Unemployment Status Subsequent to Cancer Diagnosis and Therapies: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
Objectives
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Information Sources and Search Strategies
2.3. Selection Process
2.3.1. Eligibility Criteria
- Inclusion criteria:
- Study design
- Prospective studies;
- Studies presenting one or more measures of association.
- Exposure
- Any regimen options;
- At least two-year cohort follow-up.
- Outcome
- Change in employment status.
- Publication type
- Primary studies published in peer-reviewed journals;
- Non-peer reviewed publications (e.g., government reports), if publicly available;
- In English language or available in English translation;
- Published after the year 2000.
- Esclusion criteria:
- Exposure
- Unemployable individuals, e.g., due to disease, disability, or age;
- Population limited to a specific occupation.
- Study design
- Cross-sectional (prevalence) studies without prospective elements;
- Case reports and case series;
- Retrospective studies;
- Prospective studies without measures of association and/or without confidence intervals (or data enabling their calculation);
- Studies measuring short-term effects (less than two years of follow-up) of therapies on employment status (e.g., time-series analyses).
- Publication type
2.3.2. Data Collection Process
- Number of participants;
- Observation interval (in years);
- Treatment type;
- Country;
- Type of cancer;
- Number employed;
- Number unemployed.
2.3.3. Study Risk of Bias Assessment
2.3.4. Effect Measures
2.3.5. Synthesis Methods
2.3.6. Reporting Bias Assessment
2.3.7. Certainty Assessment
3. Results
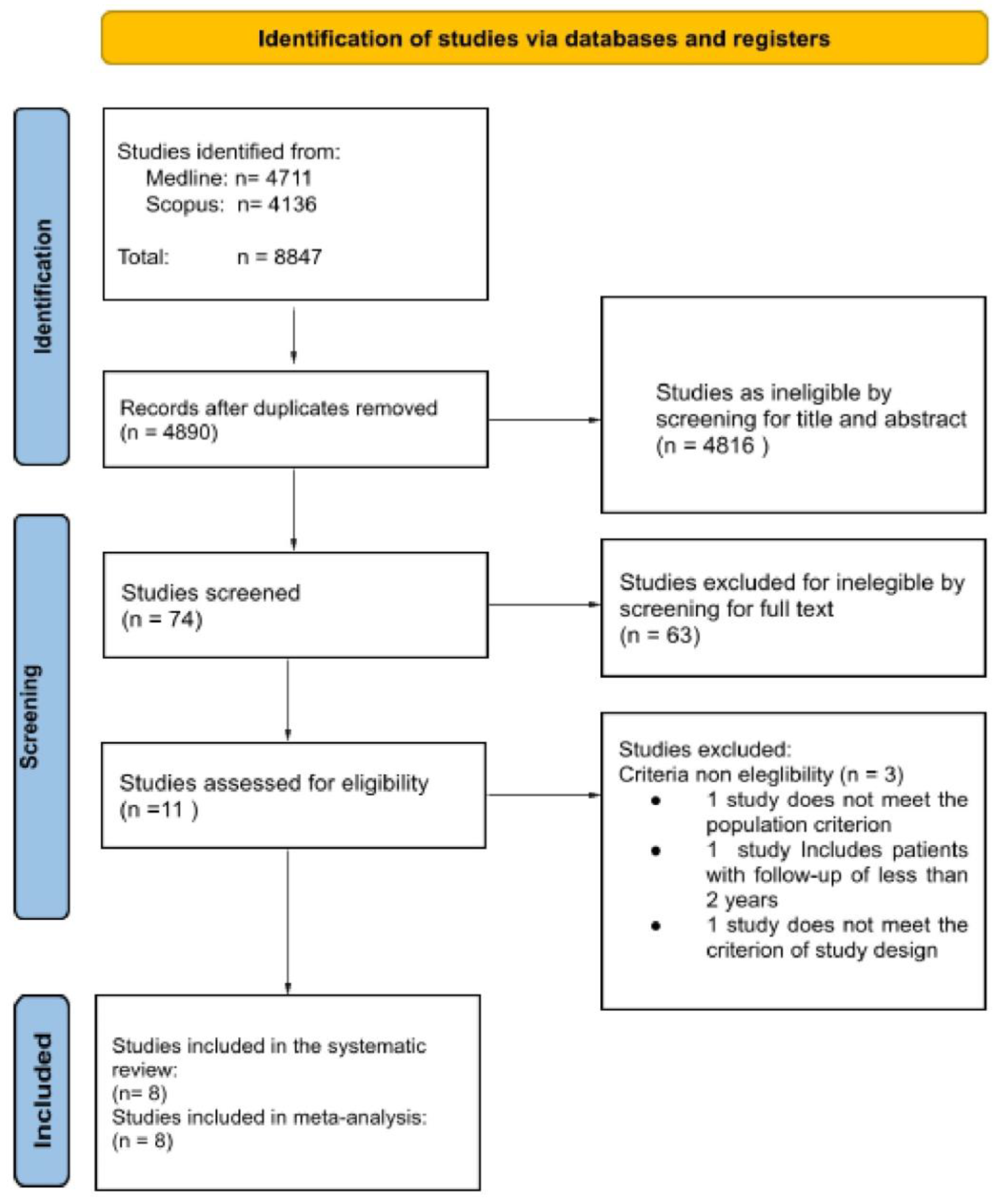
3.1. Study Selection
3.2. Study Characteristics
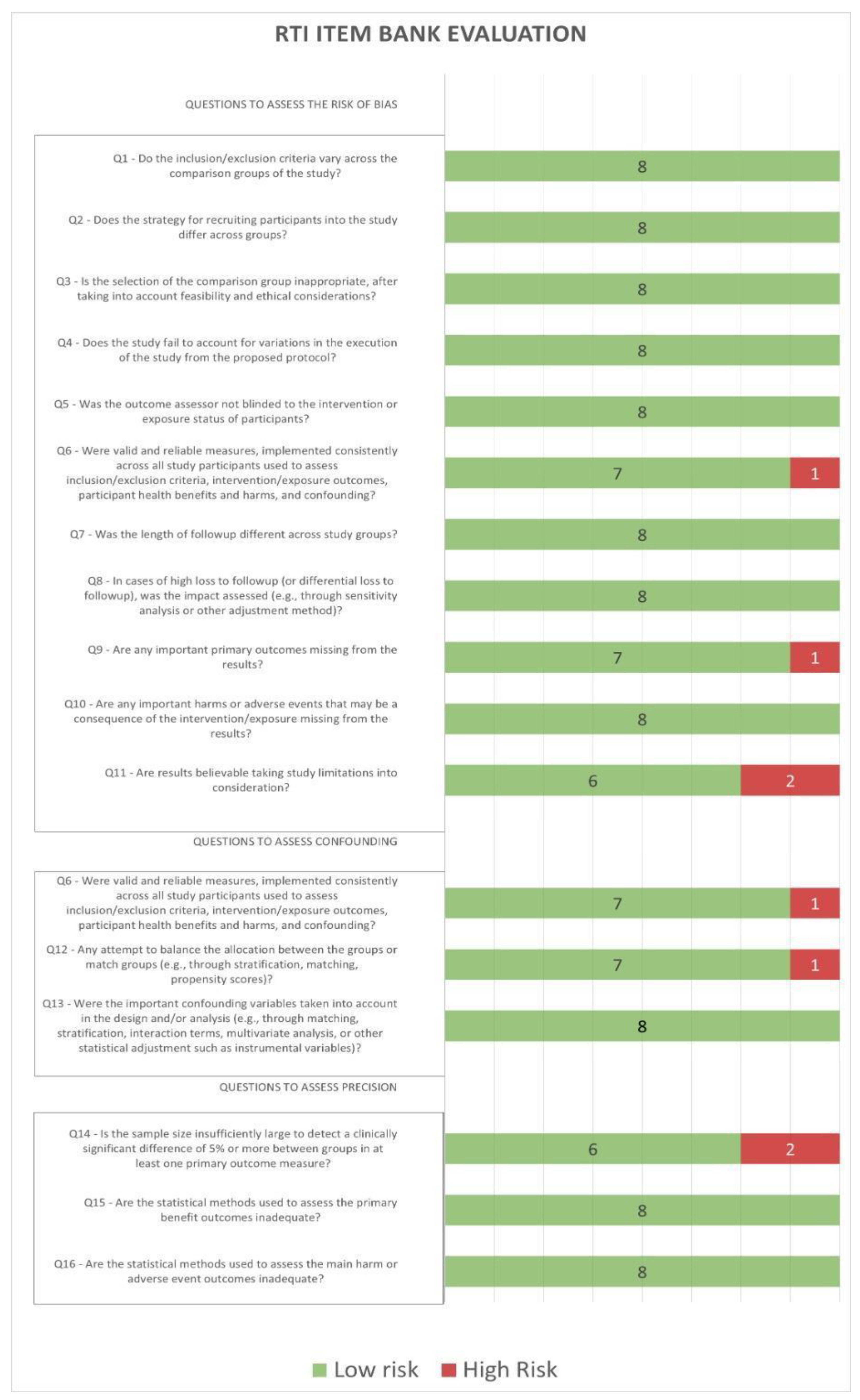
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
3.4.1. Primary Result
3.4.2. Secondary Results
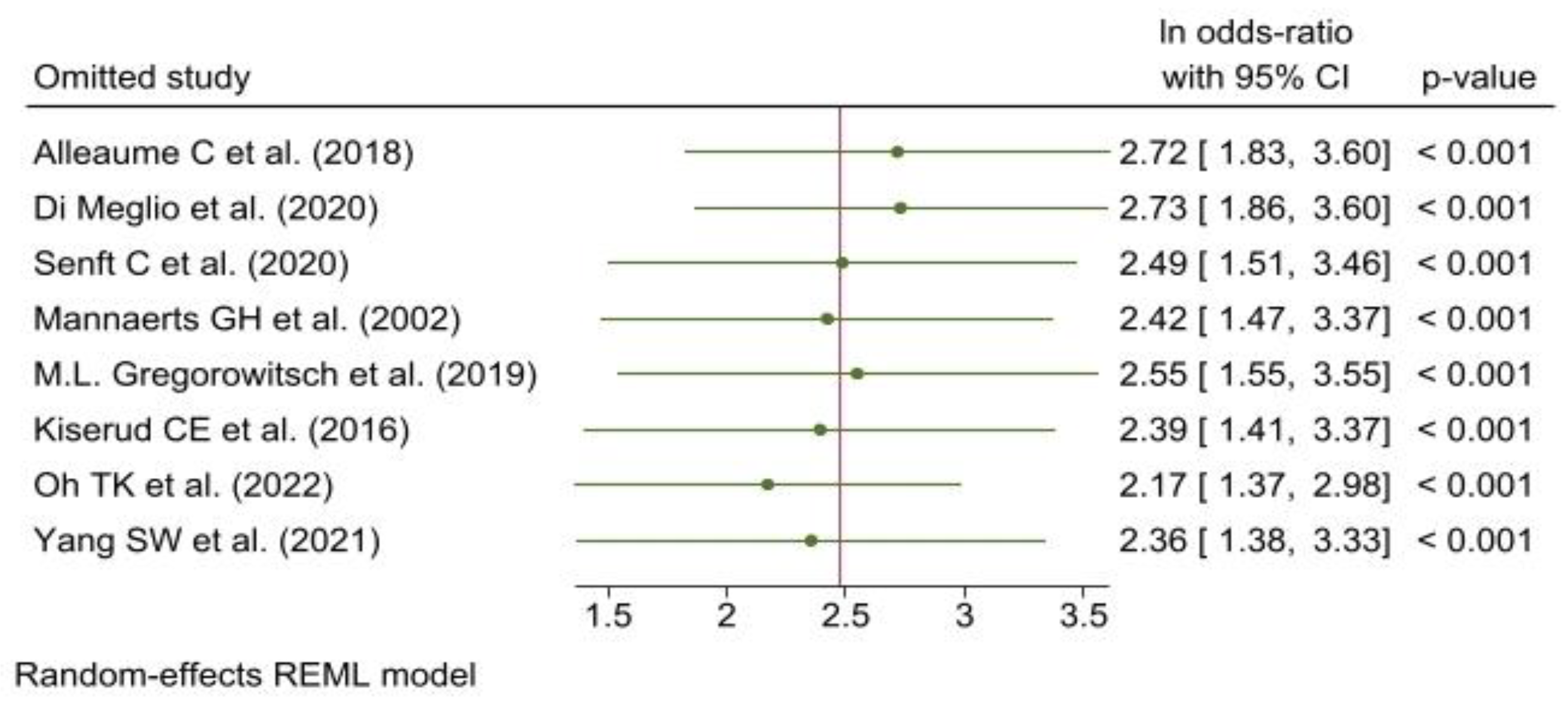
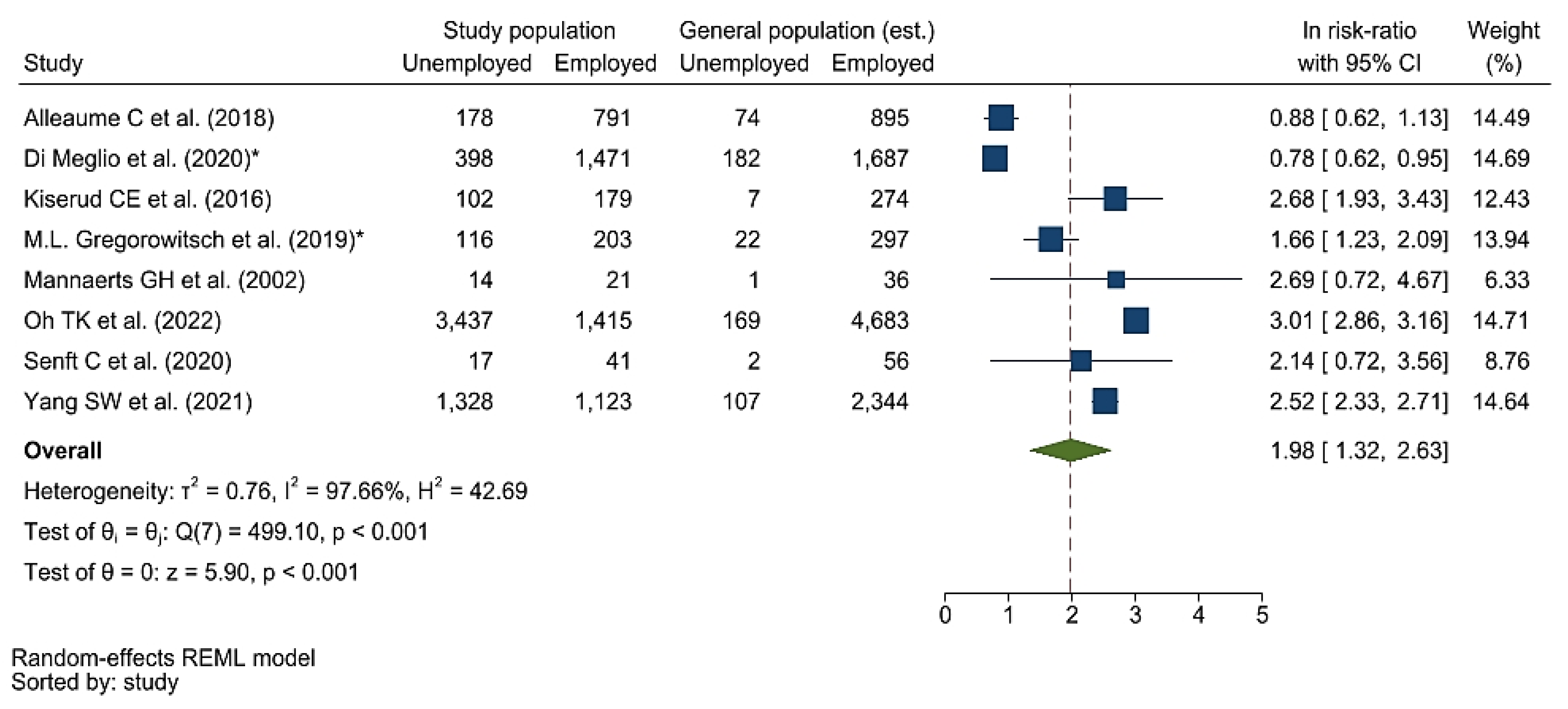
3.5. Meta-Analysis
3.5.1. Reporting Biases
3.5.2. Certainty of Evidence
4. Discussion
4.1. Results in Context
4.2. Limitations of Included Studies
4.3. Limitations of the Review Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| PICO | |
|---|---|
| Population | Employed adults aged between 18 and 65 years, in a post-cancer follow-up of at least 2 years. |
| Exposure | Any regimen option among: |
| |
| Comparison | Different therapy regimens |
| Outcome | Change in employment status:
|
| Database | Search String(s) |
|---|---|
| Medline | (cancer[MeSH Terms] OR neoplasm[Title/Abstract] OR carcinoma[Title/Abstract] OR tumor[Title/Abstract] OR oncology[Title/Abstract]) AND (radiotherapy[Title/Abstract] OR chemotherapy[Title/Abstract] OR immunotherapy[Title/Abstract] OR surgery[Title/Abstract]) AND (employment[Title/Abstract] OR unemployment[Title/Abstract] OR retirement[Title/Abstract] OR sick leave[Title/Abstract] OR sickness absence[Title/Abstract] OR absenteeism[Title/Abstract] OR presenteeism[Title/Abstract] OR work[Title/Abstract] OR occupation[Title/Abstract] OR work ability[Title/Abstract] OR work disability[Title/Abstract] OR disability management[Title/Abstract] OR rehabilitation[Title/Abstract] OR vocational[Title/Abstract]) AND (survivor[Title/Abstract] OR survival[Title/Abstract] OR long-term[Title/Abstract] OR mortality[Title/Abstract] OR dead[Title/Abstract]) |
| Scopus | (cancer OR carcinoma) AND (treatment OR therapy) AND (employment OR unemployment) |
References
- International Agency for Research on Cancer (IARC). Biennal Report 2020–2021. 2021. Available online: https://publications.iarc.fr/607 (accessed on 6 July 2022).
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef]
- Kato, D.; Kawachi, I.; Kondo, N. Complex Multimorbidity and Working beyond Retirement Age in Japan: A Prospective Propensity-Matched Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6553. [Google Scholar] [CrossRef] [PubMed]
- Retirement Ages. Available online: https://www.etk.fi/en/work-and-pensions-abroad/international-comparisons/retirement-ages/ (accessed on 21 February 2023).
- Verma, M.; Kalra, S. Epidemiological transition in South -East Asia and its Public Health Implications. J. Pak. Med. Assoc. 2020, 70, 1661–1663. [Google Scholar] [PubMed]
- Yadav, S.; Arokiasamy, P. Understanding epidemiological transition in India. Glob. Health Action 2014, 7, 23248. [Google Scholar] [CrossRef]
- Roelfs, D.J.; Shor, E.; Davidson, K.W.; Schwartz, J.E. Losing life and livelihood: A systematic review and meta-analysis of unemployment and all-cause mortality. Soc. Sci. Med. 2011, 72, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, A. Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 2011, 77, 109–130. [Google Scholar] [CrossRef]
- De Boer, A.G.; Taskila, T.; Ojajärvi, A.; van Dijk, F.J.; Verbeek, J.H. Cancer survivors and unemployment: A meta-analysis and meta-regression. JAMA 2009, 301, 753–762. [Google Scholar] [CrossRef]
- Wang, L.; Hong, B.Y.; Kennedy, S.A.; Chang, Y.; Hong, C.J.; Craigie, S.; Kwon, H.Y.; Romerosa, B.; Couban, R.J.; Reid, S.; et al. Predictors of Unemployment After Breast Cancer Surgery: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Oncol. 2018, 36, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Pálmarsdóttir, R.; Kiesbye Øvlisen, A.; Severinsen, M.T.; Glimelius, I.; Smedby, K.E.; El-Galaly, T. Socioeconomic impact of Hodgkin lymphoma in adult patients: A systematic literature review. Leuk. Lymphoma 2019, 60, 3116–3131. [Google Scholar] [CrossRef]
- Alleaume, C.; Bendiane, M.K.; Bouhnik, A.D.; Rey, D.; Cortaredona, S.; Seror, V.; Peretti-Watel, P. Chronic neuropathic pain negatively associated with employment retention of cancer survivors: Evidence from a national French survey. J. Cancer Surviv. 2018, 12, 115–126. [Google Scholar] [CrossRef]
- Frederiksen, L.E.; Mader, L.; Feychting, M.; Mogensen, H.; Madanat-Harjuoja, L.; Malila, N.; Tolkkinen, A.; Hasle, H.; Winther, J.F.; Erdmann, F. Surviving childhood cancer: A systematic review of studies on risk and determinants of adverse socioeconomic outcomes. Int. J. Cancer 2019, 144, 1796–1823. [Google Scholar] [CrossRef]
- De Boer, A.G.; Torp, S.; Popa, A.; Horsboel, T.; Zadnik, V.; Rottenberg, Y.; Bardi, E.; Bultmann, U.; Sharp, L. Long-term work retention after treatment for cancer: A systematic review and meta-analysis. J. Cancer Surviv. 2020, 14, 135–150. [Google Scholar] [CrossRef]
- Rottenberg, Y.; Ratzon, N.Z.; Jacobs, J.M.; Cohen, M.; Peretz, T.; de Boer, A.G. Unemployment risk and income change after testicular cancer diagnosis: A population-based study. Urol. Oncol. 2016, 34, e27–e33. [Google Scholar] [CrossRef]
- Ratzon, N.Z.; Uziely, B.; de Boer, A.G.; Rottenberg, Y. Unemployment Risk and Decreased Income Two and Four Years After Thyroid Cancer Diagnosis: A Population-Based Study. Thyroid 2016, 26, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Norström, F.; Waenerlund, A.K.; Lindholm, L.; Nygren, R.; Sahlén, K.G.; Brydsten, A. Does unemployment contribute to poorer health-related quality of life among Swedish adults? BMC Public Health 2019, 19, 457. [Google Scholar] [CrossRef] [PubMed]
- The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews|The BMJ. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 10 September 2022).
- Viswanathan, M.; Berkman, N.D.; Dryden, D.M.; Hartling, L. Assessing Risk of Bias and Confounding in Observational Studies of Interventions or Exposures: Further Development of the RTI Item Bank [Internet]; Report No.: 13-EHC106-EF; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2013. [Google Scholar] [PubMed]
- Database-Eurostat. Available online: https://ec.europa.eu/eurostat/web/main/data/database (accessed on 10 September 2022).
- The World Bank Data. Available online: https://data.worldbank.org/ (accessed on 10 September 2022).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Kirmayr, M.; Quilodrán, C.; Valente, B.; Loezar, C.; Garegnani, L.; Franco, J.V.A. The GRADE approach, Part 1: How to assess the certainty of the evidence. Medwave 2021, 21, e8109. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Yang, S.W.; Chen, W.L.; Wu, W.T.; Wang, C.C. Investigation on returning to work in liver cancer survivors in Taiwan: A 5-year follow-up study. BMC Public Health 2021, 21, 1846. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I.A.; Kwon, J.E.; Lee, S.; Choi, H.R.; Jeon, Y.T. Decreased income, unemployment, and disability after craniotomy for brain tumor removal: A South Korean nationwide cohort study. Support Care Cancer 2022, 30, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Behrens, M.; Lortz, I.; Wenger, K.; Filipski, K.; Seifert, V.; Forster, M.T. The ability to return to work: A patient-centered outcome parameter following glioma surgery. J. Neurooncol. 2020, 149, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kiserud, C.E.; Fagerli, U.M.; Smeland, K.B.; Fluge, Ø.; Bersvendsen, H.; Kvaløy, S.; Holte, H.; Dahl, A.A. Pattern of employment and associated factors in long-term lymphoma survivors 10 years after high-dose chemotherapy with autologous stem cell transplantation. Acta Oncol. 2016, 55, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, G.H.; Rutten, H.J.; Martijn, H.; Hanssens, P.E.; Wiggers, T. Effects on functional outcome after IORT-containing multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Gregorowitsch, M.L.; van den Bongard, H.J.G.D.; Couwenberg, A.M.; Young-Afat, D.A.; Haaring, C.; Van Dalen, T.; Schoenmaeckers, E.J.P.; Agterof, M.J.; Baas, I.O.; Sier, M.F.; et al. Self-reported work ability in breast cancer survivors; a prospective cohort study in the Netherlands. Breast 2019, 48, 45–53. [Google Scholar] [CrossRef]
- Di Meglio, A.; Menvielle, G.; Dumas, A.; Gbenou, A.; Pinto, S.; Bovagnet, T.; Martin, E.; Ferreira, A.R.; Vanlemmens, L.; Arsene, O.; et al. Body weight and return to work among survivors of early-stage breast cancer. ESMO Open 2020, 5, e000908. [Google Scholar] [CrossRef]
- Hanly, P.; Walsh, P.M.; OCéilleachair, A.; Skally, M.; Staines, A.; Kapur, K.; Fitzpatrick, P.; Sharp, L. Work-related productivity losses in an era of ageing populations: The case of colorectal cancer. J. Occup. Environ. Med. 2013, 55, 128–134. [Google Scholar] [CrossRef]
- Silvaggi, F.; Leonardi, M.; Raggi, A.; Eigenmann, M.; Mariniello, A.; Silvani, A.; Lamperti, E.; Schiavolin, S. Employment and Work Ability of Persons With Brain Tumors: A Systematic Review. Front. Hum. Neurosci. 2020, 14, 571191. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Braybrooke, J.P.; Mimoun, S.; Zarca, D.; Elia, D.; Pinder, B.; Lloyd, A.J.; Breheny, K.; Lomazzi, M.; Borisch, B. Patients’ experiences following breast cancer treatment: An exploratory survey of personal and work experiences of breast cancer patients from three European countries. Eur. J. Cancer Care 2015, 24, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Den Bakker, C.M.; Anema, J.R.; Zaman, A.G.N.M.; de Vet, H.C.W.; Sharp, L.; Angenete, E.; Allaix, M.E.; Otten, R.H.J.; Huirne, J.A.F.; Bonjer, H.J.; et al. Prognostic factors for return to work and work disability among colorectal cancer survivors; A systematic review. PLoS ONE 2018, 13, e0200720. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.J.; Yip, S.Y.C.; Chan, R.J.; Chew, L.; Chan, A. Investigating how cancer-related symptoms influence work outcomes among cancer survivors: A systematic review. J. Cancer Surviv. 2022, 16, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Fantoni, S.Q.; Peugniez, C.; Duhamel, A.; Skrzypczak, J.; Frimat, P.; Leroyer, A. Factors related to return to work by women with breast cancer in northern France. J. Occup. Rehabil. 2010, 20, 49–58. [Google Scholar] [CrossRef]
- Jacob, J.; Durand, T.; Feuvret, L.; Mazeron, J.J.; Delattre, J.Y.; Hoang-Xuan, K.; Psimaras, D.; Douzane, H.; Ribeiro, M.; Capelle, L.; et al. Cognitive impairment and morphological changes after radiation therapy in brain tumors: A review. Radiother. Oncol. 2018, 128, 221–228. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764; discussion 264–246. [Google Scholar] [CrossRef]
- Paxton, R.J.; Phillips, K.L.; Jones, L.A.; Chang, S.; Taylor, W.C.; Courneya, K.S.; Pierce, J.P. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer 2012, 118, 4024–4031. [Google Scholar] [CrossRef]
- Cavico, J.F. Appearance discrimination in employment. Equal. Divers. Incl. Int. J. 2013, 32, 83–119. [Google Scholar] [CrossRef]
- Han, E.; Norton, E.C.; Stearns, S.C. Weight and wages: Fat versus lean paychecks. Health Econ. 2009, 18, 535–548. [Google Scholar] [CrossRef]
- Lindbohm, M.L.; Kuosma, E.; Taskila, T.; Hietanen, P.; Carlsen, K.; Gudbergsson, S.; Gunnarsdottir, H. Cancer as the cause of changes in work situation (a NOCWO study). Psychooncology 2011, 20, 805–812. [Google Scholar] [CrossRef]
- Torp, S.; Gudbergsson, S.B.; Dahl, A.A.; Fosså, S.D.; Fløtten, T. Social support at work and work changes among cancer survivors in Norway. Scand. J. Public Health 2011, 39 (Suppl. S6), 33–42. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Fugazzaro, S.; Bertozzi, L.; Bassi, M.C.; Pellegrini, M.; Vicentini, M.; Mazzini, E.; Costi, S. Return to work in European Cancer survivors: A systematic review. Support Care Cancer 2018, 26, 2983–2994. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, A.; de Boer, A.; Feuerstein, M. Employment challenges for cancer survivors. Cancer 2013, 119 (Suppl. S11), 2151–2159. [Google Scholar] [CrossRef]
- World Economic Outlook Database, April 2020–2022, su IMF.org, International Monetary Fund, 19 April 2022. Available online: https://www.imf.org/en/Publications/WEO/weo-database/2022/April (accessed on 20 April 2022).
- The Legatum Prosperity Index. 2021. Available online: https://www.prosperity.com/rankings (accessed on 9 October 2022).
- Honeybul, S.; Ho, K.M. Long-term complications of decompressive craniectomy for head injury. J. Neurotrauma 2011, 28, 929–935. [Google Scholar] [CrossRef]
- Duijts, S.F.; van Egmond, M.P.; Spelten, E.; van Muijen, P.; Anema, J.R.; van der Beek, A.J. Physical and psychosocial problems in cancer survivors beyond return to work: A systematic review. Psychooncology 2014, 23, 481–492. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Data Source | Country/Region | Sample Size | Years of Observation | Unemployment Measure Used | Population Characteristics | Primary Outcome |
|---|---|---|---|---|---|---|---|
| Yang SW. 2021 [26] |
| Taiwan | 2451 | 5 | Database | Employed: 1123 Unemployed: 1328 | Return to work |
| Oh TK. 2022 [27] |
| South Korea | 4852 | 2 | Database | Employed: 1415 Unemployed: 3437 | Deterioration in quality of life, measures of unemployment |
| Senft C. 2020 [28] |
| Germany | 58 | >3 | Clinical data | Employed: 41 Unemployed: 17 | Return to work |
| Alleaume C. 2018 [12] |
| France | 969 | 5 | Telephone interviews | Employed: 791 Unemployed: 178 | Employment retention |
| Kiserud CE. 2016 [29] |
| Norway | 281 | 2 | Questionnaire | Employed: 179 Unemployed: 102 | Unemployment |
| Mannaerts GH. 2002 [30] |
| Netherlands | 76 | 5 | Questionnaire | Employed: 21 Unemployed: 14 No job preoperatively: 39 Not answered: 2 | Quality of life, return to work |
| Gregorowitsch ML. 2019 [31] |
| Netherlands | Baseline: 939 30 nonths Follow-up: 319 | 2.5 | Questionnaire | Baseline: Employed: 641 Unemployed: 298 30 Months Follow-up: Employed: 203 Unemployed: 116 | Work ability, survival |
| Di Meglio A. 2020 [32] |
| France | 1869 | 2 | Questionnaire | Employed: 1471 Unemployed: 398 | Return to work, unemployment |
| Author and Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Overall Judgement |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang SW. 2021 [26] | no | no | no | no | no | yes | No | no | no | No | yes | no | yes | no | no | No | High Quality |
| Oh TK. 2022 [27] | no | no | no | no | no | no | No | no | no | No | yes | no | yes | no | no | No | High Quality |
| Senft C. 2020 [28] | no | no | no | no | no | no | No | no | no | No | no | no | yes | yes | no | No | High Quality |
| Alleaume C. 2018 [12] | no | no | no | no | no | no | No | no | no | No | no | no | yes | no | no | No | High Quality |
| Kiserud CE. 2016 [29] | no | no | no | no | no | no | No | no | yes | No | no | yes | yes | no | no | No | High Quality |
| Mannaerts GH. 2002 [30] | no | no | no | no | no | no | No | no | no | No | no | no | yes | yes | no | No | High Quality |
| Gregorowitsch ML. 2019 [31] | no | no | no | no | no | no | No | no | no | No | no | no | yes | no | no | No | High Quality |
| Di Meglio A. 2020 [32] | no | no | no | no | no | no | No | no | no | No | no | no | yes | no | no | No | High Quality |
| Author and Year | Cancer Sites | Therapies | Induced Disabilities |
|---|---|---|---|
| Yang SW. 2021 [26] |
|
|
|
| Oh TK. 2022 [27] |
|
|
|
| Senft C. 2020 [28] |
|
|
|
| Alleaume C. 2018 [12] |
|
|
|
| Kiserud CE. 2016 [29] |
|
|
|
| Mannaerts GH. 2002 [30] |
|
|
|
| Gregorowitsch ML. 2019 [31] |
|
|
|
| Di Meglio A. 2020 [32] |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimienti, M.; Morlino, G.; Ingravalle, F.; Vinci, A.; Colarusso, E.; De Santo, C.; Formosa, V.; Gentile, L.; Lorusso, G.; Mosconi, C.; et al. Unemployment Status Subsequent to Cancer Diagnosis and Therapies: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1513. https://doi.org/10.3390/cancers15051513
Chimienti M, Morlino G, Ingravalle F, Vinci A, Colarusso E, De Santo C, Formosa V, Gentile L, Lorusso G, Mosconi C, et al. Unemployment Status Subsequent to Cancer Diagnosis and Therapies: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(5):1513. https://doi.org/10.3390/cancers15051513
Chicago/Turabian StyleChimienti, Martina, Giustino Morlino, Fabio Ingravalle, Antonio Vinci, Emilio Colarusso, Carolina De Santo, Valeria Formosa, Lavinia Gentile, Grazia Lorusso, Claudia Mosconi, and et al. 2023. "Unemployment Status Subsequent to Cancer Diagnosis and Therapies: A Systematic Review and Meta-Analysis" Cancers 15, no. 5: 1513. https://doi.org/10.3390/cancers15051513
APA StyleChimienti, M., Morlino, G., Ingravalle, F., Vinci, A., Colarusso, E., De Santo, C., Formosa, V., Gentile, L., Lorusso, G., Mosconi, C., Scaramella, M., Rosca, V., Veneziano, E., Torino, F., Emberti Gialloreti, L., & Palombi, L. (2023). Unemployment Status Subsequent to Cancer Diagnosis and Therapies: A Systematic Review and Meta-Analysis. Cancers, 15(5), 1513. https://doi.org/10.3390/cancers15051513