Melanoma Mortality Trends in 28 European Countries: A Retrospective Analysis for the Years 1960–2020
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Limitations of the Study
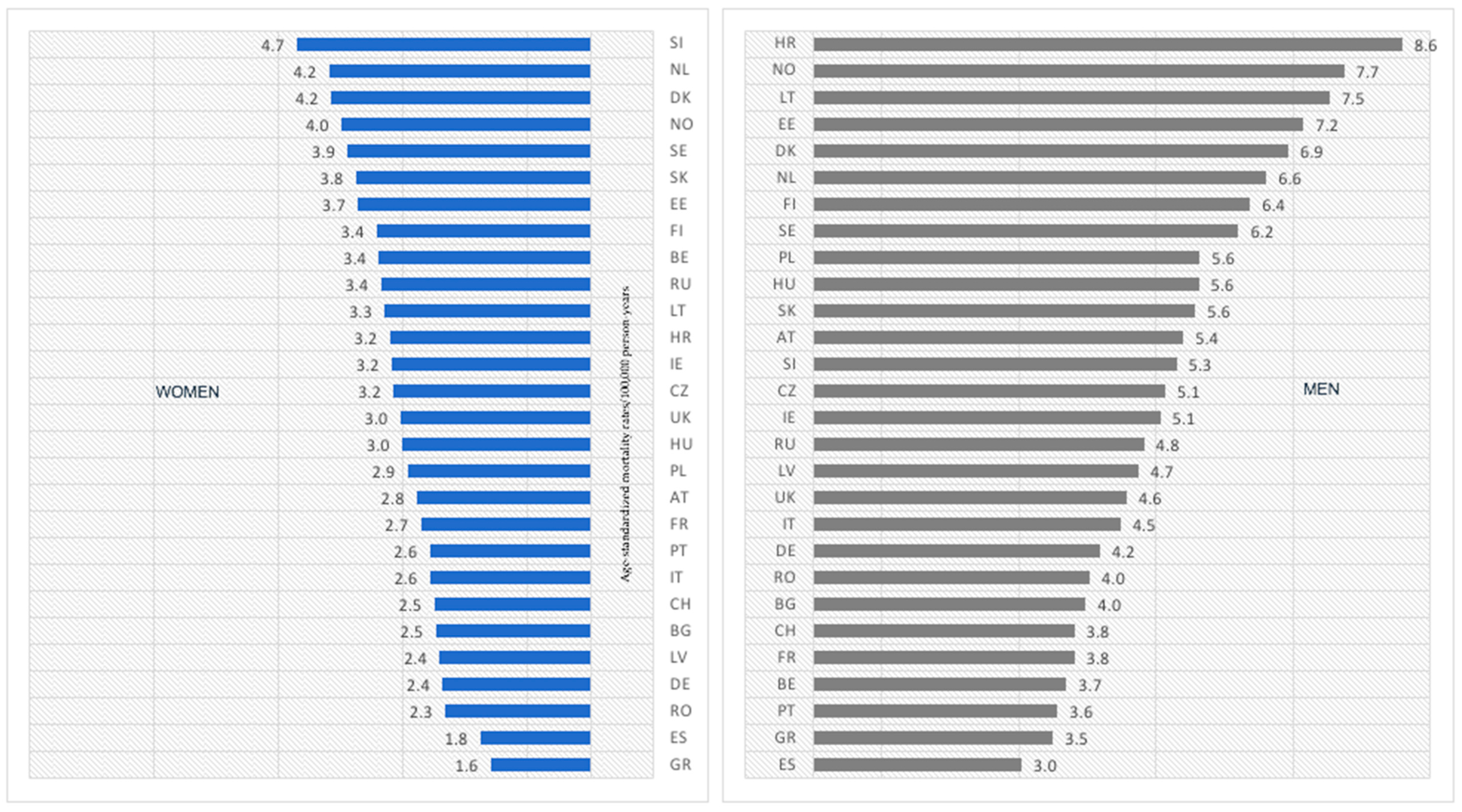
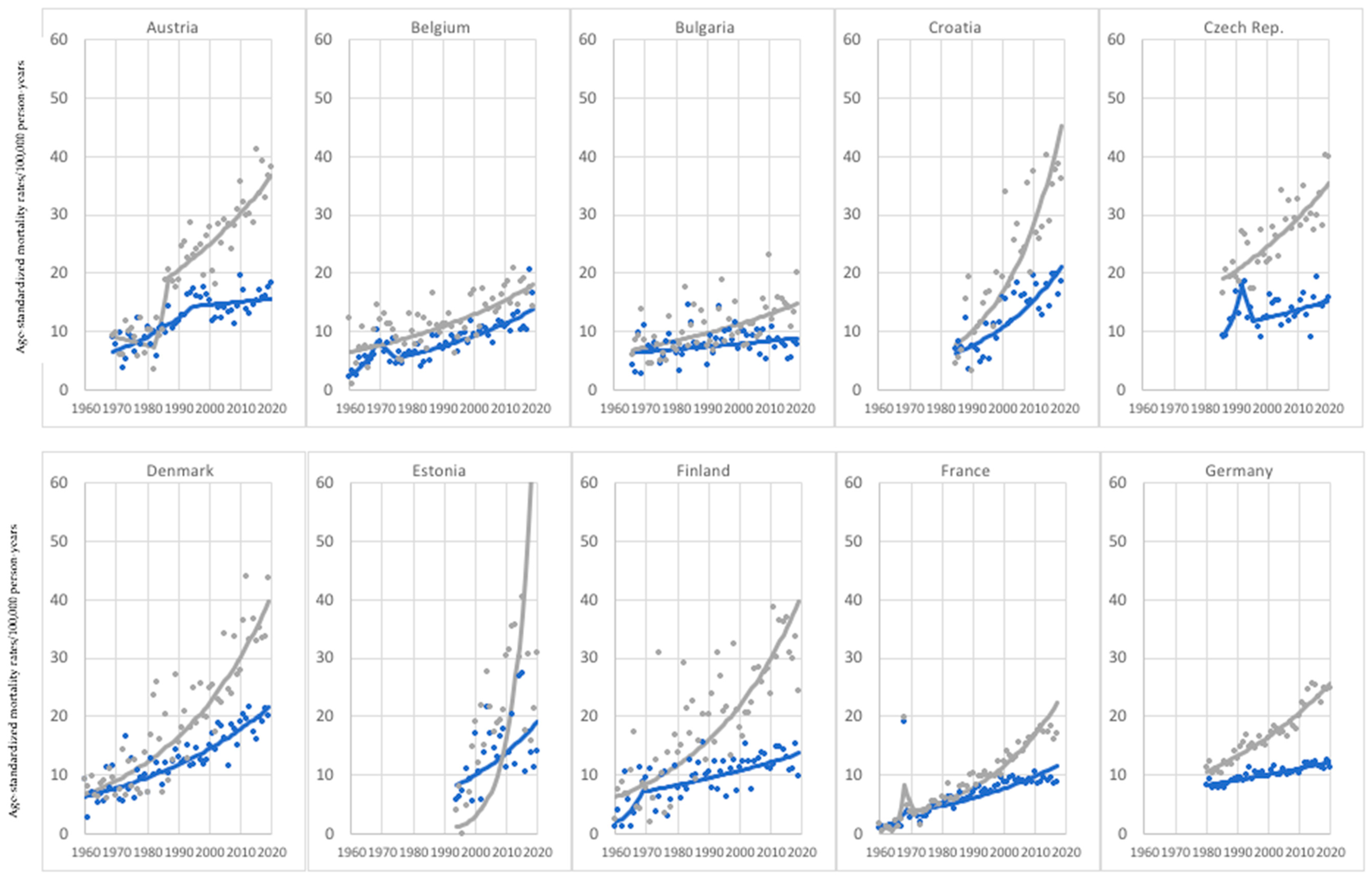
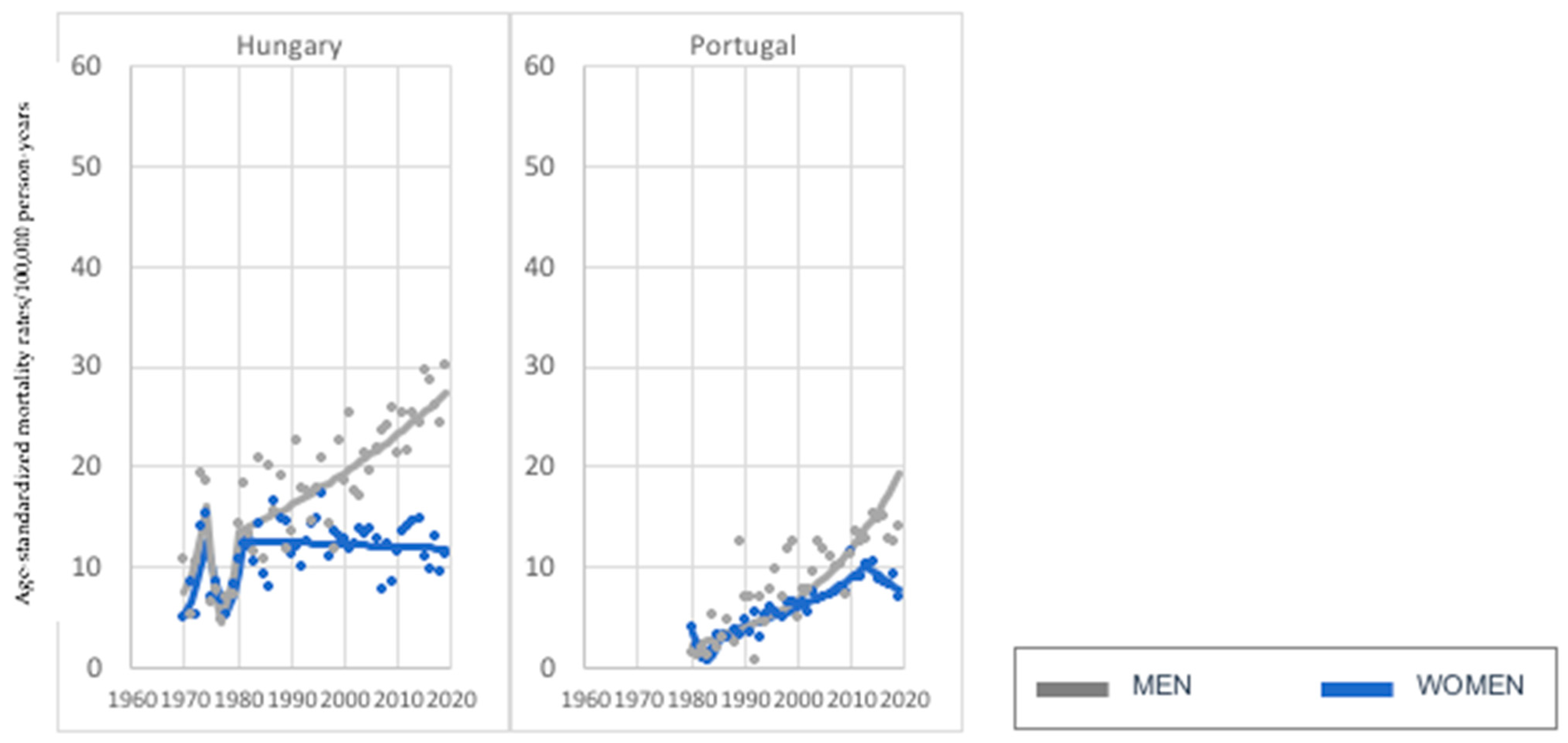
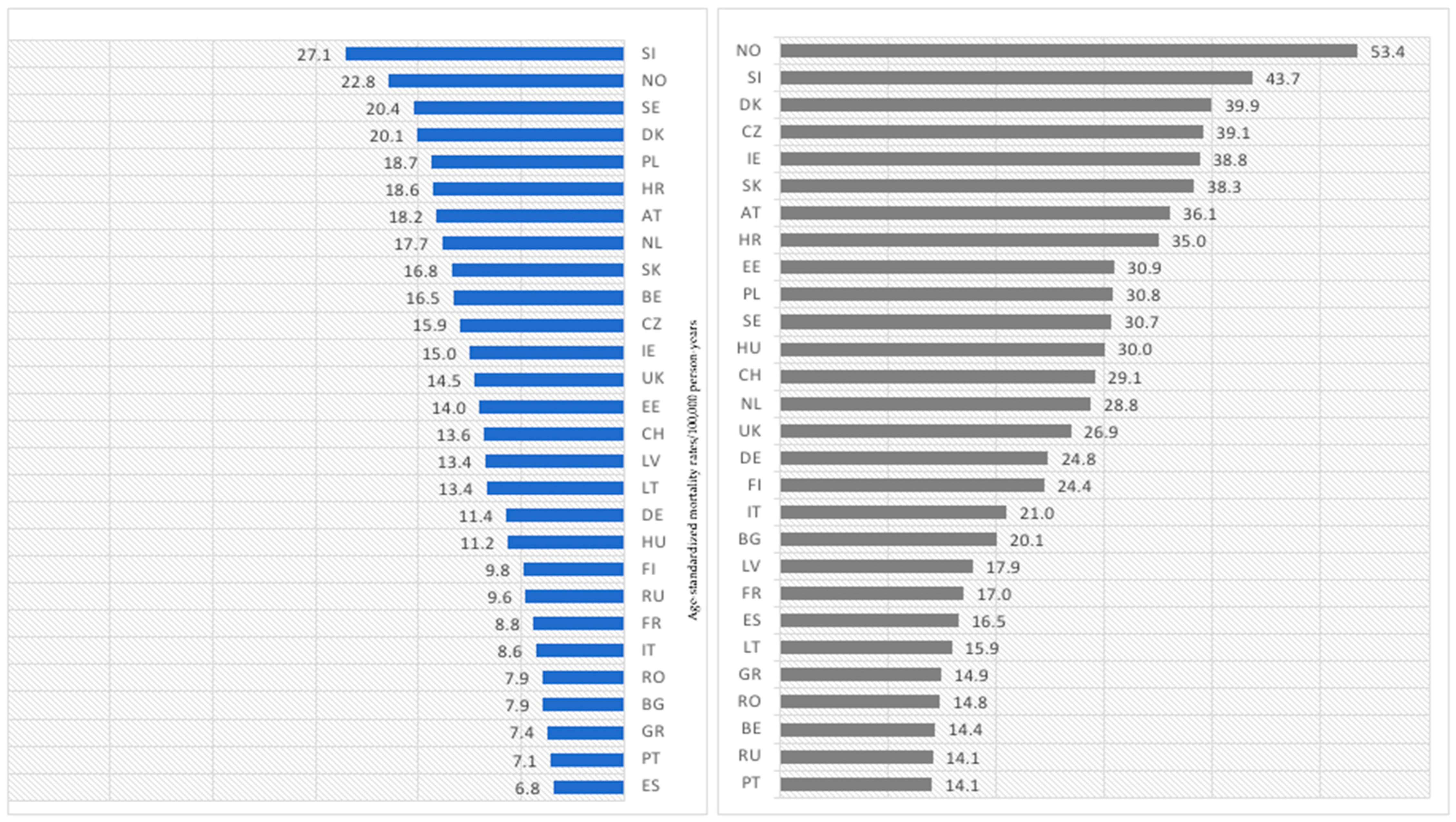
3. Results
3.1. Age Group: 45–74 Years Old
3.2. Age Group: 75+ Years Old
4. Discussion
5. Conclusions
- Investigated melanoma-mortality trends vary in individual countries and age groups; however, a highly concerning phenomenon—increasing melanoma-mortality rates in both sexes—was observed in 7 countries for the younger age group and in as much as 26 countries for the older age group. Undoubtedly, a higher cancer mortality is related to older age. However, this phenomenon raises a question regarding cancer care that is available for older people, which should be a subject of further research.
- As in most countries, melanoma-mortality-rate values are higher for men, and the pace of increase is much more rapid for men; one should consider putting more emphasis on gender-tailored preventive actions. Likewise, in the case of age groups, melanoma mortality increases with age.
- The results of our study suggest that there is not only one universal factor affecting melanoma mortality for all investigated European countries. Rather, we should define a mixture of the most significant factors that substantially impact individual European populations with different intensities. We assume that the most substantial factors are (starting with the most important) aging, the efficiency of healthcare systems, good access to innovative treatment options, and UV overexposure (mainly caused by sunbeds).
- Despite the high availability of innovative treatment options for melanoma cancer patients (in the vast majority of investigated countries, >90% of melanoma patients had access to innovative treatment), not all of these countries have undergone decreasing melanoma trends. Moreover, some of them have the highest current melanoma-mortality-rate values. This phenomenon could be possibly explained by a period that is too short since the given treatment options have been available or/and a worse efficiency of cancer care and the healthcare system in individual countries. Moreover, an assurance of innovative medicines is insufficient for a melanoma-mortality decrease during the staff shortages, underfinancing, or organizational difficulties in healthcare that many investigated countries have faced for decades. However, these hypotheses should be the subject of further studies.
- Considering the obtained results, primary and secondary actions aiming for the prevention and early detection of melanoma seem to be notably underestimated by policymakers and not effectively performed in many investigated countries—in most cases, primarily the fact that the early symptoms of melanoma can be easily recognized.
- There is a need to strengthen the efforts of medical societies to promote education on melanoma prevention, as health professionals have reliable knowledge and the highest trust among patients. We can assume that, as in the case of other highly preventable cancers (e.g., cervical cancer, where, similar to melanoma, a single risk factor plays a crucial role in elevating the risk of the disease), mortality reduction demands much broader coordinated public-health actions than primary prevention alone. The effectiveness of these systemic and individual actions will determine the success of such strategic initiatives as Europe’s Beating Cancer Plan.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(UV)-radiation-and-skin-cancer (accessed on 24 August 2022).
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 24 August 2022).
- European Comelanomaission, Skin Melanoma Burden in EU-27. Available online: https://ecis.jrc.ec.europa.eu/pdf/factsheets/Melanoma_cancer_en.pdf (accessed on 24 August 2022).
- World Health Organization Mortality Database. Available online: https://www.who.int/data/data-collection-tools/who-mortality-database (accessed on 1 June 2022).
- Eurostat Database. Available online: https://ec.europa.eu/eurostat/web/main/data/database (accessed on 1 June 2022).
- World Health Organization. Incidence of Melanoma in People Aged under 55 Years, FACT SHEET 4.2 z December 2009 z CODE: RPG4_UVrd_E1. Available online: https://www.euro.who.int/__data/assets/pdf_file/0009/97029/4.2.-Incidence-of-melanoma-EDITED_layouted.pdf (accessed on 25 August 2022).
- Segi, M. Cancer Mortality for Selected Sites in 24 Countries (1950–1957); Department of Public Health, Tohoku University of Medicine: Sendai, Japan, 1960. [Google Scholar]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351, Erratum in Stat. Med. 2001, 20, 655. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Heijnen, M.L.; Houterman, S.; Lemelanomaens, V.E.; Louwman, M.W.; Maas, H.A.; Coebergh, J.W. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit. Rev. Oncol. Hematol. 2005, 55, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ribero, S.; Stucci, L.S.; Marra, E.; Marconcini, R.; Spagnolo, F.; Orgiano, L.; Picasso, V.; Queirolo, P.; Palmieri, G.; Quaglino, P.; et al. Effect of Age on Melanoma Risk, Prognosis and Treatment Response. Acta Derm. Venereol. 2018, 98, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Robsahm, T.E.; Helsing, P.; Nilssen, Y.; Vos, L.; Rizvi, S.M.H.; Akslen, L.A.; Veierød, M.B. High mortality due to cutaneous melanoma in Norway: A study of prognostic factors in a nationwide cancer registry. Clin. Epidemiol. 2018, 10, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Rockberg, J.; Amelio, J.M.; Taylor, A.; Jörgensen, L.; Ragnhamelanomaar, P.; Hansson, J. Epidemiology of cutaneous melanoma in Sweden-Stage-specific survival and rate of recurrence. Int. J. Cancer 2016, 139, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, R.J.; van Steenbergen, L.N.; Hollestein, L.M.; de Vries, E.; Nijsten, T.; van Akkooi, A.C.; Janssen-Heijnen, M.L.; Coebergh, J.W. Conditional survival of malignant melanoma in The Netherlands: 1994–2008. Eur. J. Cancer. 2014, 50, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Bellenghi, M.; Puglisi, R.; Pontecorvi, G.; De Feo, A.; Carè, A.; Mattia, G. Sex and Gender Disparities in Melanoma. Cancers 2020, 12, 1819. [Google Scholar] [CrossRef] [PubMed]
- Shipman, A.R.; Clark, A.B.; Levell, N.J. Sunnier European countries have lower melanoma mortality. Clin. Exp. Dermatol. 2011, 36, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Suppa, M.; Gandini, S.; Njimi, H.; Bulliard, J.L.; Correia, O.; Duarte, A.F.; Peris, K.; Stratigos, A.J.; Nagore, E.; Longo, M.I.; et al. Prevalence and determinants of sunbed use in thirty European countries: Data from the Euromelanoma skin cancer prevention campaign. J. Eur. Acad Dermatol. Venereol. 2019, 33 (Suppl. S2), 13–27. [Google Scholar] [CrossRef] [PubMed]
- Bieliauskiene, G.; Holm-Schou, A.S.; Philipsen, P.A.; Murphy, G.M.; Sboukis, D.; Schwarz, T.; Young, A.R.; Wulf, H.C. Measurements of sun sensitivity in five European countries confirm the relative nature of the Fitzpatrick skin phototype scale. Photodermatol. Photoimelanomaunol. Photomed. 2020, 36, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kandolf Sekulovic, L.; Peris, K.; Hauschild, A.; Stratigos, A.; Grob, J.J.; Nathan, P.; Dumelanomaer, R.; Forsea, A.M.; Hoeller, C.; Gogas, H.; et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended melanoma first-line innovative treatments. Eur. J. Cancer 2017, 75, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.; Lindwedel, K.S.; Mathes, S.; Görig, T.; Gefeller, O. Tanning Bed Legislation for Minors: A Comprehensive International Comparison. Children 2022, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Guerra, K.C.; Crane, J.S. Sunburn. 28 August 2022. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tripp, M.K.; Peterson, S.K.; Prokhorov, A.V.; Shete, S.S.; Lee, J.E.; Gershenwald, J.E.; Gritz, E.R. Correlates of Sun Protection and Sunburn in Children of Melanoma Survivors. Am. J. Prev. Med. 2016, 51, e77–e85. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Lindqvist, P.G.; DEGruijl, F.R.; Pilz, S.; Kimball, S.M.; Grant, W.B.; Holick, M.F. A Critical Appraisal of the Recent Reports on Sunbeds from the European Comelanomaission’s Scientific Comelanomaittee on Health, Environmental and Emerging Risks and from the World Health Organization. Anticancer Res. 2018, 38, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- OECD/European Observatory on Health Systems and Policies. Slovenia: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Kandolf-Sekulovic, L.; Peris, K.; Stratigos, A.; Hauschild, A.; Forsea, A.M.; Lebbe, C.; Lallas, A.; Grob, J.J.; Harwood, C.; Gogas, H.; et al. Which medical disciplines diagnose and treat melanoma in Europe in 2019? A survey of experts from melanoma centers in 27 European countries. J. Eur. Acad Dermatol. Venereol. 2021, 35, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Koczkodaj, P.; Camacho, F.; Batten, G.P.; Anderson, R.T. Are Wellness Visits a Possible and Effective Cure for the Increasing Cancer Burden in Poland? Example of Women’s Preventive Services in the U.S. Cancers 2022, 14, 4296. [Google Scholar] [CrossRef] [PubMed]
- Micheli, A.; Coebergh, J.W.; Mugno, E.; Massimiliani, E.; Sant, M.; Oberaigner, W.; Holub, J.; Storm, H.H.; Forman, D.; Quinn, M.; et al. European health systems and cancer care. Ann. Oncol. 2003, 14 (Suppl. S5), v41–v60. [Google Scholar] [CrossRef] [PubMed]
- OECD/European Observatory on Health Systems and Policies. Greece: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Ireland: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Italy: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Lithuania: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Portugal: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- OECD/European Observatory on Health Systems and Policies. Slovak Republic: Country Health Profile 2021. In State of Health in the EU; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Eurostat Data. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20210118-1?redirect=%2Feurostat%2Fnews%2Fwhats-new (accessed on 26 November 2022).
- Forsea, A.M.; Del Marmol, V.; Stratigos, A.; Geller, A.C. Melanoma prognosis in Europe: Far from equal. Br. J. Dermatol. 2014, 171, 179–182. [Google Scholar] [CrossRef] [PubMed]
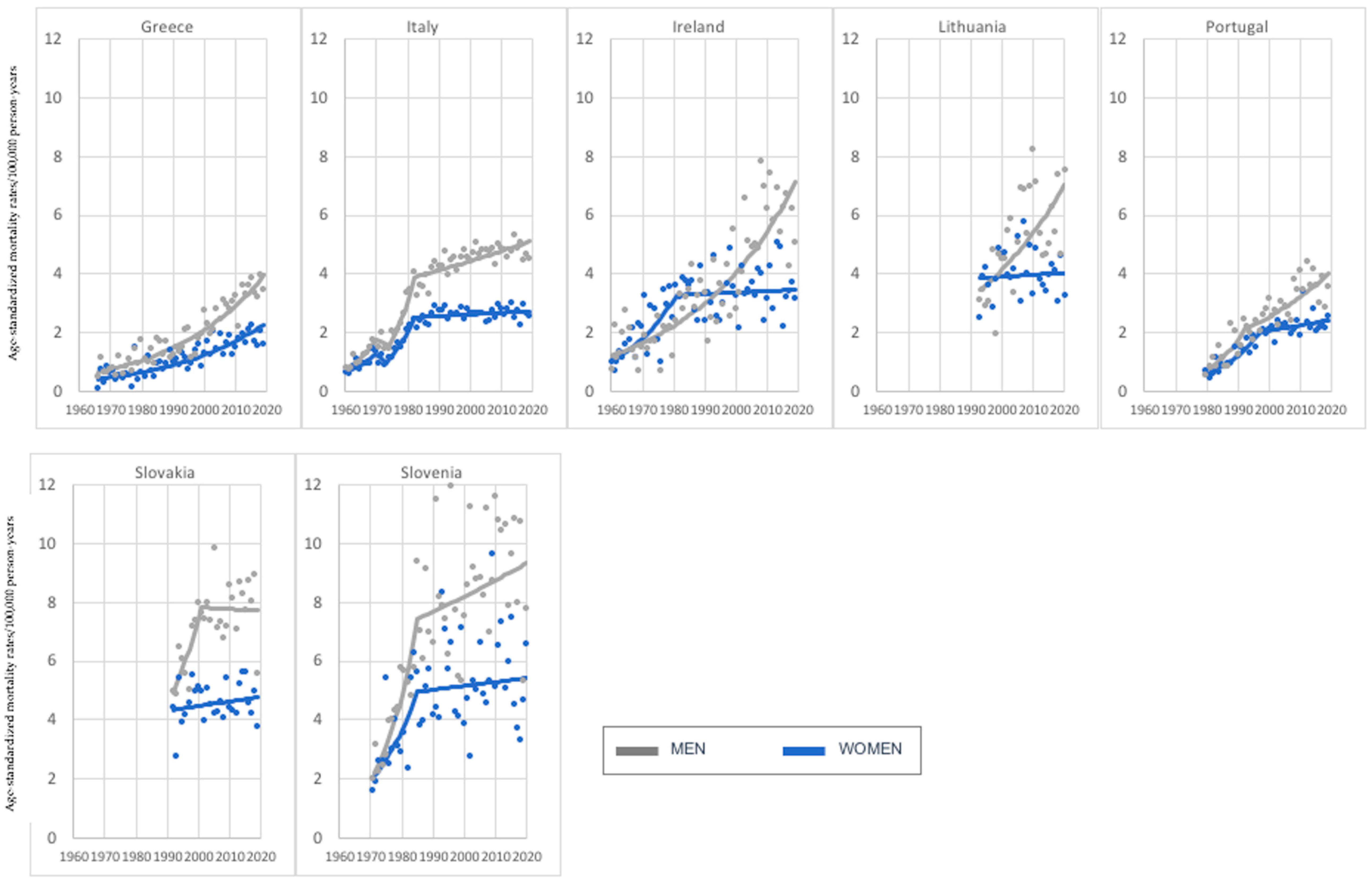
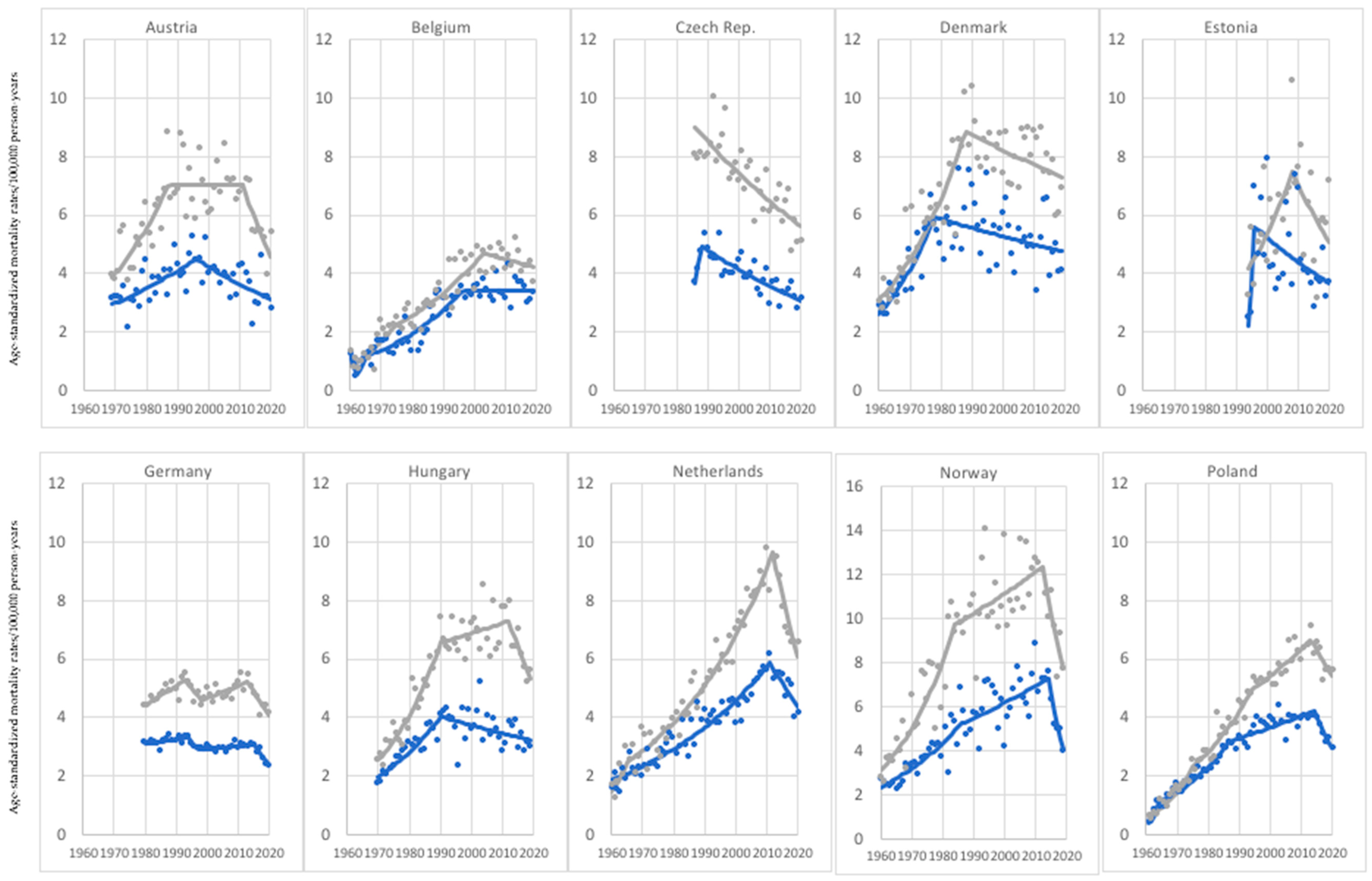

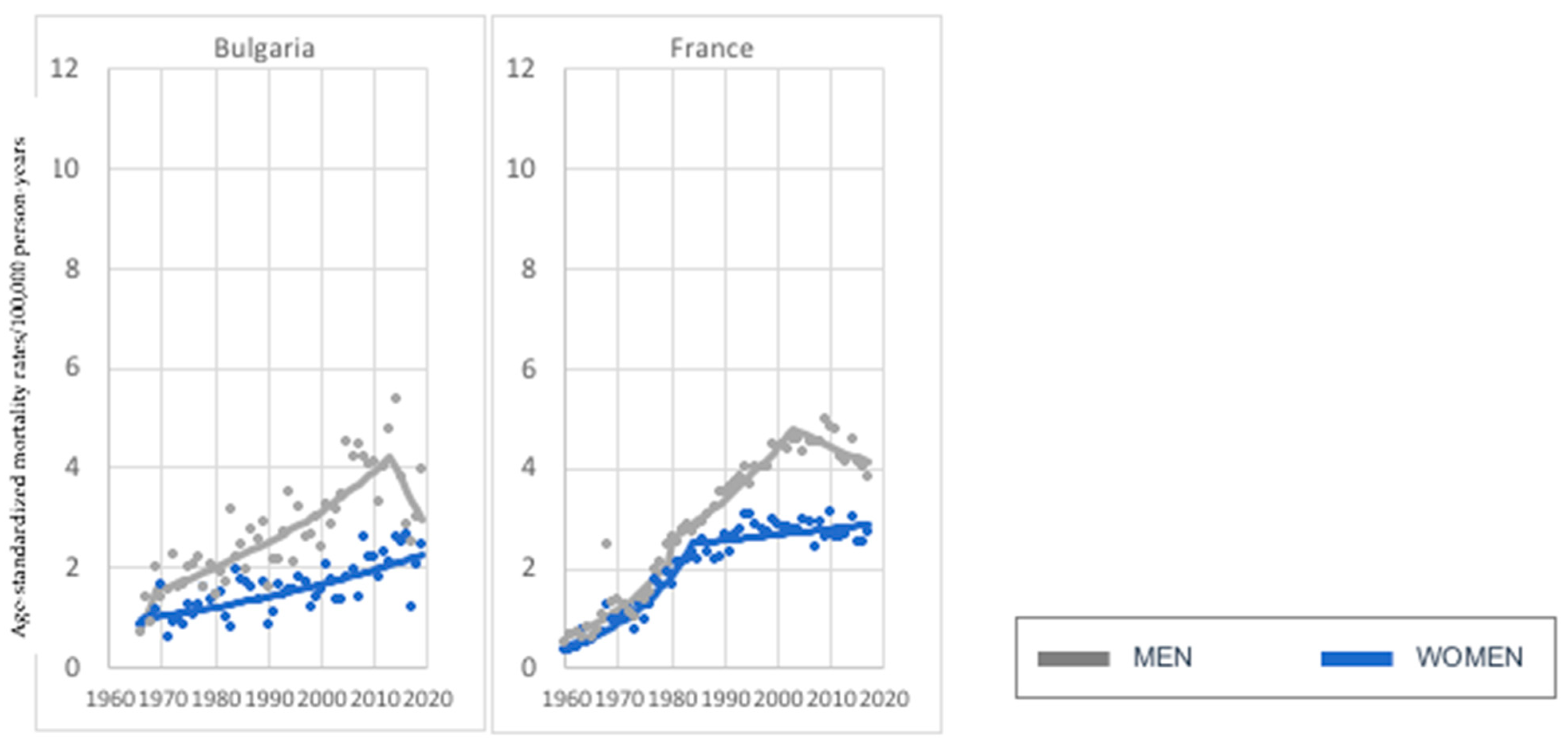
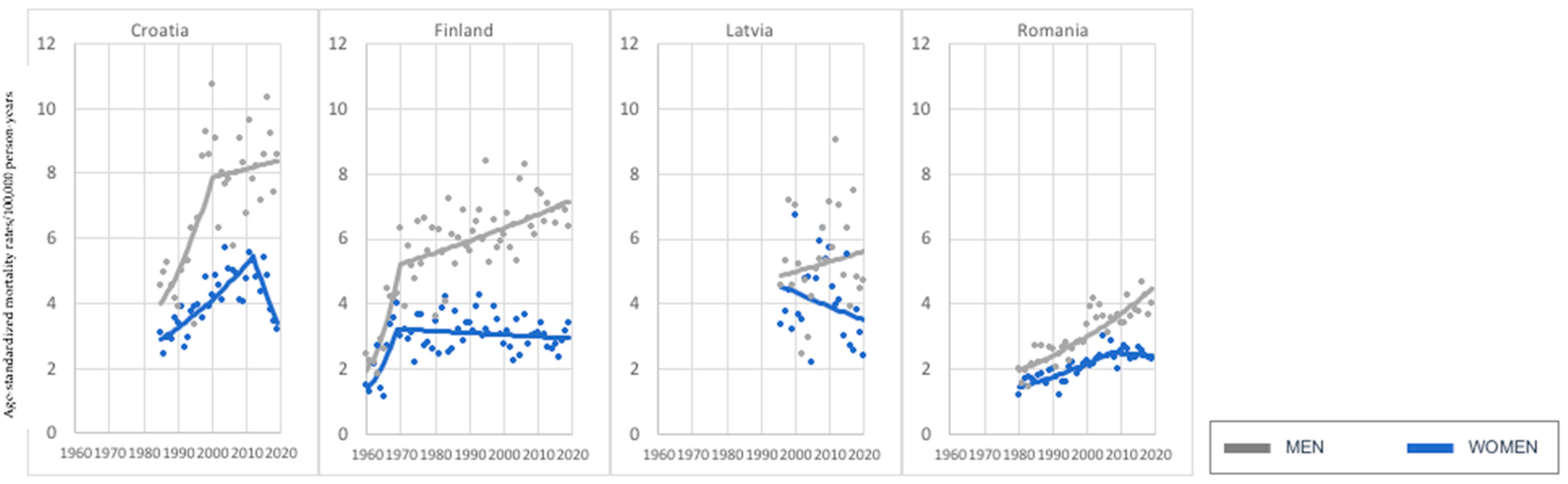


| Current Mortality Trend | Age Groups | |
|---|---|---|
| 45–74 | 75+ | |
| Increase—both sexes | Greece, Italy, Ireland, Lithuania, Portugal, Slovakia, Slovenia (7) | Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Romania, Russia, Slovakia, Slovenia, Spain, Sweden, Switzerland, United Kingdom (UK) (26) |
| Decrease—both sexes | Austria, Belgium, Czech Republic, Denmark, Estonia, Germany, Hungary, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom (UK) (15) | None (0) |
| Increase in women, decrease in men. | Bulgaria, France (2) | None (0) |
| Decrease in women, increase in men. | Croatia, Finland, Latvia, Romania (4) | Hungary, Portugal (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczkodaj, P.; Sulkowska, U.; Didkowska, J.; Rutkowski, P.; Mańczuk, M. Melanoma Mortality Trends in 28 European Countries: A Retrospective Analysis for the Years 1960–2020. Cancers 2023, 15, 1514. https://doi.org/10.3390/cancers15051514
Koczkodaj P, Sulkowska U, Didkowska J, Rutkowski P, Mańczuk M. Melanoma Mortality Trends in 28 European Countries: A Retrospective Analysis for the Years 1960–2020. Cancers. 2023; 15(5):1514. https://doi.org/10.3390/cancers15051514
Chicago/Turabian StyleKoczkodaj, Paweł, Urszula Sulkowska, Joanna Didkowska, Piotr Rutkowski, and Marta Mańczuk. 2023. "Melanoma Mortality Trends in 28 European Countries: A Retrospective Analysis for the Years 1960–2020" Cancers 15, no. 5: 1514. https://doi.org/10.3390/cancers15051514
APA StyleKoczkodaj, P., Sulkowska, U., Didkowska, J., Rutkowski, P., & Mańczuk, M. (2023). Melanoma Mortality Trends in 28 European Countries: A Retrospective Analysis for the Years 1960–2020. Cancers, 15(5), 1514. https://doi.org/10.3390/cancers15051514










