Macrophage Biomarkers sCD163 and sSIRPα in Serum Predict Mortality in Sarcoma Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Sampling
2.2. Enzyme-Linked Immunosorbent Assays (ELISA) for Macrophage Biomarkers
2.3. Monocyte Count and C-reactive Protein
2.4. Data Analysis and Statistics
2.5. Ethics
3. Results
3.1. Patients, Tumour and Treatment Characteristics
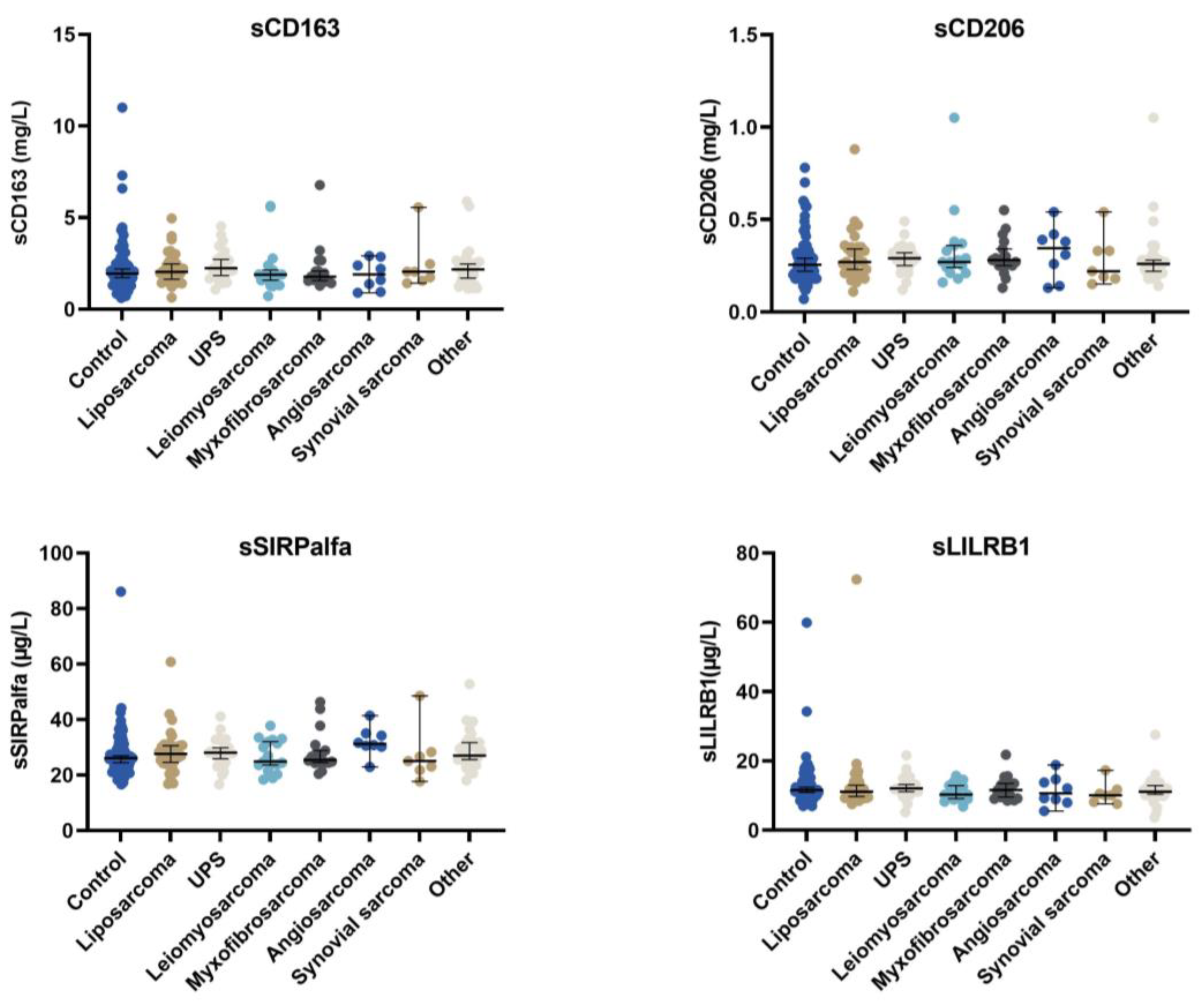
3.2. Macrophages Biomarkers in Control and Sarcoma Patients
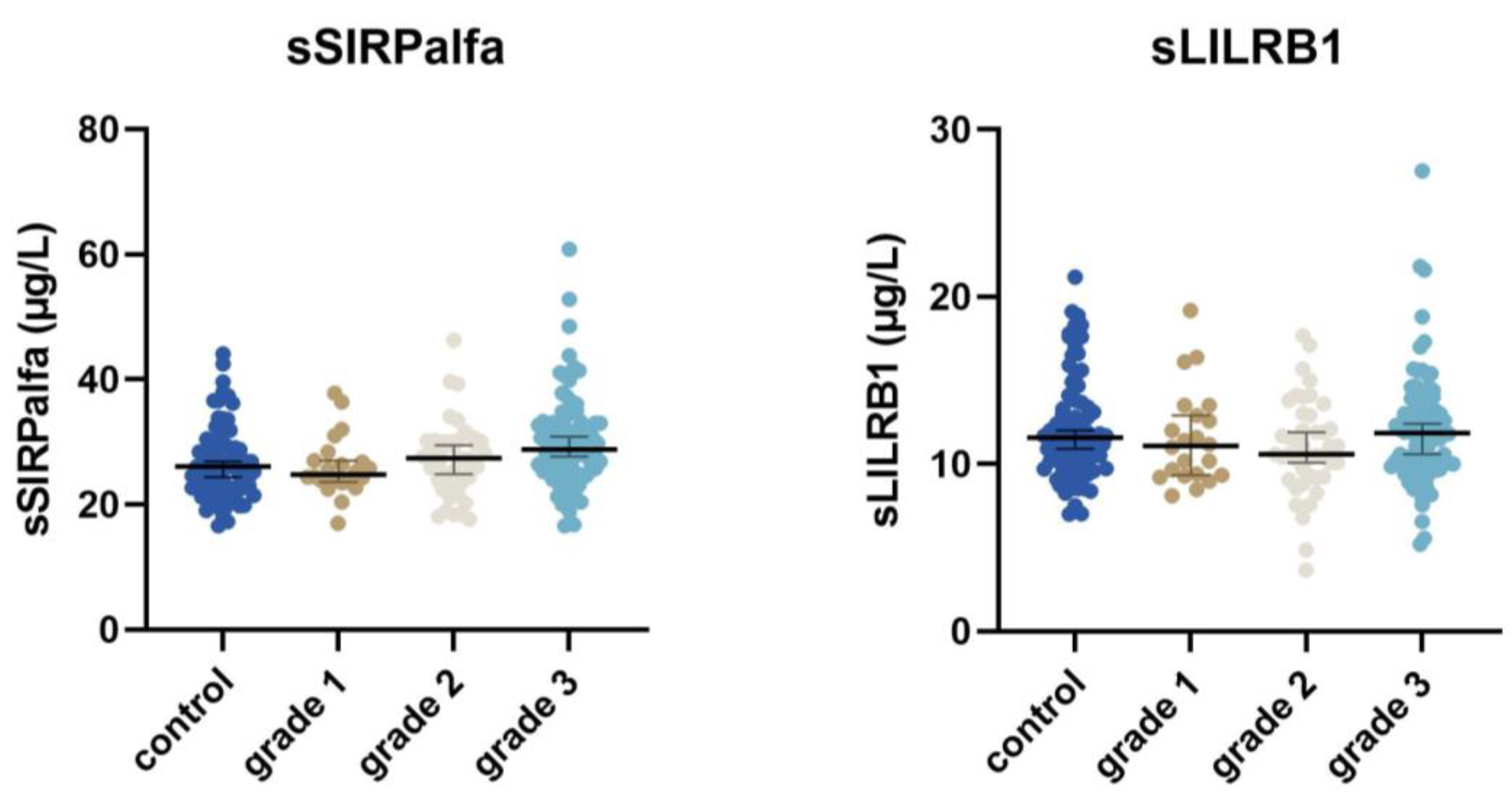
3.3. Relation of Macrophage Biomarkers to Disease Severity
3.4. Relation of Macrophage Biomarkers to Disease Relapse and Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Italiano, A.; Mathoulin-Pelissier, S.; Cesne, A.L.; Terrier, P.; Bonvalot, S.; Collin, F.; Michels, J.J.; Blay, J.Y.; Coindre, J.M.; Bui, B. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer 2011, 117, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up (✩). Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Pizzamiglio, S.; Touati, N.; Litiere, S.; Marreaud, S.; Kasper, B.; Gelderblom, H.; Stacchiotti, S.; Judson, I.; Dei Tos, A.P.; et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: Revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur. J. Cancer 2019, 109, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Leung, D.H.; Woodruff, J.; Shi, W.; Brennan, M.F. Analysis of prognostic factors in 1041 patients with localized soft tissue sarcomas of the extremities. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1996, 14, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Korytowsky, B.; Penrod, J.R.; Zhang, Y.; Le, T.K.; Batenchuk, C.; Krug, L. Real-World Clinical Impact of Immune Checkpoint Inhibitors in Patients With Advanced/Metastatic Non–Small Cell Lung Cancer After Platinum Chemotherapy. Clin. Lung Cancer 2019, 20, 287–296.e284. [Google Scholar] [CrossRef]
- Li, J.; Gu, J. Efficacy and safety of PD-1 inhibitors for treating advanced melanoma: A systematic review and meta-analysis. Immunotherapy 2018, 10, 1293–1302. [Google Scholar] [CrossRef]
- Saerens, M.; Brusselaers, N.; Rottey, S.; Decruyenaere, A.; Creytens, D.; Lapeire, L. Immune checkpoint inhibitors in treatment of soft-tissue sarcoma: A systematic review and meta-analysis. Eur. J. Cancer 2021, 152, 165–182. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- Sousa, L.M.; Almeida, J.S.; Fortes-Andrade, T.; Santos-Rosa, M.; Freitas-Tavares, P.; Casanova, J.M.; Rodrigues-Santos, P. Tumor and Peripheral Immune Status in Soft Tissue Sarcoma: Implications for Immunotherapy. Cancers 2021, 13, 3885. [Google Scholar] [CrossRef]
- Lazar, A.J.; McLellan, M.D.; Bailey, M.H.; Miller, C.A.; Appelbaum, E.L.; Cordes, M.G.; Fronick, C.C.; Fulton, L.A.; Fulton, R.S.; Mardis, E.R.; et al. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e928. [Google Scholar] [CrossRef]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254.e236. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, J.; Wang, M.; Hu, D. Pan-Cancer Molecular Characterization of m(6)A Regulators and Immunogenomic Perspective on the Tumor Microenvironment. Front. Oncol. 2020, 10, 618374. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Zheng, S.; Peng, J.; Jia, J.; Wu, T.; Cheng, X. Revealing the link between macrophage in microenvironment of osteosarcoma and poor prognosis by utilizing the Integrated analysis. J. Musculoskelet. Neuronal Interact. 2021, 21, 130–137. [Google Scholar]
- Dumars, C.; Ngyuen, J.M.; Gaultier, A.; Lanel, R.; Corradini, N.; Gouin, F.; Heymann, D.; Heymann, M.F. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget 2016, 7, 78343–78354. [Google Scholar] [CrossRef]
- Toulmonde, M.; Penel, N.; Adam, J.; Chevreau, C.; Blay, J.Y.; Le Cesne, A.; Bompas, E.; Piperno-Neumann, S.; Cousin, S.; Grellety, T.; et al. Use of PD-1 Targeting, Macrophage Infiltration, and IDO Pathway Activation in Sarcomas: A Phase 2 Clinical Trial. JAMA Oncol. 2018, 4, 93–97. [Google Scholar] [CrossRef]
- Dancsok, A.R.; Gao, D.; Lee, A.F.; Steigen, S.E.; Blay, J.Y.; Thomas, D.M.; Maki, R.G.; Nielsen, T.O.; Demicco, E.G. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology 2020, 9, 1747340. [Google Scholar] [CrossRef]
- Jaiswal, S.; Chao, M.P.; Majeti, R.; Weissman, I.L. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010, 31, 212–219. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Vladimirova, Y.V.; Mølmer, M.K.; Antonsen, K.W.; Møller, N.; Rittig, N.; Nielsen, M.C.; Møller, H.J. A New Serum Macrophage Checkpoint Biomarker for Innate Immunotherapy: Soluble Signal-Regulatory Protein Alpha (sSIRPα). Biomolecules 2022, 12, 937. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Høyer, M.; Maniecki, M.B.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J. Clin. Oncol. 2009, 27, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Rode, A.; Simonsen, K.; Villadsen, G.E.; Nicoll, A.; Møller, H.J.; Lim, L.; Angus, P.; Kronborg, I.; Arachchi, N.; et al. Macrophage activation marker soluble CD163 may predict disease progression in hepatocellular carcinoma. Scand. J. Clin. Lab. Investig. 2016, 76, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; De Vries, N.L.; Andersen, M.N.; Skovbo, A.; Tollenaar, R.; Møller, H.J.; Hokland, M.; Kuppen, P.J.K. CD163 as a Biomarker in Colorectal Cancer: The Expression on Circulating Monocytes and Tumor-Associated Macrophages, and the Soluble Form in the Blood. Int. J. Mol. Sci. 2020, 21, 5925. [Google Scholar] [CrossRef]
- No, J.H.; Moon, J.M.; Kim, K.; Kim, Y.B. Prognostic significance of serum soluble CD163 level in patients with epithelial ovarian cancer. Gynecol. Obstet. Investig. 2013, 75, 263–267. [Google Scholar] [CrossRef]
- Andersen, M.N.; Andersen, N.F.; Rødgaard-Hansen, S.; Hokland, M.; Abildgaard, N.; Møller, H.J. The novel biomarker of alternative macrophage activation, soluble mannose receptor (sMR/sCD206): Implications in multiple myeloma. Leuk. Res. 2015, 39, 971–975. [Google Scholar] [CrossRef]
- Møller, H.J.; Hald, K.; Moestrup, S.K. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand. J. Clin. Lab. Investig. 2002, 62, 293–299. [Google Scholar] [CrossRef]
- Rødgaard-Hansen, S.; Rafique, A.; Christensen, P.A.; Maniecki, M.B.; Sandahl, T.D.; Nexø, E.; Møller, H.J. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin. Chem. Lab. Med. 2014, 52, 453–461. [Google Scholar] [CrossRef]
- Murdoch, C.; Giannoudis, A.; Lewis, C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004, 104, 2224–2234. [Google Scholar] [CrossRef]
- Koo, J.; Hayashi, M.; Verneris, M.R.; Lee-Sherick, A.B. Targeting Tumor-Associated Macrophages in the Pediatric Sarcoma Tumor Microenvironment. Front. Oncol. 2020, 10, 581107. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005, 65, 5278–5283. [Google Scholar] [CrossRef]
- Wang, F.Q.; So, J.; Reierstad, S.; Fishman, D.A. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int. J. Cancer 2005, 114, 19–31. [Google Scholar] [CrossRef]
- Hagemann, T.; Robinson, S.C.; Schulz, M.; Trümper, L.; Balkwill, F.R.; Binder, C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis 2004, 25, 1543–1549. [Google Scholar] [CrossRef]
- Lewis, C.E.; Leek, R.; Harris, A.; McGee, J.O. Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. J. Leukoc. Biol. 1995, 57, 747–751. [Google Scholar] [CrossRef]
- Fujiwara, T.; Healey, J.; Ogura, K.; Yoshida, A.; Kondo, H.; Hata, T.; Kure, M.; Tazawa, H.; Nakata, E.; Kunisada, T.; et al. Role of Tumor-Associated Macrophages in Sarcomas. Cancers 2021, 13, 1086. [Google Scholar] [CrossRef]
- Yusa, T.; Yamashita, Y.I.; Okabe, H.; Nakao, Y.; Itoyama, R.; Kitano, Y.; Kaida, T.; Miyata, T.; Mima, K.; Imai, K.; et al. Survival impact of immune cells infiltrating peri-tumoral area of hepatocellular carcinoma. Cancer Sci. 2022, 113, 4048–4058. [Google Scholar] [CrossRef]
- Yoshida, C.; Kadota, K.; Yamada, K.; Fujimoto, S.; Ibuki, E.; Ishikawa, R.; Haba, R.; Yokomise, H. Tumor-associated CD163(+) macrophage as a predictor of tumor spread through air spaces and with CD25(+) lymphocyte as a prognostic factor in resected stage I lung adenocarcinoma. Lung Cancer 2022, 167, 34–40. [Google Scholar] [CrossRef]
- Cavalleri, T.; Greco, L.; Rubbino, F.; Hamada, T.; Quaranta, M.; Grizzi, F.; Sauta, E.; Craviotto, V.; Bossi, P.; Vetrano, S.; et al. Tumor-associated macrophages and risk of recurrence in stage III colorectal cancer. J. Pathol. Clin. Res. 2022, 8, 307–312. [Google Scholar] [CrossRef]
- Lee, C.H.; Espinosa, I.; Vrijaldenhoven, S.; Subramanian, S.; Montgomery, K.D.; Zhu, S.; Marinelli, R.J.; Peterse, J.L.; Poulin, N.; Nielsen, T.O.; et al. Prognostic significance of macrophage infiltration in leiomyosarcomas. Clin. Cancer Res. 2008, 14, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Briaire-de Bruijn, I.H.; Cleven, A.H.G.; Vervat, C.; Corver, W.E.; Schilham, M.W.; Van Beelen, E.; van Boven, H.; Haas, R.L.; Italiano, A.; et al. Increased infiltration of M2-macrophages, T-cells and PD-L1 expression in high grade leiomyosarcomas supports immunotherapeutic strategies. Oncoimmunology 2018, 7, e1386828. [Google Scholar] [CrossRef]
- Nabeshima, A.; Matsumoto, Y.; Fukushi, J.; Iura, K.; Matsunobu, T.; Endo, M.; Fujiwara, T.; Iida, K.; Fujiwara, Y.; Hatano, M.; et al. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br. J. Cancer 2015, 112, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Herbsthofer, L.; Goda, M.; Granegger, B.; Brcic, I.; Bergovec, M.; Scheipl, S.; Prietl, B.; El-Heliebi, A.; Pichler, M.; et al. Influence of tumor-infiltrating immune cells on local control rate, distant metastasis, and survival in patients with soft tissue sarcoma. Oncoimmunology 2021, 10, 1896658. [Google Scholar] [CrossRef]
- Edris, B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.P.; Willingham, S.B.; Contreras-Trujillo, H.; Liu, J.; Majeti, R.; West, R.B.; Fletcher, J.A.; et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 6656–6661. [Google Scholar] [CrossRef]
- Xu, J.F.; Pan, X.H.; Zhang, S.J.; Zhao, C.; Qiu, B.S.; Gu, H.F.; Hong, J.F.; Cao, L.; Chen, Y.; Xia, B.; et al. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget 2015, 6, 23662–23670. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Gao, J.; Fu, Y.; Hua, P.; Jing, Y.; Cai, M.; Wang, H.; Tong, T. The role of CD47-SIRPα immune checkpoint in tumor immune evasion and innate immunotherapy. Life Sci. 2021, 273, 119150. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Fan, D.; Tian, W.; Zhou, J.; Ji, Z.; Song, Y. Advances in the study of CD47-based bispecific antibody in cancer immunotherapy. Immunology 2022, 167, 15–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xu, X.; Liu, H.; Hao, T.; Yin, S.; Zhang, C.; He, Y. Poor Prognosis and Therapeutic Responses in LILRB1-Expressing M2 Macrophages-Enriched Gastric Cancer Patients. Front. Oncol. 2021, 11, 668707. [Google Scholar] [CrossRef]






| Number (n) | Percentage | |
|---|---|---|
| Sex | ||
| male | 68 | 45 |
| female | 84 | 55 |
| Age | ||
| median (p5–p95) | 66 (27–85) | |
| Stage at diagnosis | ||
| localised | 134 | 88 |
| metastatic * | 18 | 12 |
| Histological subtype | ||
| liposarcoma | 33 | 22 |
| UPS | 25 | 16 |
| leiomyosarcoma | 21 | 14 |
| myxofibrosarcoma | 17 | 11 |
| angiosarcoma | 9 | 6 |
| synovial sarcoma | 8 | 5 |
| others | 39 | 26 |
| Median tumour size (p5–p95) | 7 (1–18) | |
| Tumour grade | ||
| low | 23 | 15 |
| intermediate | 43 | 28 |
| high | 86 | 57 |
| Depth | ||
| superficial | 38 | 25 |
| deep | 96 | 63 |
| n/a ** | 18 | 12 |
| Treatment | ||
| surgery | 139 | 91 |
| radiation therapy | 61 | 40 |
| Treatment intent *** | ||
| curative | 137 | 90 |
| palliative | 15 | 10 |
| relapse | ||
| yes | 52 | 38 |
| no | 85 | 62 |
| Risk of Relapse | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | |
| age | 1.03 | 1.00–1.05 | 0.01 | 1.06 | 1.03–1.09 | <0.001 |
| sex | ||||||
| female | 1 | 1 | ||||
| male | 1.33 | 0.76–2.25 | 0.32 | 1.17 | 0.63–2.15 | 0.62 |
| size | 1.04 | 1.01–1.08 | 0.01 | 1.05 | 1.01–1.09 | 0.009 |
| tumour grade | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.28 | 0.38–4.24 | 0.69 | 2.60 | 0.54–12–52 | 0.234 |
| 3 | 4.25 | 1.51–11.94 | <0.01 | 8.13 | 1.94–34.12 | 0.004 |
| Serum biomarkers | ||||||
| Monocytes | 1.12 | 0.57–2.22 | 0.33 | 1.72 | 0.88–3.35 | 0.111 |
| CRP | 2.01 | 1.11–3.65 | 0.02 | 2.77 | 1.47–5.22 | 0.002 |
| sCD163 | 1.66 | 0.94–2.93 | 0.08 | 2.80 | 1.45–5.40 | 0.002 |
| sCD206 | 1.42 | 0.81–2.49 | 0.21 | 1.88 | 1.01–3.51 | 0.048 |
| sSIRPα | 1.75 | 1.00–3.07 | 0.05 | 3.42 | 1.74–6.72 | <0.001 |
| sLILRB1 | 1.32 | 0.75–2.30 | 0.33 | 1.64 | 0.89–3.04 | 0.116 |
| Predictive Accuracies of the Prognostic Models | Relapse | Survival | ||
|---|---|---|---|---|
| Model | AIC | C-index | AIC | C-index |
| Grade | 450 | 0.66 | 369 | 0.67 |
| Age | 459 | 0.61 | 365 | 0.69 |
| Tumour size | 460 | 0.62 | 380 | 0.66 |
| Grade + tumour size | 445 | 0.66 | 364 | 0.74 |
| Age + tumour size | 455 | 0.67 | 363 | 0.72 |
| sCD163 | 463 | 0.57 | 376 | 0.61 |
| sCD163 + sSIRPα | 462 | 0.60 | 370 | 0.67 |
| sCD163 + sSIRPα + grade | 451 | 0.69 | 361 | 0.73 |
| sCD163 + sSIRPα + grade + age + tumour size | 445 | 0.73 | 347 | 0.79 |
| sCD163 + sSIRPα + grade + age + tumour size + CRP | 402 | 0.73 | 311 | 0.81 |
| sSIRPα + grade + age + tumour size + CRP | 401 | 0.73 | 311 | 0.80 |
| Profile | 406 | 0.66 | 325 | 0.72 |
| Profile + age | 401 | 0.69 | 313 | 0.78 |
| Profile + age + tumour size | 403 | 0.71 | 308 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggerholm-Pedersen, N.; Friis, H.N.; Baad-Hansen, T.; Møller, H.J.; Sandfeld-Paulsen, B. Macrophage Biomarkers sCD163 and sSIRPα in Serum Predict Mortality in Sarcoma Patients. Cancers 2023, 15, 1544. https://doi.org/10.3390/cancers15051544
Aggerholm-Pedersen N, Friis HN, Baad-Hansen T, Møller HJ, Sandfeld-Paulsen B. Macrophage Biomarkers sCD163 and sSIRPα in Serum Predict Mortality in Sarcoma Patients. Cancers. 2023; 15(5):1544. https://doi.org/10.3390/cancers15051544
Chicago/Turabian StyleAggerholm-Pedersen, Ninna, Henriette Nymark Friis, Thomas Baad-Hansen, Holger Jon Møller, and Birgitte Sandfeld-Paulsen. 2023. "Macrophage Biomarkers sCD163 and sSIRPα in Serum Predict Mortality in Sarcoma Patients" Cancers 15, no. 5: 1544. https://doi.org/10.3390/cancers15051544
APA StyleAggerholm-Pedersen, N., Friis, H. N., Baad-Hansen, T., Møller, H. J., & Sandfeld-Paulsen, B. (2023). Macrophage Biomarkers sCD163 and sSIRPα in Serum Predict Mortality in Sarcoma Patients. Cancers, 15(5), 1544. https://doi.org/10.3390/cancers15051544





