Simple Summary
This multicentre randomised controlled trial investigated the added value of fluoroscopy-guided manual lymph drainage (MLD) (compared to traditional or placebo MLD) as part of the decongestive lymphatic therapy (DLT) for superficial lymphatic architecture in patients with chronic mild to moderate breast cancer-related lymphoedema (BCRL). No differences between the three groups were found regarding the change in the number of efferent superficial lymphatic vessels leaving the dermal backflow region in the total dermal backflow score, and in the number of superficial lymph nodes. As a consequence, this trial was not able to demonstrate the beneficial value of MLD for the other components of DLT on superficial lymphatic architecture in patients with chronic mild to moderate BCRL.
Abstract
The objective of this trial was to investigate the effectiveness of fluoroscopy-guided manual lymph drainage (MLD), as part of decongestive lymphatic therapy (DLT), on the superficial lymphatic architecture in patients with chronic mild to moderate breast cancer-related lymphoedema (BCRL). This trial was a multicentre, double-blind, randomised controlled trial involving 194 participants with BCRL. Participants were randomised into (1) DLT with fluoroscopy-guided MLD (intervention group), (2) DLT with traditional MLD (control group), or (3) DLT with placebo MLD (placebo group). Superficial lymphatic architecture was evaluated as a secondary outcome, visualised by ICG lymphofluoroscopy at the baseline (B0), post-intensive (P), and post-maintenance phases (P6). Variables were (1) number of efferent superficial lymphatic vessels leaving the dermal backflow region, (2) total dermal backflow score, and (3) number of superficial lymph nodes. The traditional MLD group showed a significant decrease in the number of efferent superficial lymphatic vessels at P (p = 0.026), and of the total dermal backflow score at P6 (p = 0.042). The fluoroscopy-guided MLD and placebo group showed significant decreases in the total dermal backflow score at P (p < 0.001 and p = 0.044, respectively) and at P6 (p < 0.001 and p = 0.007, respectively); the placebo MLD group showed a significant decrease in the total number of lymph nodes at P (p = 0.008). However, there were no significant between-group differences for the changes in these variables. In conclusion, based on lymphatic architecture outcomes, the added value of MLD, in addition to the other parts of DLT, could not be demonstrated in patients with chronic mild to moderate BCRL.
1. Introduction
With 2.2 million cases a year worldwide, breast cancer is the most common cancer among women [1]. The survival in general is good, but the treatment has an impact on quality of life. Its medical treatment is associated with several complications such as pain, fatigue [2], and limited shoulder range of motion, leading to a decreased quality of life [3,4]. Around one in five breast cancer survivors develop breast cancer-related lymphoedema (BCRL) at the level of the ipsilateral hand, arm, shoulder, trunk, or any combination of these areas [5]. Lymphoedema is caused by a reduced transport capacity of the lymphatic system (related to surgery, radiotherapy, or both), sometimes combined with an increase in lymph load (for example, related to infection) [6,7].
Near infrared fluorescence imaging of the lymphatic system, also called lymphofluoroscopy, is an imaging technique that can be used to evaluate the superficial lymphatic architecture [8,9]. A tracer, indocyanine green (ICG), is injected intradermally into the patient’s hand. Once excited by a near-infrared light, ICG emits a fluorescent signal and is captured by the camera. In this way, real-time video images of the superficial lymphatic vessels and lymph nodes are provided. Moreover, in patients with lymphoedema, three dysfunctional dermal backflow patterns can be distinguished [10]: (1) splash pattern, representing tortuous dermal dilated lymph capillaries; (2) stardust pattern, which demonstrates spotted fluorescent signals, representing the effusion of lymph fluid out of the lymph capillaries; and (3) diffuse pattern, by which the tracer is widely distributed without identifiable spots and lymph vessels [11]. Lymphofluoroscopy is safe, non-invasive, and has a low cost [9]. A disadvantage is that it only visualises superficial structures and tissue (maximal depth of 2 cm) [8].

Table 1.
Comparison of the characteristics of fluoroscopy-guided MLD and traditional MLD.
Table 1.
Comparison of the characteristics of fluoroscopy-guided MLD and traditional MLD.
| Fluoroscopy-Guided MLD [12,13] | Traditional MLD [12,14,15] | |
|---|---|---|
| Stimulation of lymphatic transport in general | Patient-specific, applied on the superficial lymph vessels, lymph nodes and region with dermal backflow, visualized through lymphofluoroscopy | Blind, without knowing the patient-specific lymphatic transport and architecture |
| Stimulation of resorption by lymph capillaries | Short rolling and stretching with a small surface (e.g., with the thumb) to create a relatively high local pressure | Rolling and stretching movement with the whole hand |
| Stimulation of transport through lymph collectors | Gliding gently with the medial side of the thumb and lateral side of the index over the lymph collector; light pressure with the hands and limited shear forces on the skin | Pumping with the whole hand (i.e., rolling with the hand from index to little finger) over the lymph collector; light pressure with hands |
| Stimulation of transport through area with dermal rerouting | Idem stimulation of transport through lymph collectors, but greater pressure with the hands and slower hand movements | Idem stimulation of transport through lymph collectors |
MLD = manual lymph drainage.
According to the consensus document of the International Society of Lymphology (ISL), decongestive lymphatic therapy (DLT) is recommended as the treatment for BCRL [16]. It consists of two phases: the intensive phase, which pursues the largest volume reduction, and the maintenance phase, which aims to sustain and optimise the results obtained in the intensive phase. The first phase lasts two to four weeks and consists of skin care, exercise therapy, compression by a multi-component bandage, manual lymph drainage (MLD), and education. The second phase lasts a lifetime and consists of skincare, exercise therapy, compression by a low stretch elastic sleeve, and MLD if needed. There are multiple traditional MLD techniques taught by the Vodder [15], Leduc [17], and Földi [18] MLD schools. A technique that differs from these traditional MLD methods is the Fill and Flush MLD method (developed by Belgrado et al. on a normal lymphatic system), which is also called fluoroscopy-guided MLD [13]. See Table 1 for the difference between fluoroscopy-guided MLD and traditional MLD.
Different systematic reviews show that the added value of traditional MLD in the other parts of DLT for volume reduction is limited [19,20,21,22,23]. Our RCT comparing fluoroscopy-guided MLD with traditional or placebo MLD in addition to DLT was unable to find any difference in volume reduction of the arm or in fluid accumulation at the shoulder/trunk [24]. In contrast, some studies have demonstrated the improvement of the lymphatic transport and architecture after one session of traditional MLD. In two studies, the lymph velocity was increased in healthy volunteers [25,26] and subjects with lymphoedema [26]. Another study showed more visible pathways after one session of traditional MLD [27]. One study showed a positive evolution of the stardust pattern toward a splash pattern after a short period (3 weeks) of daily traditional MLD treatment [28]. However, robust evidence on the long-lasting effect of MLD on the lymphatic architecture has not been provided thus far.
Therefore, the aim of the present study is to investigate the effectiveness of fluoroscopy-guided MLD versus traditional MLD or placebo MLD, in addition to the other components of DLT, on the superficial lymphatic architecture in breast cancer survivors with chronic mild to moderate BCRL.
We hypothesise that patients who received the fluoroscopy-guided MLD would have a significantly greater improvement in the superficial lymphatic architecture compared to the traditional MLD group or placebo MLD group after three weeks of intensive treatment (P) and after six months of maintenance treatment (P6). This means that patients receiving the fluoroscopy-guided MLD will have (1) a significantly greater increase in the number of efferent superficial lymphatic vessels leaving the dermal backflow region; (2) a significantly greater decrease in the total dermal backflow score; and (3) a significantly greater increase in the number of lymph nodes compared to the traditional MLD group or placebo MLD group.
2. Materials and Methods
2.1. Study Design and Setting
The Effort-BCRL trial is a multicentre, double-blind randomised controlled trial involving three groups. The details of the trial design are described in detail elsewhere [24]. The results of the primary outcomes and some of the secondary outcomes have already been published [24,29]. The current paper analyses one of the secondary outcomes: the lymphatic architecture visualised through lymphofluoroscopy.
The trial has been approved by the Ethical Committee of the University Hospitals of Leuven (main committee), with subsequent positive advice from the Ethical Committees of each other participating centre (i.e., University Hospital of Saint-Pierre, in General Hospital Groeninge and University Hospital of Ghent, Antwerp University Hospital; CME reference S58689, EudraCT Number 2015-004822-33). The trial has also been registered at clinicaltrials.gov (NCT02609724). The RCT is written according to the CONSORT guideline [30].
2.2. Participants
All participants were recruited between February 2016 and September 2019. The inclusion criteria were (1) unilateral lymphoedema of the arm and/or hand, developed after treatment for breast cancer; (2) chronic lymphoedema stage I to IIb (duration of >3 months); (3) at least 5% difference between the arms, adjusted for hand dominance, and/or between the hands; and (4) no active metastases. The exclusion criteria were (1) age < 18 y; (2) oedema of the upper limb from a cause other than breast cancer treatment; (3) mentally or physically unable to participate during the entire study period; (4) allergy to iodine, sodium iodine, or indocyanine green; (5) hyperthyroidism; (6) previous lymph node transplantation or lymphovenous shunt; and (7) bilateral axillary lymph node dissection. Only participants who signed the informed consent form were included in the study.
2.3. Intervention
See Figure 1 for an overview of the intervention. All participants received standard DLT consisting of education, skincare, exercises, and compression therapy. The intervention consisted of an intensive phase of 3 weeks and a maintenance phase of 6 months. The participants were randomly assigned to the intervention group receiving fluoroscopy-guided MLD, the control group receiving traditional MLD, or the placebo group receiving placebo MLD.
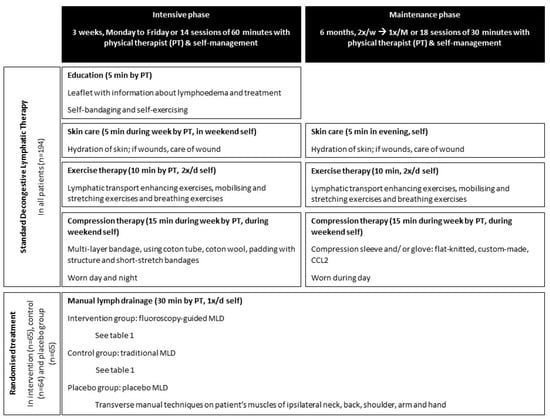
Figure 1.
Overview of the intervention in the trial (PT = physical therapist; CCL = compression class; MLD = manual lymph drainage).
Treatment sessions were performed by five different physical therapists experienced in the treatment of BCRL. At University Hospitals Leuven, patients were treated by RVH, LB, LV, and AKH; at the University Hospital of Saint-Pierre, by LV and TDV; at the General Hospital Groeninge and University Hospital of Ghent, by LV and TDV; and at the Antwerp University Hospital, by TDV). Prior to the start of the trial, the therapists had multiple training sessions to become familiar with this protocol and ensure identical treatment.
2.4. Assessments
2.4.1. Lymphofluoroscopy
To determine the superficial lymphatic architecture, all participants received a standardised lymphofluoroscopy at baseline (B0), both after 3 weeks of intensive treatment (P) and after 6 months of maintenance treatment (P6). For the fluoroscopy-guided MLD group, the baseline lymphofluoroscopy was also used to determine the procedure of MLD (i.e., which hand manoeuvres at which location). Three doctors (ST, LV, and CM) and physical therapists (ND, NG, and KD) performed the lymphofluoroscopic assessments. ST and ND performed the assessments at the University Hospitals Leuven and General Hospital Groeninghe; ST and NG at the Antwerp University Hospital,;CM and ND at the University Hospital of Ghent; and LV and KD at Sint-Pierre University Hospital.
The protocol for the lymphofluoroscopy is shown in Table 2. In summary, a solution with ICG was injected intradermally in the first and fourth web space of the affected hand. The whole procedure consisted of three phases (early phase, break, and late phase). During the early phase, the lymphatic transport was evaluated at rest (3 min), after activity (3 min of flexion/extension of the hand), and after stimulation with MLD (5 min). The second phase consisted of a 60 min break in which exercises (5 min) were alternated with rest (10 min). In the late phase, (1) after making a scan with the camera, the superficial lymphatic architecture was designed on the patient’s arm and trunk and pictures were taken of the ventral, dorsal, and lateral side of the arm and trunk; (2) the lymphatic vessels, lymph nodes, and dermal backflow patterns were designed on a body diagram; and (3) the evaluation sheet was completed.

Table 2.
Protocol of lymphofluoroscopy and the procedure to determine the different variables.
Based on the study conducted by Thomis et al. on interrater reliability of the scoring of the superficial lymphatic architecture through lymphofluoroscopy [26], the following variables were used:
- (1)
- The number of efferent superficial lymphatic vessels leaving the dermal backflow region after the break: The presence and number of efferent superficial lymphatic vessels leaving the dermal backflow region were scored.
- (2)
- The total dermal backflow score after the break: This score represents the dermal backflow patterns based on the severity staging of Yamamoto (normal pattern (score 0), splash pattern (score 1), stardust pattern (score 2), and diffuse pattern (score 3)) [27]. The body was divided into thirteen areas, and each area was scored between 0 to 3. The areas were: (1) fingers, (2) dorsal hand, (3) ventral hand, (4) dorsal distal forearm, (5) dorsal proximal forearm, (6) ventral distal forearm, (7) ventral proximal forearm, (8) dorsal distal upper arm, (9) dorsal proximal upper arm, (10) ventral distal upper arm, (11) ventral proximal upper arm, (12) breast region, and (13) scapular region. Per area, the most severe score was withheld; if only a part of an area showed dermal backflow, the dermal backflow pattern score was given for the total area. The total dermal backflow score is the sum of the dermal backflow scores of the thirteen areas of the body (score between 0 and 39). The higher the score, the larger the area with dermal backflow and the more severe the stage of dermal backflow.
- (3)
- The number of superficial lymph nodes after the break: The number of retroclavicular, axillar, cubital, and/or humeral lymph nodes were scored. The total number of superficial lymph nodes is the sum of all the different lymph nodes that were visualised.
The data of the variables were extracted from the evaluation sheets and compared with the body diagram. If there was a disagreement between the evaluation sheet and the body diagram, the information on the body diagram was preferred.
2.4.2. Other Assessments
The baseline characteristics were obtained through a clinical evaluation of the participants, including an assessment of body height and weight to determine BMI, an evaluation of the pitting status at the level of the hand, ventral and dorsal lower and upper arm, elbow, shoulder, trunk, and breast (0 = no, 1 = doubtfully present, 2 = clearly present), and this would determine a pitting score between 0 and 18 as well as the lymphoedema stage (ISL stage 1-2b). Moreover, circumferences of the affected and non-affected arms were determined and—based on the formula of the truncated cone—the volumes of the arms were calculated. The volumes of both hands were determined by the water displacement method. By adding the volume of the arm and hand, the total volume of the arm was obtained and the absolute and relative excessive volume of the arm was calculated.
During an interview, the duration of the lymphoedema was questioned. The medical file of the patient was consulted for other relevant information, such as the participant’s age, as well as breast cancer histology, stage, and treatment.
The participants registered in a diary when they performed self-exercises, self-MLD and registered the hours of wearing the compression garment. In this way, the adherence to the self-management was evaluated.
2.5. Sample Size Calculation
The study required 201 subjects (67 subjects per group) based on sample size calculation of the primary outcomes, i.e., reduction in excess volume of the arm/hand and accumulation of excess volume at the shoulder/trunk, as well as an alpha of 0.0125 and a power of 80% [24]. This number of subjects was necessary to detect a difference of 15% in the reduction of the excessive volume at the level of the arm/hand or the excessive fluid accumulation at the level of the shoulder/trunk between the three groups. The estimated drop-out rate was 5% based on a previous longitudinal study with breast cancer patients [28]. There was no sample size calculation performed for the outcome of this paper, as it demonstrates the secondary outcome of the EFforT-BCRL trial.
2.6. Randomization and Allocation Sequence Generation
All participants were assigned to one of the three groups. The allocation was randomly computer-generated by using 6-size permuted blocks, and then performed by an independent person (ADG). The randomisation was determined by an identification number, which was given to the participants after inclusion in the study.
2.7. Blinding
The assessors were blinded for the allocation of the participants to the intervention groups. In addition, the participants were blinded successfully during their allocation to the intervention groups. At the end of the follow-up, only 41 of 180 participants (23%) could indicate their allocated group correctly [19].
2.8. Statistical Analyses
Analyses of the number of efferent superficial lymphatic vessels leaving the dermal backflow region, the total dermal backflow score, and the total number of lymph nodes were based on raw scores. A multivariate linear model for longitudinal measures was used to compare the evolution of the raw scores between the three groups (fluoroscopy-guided MLD vs. traditional MLD vs. placebo MLD). An unstructured covariance matrix was used for the 3 × 3 covariance matrix of the repeated measures over time (B0, P, P6) for the three variables. At each time point, changes versus the baseline were calculated and compared between the three groups. p-values for the overall interaction (group × time) effect were measured. Given that a likelihood procedure was used, subjects with incomplete outcome information were also included in the analysis. Alpha levels were set at 5%. There were no corrections performed for multiple testing of the secondary outcomes.
All analyses were performed by using IBM SPSS Statistics software, version 27, for Windows.
3. Results
3.1. Participants
Of the 391 patients that were screened, 194 were included in the present study (UH Leuven, n = 112; UH Saint-Pierre, n = 10; UH Antwerp, n = 35; UH Ghent, n = 14; and GH Groeninge, n = 23). Among these, 65 patients were randomised to the intervention group, 64 patients to the control group, and 65 patients to the placebo group. Of all 194 included patients, 5 (of the 9 that were estimated) participants dropped out during the intensive treatment phase. Of these, 4 were lost to follow-up. Figure 2 shows the overall flow of the subjects in the trial up to 6 months of maintenance treatment. Of all participants, 65 were randomised to the intervention group, 64 to the control group, and 65 to the placebo group. Only 4 and 7 participants did not receive a lymphofluoroscopy after the intensive phase (P) and 6 months of maintenance (P6), respectively. None of the participants reported an adverse event caused by the lymphofluoroscopy. The characteristics of all participants and for each group separately are given in Table 3.
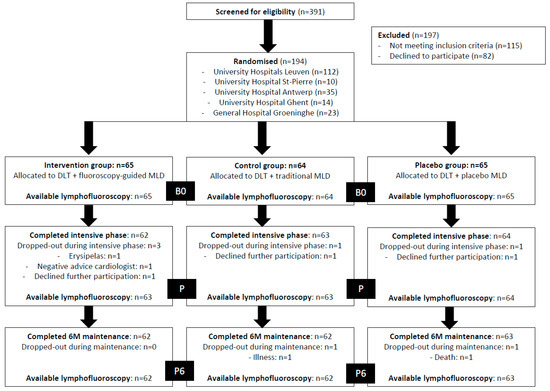
Figure 2.
Overview of the study flow (DLT = decongestive lymphatic therapy; MLD = manual lymph drainage; B0 = baseline; P = post-intensive phase; P6 = after 6 months of maintenance phase).

Table 3.
Characteristics of the participants.
3.2. Change of Superficial Lymphatic Architecture
Table 4 demonstrates the change in the lymphatic architecture over time using a comparison between the intervention group, control group, and placebo group.

Table 4.
Change of the lymphatic architecture from baseline to post-intensive phase and 6 months post-maintenance, with a comparison between the 3 groups.
3.2.1. Number of Efferent Superficial Lymphatic Vessels Leaving Dermal Backflow Region
In the whole group, the number of efferent lymphatic vessels did not significantly change between the baseline and the post-intensive phase, nor between baseline and the 6 months of maintenance time interval (p > 0.05). The change in the number of efferent lymphatic vessels from the baseline to the post-intensive phase and 6 months of maintenance was not significantly different between the 3 groups (p = 0.406). However, in the group with traditional MLD (control group), a significant decrease in the number of efferent lymphatic vessels was observed between the baseline and the post-intensive phase (p = 0.026), as well as a borderline significant decrease between the baseline and 6 months of maintenance (p = 0.089).
The hypothesis that patients receiving the fluoroscopy-guided MLD (intervention group) had a significantly greater increase in the number of efferent superficial lymphatic vessels leaving the dermal backflow region compared to patients receiving traditional MLD (control group) or placebo MLD (placebo group) could not be withheld.
3.2.2. Total Dermal Backflow Score
In the whole group, the dermal backflow score decreased significantly between the baseline and post-intensive phase (p = 0.001), as well as between baseline and 6 months of maintenance (p < 0.001). This decrease in the dermal backflow score was also found in the three groups separately. However, the change in the total dermal backflow score over time was not significantly different between the three groups (p = 0.268).
The hypothesis that patients receiving the fluoroscopy-guided MLD (intervention group) had a significantly greater decrease in the total dermal backflow score than patients receiving traditional MLD (control group) or placebo MLD (placebo group) could not be withheld.
3.2.3. Total Number of Lymph Nodes
In the whole group, the total number of lymph nodes decreased significantly from the baseline to the post-intensive timepoint (p = 0.008). The change in the total number of lymph nodes was not significantly different between the three groups either (p = 0.642). However, in the placebo group, there was a significant decrease in the number of lymph nodes between the baseline and the post-intensive phase (from 0.7 to 0.4; p = 0.008).
The hypothesis that patients receiving fluoroscopy-guided MLD (intervention group) had a significantly greater increase of the number of lymph nodes than patients receiving the traditional MLD (control group) or placebo MLD (placebo group) could not be withheld.
4. Discussion
This is the first randomised controlled trial with a large sample size investigating the short- and long-term added value of MLD to the other parts of DLT for superficial lymphatic architecture.
The present study was unable to demonstrate any added value of fluoroscopy-guided MLD compared to traditional MLD or placebo MLD for the superficial lymphatic architecture in the short term (post-intensive phase) or in the long term (after 6 months of maintenance). Consequently, the three hypotheses were rejected. There was a significant decrease in the dermal backflow score in the three groups. However, this change was not significantly different between the three groups. Similarly to our study, Medina-Rodríguez et al. performed intensive DLT consisting of MLD, pneumatic compression, multi-layer bandaging, and exercises over the course of 3 weeks. They also found an improvement of the dermal backflow pattern [23]. This is the only study apart from ours that has investigated the effect of different sessions of DLT on changes in the lymphatic architecture. However, they did not include a control group, as all participants received MLD. As a consequence, they were unable to state that the improvement of the dermal backflow pattern was due to MLD. Moreover, follow-up was limited to 3 weeks, and the number of patients was small (n = 19). All other studies investigated the effect of only one session of MLD on the lymphatic transport/architecture [20,21,22,29]. In the present study, no significant between-group differences for the change of the number of efferent superficial lymphatic vessels, nor for the number of superficial lymph nodes over time, was found. On the contrary, a decrease was observed in the number of lymph vessels outside of the dermal backflow region for the traditional MLD group (control group), as was a decrease in the number of superficial lymph nodes for the placebo group, which may indicate deterioration of the lymphatic transport. The results of these within-group differences must be interpret with caution, because there were no corrections performed for multiple testing.
4.1. Strengths and Limitations
The study has several strengths. First, the risk of performance bias was minimal. More than 75% of the patients were blinded for the allocation to the treatment group [19]. Second, the medical doctors/physical therapists performing the lymphofluoroscopy were blinded for the treatment allocation as well. Third, interrater reliability of the scoring of the lymphatic architecture by ICG lymphofluoroscopy was proven by the investigator team [26]. Fourth, to guarantee standardization of the treatments, they were performed by a group of physical therapists who were trained before the start of the study. Fifth, the drop-out rate was very low. Sixth, patients were taught how to perform self-MLD (according to their method, i.e., fluoroscopy-guided MLD, traditional MLD, or placebo MLD) during the maintenance phase, when there was no treatment given by a therapist. In this way the study obtains the maximum benefit from the MLD treatment. Finally, in comparison with other randomised controlled trials, this study has a large sample size and a follow-up period of 6 months. A limitation of this study is that the calculation of the sample size was based on the primary outcomes of the EFforT-BCRL trial [24], not on the secondary endpoint reported in this trial. Therefore, the significance of results in this paper should be interpreted with caution.
4.2. Clinical Implications
Analyses of the primary outcomes of the EFforT-BCRL trial showed no additional effect of MLD (neither fluoroscopy-guided nor traditional) on arm volume reduction and changes in lymph stagnation at the level of the shoulder and trunk, in addition to the other components of DLT [19]. These results are in line with the results of previous systematic reviews [14,15,16,17,18]. Analyses of our secondary outcomes showed no additional effects of MLD on the quality of life, problems with functioning, local tissue water, extracellular fluid, skin thickness, or skin elasticity at the arm or trunk level in the short or long term [30]. In line with the primary analyses and other secondary analyses, the current paper was unable to demonstrate an added value of MLD for superficial lymphatic architecture. This means that in patients with chronic BCRL, MLD does not provide a clinically relevant additional benefit nor a physiological benefit when added to other components of DLT. As a consequence, there is no evidence to include (fluoroscopy-guided or traditional) MLD in the treatment of patients with chronic BCRL.
5. Conclusions
This trial was not able to demonstrate any beneficial value of MLD for the other components of DLT in terms of outcomes regarding the superficial lymphatic architecture of patients with chronic, mild to moderate BCRL.
Author Contributions
Conceptualization, N.D. and T.D.V.; writing—original draft preparation, N.D. and T.D.V.; writing—review and editing, S.T., A.D.G., A.-K.H., I.N., N.G., W.A.A.T., J.-P.B., C.M. and M.H.; funding acquisition, N.D., S.T., N.G., W.A.A.T. and J.-P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This trial was funded by the Agency for Innovation by Science and Technology (Applied Biomedical Research) (IWT 60519).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and conducted with informed consent from all participants, under a protocol approved by the Ethical Committee of the University Hospitals of Leuven on the 10 February 2016 (main committee) and with subsequent positive advice from the Ethical Committees of each other participating centre (i.e., University Hospital of Saint-Pierre, in General Hospital Groeninge, and University Hospital of Ghent, Antwerp University Hospital; CME reference S58689, EudraCT Number 2015-004822-33).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting the reported results can be made available upon request.
Acknowledgments
The authors are very grateful to the different hospitals and research teams who collaborated on this study, and to the study participants and the nurses and medical staff of the multidisciplinary breast centres and lymphoedema centres of the different participating hospitals.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, H.J.G.; Gielissen, M.F.M.; Schmits, I.C.; Verhagen, C.; Rovers, M.M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. J. Midwifery Womens Health 2019, 64, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, H.; Gebruers, N.; Eeckhout, F.M.; Verlinden, K.; Tjalma, W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: A systematic review. Breast Cancer Res. Treat. 2014, 144, 21–31. [Google Scholar] [CrossRef] [PubMed]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and clinical manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef]
- Mortimer, P.S. The pathophysiology of lymphedema. Cancer 1998, 83, 2798–2802. [Google Scholar] [CrossRef]
- Abbaci, M.; Conversano, A.; De Leeuw, F.; Laplace-Builhe, C.; Mazouni, C. Near-infrared fluorescence imaging for the prevention and management of breast cancer-related lymphedema: A systematic review. Eur. J. Surg. Oncol. 2019, 45, 1778–1786. [Google Scholar] [CrossRef]
- Dip, F.; Alexandru, N.; Amore, M.; Becker, C.; Belgrado, J.P.; Bourgeois, P.; Chang, E.I.; Koshima, I.; Liberale, G.; Masia, J.; et al. Use of fluorescence imaging during lymphatic surgery: A Delphi survey of experts worldwide. Surgery 2022, 172, S14–S20. [Google Scholar] [CrossRef]
- Belgrado, J.P.; Vandermeeren, L.; Vankerckhove, S. VAS European Book on Angiology/Vascular Medicine; Aracne: Canterano, Italy, 2018. [Google Scholar]
- Narushima, M.; Yamamoto, T.; Ogata, F.; Yoshimatsu, H.; Mihara, M.; Koshima, I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J. Reconstr. Microsurg. 2016, 32, 72–79. [Google Scholar] [CrossRef]
- De Vrieze, T.; Vos, L.; Gebruers, N.; Tjalma, W.A.A.; Thomis, S.; Neven, P.; Nevelsteen, I.; De Groef, A.; Vandermeeren, L.; Belgrado, J.P.; et al. Protocol of a randomised controlled trial regarding the effectiveness of fluoroscopy-guided manual lymph drainage for the treatment of breast cancer-related lymphoedema (EFforT-BCRL trial). Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 221, 177–188. [Google Scholar] [CrossRef]
- Belgrado, J.P.; Vandermeeren, L.; Vankerckhove, S.; Valsamis, J.B.; Malloizel-Delaunay, J.; Moraine, J.J.; Liebens, F. Near-Infrared Fluorescence Lymphatic Imaging to Reconsider Occlusion Pressure of Superficial Lymphatic Collectors in Upper Extremities of Healthy Volunteers. Lymphat. Res. Biol. 2016, 14, 70–77. [Google Scholar] [CrossRef]
- Lee, B.-B.; Bergan, J.J.; Rockson, S.G. Lymphedema: A Concise Compendium of Theory and Practice; Springer: London, UK, 2011; pp. 423–424. [Google Scholar]
- Kasseroller, R.G. The Vodder School: The Vodder method. Cancer 1998, 83, 2840–2842. [Google Scholar] [CrossRef]
- Executive Committee of the International Society of, L. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Leduc, O.; Leduc, A.; Bourgeois, P.; Belgrado, J.P. The physical treatment of upper limb edema. Cancer 1998, 83, 2835–2839. [Google Scholar] [CrossRef]
- Földi, M.; Földi, E.; Strössenreuther, R.H.K.; Kubik, S. Földi’s Textbook of Lymphology for Physicians and Lymphedema Therapists, 2nd ed.; Elsevier Urban & Fischer: Munich, Germany, 2006. [Google Scholar]
- Ezzo, J.; Manheimer, E.; McNeely, M.L.; Howell, D.M.; Weiss, R.; Johansson, K.I.; Bao, T.; Bily, L.; Tuppo, C.M.; Williams, A.F.; et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst. Rev. 2015, 5, CD003475. [Google Scholar] [CrossRef]
- Huang, T.W.; Tseng, S.H.; Lin, C.C.; Bai, C.H.; Chen, C.S.; Hung, C.S.; Wu, C.H.; Tam, K.W. Effects of manual lymphatic drainage on breast cancer-related lymphedema: A systematic review and meta-analysis of randomized controlled trials. World J. Surg. Oncol. 2013, 11, 15. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Q.; Peng, K.; Deng, L.; He, L.; Hou, Y.; Zhang, Y.; Guo, J.; Mei, Z.; Li, L. Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020, 99, e23192. [Google Scholar] [CrossRef]
- Thompson, B.; Gaitatzis, K.; Janse de Jonge, X.; Blackwell, R.; Koelmeyer, L.A. Manual lymphatic drainage treatment for lymphedema: A systematic review of the literature. J. Cancer Surviv. 2021, 15, 244–258. [Google Scholar] [CrossRef]
- Wanchai, A.; Armer, J.M. Manual Lymphedema Drainage for Reducing Risk for and Managing Breast Cancer-Related Lymphedema After Breast Surgery: A Systematic Review. Nurs. Womens Health 2021, 25, 377–383. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Fieuws, S.; Thomis, S.; De Groef, A.; Tjalma, W.A.; Belgrado, J.P.; Vandermeeren, L.; Monten, C.; et al. Manual lymphatic drainage with or without fluoroscopy guidance did not substantially improve the effect of decongestive lymphatic therapy in people with breast cancer-related lymphoedema (EFforT-BCRL trial): A multicentre randomised trial. J. Physiother. 2022, 68, 110–122. [Google Scholar] [CrossRef]
- Lopera, C.; Worsley, P.R.; Bader, D.L.; Fenlon, D. Investigating the Short-Term Effects of Manual Lymphatic Drainage and Compression Garment Therapies on Lymphatic Function Using Near-Infrared Imaging. Lymphat. Res. Biol. 2017, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.C.; Maus, E.A.; Rasmussen, J.C.; Marshall, M.V.; Adams, K.E.; Fife, C.E.; Smith, L.A.; Chan, W.; Sevick-Muraca, E.M. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys. Med. Rehabil. 2011, 92, 756–764.e751. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, M.E.; de-la-Casa-Almeida, M.; Martel-Almeida, E.; Ojeda-Cardenes, A.; Medrano-Sanchez, E.M. Visualization of Accessory Lymphatic Pathways, before and after Manual Drainage, in Secondary Upper Limb Lymphedema Using Indocyanine Green Lymphography. J. Clin. Med. 2019, 8, 1917. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, M.E.; de-la-Casa-Almeida, M.; Gonzalez Martin, J.; Hermida Anllo, M.; Medrano-Sanchez, E.M. Changes in Indocyanine Green Lymphography Patterns after Physical Treatment in Secondary Upper Limb Lymphedema. J. Clin. Med. 2020, 9, 306. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Thomis, S.; De Groef, A.; Tjalma, W.A.A.; Belgrado, J.P.; Vandermeeren, L.; Monten, C.; Hanssens, M.; et al. Does Manual Lymphatic Drainage Add Value in Reducing Arm Volume in Patients With Breast Cancer-Related Lymphedema? Phys. Ther. 2022, 102, pzac137. [Google Scholar] [CrossRef]
- Cuschieri, S. The CONSORT statement. Saudi J. Anaesth. 2019, 13, S27–S30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).