Long-Term Follow-Up of Lentigo Maligna Patients Treated with Imiquimod 5% Cream
Abstract
Simple Summary
Abstract
1. Introduction
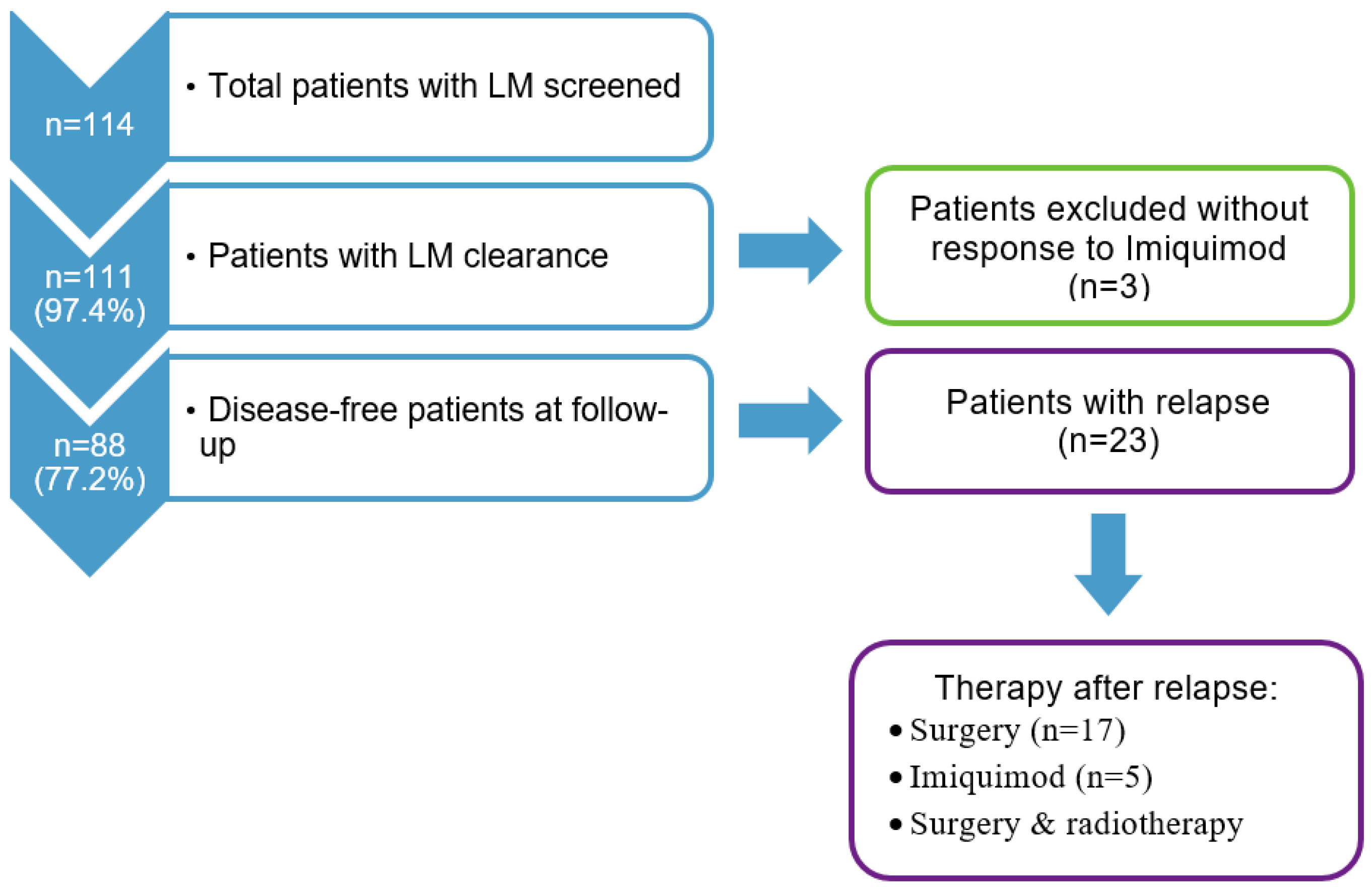
2. Materials and Methods
2.1. Study Population
2.2. Collected Data
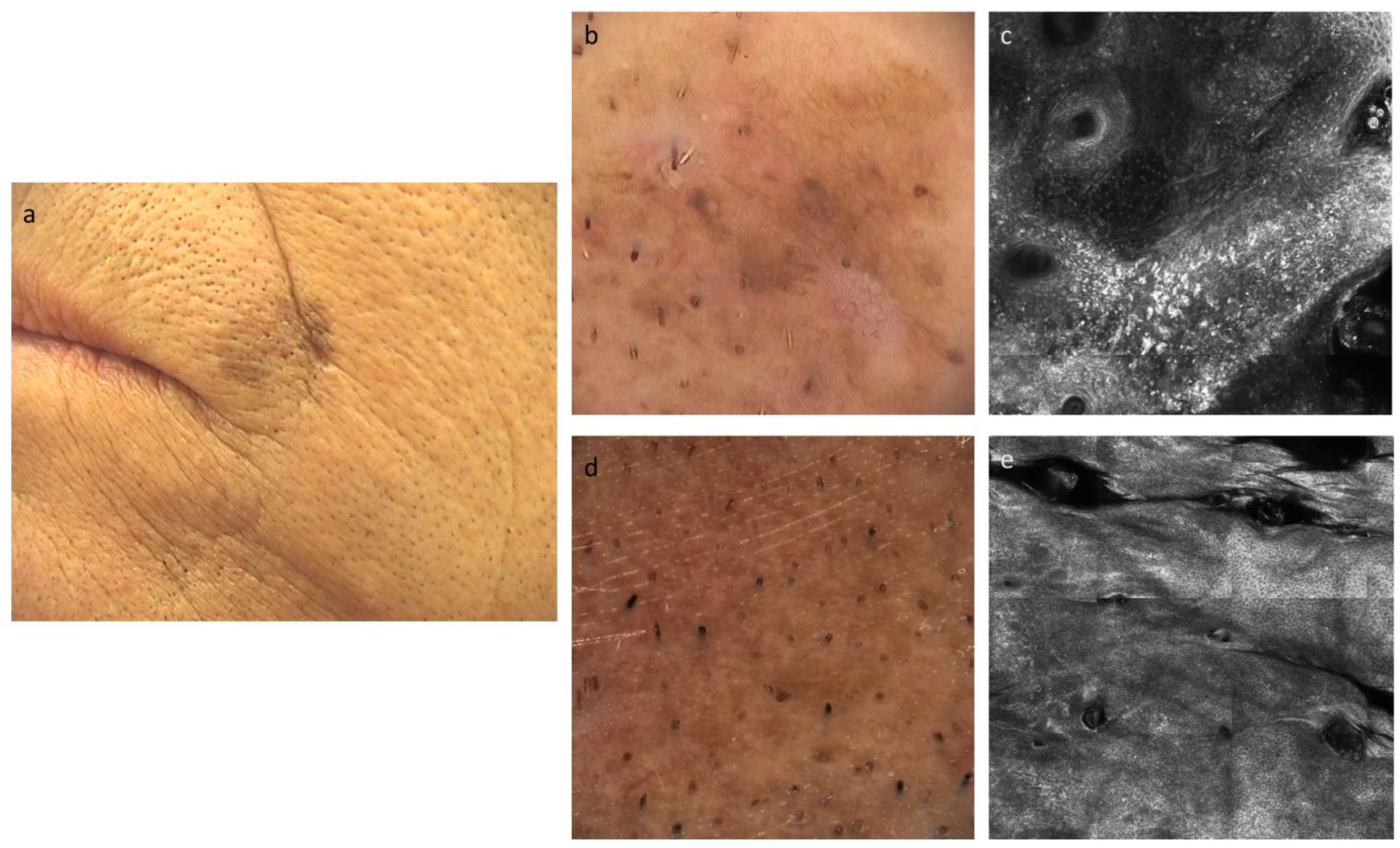
2.3. Procedures
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
3.2. Imiquimod Therapy
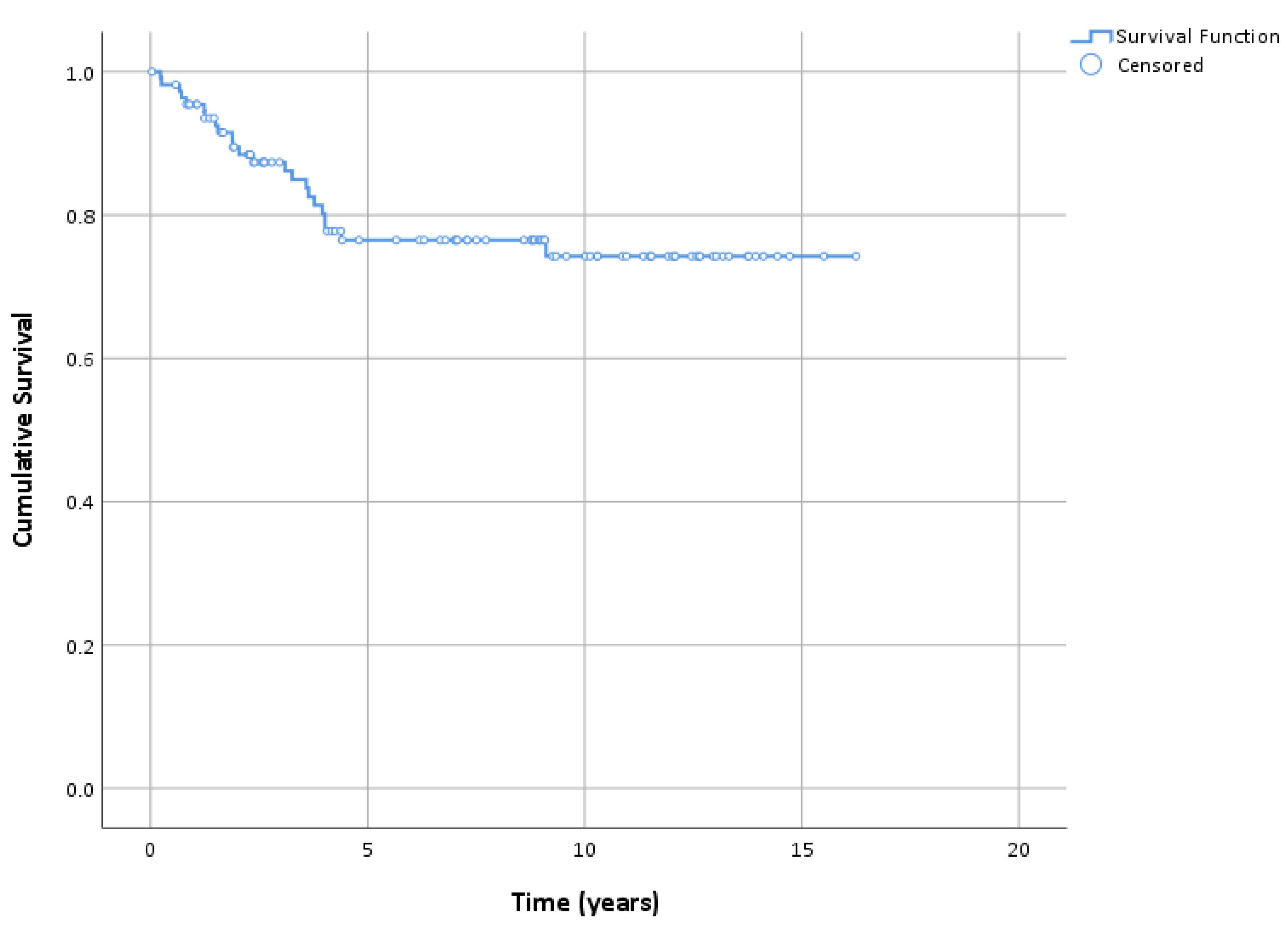
3.3. Follow-up and Survival
3.4. Prognostic Factors for DFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mocellin, S.; Nitti, D. Cutaneous melanoma in situ: Translational evidence from a large population-based study. Oncologist 2011, 16, 896–903. [Google Scholar] [CrossRef]
- Penneys, N.S. Microinvasive lentigo maligna melanoma. J. Am. Acad. Dermatol. 1987, 17, 675–680. [Google Scholar] [CrossRef]
- Brand, F.L.; Seyed Jafari, S.M.; Hunger, R.E. Confocal Microscopy and Lentigo Maligna: An in vivo Pilot Study for the Assessment of Response to Imiquimod Therapy. Dermatology 2019, 235, 150–155. [Google Scholar] [CrossRef]
- Alarcon, I.; Carrera, C.; Alos, L.; Palou, J.; Malvehy, J.; Puig, S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J. Am. Acad. Dermatol. 2014, 71, 49–55. [Google Scholar] [CrossRef]
- Farshad, A.; Burg, G.; Panizzon, R.; Dummer, R. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. Br. J. Dermatol. 2002, 146, 1042–1046. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Halpern, A.C.; Johnson, T.M.; Foote Hood, A.; Grichnik, J.M.; Swetter, S.M.; Tsao, H.; Barbosa, V.H.; Chuang, T.Y.; Duvic, M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2011, 65, 1032–1047. [Google Scholar] [CrossRef]
- Fikrle, T.; Divišová, B.; Šuchmannová, J.; Pizinger, K. The use of 2940-nm ER:YAG laser for the treatment of lentigo maligna. J. Dtsch. Dermatol. Ges. 2019, 17, 425–431. [Google Scholar] [CrossRef]
- Bilu, D.; Sauder, D.N. Imiquimod: Modes of action. Br. J. Dermatol. 2003, 149 (Suppl. S66), 5–8. [Google Scholar]
- Cotter, M.A.; McKenna, J.K.; Bowen, G.M. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol. Surg. 2008, 34, 147–151. [Google Scholar] [CrossRef]
- Gautschi, M.; Oberholzer, P.A.; Baumgartner, M.; Gadaldi, K.; Yawalkar, N.; Hunger, R.E. Prognostic markers in lentigo maligna patients treated with imiquimod cream: A long-term follow-up study. J. Am. Acad. Dermatol. 2016, 74, 81–87. [Google Scholar] [CrossRef]
- Fay, M.P.; Brittain, E.H.; Proschan, M.A. Pointwise confidence intervals for a survival distribution with small samples or heavy censoring. Biostatistics 2013, 14, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.D.; Woolford, K.M.; Neumeister, M.W. Lentigo Maligna. Clin. Plast. Surg. 2021, 48, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Tannous, Z.S.; Lerner, L.H.; Duncan, L.M.; Mihm, M.C., Jr.; Flotte, T.J. Progression to invasive melanoma from malignant melanoma in situ, lentigo maligna type. Hum. Pathol. 2000, 31, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Read, T.; Noonan, C.; David, M.; Wagels, M.; Foote, M.; Schaider, H.; Soyer, H.P.; Smithers, B.M. A systematic review of non-surgical treatments for lentigo maligna. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P. Diagnosis and Management of Lentigo Maligna: Clinical Presentation and Comprehensive Review. J. Skin Cancer 2021, 2021, 7178305. [Google Scholar] [CrossRef] [PubMed]
- Bosbous, M.W.; Dzwierzynski, W.W.; Neuburg, M. Lentigo maligna: Diagnosis and treatment. Clin. Plast. Surg. 2010, 37, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.Z.; Cohen, J.L.; High, W.; Stewart, L. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: Review of the literature. Dermatol. Surg. 2012, 38, 937–946. [Google Scholar] [CrossRef]
- Sauder, D.N. Immunomodulatory and pharmacologic properties of imiquimod. J. Am. Acad. Dermatol. 2000, 43, S6–S11. [Google Scholar] [CrossRef]
- Michalopoulos, P.; Yawalkar, N.; Brönnimann, M.; Kappeler, A.; Braathen, L.R. Characterization of the cellular infiltrate during successful topical treatment of lentigo maligna with imiquimod. Br. J. Dermatol. 2004, 151, 903–906. [Google Scholar] [CrossRef]
- Chambers, M.; Swetter, S.M.; Baker, C.; Saunders, E.; Chapman, M.S. Topical Imiquimod for Lentigo Maligna: Survival Analysis of 103 Cases with 17 Years Follow-up. J. Drugs Dermatol. 2021, 20, 346–348. [Google Scholar] [CrossRef]
- Powell, A.M.; Robson, A.M.; Russell-Jones, R.; Barlow, R.J. Imiquimod and lentigo maligna: A search for prognostic features in a clinicopathological study with long-term follow-up. Br. J. Dermatol. 2009, 160, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.F.; Crowson, N.; Kuwahara, R.; Teague, K.; Garcia, C.; Mackinnis, C.; Haque, R.; Odom, C.; Jankey, C.; Cornelison, R.L. Treatment of lentigo maligna with topical imiquimod. Br. J. Dermatol. 2003, 149, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, M.; Lawrence, C.M. Long-term outcomes of imiquimod-treated lentigo maligna. Clin. Exp. Dermatol. 2019, 44, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Daayana, S.; Elkord, E.; Winters, U. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer 2010, 102, 1129–1136. [Google Scholar] [CrossRef]
- Liu, X.S. Non-responders to topical imiquimod followed by vaccination therapy in VIN patients may be due to the level of IL10. Br. J. Cancer 2010, 103, 595–596. [Google Scholar] [CrossRef]
- Tio, D.; van der Woude, J.; Prinsen, C.A.C.; Jansma, E.P.; Hoekzema, R.; van Montfrans, C. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma: Need for standardization of treatment schedule and outcome measures. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 616–624. [Google Scholar] [CrossRef]
- Ng, J.C.; Swain, S.; Dowling, J.P.; Wolfe, R.; Simpson, P.; Kelly, J.W. The Impact of Partial Biopsy on Histopathologic Diagnosis of Cutaneous Melanoma Experience of an Australian Tertiary Referral Service. Arch. Dermatol. 2010, 146, 234–239. [Google Scholar] [CrossRef]
| n = 111 * | ||
|---|---|---|
| Age (years) | Median, range | 72.0 (38.0–93.0) |
| <70.0 | 42 (37.8%) | |
| 70.0–79.9 | 41 (36.9%) | |
| 80.0+ | 28 (25.2%) | |
| Sex | Male | 43 (38.7%) |
| Female | 68 (61.3%) | |
| Fitzpatrick skin type | I | 10 (9.1%) |
| II | 66 (60.0%) | |
| III | 34 (30.9%) | |
| Localization ** | Cheek | 49 (44.1%) |
| Nasal region | 32 (28.8%) | |
| Temporal/auricular region | 14 (12.6%) | |
| Frontal region | 6 (5.4%) | |
| Orbital region | 5 (4.5%) | |
| Other face/head areas | 3 (2.7%) | |
| Other locations | 5 (4.5%) | |
| Major diameter (mm) | Median, range | 18.0 (5.0–60.0) |
| <10.0 | 17 (15.5%) | |
| 10.0–19.0 | 40 (36.4%) | |
| 20.0+ | 53 (48.2%) | |
| Area (cm2) *** | Median, range | 1.7 (0.1–19.6) |
| <1.0 | 42 (38.2%) | |
| 1.0–2.9 | 30 (27.3%) | |
| 3.0+ | 38 (34.5%) | |
| Duration of application (weeks) | Median, range | 4.1 (1.1–55.3) |
| <3.0 | 36 (32.4%) | |
| 3.0–5.9 | 33 (29.7%) | |
| 6.0+ | 42 (37.8%) | |
| No. applications/day | Median, range | 2.0 (0.7–2.0) |
| ≤1 | 43 (38.7%) | |
| >1 | 68 (61.3%) | |
| Maximal reaction | None | 2 (1.8%) |
| Skin redness | 14 (12.6%) | |
| Erosions, oozing, or eschars | 95 (85.6%) |
| N Events, Survival (95% CI) | |||
|---|---|---|---|
| 3 Years | 5 Years | 10 Years | |
| LM relapse | 13, 87.4% (80.9–93.8) | 22, 76.5% (67.8–85.2) | 23, 74.3% (64.8–83.8) |
| Overall death | 8, 92.1% (86.9–97.4) | 14, 85.5% (78.5–92.6) | 25, 70.4% (60.3–80.5) |
| LM-related death | 0, 100% (95.9–100) | 0, 100% (95.1–100) | 0, 100% (90.5–100) |
| Univariate Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| N Events | 10 y Survival * | p ** | HR (95% CI) | p *** | |||
| Age (years) | <70.0 | 4 | 82.3% | 0.20 | 1 | ||
| 70.0–79.9 | 16 | 68.1% | 2.17 (0.88–5.37) | 0.09 | |||
| 80.0+ | 3 | 83.4% | 0.99 (0.26–2.79) | 0.99 | |||
| Sex | Male | 8 | 73.0% | 0.62 | 1 | ||
| Female | 15 | 74.6% | 1.51 (0.62–3.63) | 0.36 | |||
| Fitzpatrick skin type | I | 1 | 88.9% | 0.42 | 1 | ||
| II | 12 | 77.9% | 1.69 (0.21–13.45) | 0.62 | |||
| III | 10 | 64.4% | 2.47 (0.31–20.06) | 0.40 | |||
| Localization | Cheek | No | 14 | 71.9% | 0.60 | 1 | |
| Yes | 9 | 77.5% | 0.92 (0.38–2.21) | 0.85 | |||
| Nasal region | No | 14 | 79.7% | 0.09 | 1 | ||
| Yes | 9 | 57.6% | 2.66 (1.06–6.64) | 0.04 | |||
| Temporal/auricular region | No | 20 | 74.2% | 0.99 | 1 | ||
| Yes | 3 | 74.6% | 0.80 (0.23–2.77) | 0.73 | |||
| Other face/head areas | No | 20 | 74.0% | 0.98 | 1 | ||
| Yes | 3 | 75.7% | 0.84 (0.25–2.83) | 0.77 | |||
| Major diameter (mm) | <10.0 | 4 | 75.5% | 0.71 | 1 | ||
| 10.0–19.0 | 10 | 71.2% | 1.12 (0.35–3.60) | 0.84 | |||
| 20.0+ | 9 | 75.6% | 0.75 (0.22–2.49) | 0.64 | |||
| Area (cm2) | <1.0 | 8 | 78.8% | 0.10 | 1 | ||
| 1.0–2.9 | 11 | 56.4% | 1.89 (0.76–4.70) | 0.17 | |||
| 3.0+ | 4 | 86.1% | 0.53 (0.16–1.77) | 0.30 | |||
| Duration of imiquimod application (weeks) | <3.0 | 8 | 70.3% | 0.88 | 1 | ||
| 3.0–5.9 | 6 | 79.6% | 0.77 (0.27–2.24) | 0.63 | |||
| 6.0+ | 9 | 74.4% | 0.81 (0.30–2.16) | 0.68 | |||
| No. applications/day | ≤1 | 9 | 75.0% | 0.87 | 1 | ||
| >1 | 14 | 74.2% | 1.03 (0.44–2.43) | 0.94 | |||
| Maximal reaction | None/skin redness | 3 | 76.2% | 0.62 | 1 | ||
| Erosions, oozing or eschars | 20 | 74.8% | 1.54 (0.45–5.24) | 0.49 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyed Jafari, S.M.; Folini-Huesser, F.; Cazzaniga, S.; Hunger, R.E. Long-Term Follow-Up of Lentigo Maligna Patients Treated with Imiquimod 5% Cream. Cancers 2023, 15, 1546. https://doi.org/10.3390/cancers15051546
Seyed Jafari SM, Folini-Huesser F, Cazzaniga S, Hunger RE. Long-Term Follow-Up of Lentigo Maligna Patients Treated with Imiquimod 5% Cream. Cancers. 2023; 15(5):1546. https://doi.org/10.3390/cancers15051546
Chicago/Turabian StyleSeyed Jafari, S. Morteza, Flavia Folini-Huesser, Simone Cazzaniga, and Robert E. Hunger. 2023. "Long-Term Follow-Up of Lentigo Maligna Patients Treated with Imiquimod 5% Cream" Cancers 15, no. 5: 1546. https://doi.org/10.3390/cancers15051546
APA StyleSeyed Jafari, S. M., Folini-Huesser, F., Cazzaniga, S., & Hunger, R. E. (2023). Long-Term Follow-Up of Lentigo Maligna Patients Treated with Imiquimod 5% Cream. Cancers, 15(5), 1546. https://doi.org/10.3390/cancers15051546






