2 (IDH1 and 2) and products of fusions of the fibroblast growth factor receptor 2 (FGFR2) gene [17]. In 2019, the US Food and Drug Administration (FDA) granted accelerated approval for pemigatinib, an FGFR2 inhibitor, as the first targeted therapy for locally advanced or metastatic iCCA with FGFR2 fusions or rearrangement [18]. Another approval for a targeted therapy specifically for CCA was on 28 May 2021 [19]. The FDA granted accelerated approval to infigratinib, a kinase inhibitor approved for adults with previously treated, locally advanced, unresectable, or metastatic CCA with an FGFR2 gene fusion or rearrangement [19]. This specific approval for CCA required confirmation that an FDA-approved diagnostic test detected FGFR2 gene fusion or rearrangement in CCA [19]. More recently, the FDA granted accelerated approval to futibatinib, an irreversible FGFR1-4 Inhibitor, for previously treated, locally advanced, unresectable, or metastatic CCA with an FGFR2 gene fusion or rearrangement on 30 September 2022 [20]. In support of the recent developments in targeted therapies, in August 2021, the FDA approved ivosidenib as a targeted therapy for adult patients with unresectable locally advanced or metastatic CCA with a mutation in the IDH1 gene detected by an FDA-approved diagnostic test [21]. Finally, ALK and ROS1 mutations occur in between 3% and 9% of patients with CCA, and ALK-positive and ROS1-positive CCA may also be treated with ALK inhibitors [22].
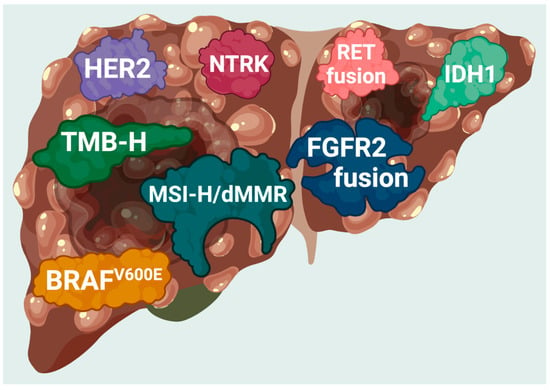
Figure 1.
FDA-approved targets found in patients with cholangiocarcinoma. Current genomic targets with FDA-approved therapies in cholangiocarcinoma. MSI-H, microsatellite instability-high; dMMR, deficiency in mismatch repair; TMB-H, tumor mutation burden-high (≥10 mutations/mb).

Table 1.
The frequency of genetic alterations according to the anatomic location of CCA.
There are also several tumor-agnostic approvals that encompass CCA. For example, in May 2017, the FDA granted accelerated approval for the therapeutic monoclonal antibody, pembrolizumab, for microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) unresectable or metastatic solid tumors that have progressed despite prior treatment [36]. In 2020, pembrolizumab received FDA approval for adults and children with high tumor mutational burden (TMB-H) solid tumors [37]. In August 2021, accelerated approval was granted for dostarlimab for adult patients with recurrent or advanced solid tumors identified as mismatch repair deficient (dMMR) as determined by an FDA-approved diagnostic test [38]. In 2018 and 2019, two therapies targeting neurotrophic tyrosine receptor kinase (NTRK) gene fusion, entrectinib and larotrectinib, were approved for locally advanced or metastatic solid tumors [39,40]. In June 2022, the FDA approved dabrafenib combined with trametinib to treat unresectable or metastatic solid tumors with a BRAFV600E mutation [41].
This review aims to present and discuss the advances in molecular targeted therapy for patients with advanced CCA.
2. The Options for Molecular Targeted Therapy
2.1. Neurotrophic Tyrosine Receptor Kinase (NTRK) Gene Fusion-Positive Cholangiocarcinoma (CCA)
Molecular profiling of solid tumors has identified clinically actionable fusions of the NTRK1, NTRK2, and NTRK3 genes, which encode neurotrophic tropomyosin receptor kinase (NTRK) [42]. NTRK fusion products activate the TRK gene and, subsequently, the downstream signaling pathways, PI3K and MAPK, leading to tumor cell proliferation and invasion [43,44]. Therefore, NTRK inhibitors are promising targeted therapies for patients with NTRK fusion-positive cancers and have shown antitumor responses in NCSLC, melanoma, and other tumors [43,44]. NTRK gene fusions have been identified in 1–3% of patients with CCA [45]. Table 2 shows the results of pivotal clinical trials that assessed the outcomes of NTRK inhibitors in NTRK fusion-positive metastatic or unresectable locally advanced solid tumors.

Table 2.
Examples of targeted therapy trials.
Larotrectinib is a first-generation, highly selective pan-NTRK competitive inhibitor that suppresses cancer cell proliferation [62]. It has shown immediate, robust, and long-lasting anticancer efficacy in pediatric and adult patients with solid tumors harboring TRK fusions [49]. Drilon and colleagues conducted a phase 1 trial in adults (NCT02122913), a phase 1/2 trial in children (NCT02637687), and a phase 2 trial in children and adults (NCT02576431) with locally advanced or metastatic solid tumors who had received previous standard systemic therapy and were then given larotrectinib (100 mg twice daily), see Table 1 [49]. A total of 55 patients with 17 TRK fusion-positive tumor types included two patients (4%) with CCA [49]. The objective response rate (ORR) was high, at 80% (95% CI, 67–90) [49]. At one year, 55% of patients were still progression-free [49]. The median duration of response (MDR) and the progression-free survival (PFS) remain unmet [49]. The most common toxicities ≥ grade 3 included a raised ALT or AST level (9%), anemia (3.6%), reduced neutrophil count (3.6%), and nausea (3.6%) [49]. Based on these findings, larotrectinib was granted accelerated approval by the FDA in November 2018 for adult and pediatric patients with NTRK-positive solid malignant tumors, either metastatic or where surgical resection is unfeasible due to severe morbidity, who have progressed on systematic therapy [39]. Larotrectinib is also approved for patients with no satisfactory alternative treatments [39]. Currently, the MD Anderson Cancer Center and the National Cancer Institute (NCI) are conducting a phase 2 trial to investigate the efficacy of larotrectinib in previously treated patients with locally advanced or metastatic solid tumors and NTRK gene amplification (NCT04879121). Another ongoing phase 2 trial aims to assess the efficacy of larotrectinib in pediatric patients with relapsed or refractory advanced solid tumors with NTRK gene fusion (NCT03213704), Table 3.

Table 3.
Examples of ongoing trials in CCA.
Entrectinib is another selective pan-TRK inhibitor with activity against ROS1 and ALK [63,64]. In two phase I studies (ALKA-372–001 and STARTRK-1), entrectinib was administered to 119 patients with relapsed or refractory advanced/metastatic solid tumors harboring NTRK1/2/3, ROS1, or ALK gene fusions [47]. Entrectinib was well-tolerated and only 15% of patients required a dose modification [47]. The most common grade ≥ 3 toxicities included fatigue/asthenia (4%), weight increase (2%), diarrhea (1%), and eosinophilic myocarditis (1%), Table 2 [47].
The phase 2 STARTRK-2 trial was an open-label, multicenter, global basket study that included patients with solid tumors harboring NTRK1/2/3, ROS1, or ALK gene fusions (NCT02568267). A focused integrated analysis on NTRK fusion-positive tumors showed that at a median follow-up of 12.9 months (interquartile range (IQR), 8.77–18.76), the median duration of response was 10 months (95% confidence interval (CI), 7.1–not reached) and the objective response rate (ORR) was 57% (95% CI, 43.2–70.8) [48]. The median overall survival (OS) was 21 months (95% CI, 14.9–NE) and the median progression-free survival (PFS) was 11.2 months (95% CI, 8.0–14.9) [48]. Major adverse events (≥3 grade) were reported in 61.6% of patients and included anemia (12%), an increase in weight (10%), and fatigue (7%), with no patient mortality, Table 2 [48]. Based on these findings, entrectinib gained accelerated approval by the FDA in August 2019 at a dose of 600 mg once daily [40]. Approval was for use in patients with NTRK gene fusion and metastatic or unresectable locally advanced solid tumors, who have progressed on systemic therapy or have no satisfactory alternative treatment [40].
The approval of entrectinib provides an additional treatment option for patients with advanced cancer, potentially creating the opportunity for patients and their physicians to choose between different therapies [65]. Larotrectinib is available in a liquid formulation approved for children younger than 12 years old, whereas entrectinib is not. However, entrectinib may be effective for children with brain tumors, while the efficacy of larotrectinib for primary and metastatic brain tumors is currently being evaluated [65]. The two drugs also have different side effect profiles. The adverse events seen most frequently in the larotrectinib trials include increased ALT or AST, fatigue, and vomiting. Warnings and precautions for larotrectinib include neurotoxicity, hepatotoxicity, and embryo–fetal toxicity. Entrectinib has additional warnings and precautions, including congestive heart failure, CNS effects, and skeletal fractures [65]. The National Comprehensive Cancer Network (NCCN) guidelines recommend larotrectinib and entrectinib as first-line or subsequent-line (following disease progression) treatment options for unresectable or metastatic iCCA and eCCA with NTRK gene fusions. Both entrectinib and larotrectinib are approved in the United States and Europe for the treatment of unresectable or metastatic solid tumors with NTRK gene fusion and progression after previous therapy [66,67]. However, there are currently limited data for patients with CCA.
2.2. Cholangiocarcinoma (CCA) with BRAFV600E Mutations
Mitogen-activated protein kinase (MAPK) signaling is essential for cell growth and survival through the RAS/RAF/MEK/ERK pathway [68]. The BRAF gene is an oncogene whose protein product upregulates the RAS/RAR/MEK pathway [68]. BRAF mutations have been identified in several solid malignancies, including colorectal cancer, NSCLC, and melanoma [69,70]. More than 50 different BRAF mutations have been reported, with the V600E point mutation being the most common mutation (BRAFV600E) [71]. In BRAFV600E, valine (V) is substituted by glutamic acid (E) at amino acid 600, resulting in activating BRAF with subsequent tumor growth and spread [72]. In CCA, BRAF mutations are uncommon, occurring almost exclusively in iCCAs, with a prevalence ranging between 5% and 7%, and with the BRAFV600E mutation in 1.5% of patients with iCCA [26,73].
Dabrafenib is a competitive inhibitor of the RAF protein, which causes apoptosis by decreasing downstream phosphorylation of MEK and ERK, arresting the cell cycle in G1, and activating caspase-3/7 [74,75]. Even if a tumor initially responds to dabrafenib alone, it may eventually become resistant to treatment if another pathway activates the MEK protein [75]. Trametinib is a selective inhibitor of MEK1/MEK2 and is used with dabrafenib, which prevents tumors from using this escape mechanism [76]. Trametinib reduces cell proliferation, causes G1 cell-cycle arrest, and induces apoptosis [77]. The combination of these drugs in targeting MEK and BRAF has yielded promising results (Table 2). In the phase 2, single-arm, open-label trial ROAR, the BRAF cohort included 43 adult patients with BRAFV600E-mutated CCA with metastatic, locally advanced, unresectable, or recurrent disease that had progressed on prior therapy [50]. Patients received trametinib 2 mg once daily and dabrafenib 150 mg twice daily, with a mean follow-up of 10 months [50]. The ORR was 51% (95% CI, 36–67), the mean OS was 14 months (95% CI, 10–33), the mean PFS was 9 months (95% CI, 5–10), and the MDR was 9 months (95% CI, 6–14) [50]. Increased gamma-glutamyl transferase (GGT) (12%), low WBC count (7%), and pyrexia (7%) were the most common grade ≥ 3 adverse events, Table 2 [50].
Salama et al. reported results from the NCI-MATCH trial, a single-arm, open-label study that enrolled 29 patients with different solid tumors that progressed on standard lines of therapy, including four patients with iCCA [51]. Patients were given a continued dosing of dabrafenib (150 mg twice a day) and trametinib (2 mg once daily) [51]. The ORR was 38% (90% CI, 22.9 –54.9%), the median OS was 28.6 months, the median PFS was 11.4 months (90% CI, 8.4–16.3), and the median MDR was 25.1 months (90% CI, 12.8–NE) [51]. Three of the four patients with CCA had a partial response and grade ≥ 3 adverse events occurred in 65.7% of the enrolled patients [51]. Fatigue (11.4%), decreased neutrophil count (8.6%), and decreased WBC count (8.6%) were the most frequently reported grade ≥ 3 adverse events associated with the treatment, Table 2 [51]. The results of these trials supported the FDA approval of the combination of dabrafenib and trametinib for treating adults and children >6 years with BRAFV600E mutation-positive, unresectable, or metastatic solid tumors who have progressed on prior therapy [41].
Other BRAF Inhibitors Currently Undergoing Clinical Trials
In a phase 1 study, patients with BRAFV600-mutated solid tumors (including CCA) are currently being evaluated for a response to ABM-1310, a selective inhibitor of BRAFV600E mutation tumors (NCT04190628). Patients with advanced solid tumors, including biliary tract cancers, with BRAF mutations, are the focus of a phase 1 study of BGB-3245, a second-generation BRAF inhibitor (NCT04249843), Table 3. Combining the selective ERK1/2 inhibitor JSI-1187 with a BRAF inhibitor is another potential study strategy.
Despite the significant advances in managing patients with BRAFV600 mutations, further studies are required. For example, in studying the efficacy of dabrafenib and trametinib and concurrent mutations of TP53 and BRAFV600E, early studies reported that this was associated with a more aggressive disease, resulting in less clinical benefits from dabrafenib and trametinib [78]. In addition, patients with BRAFV600E/TP53 mutations were associated with reduced PFS and OS [79].
2.3. Cholangiocarcinoma (CCA) with Fibroblast Growth Factor Receptor 2 (FGFR2) Gene Fusion or Rearrangement
Alterations in the FGFR gene and dysregulated FGFR signaling play a role in the development and progression of several types of cancer, including CCA. Four receptors belong to the FGFR family, including FGFR1, 2, 3, and 4, which share a cytoplasmic tyrosine kinase domain [80]. FGFR2 alterations include rearrangements, amplifications, and mutations, present in 10–16% of patients with iCCA [33]. These alterations activate mitogen-activated protein kinases (MAPKs), triggering constitutive signaling cascades that prompt tumor cell proliferation, survival, migration, and angiogenesis [81,82]. Therefore, FGFR inhibitors are promising targeted therapies that can potentially improve the survival of patients with CCA. Initially, non-selective tyrosine kinase inhibitors (TKIs) were investigated in phase 1/2 clinical trials and showed low antitumor activity and a limited survival benefit [83,84,85]. More recently, selective FGFR inhibitors were introduced for patients with FGFR2 fusion-positive iCCA and resulted in a significant clinical response, prompting phase 2/3 trials and accelerated FDA approvals [18].
Pemigatinib is a selective inhibitor of FGFR1, 2, and 3 that competitively inhibits the autophosphorylation and FGFR-mediated signaling cascades in tumor cells [86]. In the phase 2, multicenter, open-label FIGHT-202 trial, previously treated patients with metastatic CCA with FGFR2 fusions or FGFR2 rearrangements (n = 107), other FGFR mutations (n = 20), or wild-type FGFR (n = 18) received 13.5 mg of pemigatinib once daily on day 1–14 of a 21-day cycle [52]. In patients with FGFR2 fusions or FGFR2 rearrangements, the objective response rate (ORR) was 35.5% (95% CI, 26.5–45.4%) [52]. The median PFS and OS were 6.9 and 21.1 months, respectively [52]. Up to 64% of the patients had grade ≥ 3 toxicities that included hyper/hypophosphatemia (12%), arthralgia (6%), fatigue (5%), and retinal detachment (4%), Table 2 [52]. Based on these results, pemigatinib became the first FDA-approved targeted therapy for previously treated metastatic CCA with FGFR2 fusions or FGFR2 rearrangements [18]. Currently, FIGHT-302 is an ongoing phase 3 clinical study comparing pemigatinib with gemcitabine and cisplatin chemotherapy to determine the drug’s efficacy in the first-line treatment of CCA (NCT03656536), Table 3.
Infigratinib is another highly selective ATP-competitive FGFR1–3 inhibitor that showed promising antitumor activity in patients with FGFR2 fusions in early-phase trials [87]. Patients with advanced iCCA resistant to chemotherapy were enrolled in a phase 2 study of infigratinib in a multicenter, open-label, phase 2 trial [53]. Of the 122 enrolled patients, 108 had positive FGFR2 fusions or FGFR2 rearrangements [53]. Patients received 25 mg once daily infigratinib for 21 days in a 28-day cycle [53]. In patients with FGFR2 fusions or FGFR2 rearrangements, the ORR was 23.1% (95% CI, 15.6–32.3%), indicating clinically significant activity after treatment [53]. In this trial, one patient had a complete response (CR) and 24 patients had partial responses [53]. The median duration of response (DOR) was five months (95% CI, 3.7–9.3), and eight patients had a maintained response for more than six months [53]. Two-thirds of the patients (66.2%) had grade ≥ 3 toxicities, with hypophosphatemia (14.1%) and hyperphosphatemia (12.7%) being the most common adverse events, Table 2 [53]. A phase 1 dose-escalation study showed that the risk of hyperphosphatemia in patients receiving infigratinib increased with higher drug exposure and was associated with higher antitumor activity [88]. On May 2021, the FDA granted accelerated approval to infigratinib for previously treated locally advanced CCA or for patients with metastatic CCA with FGFR2 fusions or FGFR2 rearrangements [19].
Infigratinib is also a potential targeted therapy for patients with untreated CCA with FGFR2 fusions or FGFR2 rearrangements. PROOF-301 is an ongoing phase 3 trial that recruited patients with untreated locally advanced CCA or patients with metastatic CCA with FGFR2 fusions or FGFR2 rearrangements [89]. In PROOF-301, patients received either infigratinib or a standard chemotherapy regimen of gemcitabine and cisplatin [89]. Infigratinib may potentiate the apoptotic activities of chemotherapies in multidrug-resistant tumor cells [90]. Therefore, there is a potential future role for infigratinib in combination therapy for patients with advanced CCA, and the results of further clinical trials are awaited with interest.
Futibatinib is an irreversible FGFR1-4 inhibitor that binds to the conserved cysteine residues of the P-loop of the kinase domain [91]. Previous studies showed that futibatinib exhibited highly selective antitumor activities against tumor cells harboring FGFR mutations, particularly against mutations commonly associated with resistance to ATP-dependent FGFR inhibitors [91]. In addition, the oral futibatinib molecule was associated with a comparatively lower number of drug-resistant clones than ATP-dependent FGFR inhibitors [91,92]. Futibatinib was studied in a phase I trial that recruited 197 patients with previously-treated advanced solid tumors, 83 of which had CCA with a mutation, fusion, or amplification of FGFR2 (NCT02052778) [92]. In this trial, 64 patients were treated with futibatinib at 20 mg and 19 patients at 16 mg [92]. The results showed that futibatinib at 20 mg daily resulted in an ORR of 15.6%, a disease control rate (CDR) of 71.9%, a median DOR of 5.3 months, and a median PFS of 5.1 months [92]. The updated analysis of the single-arm FOENIX-CCA2 phase 2 trial included 103 patients with previously-treated advanced or metastatic iCCA with FGFR2 fusions or FGFR2 rearrangements (NCT02052778) [54]. Futibatinib 20 mg daily led to an ORR of 41.7% at a median follow-up period of 25.0 months [54]. The DC was 82.5%, the median PFS was 8.9 months, and the median OS was 20.0 months, Table 2 [54]. Based on these findings, on September 30, 2022, the FDA granted accelerated approval for futibatinib for adult patients with previously treated locally advanced or metastatic iCCA with FGFR2 gene fusion or rearrangement [20].
Derazantinib is another potent anti-FGFR1-3 that showed promising antitumor activity in iCCA harboring FGFR2 fusions or FGFR2 rearrangement. In the phase 2 FIDES, 143 patients with iCCA harboring FGFR2 fusions (n = 103) or FGFR2 mutations or amplifications (n = 40) received derazantinib 300 mg once daily [93]. In the cohort with FGFR2 fusions, the ORR was 21.4% (95% CI 13.9–30.5), with a median PFS and OS of 8.0 (95% CI 5.5–8.3) and 17.2 (95% CI 12.5–22.4) months, respectively [93]. In the FGFR2 mutations or amplifications cohort, the ORR and DCR were 6.5% (95% CI 0.8–21.4) and 58.1% (95% CI 39.1–75.5). The median PFS was 8.3 (95% CI 1.9–16.7) and the median OS was 15.9 (95% CI 8.4–not estimated) [93]. The most common grade ≥ 3 adverse events in the overall cohort were hyperphosphatemia (3%), asthenia/fatigue (5%), nausea (1%), and transaminase elevations (12%) [93].
The phase 1/2 ReFocus trial evaluated RLY-4008, a selective FGFR2 inhibitor that can target FGFR resistance mutations, in CCA patients with FGFR2 fusions or FGFR2 rearrangements who did not receive FGFR inhibitors before. The preliminary analysis of 38 patients showed an ORR of 63.2% (95% CI 46.0–78.2) and a DCR of 94.7%. There was no observation of grade 4/5 adverse events [94].
Despite the promising findings of selective FGFR inhibitors in patients with CCA and FGFR2 fusions or FGFR2 rearrangements, several issues remained unanswered. More than 150 fusion partners are associated with FGFR2 gene rearrangement, which results in significant molecular diversity in patients with FGFR2 fusions and rearrangements [95,96]. Furthermore, nearly 50% of the gene fusion and rearrangement partners are present within the same chromosome of the FGFR2 gene [95,96].
It is still unclear which patients might respond to FGFR2-targeted therapies and whether fusion partners can affect the response and survival with FGFR2-targeted therapies [96]. Therefore, future research should focus on the effect of combined genetic alterations on the responses to FGFR2 inhibitors and their role in the development of acquired resistance, as well as identifying reliable response biomarkers for FGFR2-targeted therapies [96,97].
2.4. High Tumor Mutational Burden (TMB-H) as a Predictive and Prognostic Biomarker
TMB is a recently identified biomarker of the response to immune checkpoint inhibitors (ICIs) in several types of cancer [97]. The TMB is the number of somatic mutations per megabase (Mb) of the genomic sequence of a tumor [97]. A TMB score of ≥10 mutations/Mb has been proposed as a threshold with a high likelihood of neoantigen formation and represents TMB-H status [97]. In patients with several tumor types, including melanoma, NSCLC, and bladder cancer, patients with TMB-H had better outcomes when treated with programmed death protein-1 and programmed cell death ligand-1 (PD-1/PD-L1) checkpoint inhibitors, or a cytotoxic T lymphocyte antigen 4 (CTLA-4) blockade [98,99,100]. TMB-H has been detected in 27.3% of patients with iCCA [101].
Pembrolizumab is a humanized antibody that inhibits the PD-1 receptors on lymphocytes by inhibiting the ligands that would block the receptor and inhibit an immune response [102,103,104]. In a subgroup analysis of 102 patients with TMB-H, who were enrolled in the phase 2 KEYNOTE-158 trial and received pembrolizumab, the ORR was 29% (95% CI, 21–39%), the median OS was 11.7 months (95% CI, 9.1–19.1), and the median PFS was 2.1 months (95% CI, 2.1–4.1) [105]. Notably, there were no patients with CCA in the TMB-H subgroup of this trial [105]. However, based on these findings, in 2017, the FDA approved pembrolizumab to treat adult and pediatric patients with unresectable or metastatic solid tumors (including CCA) [36]. The indications for this approval also required that the tumor tissue was TMB-H (≥10 mutations/megabase), and that the patients had progressed following prior therapy and for whom there were no satisfactory alternative treatment options [36].
However, it is unclear if the TMB levels for predicting the response to the PD-1 blockade are consistent throughout the spectrum of solid tumors. There are situations in which a high TMB does not indicate a response. Therefore, novel biomarkers are required that reflect the complexity of the tumor immune microenvironment and consider the effects of tumor mutations on the immune response.
2.5. High Microsatellite Instability and Mismatch Repair Deficient (MSI-H/dMMR) Cholangiocarcinoma (CCA)
The production of neoantigens and CD8+ T cell infiltrations into the tumor microenvironment are both increased in tumors with dMMR or high levels of MSI [106]. MSI-H/dMMR makes the errors produced during DNA replication difficult to repair, which leads to mutations [106]. The prevalence of MSI-H/dMMR in patients with iCCA ranges from 4.7–18.2% [35].
As part of the phase 2 KEYNOTE-158 study, pembrolizumab 200 mg intravenously once every three weeks was administered to 233 patients with advanced solid tumors and confirmed MSI-H/dMMR [56]. In this study, 22 (9.4%) patients had CCA [56]. With a median follow-up of 13.4 months, the ORR was 34.3% (95% CI, 28.3–40.8%), the median OS was 23.5 months (95% CI, 13.5–NR), and the median PFS was 4.1 months (95% CI, 2.4–4.9 months [56]. The median duration of the response was not reached (range, 2.9–31.3 months) [56]. A subgroup analysis of CCA showed that the ORR was 40.9% (95% CI, 20.7–63.6), the median OS was 24.3 months (95% CI: 6.5–NE), and the median PFS was 4.2 months (95% CI, 2.1–NE) [56]. Adverse events ≥ grade 3 occurred in 34 patients (14.6%) [56]. Pneumonitis (1.3%), severe skin reactions (1.3%), and colitis (0.9%) were the most common adverse events, Table 2 [56].
In an open-label, single-arm, phase 2 clinical trial, 86 patients with 12 types of cancer, including four patients with CCA, with at least one prior cancer therapy and MSI-H/dMMR mutations, were included [55]. The patients received 10 mg/kg of pembrolizumab every 14 days [55]. The ORR was 53% (95% CI, 42–64%), the 2-year OS was 64% (95% CI, 53–78%), and the 2-year PFS was 53% (95% CI, 42–68%) [55]. A subgroup analysis of patients with CCA showed that the ORR was 50%, the CR was 25%, the SD was 75%, and the disease control rate was 100% [55]. Minor adverse events were reported in 20%, with the most common being diarrhea/colitis (6%), pancreatitis/hyperamylasemia (6%), fatigue (2%), and anemia (2%), Table 2 [55]. Based on these findings, in 2020, the FDA approved the use of pembrolizumab in adults and pediatric patients who have unresectable or metastatic MSI-H/dMMR solid tumors that have progressed despite prior treatment, without satisfactory alternative treatment options [37]. The results of other trials for MSI-H patients in solid tumors are awaited. For example, a phase 1/2 trial of pevonedistat, a selective NEDD8 inhibitor, in combination with pembrolizumab in patients with dMMR/MSI-H solid cancers is ongoing (NCT04800627), Table 3.
Dostarlimab is another anti-PD-1 monoclonal antibody [107]. The phase 1 GARNET study was a non-randomized, multicenter, open-label trial to evaluate the safety and efficacy of dostarlimab (500 mg every 3 weeks for four cycles, then 1000 mg every 6 weeks) in 106 patients with advanced solid tumors (two of whom had CCA) with confirmed MSI-H/dMMR, one of whom had a biliary tract cancer [57]. The ORR was 38.7% (95% CI, 29.4–48.6) [57]. With a median duration of follow-up of 12.4 months, the median DOR was not reached [57]. At 12 months, the Kaplan–Meier estimate for the chance of response preservation was 91%, and at 18 months it was 80% [57]. Approximately 8.3% of patients reported at least one grade 3 adverse event, including anemia (3.9%), elevated lipase (2.3%), elevated ALT (1.6%), and diarrhea (1.6%), Table 2 [57]. Based on the findings from this study, the FDA approved dostarlimab in adult patients with MSI-H/dMMR recurrent or advanced solid tumors that progressed on or following prior treatment and were not candidates for satisfactory alternative treatment options [38].
Given the limited single-agent activity and the limited number of patients with CCA included in these clinical trials, further studies are required to investigate novel immunotherapy combinations to enhance the treatment efficacy in patients with CCA.
2.6. Isocitrate Dehydrogenase Isoenzyme (IDH1) Gene Mutations in Cholangiocarcinoma (CCA)
IDH1 gene mutations commonly occur in CCA [108]. Missense mutations in the IDH1 R132 codon leads to the overproduction of the oncometabolite R-2-hydroxyglutarate (R-2HG) [109]. In tumor progenitor cells, an increase in the R-2HG levels inhibits cellular differentiation and drives oncogenesis by promoting histone methylation and DNA methylation [110]. The prevalence of an IDH1 mutation in iCCA has been estimated at 13% [34].
Ivosidenib is a small molecule inhibitor of IDH1. In a phase 1, multicenter, open-label study, 73 patients with IDH1-mutant CCA (89% iCCA and 11% eCCA), refractory to other systemic therapy, were enrolled and received ivosidenib (200–1200 mg daily in 28-day cycles) [58]. The ORR was 5% (95% CI, 1.5–13.4), the median OS was 13.8 months (95% CI, 11.1–29.3), and the median PFS was 3.8 months (95% CI, 3.6–7.3) [58]. Approximately 23% of the included patients had grade ≥ 3 adverse events, including ascites (5%), anemia (4%), and fatigue (3%), Table 2 [58]. This trial led to a multicenter, randomized, double-blind, placebo-controlled phase 3 study (ClarIDHy), which included 185 adult patients with advanced CCA with IDH1 mutations who had progressed on previous therapy [34]. Patients were randomly assigned to oral ivosidenib 500 mg or matched placebo once daily in continuous 28-day cycles [34]. In the intervention group, with a median follow-up of 6.9 months, the ORR was 2% (95% CI, 0.5–6.9), and the median PFS was significantly improved (median PFS 2.7 months; 95% CI, 1.6–4.2) [34]. The median OS after accounting for the cross-over was 10.3 months (95% CI, 7.8–12.4), which was significantly better than the placebo (median OS = 5.1 months) [34]. Grade ≥ 3 adverse events were reported in 30% of the patients [34]. The most frequently reported adverse event grades ≥ 3 were ascites (7%), increased AST (5%), anemia (3%), and fatigue (3%), Table 2 [34]. Based on this trial, the FDA approved ivosidenib for the treatment of adult patients with unresectable locally advanced or metastatic IDH1-mutated CCA who had been previously treated [21].
PARP inhibitors are also being studied in this subgroup of patients as they exhibit dysregulated homologous recombination repair. The National Cancer Institute (NCI) is conducting a phase 2 clinical trial that aims to investigate the safety and efficacy of olaparib as a subsequent line therapy for patients with advanced solid tumors (including CCA) with IDH1 or IDH2 mutations (NCT03212274). Another ongoing study combined ceralasertib and olaparib in patients with refractory CCA and advanced solid tumors with IDH1/2 (NCT03878095), Table 3.
The use of IDH1 inhibitors as single treatments has shown promising results in targeted therapy of malignancies harboring IDH1 mutations in preclinical and clinical settings. However, these studies are preliminary and currently include small numbers of patients with CCA.
2.7. Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2)/Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Cholangiocarcinoma (CCA)
The ERBB2 gene encodes HER2, a receptor tyrosine kinase found in the plasma membrane [111]. ERBB2 triggers several signaling pathways involved in tumor growth [112]. Tumorigenesis is associated with HER2 and MAPK pathway dysregulation [32,113]. The overexpression of HER2 has been reported in 5.8% of iCCA and 13–20% of eCCA [31,32].
Pertuzumab and trastuzumab are monoclonal antibodies that target HER2 and are used to treat HER2-positive malignancies [114]. The combination of pertuzumab and trastuzumab suppresses HER2-AKT signaling by inhibiting both the ligand-induced and ligand-independent HER2-HER3 complex formations [114]. A phase 2, single-arm, multicenter trial called MyPathway included 39 patients with previously treated HER2-positive metastatic CCA [59]. Patients were treated with pertuzumab (840 mg loading dose and 420 mg every three weeks) plus trastuzumab (8 mg/kg loading dose, 6 mg/kg every three weeks) [59]. The ORR was 23% (95% CI, 11–39), the median PFS was 4.0 months (95% CI, 1.8–5.7), and the MDR was 10.8 months (95% CI, 0.7–25.4) [59]. Grade ≥3 adverse events were reported in 46% of patients, with the most common being an increase in the ALT and AST (13%) and an increase in ALP (10%), Table 2 [59]. These were promising results for patients with HER2-mutated CCA. However, regulatory approval for patients with CCA is awaited.
More recently, trastuzumab deruxtecan (T-DXd) showed promising antitumor activity in HER2-positive advanced solid tumors, including CCA. In a phase 1 trial of 60 patients with advanced, non-breast/non-gastric, HER2-mutant solid tumors (NCT02564900), the ORR was 28.3% and the median PFS was 7.2 (95% CI 4.8–11.1) months [115]. The phase 2 HERB trial recruited 32 patients (24 with HER2-positive and eight with HER2-low) BTCs who received T-DXd. The efficacy cohort included 22 patients (9 had CCA). In patients with HER2-positive BTCs, the ORR was 36.4%, the median PFS was 4.4 months, and the median OS was 7.1 months. In the HER2-low group, the ORR, median PFS, and median OS were 12.5%, 4.2 months, and 8.9 months, respectively. The rate of grade ≥ 3 adverse events was 81.3% [116].
Several ongoing studies aim to investigate targeted HER2 agents in treating patients with CCA with ERBB2 mutations (Table 3). In the front-line setting, gemcitabine combined with cisplatin and trastuzumab and the combination of gemcitabine, cisplatin, and varlitinib are currently being studied in two clinical trials (NCT03613168 and NCT02992340). In addition, HER2-targeting agents are currently being studied for subsequent lines of therapy, either as monotherapy or in combination with standard chemotherapy agents. Ongoing trials include: the oral HER2 covalent inhibitor, TAS0728, (NCT03410927); the HER2 antibody-drug conjugate, trastuzumab deruxtecan (NCT04482309 and JMA-IIA00423); the antibody-drug conjugate, RC48-ADC, (NCT04329429); capecitabine plus the dual HER2 and EGFR inhibitor, varlitinib (NCT03093870); chemotherapy (5-FU or IRI or Cape) plus trastuzumab (NCT03185988); and chemotherapy plus the HER2-targeted bispecific antibody, zanidatamab (NCT02892123).
The results of these ongoing trials may provide multiple options for targeted treatments for patients with CCA in the molecular subgroup and improve patient survival.
2.8. RET Gene Fusion-Positive Cholangiocarcinoma
More than three decades have passed since the discovery of the gene encoding the receptor tyrosine kinase, RET [117]. RET rearrangements and mutations are recognized as treatable drivers of oncogenesis [117]. Certain RET fusion proteins and activating point mutations can drive oncogenesis and tumor progression by activating downstream signaling pathways, leading to uncontrolled cell proliferation [117]. RET gene fusions are present in between 1% and 2% of NSCLC and thyroid cancers and represent potential targets for therapeutic inhibition of RET kinase [118]. However, RET fusions seem rare in CCA, and data regarding their exact prevalence in CCA are limited [119].
Pralsetinib is a selective inhibitor of the RET receptor tyrosine kinase. In the phase 1/2, open-label ARROW trial, 29 patients with RET fusion-positive solid tumors were included, with three patients with CCA [60]. Patients received a starting dose of pralsetinib of 400 mg QD [60]. The ORR was 57% (95% CI, 35–77%), the median OS was 13.6 months (95% CI, 7.5–NE), the median PFS was 7.4 months (95% CI, 5.1–13.6), and the median duration of response was 11.7 months (95% CI, 5.5–19.0) [60]. Altogether, 69% of patients had grade ≥ 3 adverse events that included neutropenia (31%), anemia (14%), and increased AST (10%), Table 2 [60]. Based on the findings of this trial, the FDA approved pralsetinib for adult and pediatric patients with advanced or metastatic RET-fusion-positive lung and thyroid cancers for whom systemic therapy is indicated [120].
Selpercatinib is another highly selective inhibitor of the RET receptor tyrosine kinase, with CNS activity [121]. In the phase 1/2, open-label LIBRETTO-001 trial, 45 patients with RET fusion-positive solid tumors other than lung or thyroid tumors were included, with two patients with CCA [61]. Of the 45 patients, 43 received a starting recommended dose of 160 mg BID. In the 41 patients who were evaluated for efficacy, the ORR was 43.9% (95% CI, 28.5–60.3), the median OS was 18.0 months (95% CI, 10.7– NE), the median PFS was 13.2 months (95% CI, 7.4–26.2), and the median duration of response was 24.5 months (95% CI: 9.2–NE). One patient with CCA was evaluated for efficacy; the ORR was 100% and the duration of response was 5.6 months [61]. Altogether, 49% of patients had grade ≥ 3 adverse events that included hypertension (22%), increased ALT (16%), and increased AST (13%), Table 2 [61]. Selpercatinib is currently approved for locally advanced or metastatic RET-fusion-positive solid tumors [122].
Again, further studies are needed to focus on CCA to validate these findings in this group of patients.
3. Liquid Biopsy for Assessment of Circulating Tumor DNA (ctDNA) and Cholangiocarcinoma (CCA)
Liquid biopsies refer to blood sampling to detect circulating tumor DNA (ctDNA), circulating cell-free RNA (ccfRNA), and cell-free DNA (cfDNA) [123,124]. Liquid biopsy as an adjunctive diagnostic method has gained popularity over the last decade due to its potential benefits for cancer patients [123]. The most commonly interrogated element in liquid biopsies is ctDNA or DNA fragments produced from tumors [125]. Treatment resistance tracking, response monitoring, target selection, relapse detection, and early diagnosis are the potential benefits of identifying tumor-derived material in liquid biopsies [126].
Although liquid biopsy is a potentially useful diagnostic tool for patients with CCA, this modality remains underexplored in CCA. Advances in this diagnostic area for patients with CCA have been limited by several practical factors, including the small amounts of ctDNA shed into the bloodstream in patients with localized tumors [127]. On the other hand, CCA is an internal malignancy and it is often difficult to obtain a tissue biopsy. It is also challenging to acquire sufficient tumor cells for diagnosis on aspiration cytology, and these challenges can prevent adequate molecular tumor profiling for CCA [128]. Therefore, blood-derived ctDNA may play an important role in identifying molecular alterations in patients with CCA who may not have an adequate biopsy or cytology sample available for analysis [129]. Consistent mutation findings between tumor tissue and ctDNA were reported in 2015 by Zill et al. in a prospective study of 26 pancreaticobiliary cancers, including eight patients with CCA [130]. In this preliminary study, 93% of mutations found in tissue samples were also identified by cfDNA [130]. In 2019, Mody et al. analyzed ctDNA from 138 patients with biliary tract cancers and identified genetic alterations in 89% of the samples [131].
Recently, Kumari et al. evaluated the diagnostic role of cfDNA in gallbladder carcinoma [132]. Serum was obtained from 34 patients with gallbladder carcinoma and 39 matched controls without malignancy [132]. This study showed that the cfDNA levels were lower in healthy individuals than in patients with gallbladder cancer [132]. In addition, there was a significant correlation between the cfDNA and the presence of jaundice, lymph node metastases, and overall disease TNM stage [132]. Therefore, the quantitative analysis of cfDNA may have the potential to be a unique marker for the molecular detection of targeted therapies in CCA. Furthermore, the analysis of cfDNA may have a role in distinguishing between neoplastic and inflammatory conditions of the gallbladder and biliary tract identified by imaging but without available biopsy material. However, concerns remain about the overall sensitivity of ctDNA mutations for diagnosing early-stage CCA [133].
An additional feature of ctDNA/cfDNA is to track the development of resistance to chemotherapy and targeted treatments [134]. In 2019, Ettrich and colleagues sequenced 15 common gene mutations in ctDNA samples from patients with biliary tract cancer throughout their chemotherapy [135]. In the iCCA cohort (n = 13), there was a 92% agreement between tissue samples and blood-derived ctDNA [135]. The level of agreement for the overall cohort was 74% [135]. A change in the mutational profile was also seen in 63% of chemotherapy-naive individuals after treatment [135]. A study reported in 2017 showed that the integrative genomic analysis of cfDNA could identify acquired treatment resistance due to multiple recurrent point mutations in the FGFR2 kinase domain during tumor progression [136].
4. Conclusions
Until recently, clinical studies considered CCA a homogeneous entity, which may have led to the limited antitumor activity of conventional treatment regimens. It is now apparent that the behavior and responses of CCA vary substantially according to the underlying molecular profile. These relatively recent findings highlight the crucial role of precision medicine in guiding the treatment selection for patients with CCA.
The number of investigational and approved targeted therapies for CCA has increased exponentially in the past decade. Several targeted agents are now approved as first-line and subsequent treatments for patients with locally advanced or metastatic CCA. The selective FGFR inhibitors pemigatinib and infigratinib and the IDH1 inhibitor ivosidenib are now approved for previously treated patients with FGFR2 fusions or FGFR2 rearrangements and IDH1 mutations, respectively. These gene fusions, rearrangements, and mutations are present in a subset of patients with iCCA. Pemigatinib and infigratinib are also currently being investigated in the first-line setting. Other targeted therapies are available across solid tumors, including CCA, and include: NTRK inhibitors (entrectinib and larotrectinib); a BRAF/MEK inhibitor combination (dabrafenib and trametinib); pembrolizumab (for high ≥ 10 mutations/mb tumor mutational burden or mismatch repair defect cancers); and RET inhibitors (selpercatinib). Targeted therapies for other genomic alterations in CCA and solid tumors in general continue to be developed and explored.
Further therapeutic possibilities for patients with CCA may emerge as our knowledge of the tumor microenvironment and its impact on tumor growth increases. Combined treatments that target both actionable mutations and the tumor microenvironment is another approach that assists with patient selection for the most appropriate molecular targeted therapy.
The value of blood-derived ctDNA in the clinic is currently being explored in CCA, especially since tissue biopsies can be difficult in this type of cancer.
5. Future Directions
Despite multiple FDA approved targeted therapies for patients with CCA, the overall survival for these patients remains dismal. Utilizing combination approaches with targeted therapies may offer some patients more benefit than single agents [137,138,139]. Mechanisms of resistance to single agent therapies may include RNA silencing [140] as well as multiple gene driven pathogenesis, which could be overcome with ‘multiomic’ patient diagnostics using genomic, proteomic, transcriptomic, and immunomic data as well as n-of-1 customized combination therapeutic approaches [141,142,143,144]. Further, using patient-specific immunomic data may allow for a tailored immunotherapy-targeted treatment as well as being informed by specific genomic aberrations and the most logical immunotherapeutic target [145]. A next step in improving outcomes in these patients likely involves identifying more therapeutic targets as well as overcoming secondary resistance mechanisms via targeting as many genomic alterations present within a specific tumor as possible [144,146,147].
Author Contributions
Conceptualization, J.J.A. Methodology, A.G. and J.J.A.; data curation, A.G.; writing—original draft preparation, A.G., R.K. and J.J.A.; writing—review and editing, A.G., R.K. and J.J.A.; visualization, J.J.A.; supervision, J.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
RK is funded in part by 5U01CA180888-08 and 5UG1CA233198-05, 3/1/2023-2/28/2024.
Conflicts of Interest
Amol Gupta declares no conflict of interest. Jacob J. Adashek serves on the advisory board for CureMatch, Inc. Kurzrock has received research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance and from the NCI; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Inc., Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, EISAI, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc. and IDbyDNA; serves on the Board of CureMatch and CureMetrix, and is a co-founder of CureMatch.
References
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.A.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.-M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R.; et al. Personalized Medicine in a Phase I Clinical Trials Program: The MD Anderson Cancer Center Initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; Stewart, D.J.; Kurzrock, R. Targeted therapy in non-small-cell lung cancer--is it becoming a reality? Nat. Rev. Clin. Oncol. 2010, 7, 401–414. Available online: https://pubmed.ncbi.nlm.nih.gov/20551945/ (accessed on 1 December 2022). [CrossRef]
- Huang, R.S.P.; Severson, E.; Haberberger, J.; Duncan, D.L.; Hemmerich, A.; Edgerly, C.; Ferguson, N.L.; Frampton, G.; Owens, C.; Williams, E.; et al. Landscape of Biomarkers in Non-small Cell Lung Cancer Using Comprehensive Genomic Profiling and PD-L1 Immunohistochemistry. Pathol. Oncol. Res. 2021, 27, 592997. [Google Scholar] [CrossRef]
- Gower, A.; Wang, Y.; Giaccone, G. Oncogenic drivers, targeted therapies, and acquired resistance in non-small-cell lung cancer. J. Mol. Med. 2014, 92, 697–707. [Google Scholar] [CrossRef]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J. Mol. Diagn. 2017, 19, 187. Available online: /pmc/articles/PMC5971222/ (accessed on 21 August 2022). [CrossRef]
- Dobashi, Y.; Goto, A.; Kimura, M.; Nakano, T. Molecularly Targeted Therapy: Past, Present and Future. Chemother. Open Access 2012, 1, 1–12. Available online: https://www.longdom.org/open-access/molecularly-targeted-therapy-past-present-and-future-19552.html (accessed on 9 September 2022).
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted therapy in advanced non-small cell lung cancer: Current advances and future trends. J. Hematol. Oncol. 2021, 14, 1–20. [Google Scholar] [CrossRef]
- De Mello, R.A.; Neves, N.M.; Tadokoro, H.; Amaral, G.A.; Castelo-Branco, P.; de Zia, V.A. New Target Therapies in Advanced Non-Small Cell Lung Cancer: A Review of the Literature and Future Perspectives. J. Clin. Med. 2020, 9, 3543. Available online: https://pubmed.ncbi.nlm.nih.gov/33153004/ (accessed on 9 September 2022). [CrossRef]
- D’Angelo, A.; Sobhani, N.; Chapman, R.; Bagby, S.; Bortoletti, C.; Traversini, M.; Ferrari, K.; Voltolini, L.; Darlow, J.; Roviello, G. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies. Cancers 2020, 12, 3293. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef]
- Everhart, J.E.; Ruhl, C.E. Burden of Digestive Diseases in the United States Part III: Liver, Biliary Tract, and Pancreas. Gastroenterology 2009, 136, 1134–1144. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangio-carcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Oliveira, D.V.; Zhang, S.; Chen, X.; Calvisi, D.F.; Andersen, J.B. Molecular profiling of intrahepatic cholangiocarcinoma: The search for new therapeutic targets. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 349–356. [Google Scholar] [CrossRef]
- Sia, D.; Tovar, V.; Moeini, A.; Llovet, J.M. Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for molecular therapies. Oncogene 2013, 32, 4861–4870. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Grants Accelerated Approval to Pemigatinib for Cholangiocarcinoma with an FGFR2 Rearrangement or Fusion; US Food and Drug Administration: Silver Spring, MD, USA, 2020; Volume 7, pp. 18–20. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (accessed on 30 September 2022).
- US Food and Drug Administration. FDA Grants Accelerated Approval to Infigratinib for Metastatic Cholangiocarcinoma; US Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (accessed on 22 October 2022).
- FDA. Grants Accelerated Approval to Futibatinib for Cholangiocarcinoma; US Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma (accessed on 1 December 2022).
- Casak, S.J.; Pradhan, S.; Fashoyin-Aje, L.A.; Ren, Y.; Shen, Y.-L.; Xu, Y.; Chow, E.C.Y.; Xiong, Y.; Zirklelbach, J.F.; Liu, J.; et al. FDA Approval Summary: Ivosidenib for the Treatment of Patients with Advanced Unresectable or Metastatic, Chemotherapy Refractory Cholangiocarcinoma with an IDH1 Mutation. Clin. Cancer Res. 2022, 28, 2733–2737. [Google Scholar] [CrossRef]
- Ambrosini, M.; Del Re, M.; Manca, P.; Hendifar, A.; Drilon, A.; Harada, G.; Ree, A.H.; Klempner, S.; Mælandsmo, G.M.; Flatmark, K.; et al. ALK Inhibitors in Patients with ALK Fusion–Positive GI Cancers: An International Data Set and a Molecular Case Series. JCO Precis. Oncol. 2022, 6, e2200015. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive molecular profiling of intra- and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin. Cancer Res. 2018, 24, 4154. Available online: /pmc/articles/PMC6642361/ (accessed on 12 January 2023). [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020. [Google Scholar] [CrossRef]
- Li, W.; Cui, Y.; Yin, F.; Peng, L.; Liu, X.; Shen, Y.; Guo, Y.; Wen, S.; Shi, J.; Lei, M.; et al. BRAF mutation in Chinese biliary tract cancer patients. J. Clin. Oncol. 2020, 38, e16678. [Google Scholar] [CrossRef]
- Spizzo, G.; Puccini, A.; Xiu, J.; Goldberg, R.M.; Grothey, A.; Shields, A.F.; Arora, S.P.; Khushmann, M.; Salem, M.E.; Battaglin, F.; et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open 2020, 5, e000682. [Google Scholar] [CrossRef]
- Endo, K.; Ashida, K.; Miyake, N.; Terada, T. E-Cadherin Gene Mutations in Human Intrahepatic Cholangiocarcinoma. Available online: https://onlinelibrary.wiley.com/doi/10.1002/1096-9896 (accessed on 12 January 2023).
- Lee, H.; Wang, K.; Johnson, A.; Jones, D.M.; Ali, S.M.; Elvin, J.A.; Yelensky, R.; Lipson, D.; Miller, V.A.; Stephens, P.J.; et al. Comprehensive genomic profiling of extrahepatic cholangio-carcinoma reveals a long tail of therapeutic targets. J. Clin. Pathol. 2016, 69, 403–408. Available online: https://jcp.bmj.com/content/69/5/403 (accessed on 13 January 2023). [CrossRef]
- Xue, L.; Guo, C.; Zhang, K.; Jiang, H.; Pang, F.; Dou, Y.; Liu, X.; Lin, H.; Dong, X.; Zhao, S.; et al. Comprehensive molecular profiling of extrahepatic cholangiocarcinoma in Chinese population and potential targets for clinical practice. HepatoBiliary Surg. Nutr. 2019, 8, 615–622. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef]
- Kim, H.; Kim, R.; Kim, H.R.; Jo, H.; Kim, H.; Ha, S.Y.; Park, J.O.; Park, Y.S.; Kim, S.T. HER2 Aberrations as a Novel Marker in Advanced Biliary Tract Cancer. Front Oncol. 2022, 12, 834104. [Google Scholar] [CrossRef]
- Silverman, I.M.; Hollebecque, A.; Friboulet, L.; Owens, S.; Newton, R.C.; Zhen, H.; Féliz, L.; Zecchetto, C.; Melisi, D.; Burn, T.C. Clinicogenomic Analysis of FGFR2-Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discov. 2021, 11, 326–339. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemothera-py-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Naganuma, A.; Sakuda, T.; Murakami, T.; Aihara, K.; Watanuki, Y.; Suzuki, Y.; Shibasaki, E.; Masuda, T.; Uehara, S.; Yasuoka, H.; et al. Microsatellite Instability-high Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Intern. Med. 2020, 59, 2261–2267. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (accessed on 4 April 2021).
- US Food and Drug Administration. FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors; US Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (accessed on 1 October 2022).
- US Food and Drug Administration. FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Advanced Solid Tumors; US Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors (accessed on 2 October 2022).
- US Food and Drug Administration. FDA Approves an Oncology Drug That Targets a Key Genetic Driver of Cancer, Rather Than a Specific Type of Tumor. Case Medical Research; US Food and Drug Administration: Silver Spring, MD, USA, 2018.
- US Food and Drug Administration. FDA Approves Entrectinib for NTRK Solid Tumors and ROS-1 NSCLC; US Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (accessed on 1 October 2022).
- US Food and Drug Administration. FDA Grants Accelerated Approval to Dabrafenib in Combination with Trametinib for Unre-Sectable or Metastatic Solid Tumors with BRAF V600E Mutation; US Food and Drug Administration: Silver Spring, MD, USA, 2022.
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Liu, F.; Wei, Y.; Zhang, H.; Jiang, J.; Zhang, P.; Chu, Q. NTRK Fusion in Non-Small Cell Lung Cancer: Diagnosis, Therapy, and TRK Inhibitor Resistance. Front. Oncol. 2022, 12, 864666. [Google Scholar] [CrossRef]
- Demols, A.; Rocq, L.; Charry, M.; De Nève, N.; Verrellen, A.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Salmon, I.; D’Haene, N. NTRK gene fusions in biliary tract cancers. J. Clin. Oncol. 2020, 38, 574. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New Routes to Targeted Therapy of Intrahepatic Cholangio-carcinomas Revealed by Next-Generation Sequencing. Oncologist 2014, 19, 235–242. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.-I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; McShane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors With BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Kelley, R.; Roychowdhury, S.; Weiss, K.; Abou-Alfa, G.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.; Goyal, L.; et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann. Oncol. 2018, 29, viii720. [Google Scholar] [CrossRef]
- Mohler, M.; Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Morizane, C.; Valle, J.W.; Karasic, T.B.; Abrams, T.A.; Kelley, R.K.; Cassier, P.; et al. Abstract CT010: Primary results of phase 2 FOENIX-CCA2: The irreversible FGFR1-4 inhibitor futibatinib in intrahepatic cholangiocarcinoma (iCCA) with FGFR2 fu-sions/rearrangements. Cancer Res. 2021, 81 (Suppl. 13), CT010. [Google Scholar]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEY-NOTE-158 Study. J. Clin. Oncol. 2019, 38, 1–10. [Google Scholar] [CrossRef]
- Andre, T.; Berton, D.; Curigliano, G.; Ellard, S.; Trigo Pérez, J.M.; Arkenau, H.T.; Abdeddaim, C.; Moreno, V.; Guo, W.; Im, E.; et al. Safety and efficacy of anti–PD-1 antibody dostarlimab in patients (pts) with mismatch repair-deficient (dMMR) solid cancers: Results from GARNET study. J. Clin. Oncology. 2021, 39 (Suppl. 3), 9. [Google Scholar] [CrossRef]
- Lowery, M.A.; Burris, H.A.; Janku, F.; Shroff, R.T.; Cleary, J.M.; Azad, N.S.; Goyal, L.; Maher, E.A.; Gore, L.; Hollebecque, A.; et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol. Hepatol. 2019, 4, 711–720. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. Available online: http://www.thelancet.com/article/S1470204522005411/fulltext (accessed on 23 February 2023). [CrossRef] [PubMed]
- Federman, N.; McDermott, R. Larotrectinib, a highly selective tropomyosin receptor kinase (TRK) inhibitor for the treatment of TRK fusion cancer. Expert Rev. Clin. Pharmacol. 2019, 12, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Ullah, M.; de la Cruz, C.C.; Hunsaker, T.; Senn, C.; Wirz, T.; Wagner, B.; Draganov, D.; Vazvaei, F.; Donzelli, M.; et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: Differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020, 22, 819–829. [Google Scholar] [CrossRef]
- Frampton, J.E. Entrectinib: A Review in NTRK+ Solid Tumours and ROS1+ NSCLC. Drugs 2021, 81, 697–708. [Google Scholar] [CrossRef]
- Dunn, D.B. Larotrectinib and Entrectinib: TRK Inhibitors for the Treatment of Pediatric and Adult Patients with NTRK Gene Fusion. J. Adv. Pr. Oncol. 2020, 11, 418–423. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Rozlytrek (Entrectinib) Capsules, for Oral Use; Prescribing Information; US Food and Drug Administration: Silver Spring, MD, USA, 2021.
- European Medicines Agency. Rozlytrek (Entrectinib). Summary of Product Characteristics; European Medicines Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Schaider, H.; Sturm, R. The evolving universe of BRAF mutations in melanoma. Br. J. Dermatol. 2017, 177, 893. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. Encorafenib and binimetinib for the treatment of BRAF V600E/K-mutated melanoma. Drugs Today 2019, 55, 247–264. [Google Scholar] [CrossRef]
- Tol, J.; Nagtegaal, I.D.; Punt, C.J.A. BRAF mutation in metastatic colorectal cancer. N. Engl. J. Med. 2009, 361, 98–99. [Google Scholar] [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600–Mutant Advanced Melanoma Treated with Vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Barletta, J.A. BRAF V600E mutation-specific antibody: A review. Semin. Diagn. Pathol. 2015, 32, 400–408. [Google Scholar] [CrossRef]
- Adashek, J.J.; Menta, A.K.; Reddy, N.K.; Desai, A.P.; Roszik, J.; Subbiah, V. Tissue-Agnostic Activity of BRAF plus MEK Inhibitor in BRAF V600–Mutant Tumors. Mol. Cancer Ther. 2022, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Menzies, A.M.; Long, G. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des. Dev. Ther. 2012, 6, 391–405. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Arnone, M.R.; Bleam, M.R.; Moss, K.G.; Yang, J.; Fedorowicz, K.E.; Smitheman, K.N.; Erhardt, J.A.; Hughes-Earle, A.; Kane-Carson, L.S.; et al. Dabrafenib; Preclinical Characterization, Increased Efficacy when Combined with Trametinib, while BRAF/MEK Tool Combination Reduced Skin Lesions. PLoS ONE 2013, 8, e67583. [Google Scholar] [CrossRef]
- Kim, K.B.; Kefford, R.; Pavlick, A.C.; Infante, J.R.; Ribas, A.; Sosman, J.A.; Fecher, L.A.; Millward, M.; McArthur, G.A.; Hwu, P.; et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J. Clin. Oncol. 2013, 31, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Solit, D.B.; Rosen, N. Combination of RAF and MEK Inhibition for the Treatment of BRAF-Mutated Melanoma: Feedback Is Not Encouraged. Cancer Cell 2014, 26, 603–604. [Google Scholar] [CrossRef]
- Petitjean, A.; Achatz, M.I.W.; Borresen-Dale, A.L.; Hainaut, P.; Olivier, M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene 2007, 26, 2157–2165. [Google Scholar] [CrossRef]
- Sen, S.; Meric-Bernstam, F.; Hong, D.S.; Hess, K.R.; Subbiah, V. Co-occurring Genomic Alterations and Association with Progres-sion-Free Survival in BRAFV600-Mutated Nonmelanoma Tumors. J. Natl. Cancer Inst. 2017, 109, 10. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Esposito Abate, R.; Rachiglio, A.M.; Maiello, M.R.; Esposito, C.; Schettino, C.; Izzo, F.; Nasti, G.; Normanno, N. FGFR Fusions in Cancer: From Diag-nostic Approaches to Therapeutic Intervention. Int. J. Mol. Sci. 2020, 21, 6856. [Google Scholar] [CrossRef]
- Borad, M.J.; Champion, M.D.; Egan, J.B.; Liang, W.S.; Fonseca, R.; Bryce, A.H.; McCullough, A.E.; Barrett, M.T.; Hunt, K.; Patel, M.; et al. Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma. PLoS Genet. 2014, 10, e1004135. [Google Scholar] [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2013, 59, 1427–1434. [Google Scholar] [CrossRef]
- Shroff, R.T.; Yarchoan, M.; O’Connor, A.; Gallagher, D.; Zahurak, M.L.; Rosner, G.; Ohaji, C.; Sartorius-Mergenthaler, S.; Subbiah, V.; Zinner, R.; et al. The oral VEGF receptor tyrosine kinase in-hibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. Br. J. Cancer 2017, 116, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: Primary analysis results. BMC Cancer 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Madi, A.; Jeffels, M.; Richly, H.; Nokay, B.; Rubin, S.; Ball, H.A.; Weller, S.; Botbyl, J.; Gibson, D.M.; et al. A Phase I study of pazopanib in combination with gemcitabine in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 71, 93–101. [Google Scholar] [CrossRef]
- Merz, V.; Zecchetto, C.; Melisi, D. Pemigatinib, a potent inhibitor of FGFRs for the treatment of cholangiocarcinoma. Futur. Oncol. 2021, 17, 389–402. [Google Scholar] [CrossRef]
- Botrus, G.; Raman, P.; Oliver, T.; Bekaii-Saab, T. Infigratinib (BGJ398): An investigational agent for the treatment of FGFR-altered intrahepatic cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 309–316. [Google Scholar] [CrossRef]
- Nogova, L.; Sequist, L.V.; Garcia, J.M.P.; Andre, F.; Delord, J.-P.; Hidalgo, M.; Schellens, J.H.; Cassier, P.A.; Camidge, D.R.; Schuler, M.; et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients with Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study. J. Clin. Oncol. 2017, 35, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Borbath, I.; Goyal, L.; Lamarca, A.; Macarulla, T.; Oh, D.Y.; Roychowdhury, S.; Sadeghi, S.; Shroff, R.T.; Li, A.; et al. PROOF 301: A multicenter, open-label, randomized, phase 3 trial of infigratinib versus gemcitabine plus cisplatin in patients with advanced cholangiocarcinoma with an FGFR2 gene fusion/rearrangement. J. Clin. Oncol. 2022, 40 (Suppl. 16), TPS4171. [Google Scholar] [CrossRef]
- Boichuk, S.; Dunaev, P.; Mustafin, I.; Mani, S.; Syuzov, K.; Valeeva, E.; Bikinieva, F.; Galembikova, A. Infigratinib (BGJ 398), a Pan-FGFR Inhibitor, Targets P-Glycoprotein and Increases Chemotherapeutic-Induced Mortality of Multidrug-Resistant Tumor Cells. Biomedicines 2022, 10, 601. [Google Scholar] [CrossRef]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib Is a Novel Irreversible FGFR 1–4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res 2020, 80, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1–4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef]
- Borad, M.; Javle, M.; Shaib, W.L.; Mody, K.; Bergamo, F.; Harris, W.P.; Damjanov, N.; Macarulla, T.; Brandi, G.; Masi, G.; et al. 59P Efficacy of derazantinib in intrahepatic cholangio-carcinoma (iCCA) patients with FGFR2 fusions, mutations or amplifications. Ann. Oncol. 2022, 33, S567–S568. [Google Scholar] [CrossRef]
- Hollebecque, A.; Borad, M.; Goyal, L.; Schram, A.; Park, J.O.; Cassier, P.A.; Kamath, S.D.; Meng, D.W.; Dotan, E.; Kim, R.; et al. LBA12 - Efficacy of RLY-4008, a highly selective FGFR2 inhibitor in patients (pts) with an FGFR2-fusion or rearrangement (f/r), FGFR inhibitor (FGFRi)-naïve cholangiocarcinoma (CCA): ReFocus trial. Ann. Oncol. 2022, 33, S808–S869. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress/efficacy-of-rly-4008-a-highly-selective-fgfr2-inhibitor-in-patients-pts-with-an-fgfr2-fusion-or-rearrangement-f-r-fgfr-inhibitor-fgfri-naiv (accessed on 26 February 2023). [CrossRef]
- Hollebecque, A.; Silverman, I.; Owens, S.; Féliz, L.; Lihou, C.; Zhen, H.; Newton, R.; Burn, T.; Melisi, D. Comprehensive genomic profiling and clinical outcomes in patients (pts) with fibroblast growth factor receptor rearrangement-positive (FGFR2+) cholangiocarcinoma (CCA) treated with pemigatinib in the fight-202 trial. Ann. Oncol. 2019, 30, v276. [Google Scholar] [CrossRef]
- Javle, M.M.; Murugesan, K.; Shroff, R.T.; Borad, M.J.; Abdel-Wahab, R.; Schrock, A.B.; Chung, J.; Goyal, L.; Frampton, G.M.; Kelley, R.K.; et al. Profiling of 3,634 cholangiocarcinomas (CCA) to identify genomic alterations (GA), tumor mutational burden (TMB), and genomic loss of heterozygosity (gLOH). J. Clin. Oncol. 2019, 37, 4087. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Riaz, N.; Havel, J.J.; Makarov, V.; Desrichard, A.; Urba, W.J.; Sims, J.S.; Hodi, F.S.; Martín-Algarra, S.; Mandal, R.; Sharfman, W.H.; et al. Tumor and Microenvironment Evolution during Immu-notherapy with Nivolumab. Cell 2017, 171, 934–949.e16. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular Determinants of Response to An-ti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients with Non-Small-Cell Lung Cancer Profiled with Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Kim, R.; Jo, H.; Kim, H.R.; Hong, J.; Park, J.O.; Park, Y.S.; Kim, S.T. Tumor Mutational Burden as a Biomarker for Advanced Biliary Tract Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211062324. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the mul-ticohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Park, U.B.; Jeong, T.J.; Gu, N.; Lee, H.T.; Heo, Y.-S. Molecular basis of PD-1 blockade by dostarlimab, the FDA-approved antibody for cancer immunotherapy. Biochem. Biophys. Res. Commun. 2022, 599, 31–37. [Google Scholar] [CrossRef]
- Xiang, X.; Liu, Z.; Zhang, C.; Li, Z.; Gao, J.; Zhang, C.; Cao, Q.; Cheng, J.; Liu, H.; Chen, D.; et al. IDH Mutation Subgroup Status Associates with Intratumor Heterogeneity and the Tumor Microenvironment in Intrahepatic Cholangiocarcinoma. Adv. Sci. 2021, 8, e2101230. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholan-giocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB receptors: From oncogenes to targeted cancer therapies. J. Clin. Investig. 2007, 117, 2051–2058. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab—Mechanism of Action and Use in Clinical Practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Phillips, G.D.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting her2 with trastuzumab deruxtecan: A dose-expansion, phase i study in multiple advanced solid tumors. Cancer Discov. 2020, 10, 688–701. Available online: https://aacrjournals.org/cancerdiscovery/article/10/5/688/2521/Targeting-HER2-with-Trastuzumab-Deruxtecan-A-Dose (accessed on 27 February 2023). [CrossRef] [PubMed]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J. Clin. Oncol. 2022, 40, 4006. [Google Scholar] [CrossRef]
- Paratala, B.S.; Chung, J.H.; Williams, C.B.; Yilmazel, B.; Petrosky, W.; Williams, K.; Schrock, A.B.; Gay, L.M.; Lee, E.; Dolfi, S.C.; et al. RET rearrangements are actionable alterations in breast cancer. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Drusbosky, L.M.; Rodriguez, E.; Dawar, R.; Ikpeazu, C.V. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J. Hematol. Oncol. 2021, 14, 1–8. [Google Scholar] [CrossRef]
- Adashek, J.J.; Desai, A.P.; Andreev-Drakhlin, A.Y.; Roszik, J.; Cote, G.J.; Subbiah, V. Hallmarks of RET and Co-occuring Genomic Alterations in RET-aberrant Cancers. Mol. Cancer Ther. 2021, 20, 1769–1776. [Google Scholar] [CrossRef]
- Kim, J.; Bradford, D.; Larkins, E.; Pai-Scherf, L.H.; Chatterjee, S.; Mishra-Kalyani, P.S.; Wearne, E.; Helms, W.S.; Ayyoub, A.; Bi, Y.; et al. FDA Approval Summary: Pralsetinib for the Treatment of Lung and Thyroid Cancers with RET Gene Mutations or Fusions. Clin. Cancer Res. 2021, 27, 5452–5456. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Oxnard, G.R.; Tan, D.S.; Owen, D.H.; Cho, B.C.; Loong, H.H.; McCoach, C.E.; Weiss, J.; Kim, Y.J.; et al. Intracranial Efficacy of Selpercatinib in RET Fu-sion-Positive Non-Small Cell Lung Cancers on the LIBRETTO-001. Trial. Clin Cancer Res. 2021, 27, 4160–4167. Available online: https://pubmed.ncbi.nlm.nih.gov/34088726/ (accessed on 12 February 2023). [CrossRef]
- FDA Approves Selpercatinib for Locally Advanced or Metastatic RET Fusion-Positive Solid Tumors|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-solid-tumors (accessed on 12 February 2023).
- Adashek, J.; Janku, F.; Kurzrock, R. Signed in Blood: Circulating Tumor DNA in Cancer Diagnosis, Treatment and Screening. Cancers 2021, 13, 3600. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, A.D.; Holdgaard, P.C.; Spindler, K.-L.G.; Pallisgaard, N.; Jakobsen, A. The correlation between cell-free DNA and tumour burden was estimated by PET/CT in patients with advanced NSCLC. Br. J. Cancer 2013, 110, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp-Demtröder, K.; Christensen, E.; Nordentoft, I.; Knudsen, M.; Taber, A.; Høyer, S.; Lamy, P.; Agerbæk, M.; Jensen, J.B.; Dyrskjøt, L. Monitoring Treatment Response and Metastatic Relapse in Advanced Bladder Cancer by Liquid Biopsy Analysis. Eur. Urol. 2018, 73, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, M.; Pizzo, C.; Botticelli, A.; Hahne, J.C.; Passalacqua, R.; Tomasello, G.; Petrelli, F. Biliary tract cancer: Current challenges and future prospects. Cancer Manag. Res. 2018, ume 11, 379–388. [Google Scholar] [CrossRef]
- Tamada, K.; Ushio, J.; Sugano, K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J. Clin. Oncol. 2011, 2, 203–216. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.C.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Zill, O.A.; Greene, C.; Sebisanovic, D.; Siew, L.M.; Leng, J.; Vu, M.; Hendifar, A.E.; Wang, Z.; Atreya, C.E.; Kelley, R.K.; et al. Cell-Free DNA Next-Generation Sequencing in Pancreatobiliary Carcinomas. Cancer Discov. 2015, 5, 1040–1048. [Google Scholar] [CrossRef]
- Mody, K.; Kasi, P.M.; Yang, J.; Surapaneni, P.K.; Bekaii-Saab, T.; Ahn, D.H.; Mahipal, A.; Sonbol, M.B.; Starr, J.S.; Roberts, A.; et al. Circulating Tumor DNA Profiling of Advanced Biliary Tract Cancers. JCO Precis. Oncol. 2019, 1–9. [Google Scholar] [CrossRef]
- Kumari, S.; Tewari, S.; Husain, N.; Agarwal, A.; Pandey, A.; Singhal, A.; Lohani, M. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol. Oncol. Res. 2016, 23, 91–97. [Google Scholar] [CrossRef]
- Eaton, J.E.; Gossard, A.A.; Talwalkar, J.A. Recall processes for biliary cytology in primary sclerosing cholangitis. Curr. Opin. Gastroenterol. 2014, 30, 287–294. [Google Scholar] [CrossRef]
- Lucci, A.; Hall, C.S.; Lodhi, A.K.; Bhattacharyya, A.; Anderson, A.E.; Xiao, L.; Bedrosian, I.; Kuerer, H.M.; Krishnamurthy, S. Circulating tumour cells in non-metastatic breast cancer: A prospective study. Lancet Oncol. 2012, 13, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Schwerdel, D.; Dolnik, A.; Beuter, F.; Blätte, T.J.; Schmidt, S.A.; Stanescu-Siegmund, N.; Steinacker, J.; Marienfeld, R.; Kleger, A.; et al. Genotyping of circulating tumor DNA in cholangi-ocarcinoma reveals diagnostic and prognostic information. Sci. Rep. 2019, 9, 13261. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal Secondary FGFR2 Mutations Drive Ac-quired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion–Positive Cholangiocarcinoma. Cancer Discov. 2017, 7, 252–263. [Google Scholar] [CrossRef]
- Kato, S.; Okamura, R.; Adashek, J.J.; Khalid, N.; Lee, S.; Nguyen, V.; Sicklick, J.K.; Kurzrock, R. Targeting G1/S phase cell-cycle genomic alterations and accompanying co-alterations with individualized CDK4/6 inhibitor-based regimens. JCI Insight 2021, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Adashek, J.J.; Shaya, J.; Okamura, R.; Jimenez, R.E.; Lee, S.; Sicklick, J.K.; Kurzrock, R. Concomitant MEK and Cyclin Gene Alterations: Implications for Response to Targeted Therapeutics. Clin. Cancer Res. 2021, 27, 2792–2797. [Google Scholar] [CrossRef]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Adashek, J.J.; Kato, S.; Parulkar, R.; Szeto, C.W.; Sanborn, J.Z.; Vaske, C.J.; Benz, S.C.; Reddy, S.K.; Kurzrock, R. Transcriptomic silencing as a potential mechanism of treatment resistance. J. Clin. Investig. 2020, 5. [Google Scholar] [CrossRef]
- Kato, S.; Gumas, S.; Adashek, J.J.; Okamura, R.; Lee, S.; Sicklick, J.K.; Kurzrock, R. Multi-omic analysis in carcinoma of unknown primary (CUP): Therapeutic impact of knowing the unknown. Mol. Oncol. 2022. [Google Scholar] [CrossRef]
- Adashek, J.J.; Subbiah, V.; Kurzrock, R. From Tissue-Agnostic to N-of-One Therapies: (R)Evolution of the Precision Paradigm. Trends Cancer 2020, 7, 15–28. [Google Scholar] [CrossRef]
- Wahida, A.; Buschhorn, L.; Fröhling, S.; Jost, P.J.; Schneeweiss, A.; Lichter, P.; Kurzrock, R. The coming decade in precision oncology: Six riddles. Nat. Rev. Cancer 2022, 23, 43–54. [Google Scholar] [CrossRef]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A.; et al. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.W.; Kurzrock, R.; Kato, S.; Goloubev, A.; Veerapaneni, S.; Preble, A.; Reddy, S.K.; Adashek, J.J. Association of differential expression of immuno-regulatory molecules and presence of targetable mutations may inform rational design of clinical trials. ESMO Open 2022, 7, 100396. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.; Subbiah, V.; Westphalen, C.; Naing, A.; Kato, S.; Kurzrock, R. Cancer: Slaying the nine-headed Hydra. Ann. Oncol. 2022, 34, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Goloubev, A.; Kato, S.; Kurzrock, R. Missing the target in cancer therapy. Nat. Cancer 2021, 2, 369–371. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).