Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
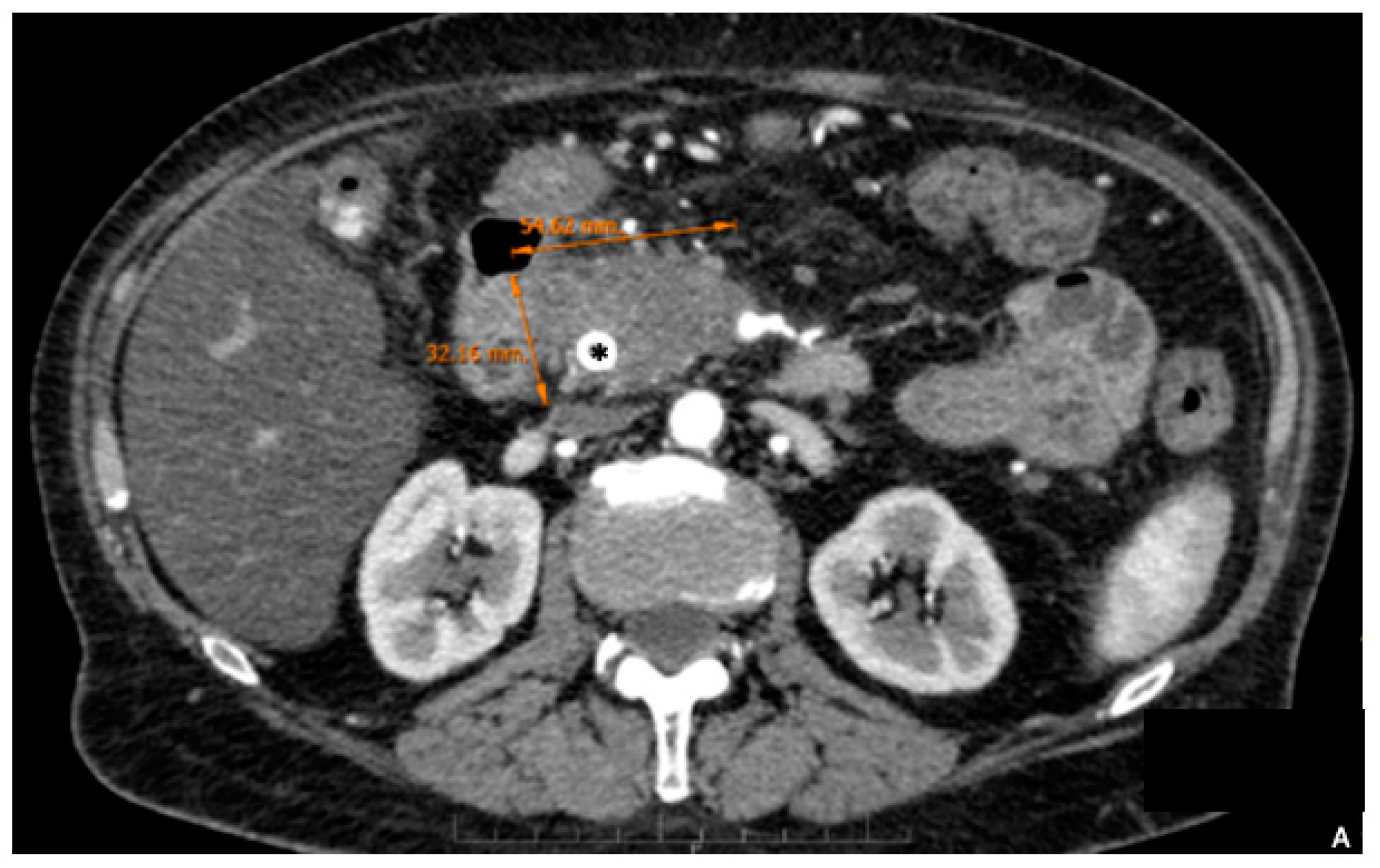
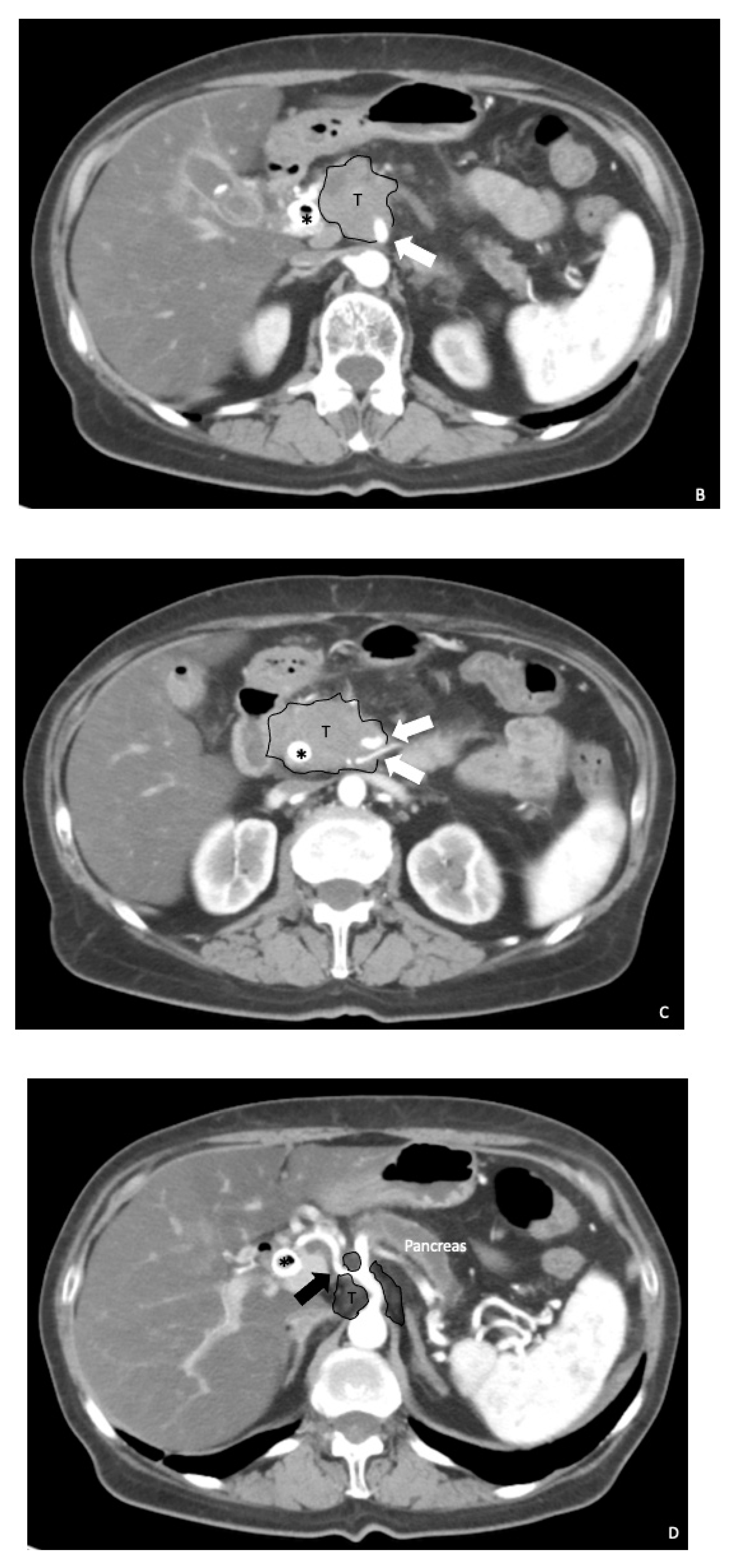
2. Preoperative Surgical Planning
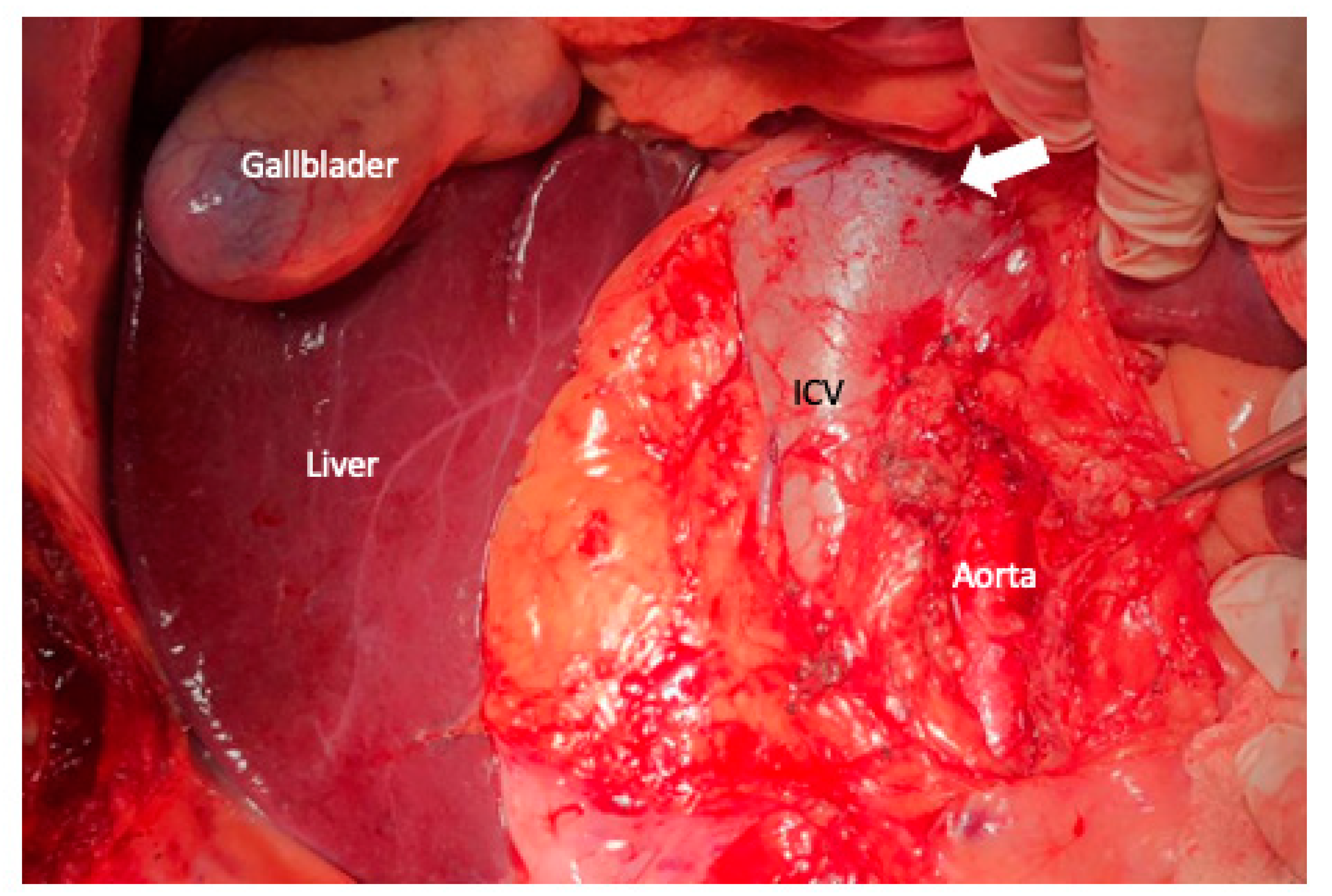
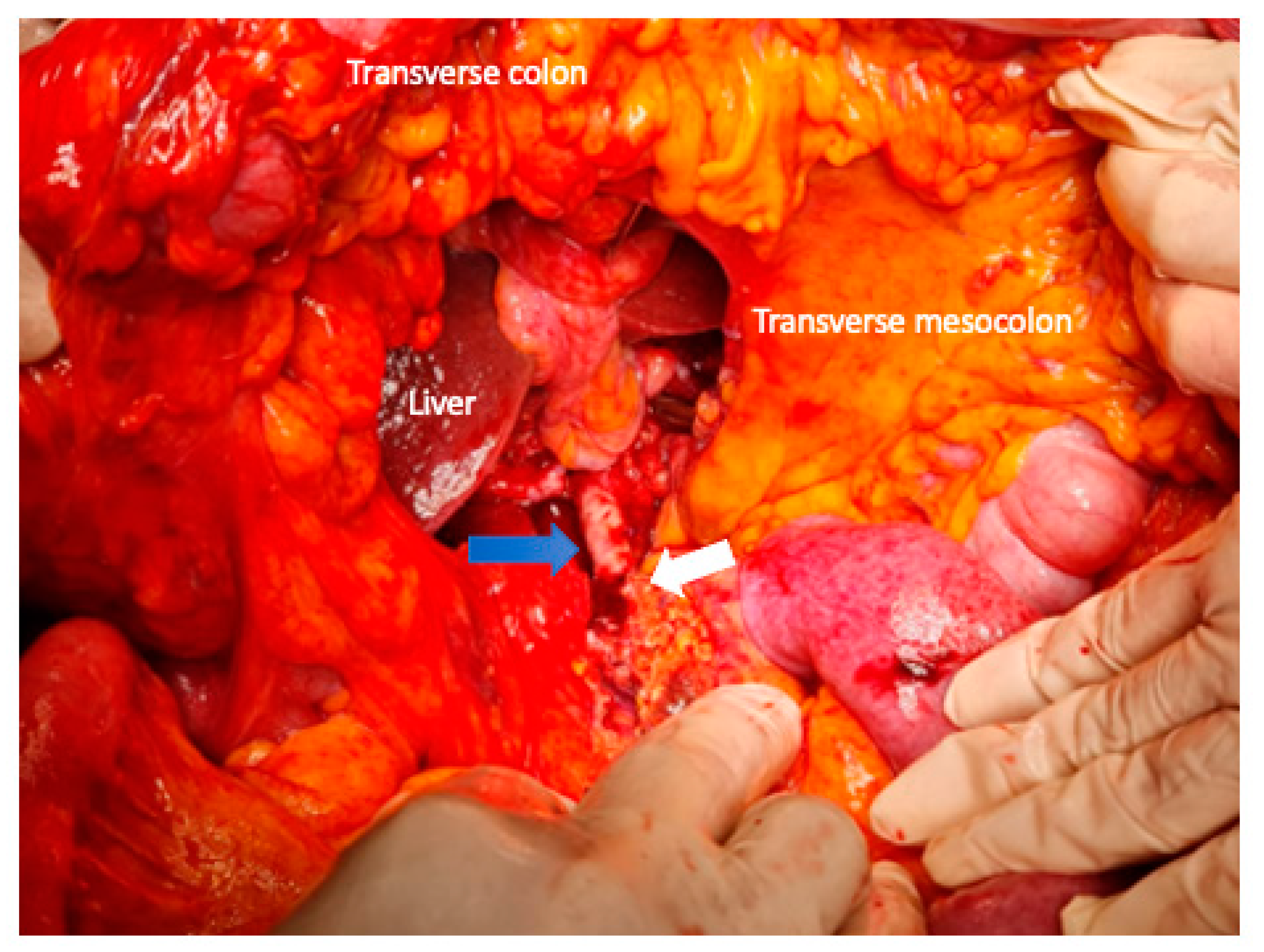
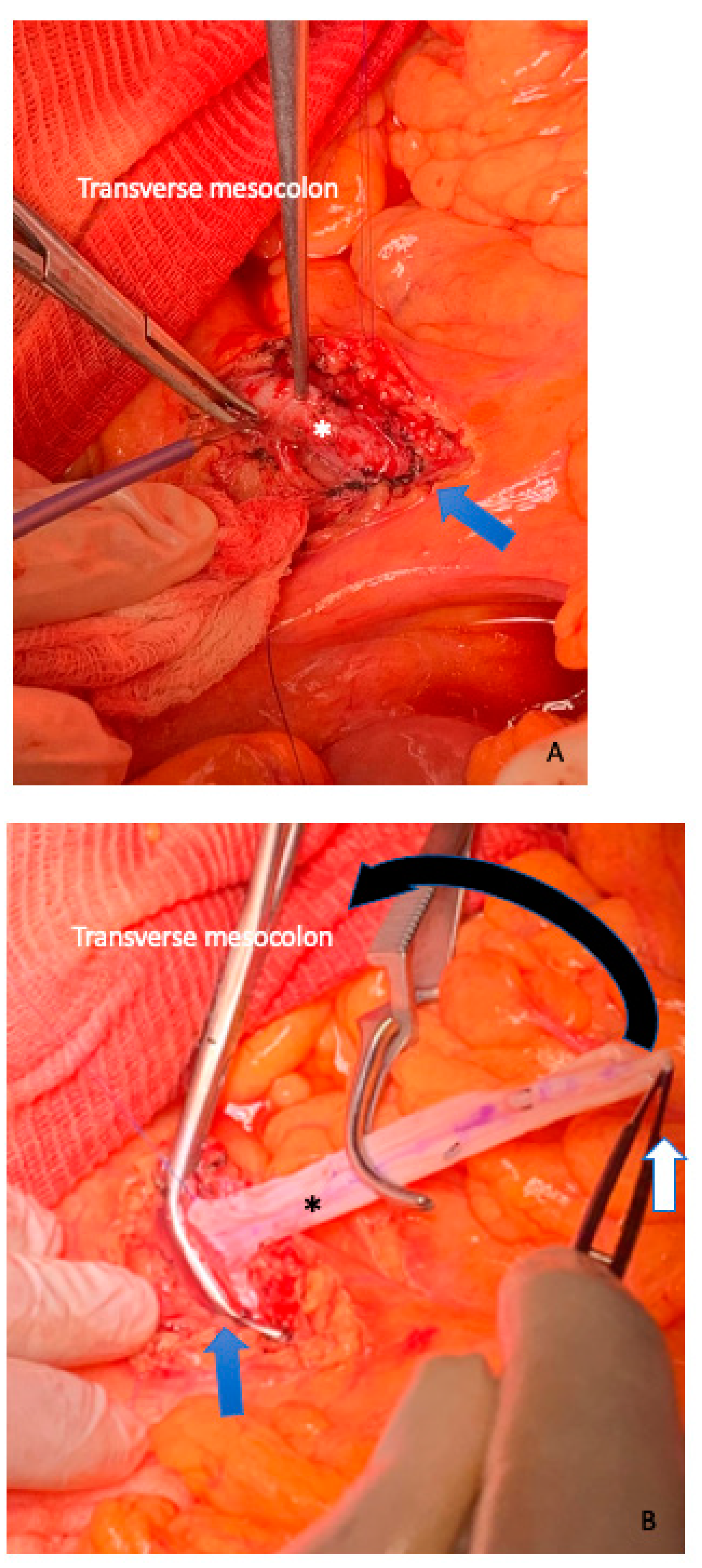
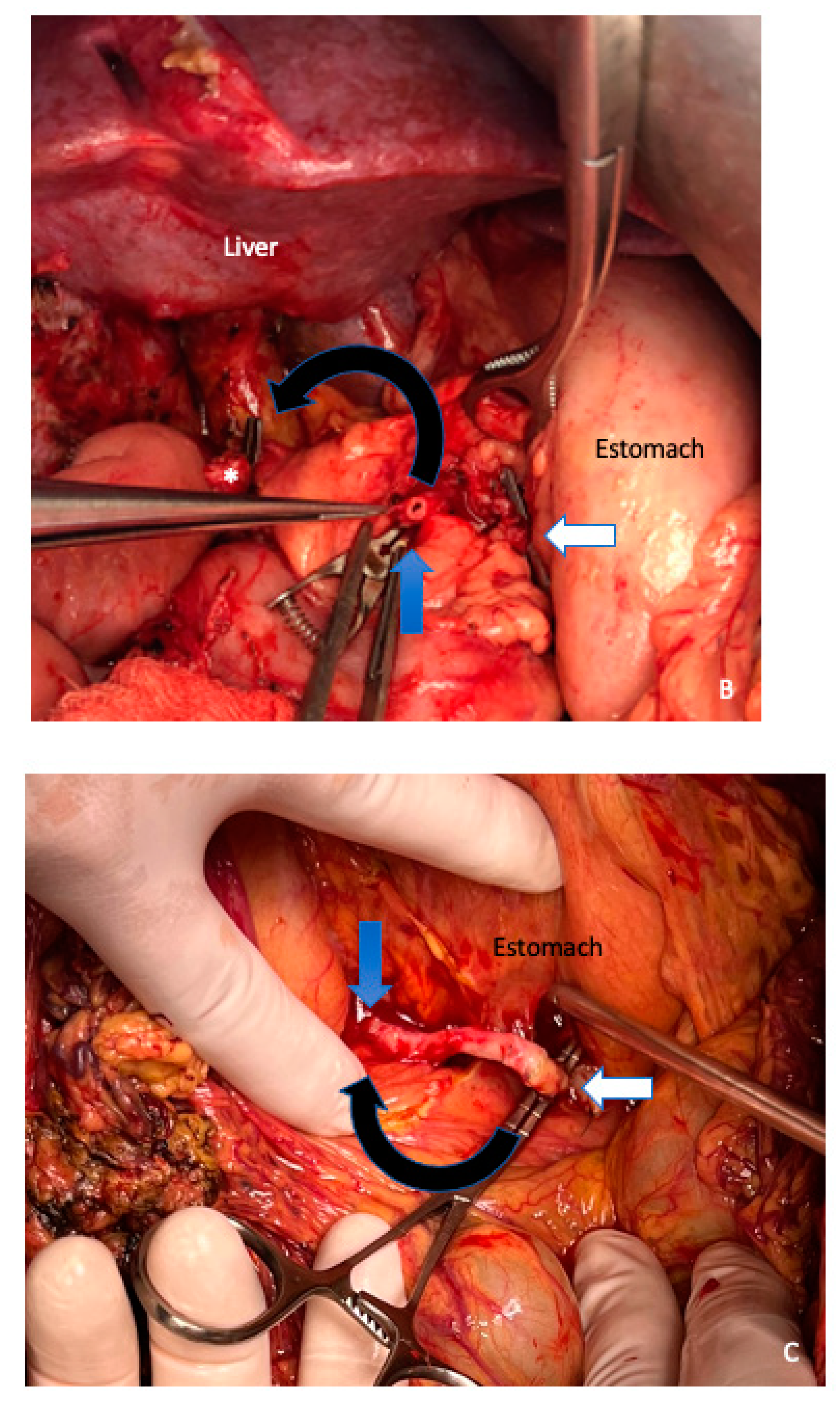
3. Surgical Aspects
4. Extended Pancreatectomy
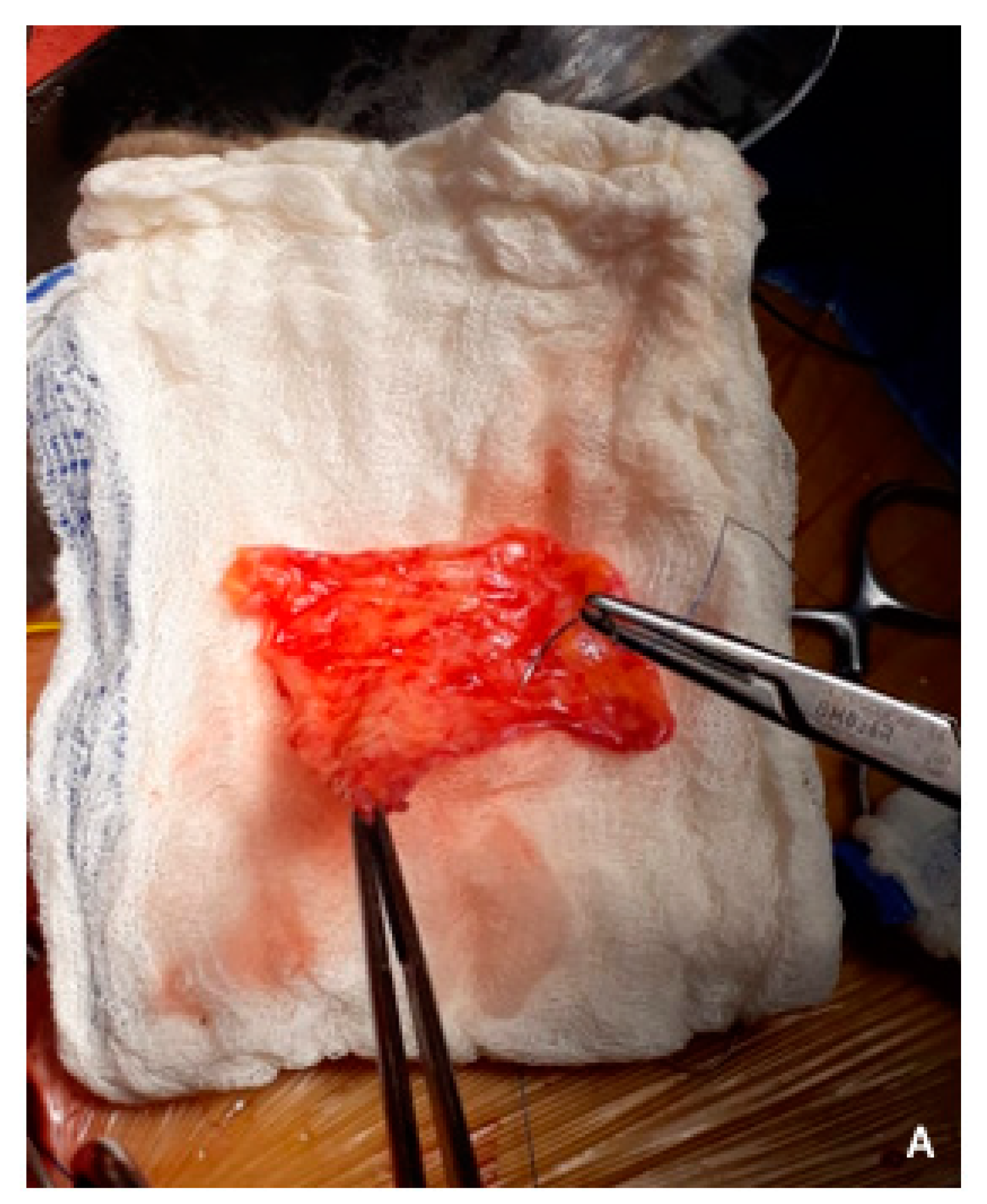
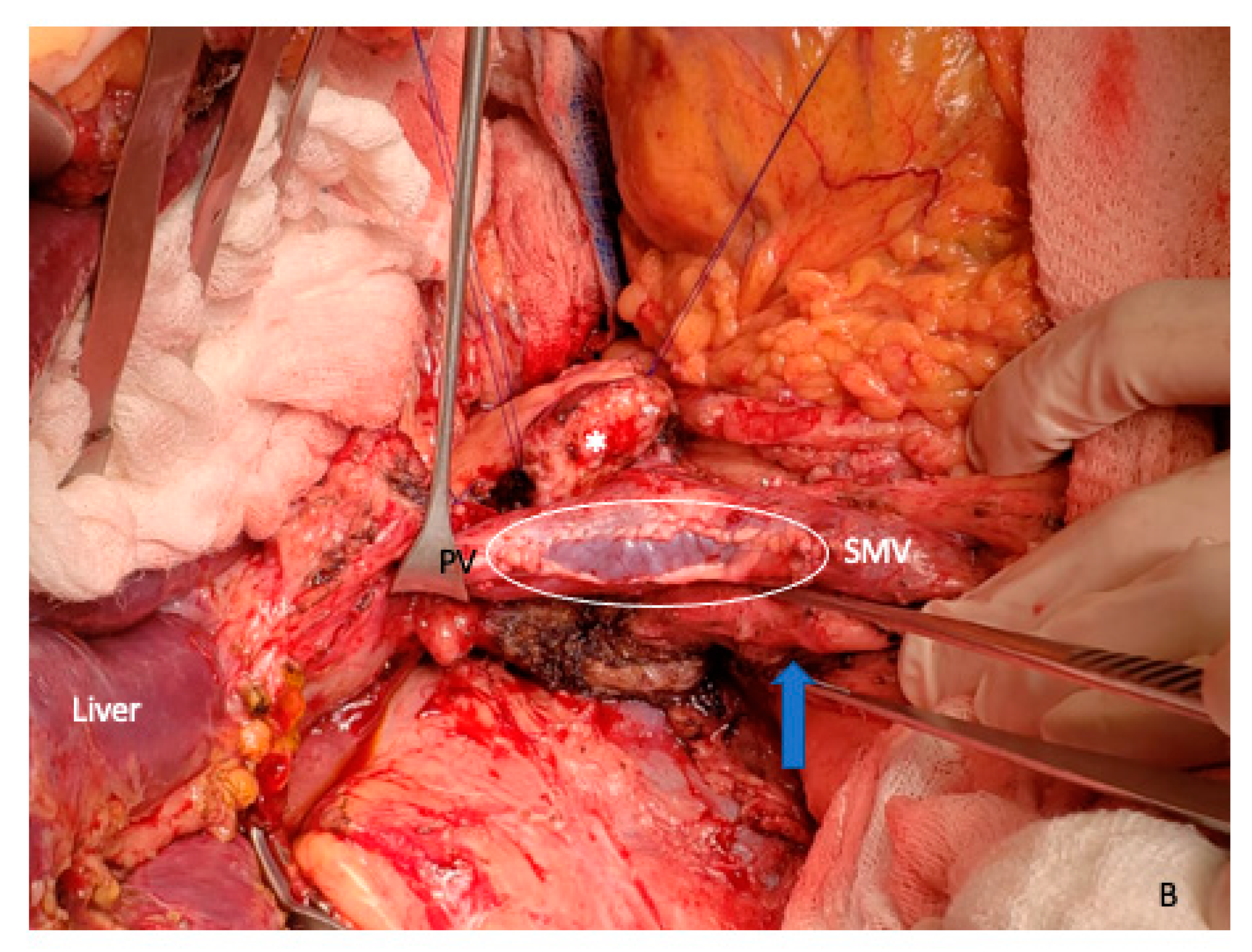
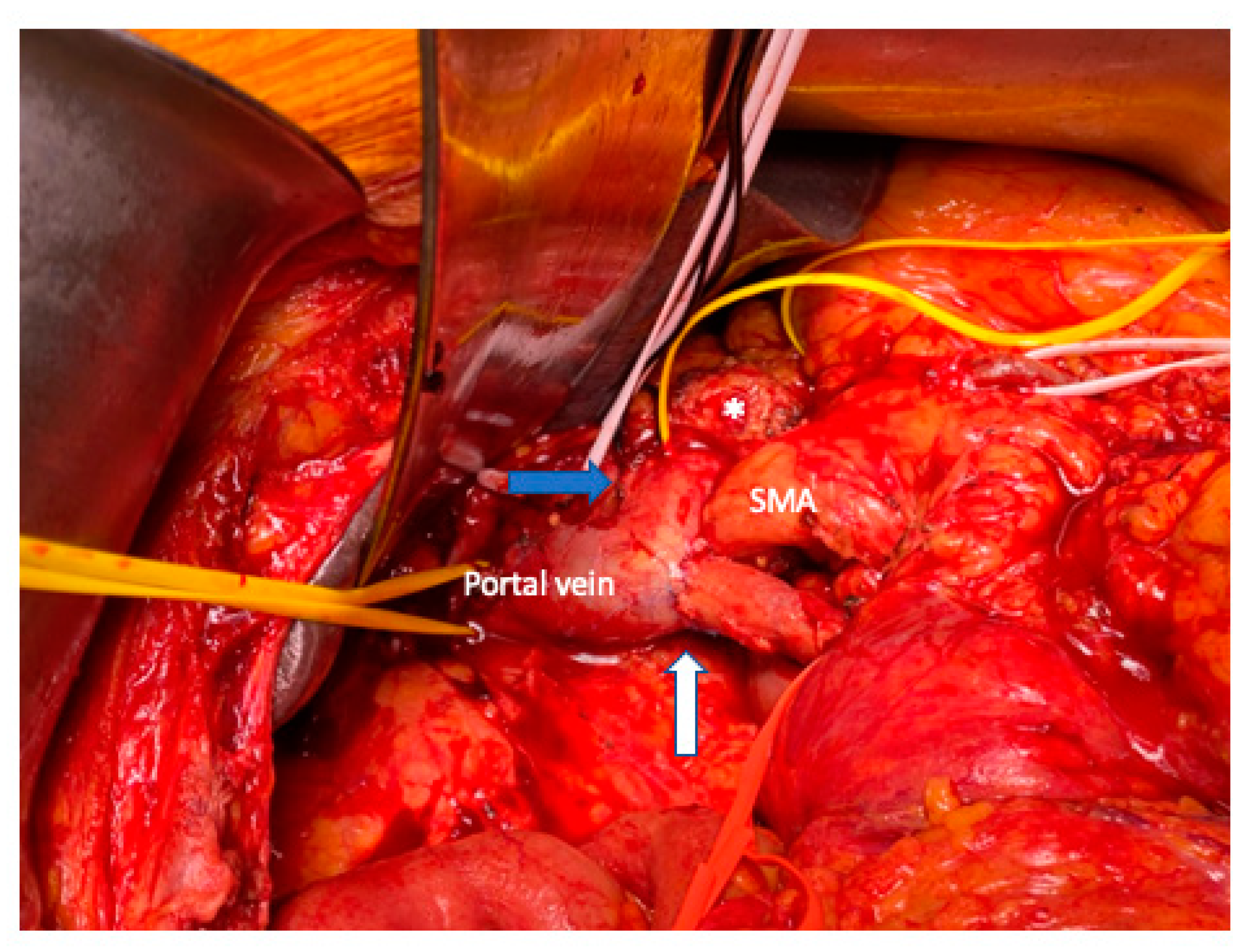
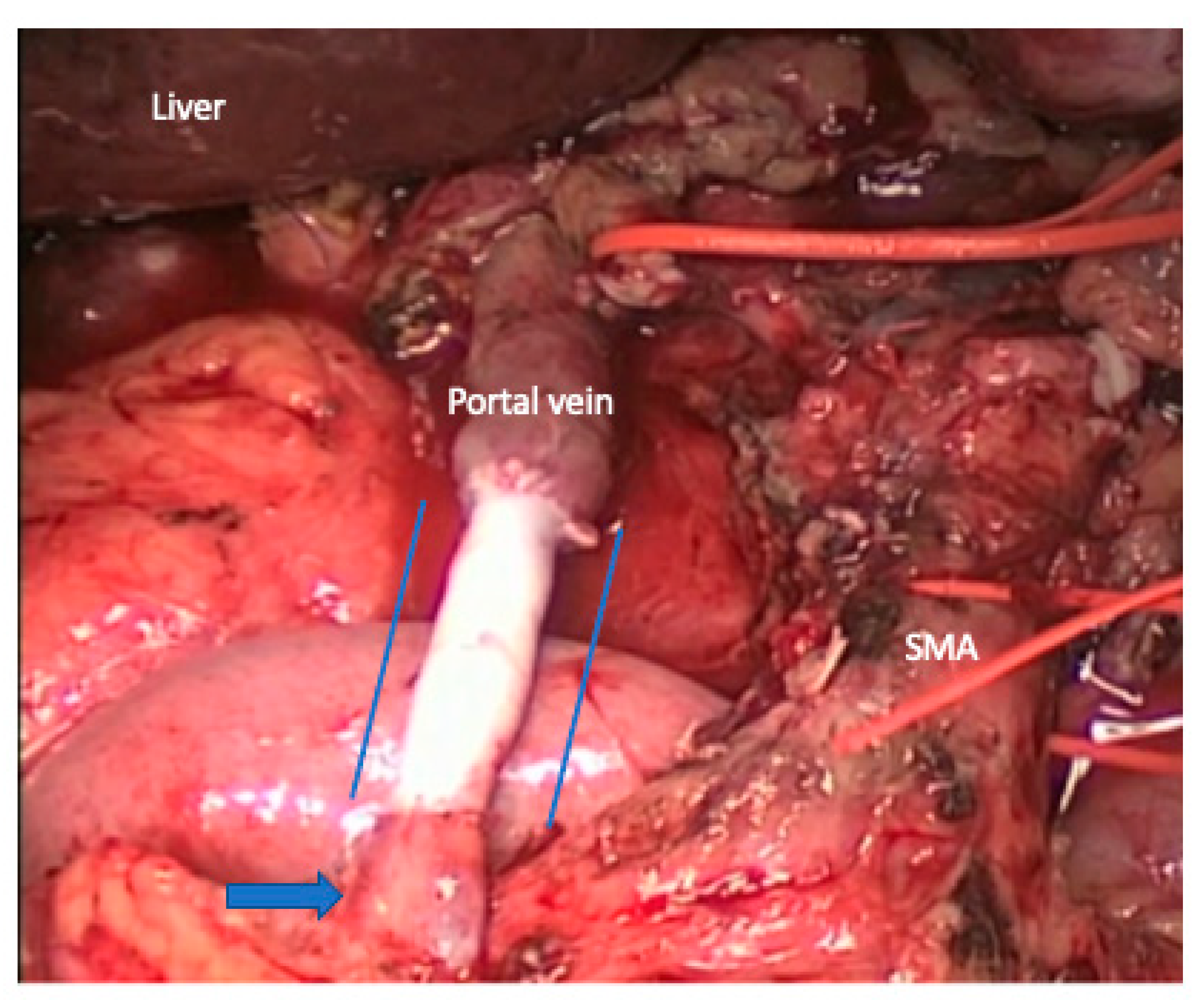
5. Vein Resection and Reconstruction
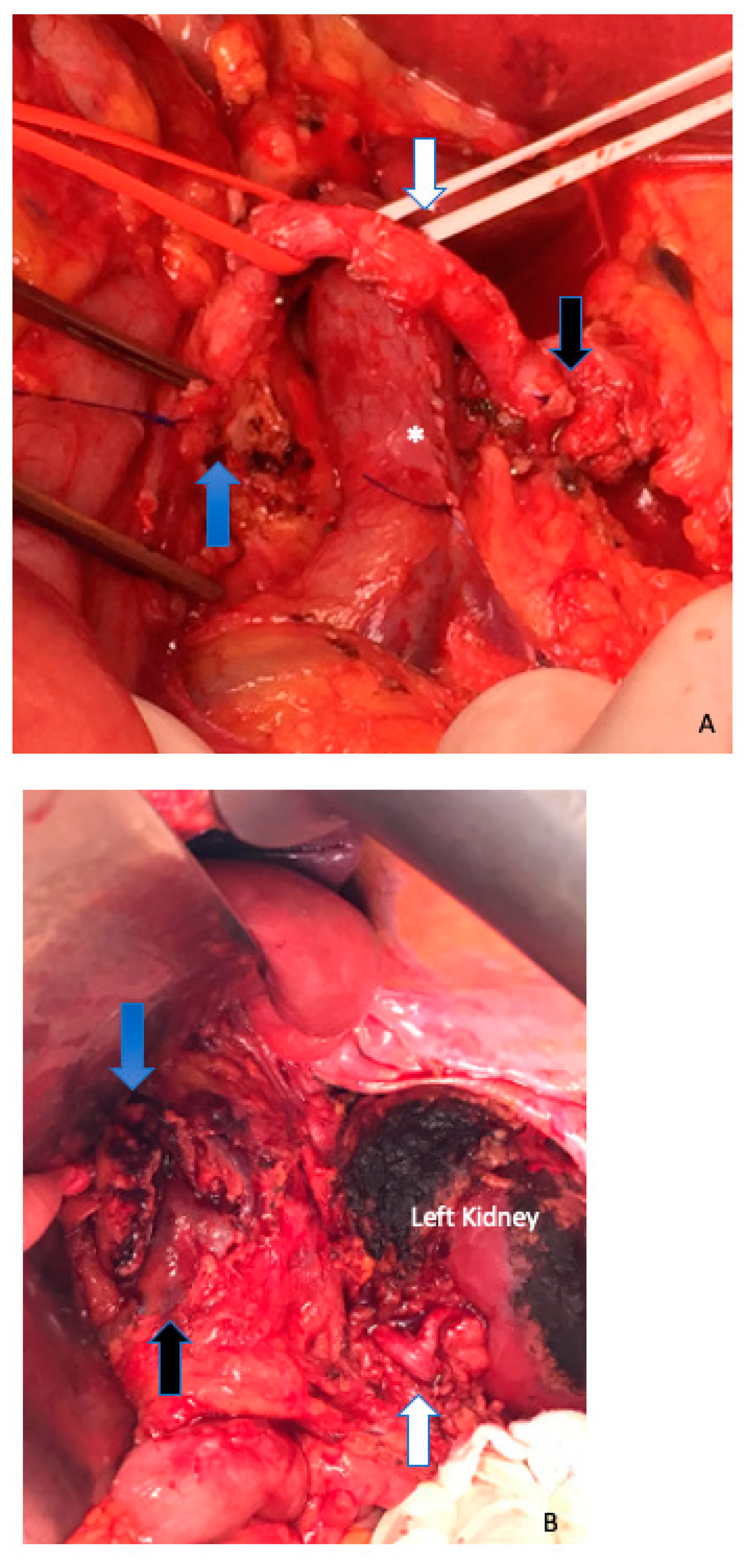
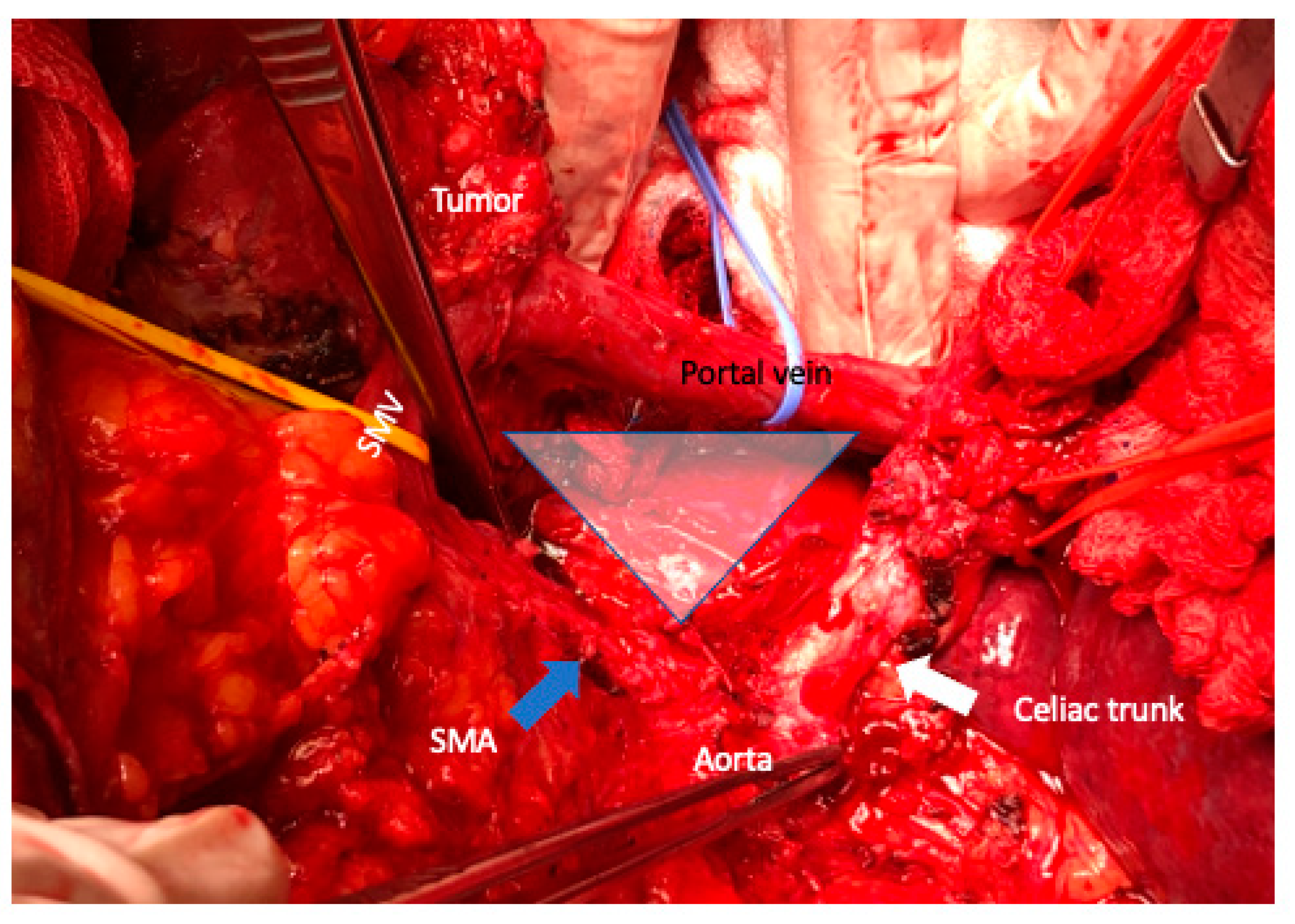
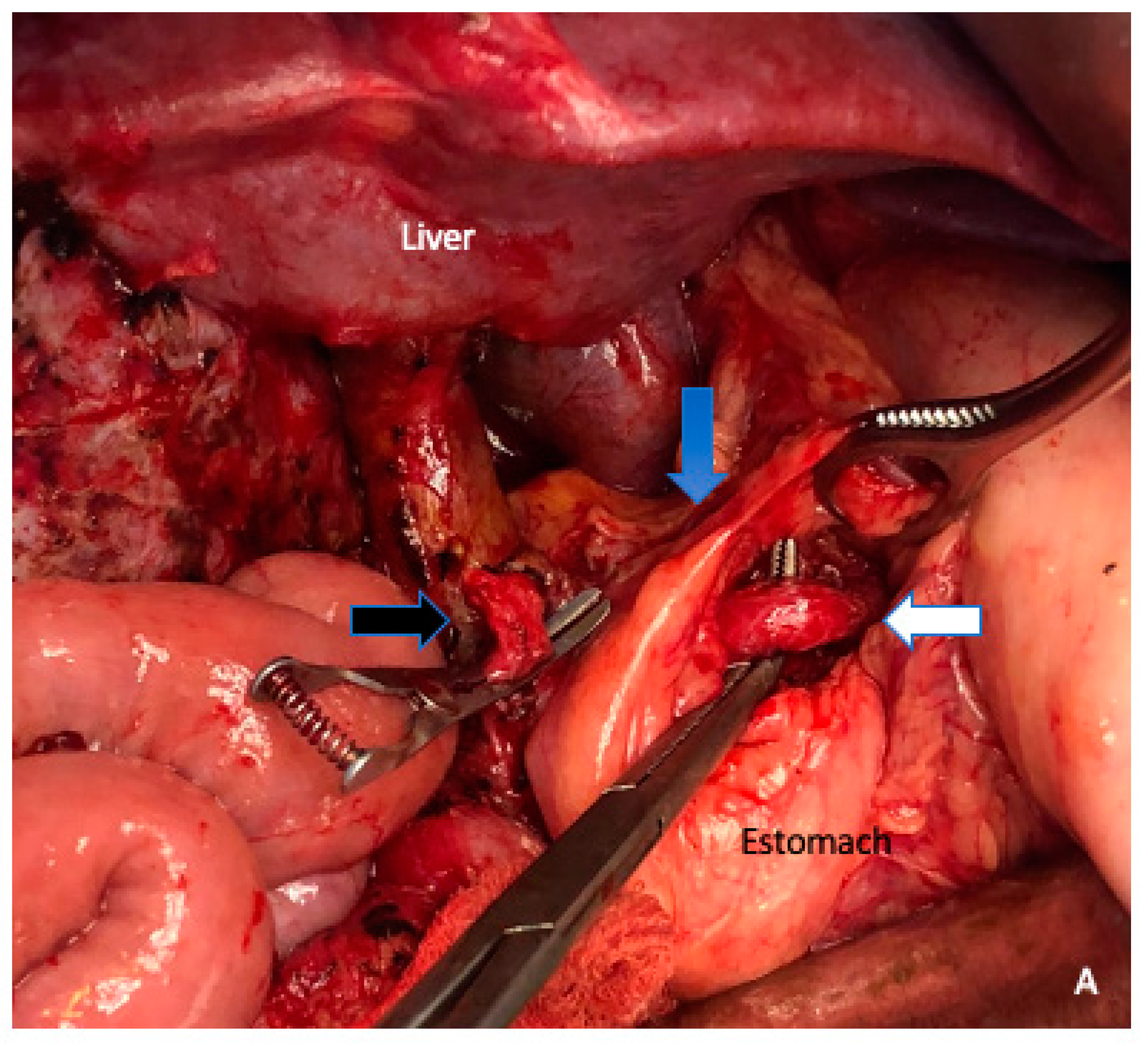
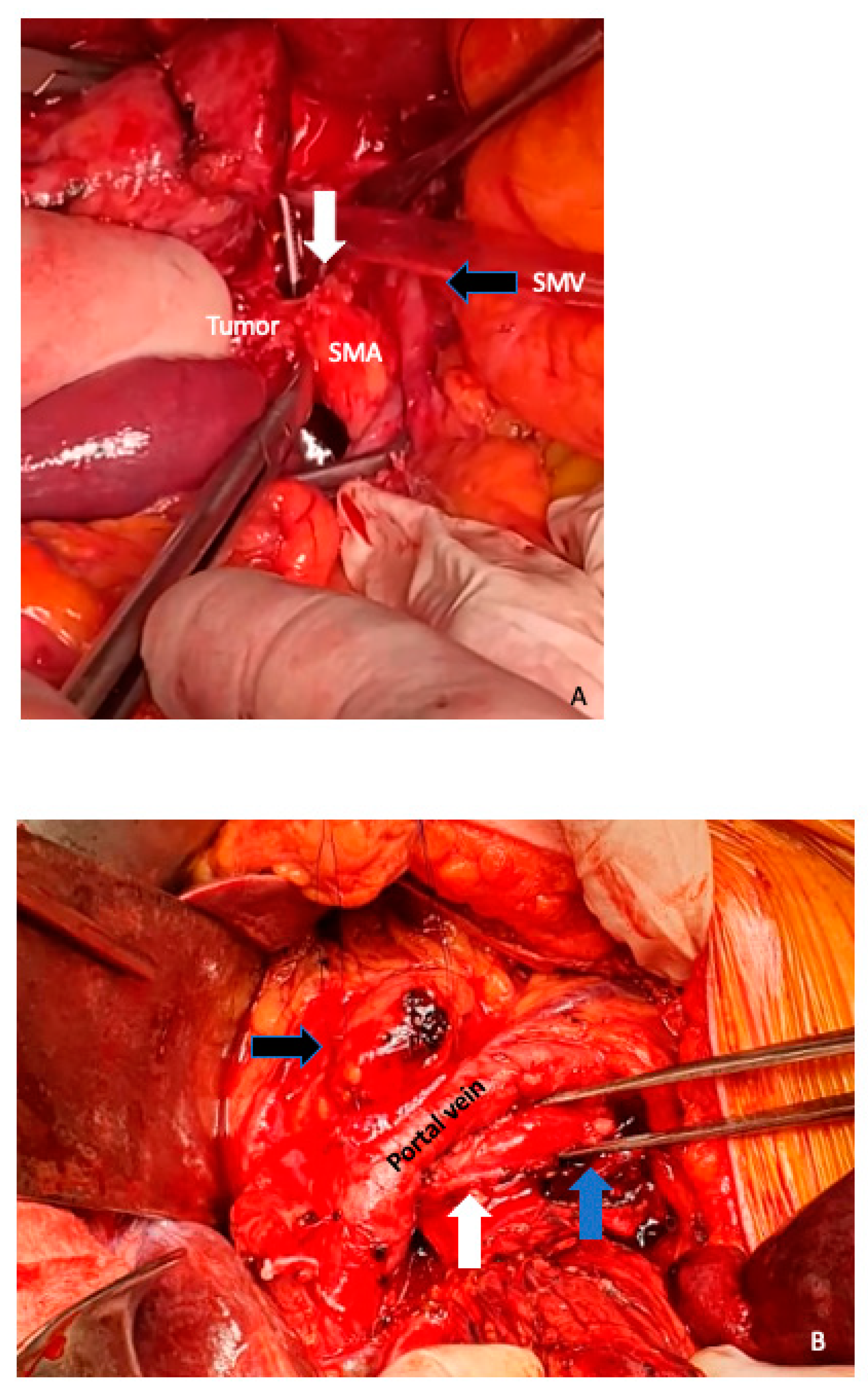
6. Arterial Resection
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Klaiber, U.; Schnaidt, E.S.; Hinz, U.; Gaida, M.M.; Heger, U.; Hank, T.; Strobel, O.; Neoptolemos, J.P.; Mihaljevic, A.L.; Büchler, M.W.; et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann. Surg. 2021, 273, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Bockhorn, M.; Uzunoglu, F.G.; Adham, M.; Imrie, C.; Milicevic, M.; Sandberg, A.A.; Asbun, H.J.; Bassi, C.; Büchler, M.; Charnley, R.M.; et al. International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: A consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014, 155, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Kirkegård, J.; Aahlin, E.K.; Al-Saiddi, M.; Bratlie, S.O.; Coolsen, M.; de Haas, R.J.; den Dulk, M.; Fristrup, C.; Harrison, E.M.; Mortensen, M.B.; et al. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br. J. Surg. 2019, 106, 756–764. [Google Scholar] [CrossRef]
- Oba, A.; Del Chiaro, M.; Satoi, S.; Kim, S.W.; Takahashi, H.; Yu, J.; Hioki, M.; Tanaka, M.; Kato, Y.; Ariake, K.; et al. New criteria of resectability for pancreatic cancer: A position paper by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). J. Hepatobiliary Pancreat. Sci. 2022, 29, 725–731. [Google Scholar] [CrossRef]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy with Folfirinox Results in Resectability in 60% of the Patients. Ann. Surg. 2016, 264, 457–463. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef]
- Javed, A.A.; Young, R.W.C.; Habib, J.R.; Kinny-Köster, B.; Cohen, S.M.; Fishman, E.K.; Wolfgang, C.L. Cinematic Rendering: Novel Tool for Improving Pancreatic Cancer Surgical Planning. Curr. Probl. Diagn. Radiol. 2022, 51, 878–883. [Google Scholar] [CrossRef]
- Stoop, T.F.; van Veldhuisen, E.; van Rijssen, L.B.; Klaassen, R.; Gurney-Champion, O.J.; de Hingh, I.H.; Busch, O.R.; van Laarhoven, H.W.M.; van Lienden, K.P.; Stoker, J.; et al. Added value of 3T MRI and the MRI-halo sign in assessing resectability of locally advanced pancreatic cancer following induction chemotherapy (IMAGE-MRI): Prospective pilot study. Langenbecks Arch. Surg. 2022, 407, 3487–3499. [Google Scholar] [CrossRef]
- Park, S.; Jang, J.K.; Byun, J.H.; Kim, J.H.; Lee, S.S.; Kim, H.J.; Hong, S.B.; Park, S.H. CT in the prediction of margin-negative resection in pancreatic cancer following neoadjuvant treatment: A systematic review and meta-analysis. Eur. Radiol. 2021, 31, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Motoi, F.; Murakami, Y.; Sho, M.; Satoi, S.; Honda, G.; Uemura, K.; Okada, K.I.; Matsumoto, I.; Nagai, M.; et al. Multicenter Study Group of Pancreatobiliary Surgery (MSG-PBS). Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: A multicenter case-control study of 240 patients. BMC Cancer 2019, 19, 252. [Google Scholar] [CrossRef]
- Rose, J.B.; Edwards, A.M.; Rocha, F.G.; Clark, C.; Alseidi, A.A.; Biehl, T.R.; Lin, B.S.; Picozzi, V.J.; Helton, W.S. Sustained Carbohydrate Antigen 19-9 Response to Neoadjuvant Chemotherapy in Borderline Resectable Pancreatic Cancer Predicts Progression and Survival. Oncologist 2020, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Heckler, M.; Mihaljevic, A.L.; Ei, S.; Klaiber, U.; Heger, U.; Büchler, M.W.; Hackert, T. Induction Chemotherapy with FOLFIRINOX for Locally Advanced Pancreatic Cancer: A Simple Scoring System to Predict Effect and Prognosis. Ann. Surg. Oncol. 2022, 1–8. [Google Scholar] [CrossRef]
- Yokose, T.; Kitago, M.; Matsusaka, Y.; Masugi, Y.; Shinoda, M.; Yagi, H.; Abe, Y.; Oshima, G.; Hori, S.; Endo, Y.; et al. Usefulness of 18 F-fluorodeoxyglucose positron emission tomography/computed tomography for predicting the prognosis and treatment response of neoadjuvant therapy for pancreatic ductal adenocarcinoma. Cancer Med. 2020, 9, 4059–4068. [Google Scholar] [CrossRef]
- Abdelrahman, A.M.; Goenka, A.H.; Alva-Ruiz, R.; Yonkus, J.A.; Leiting, J.L.; Graham, R.P.; Merrell, K.W.; Thiels, C.A.; Hallemeier, C.L.; Warner, S.G.; et al. FDG-PET Predicts Neoadjuvant Therapy Response and Survival in Borderline Resectable/Locally Advanced Pancreatic Adenocarcinoma. J. Natl. Compr. Canc. Netw. 2022, 20, 1023–1032.e3. [Google Scholar] [CrossRef]
- Mihaljevic, A.L.; Hackert, T.; Loos, M.; Hinz, U.; Schneider, M.; Mehrabi, A.; Hoffmann, K.; Berchtold, C.; Müller-Stich, B.P.; Diener, M.; et al. Not all Whipple procedures are equal: Proposal for a classification of pancreatoduodenectomies. Surgery 2021, 169, 1456–1462. [Google Scholar] [CrossRef]
- Borja-Cacho, D.; Parsons, H.M.; Habermann, E.B.; Rothen- Berger, D.A.; Henderson, W.G.; Al-Refaie, W.B. Assessment of ACS NSQIP’S predictive ability for adverse events after major cancer surgery. Ann. Surg. Oncol. 2010, 17, 2274–2282. [Google Scholar] [CrossRef]
- Schneider, M.; Strobel, O.; Hackert, T.; Büchler, M.W. Pancreatic resection for cancer-the Heidelberg technique. Langenbecks Arch. Surg. 2019, 404, 1017–1022. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Segersvärd, R.; Rangelova, E.; Coppola, A.; Scandavini, C.M.; Ansorge, C.; Verbeke, C.; Blomberg, J. Cattell-Braasch Maneuver Combined with Artery-First Approach for Superior Mesenteric-Portal Vein Resection During Pancreatectomy. J. Gastrointest. Surg. 2015, 19, 2264–2268. [Google Scholar] [CrossRef]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S.V.; Windsor, J.A. “Artery-first” approaches to pancreatoduodenectomy. Br. J. Surg. 2012, 99, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Rahbari, N.; Koch, M.; Büchler, M.W. The “artery first” approach for resection of pancreatic head cancer. J. Am. Coll. Surg. 2010, 210, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Saiura, A.; Yoshioka, R.; Ono, Y.; Takahashi, M.; Arita, J.; Takahashi, Y.; Koga, R. Pancreatoduodenectomy with Systematic Mesopancreas Dissection Using a Supracolic Anterior Artery-first Approach. Ann. Surg. 2015, 262, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Ironside, N.; Barreto, S.G.; Loveday, B.; Shrikhande, S.V.; Windsor, J.A.; Pandanaboyana, S. Meta-analysis of an artery-first approach versus standard pancreatoduodenectomy on perioperative outcomes and survival. Br. J. Surg. 2018, 105, 628–636. [Google Scholar] [CrossRef]
- Christein, J.D.; Kendrick, M.L.; Iqbal, C.W.; Nagorney, D.M.; Farnell, M.B. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J. Gastrointest. Surg. 2005, 9, 922–927. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Drebin, J.A.; Linehan, D. Radical antegrade modular pancreatosplenectomy. Surgery 2003, 133, 521–527. [Google Scholar] [CrossRef]
- Hackert, T.; Strobel, O.; Michalski, C.W.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.; Berchtold, C.; Ulrich, A.; Büchler, M.W. The TRIANGLE operation—Radical surgery after neoadjuvant treatment for advanced pancreatic cancer: A single arm observational study. HPB 2017, 19, 1001–1007. [Google Scholar] [CrossRef]
- Hartwig, W.; Vollmer, C.M.; Fingerhut, A.; Yeo, C.J.; Neoptolemos, J.P.; Adham, M.; Andrén-Sandberg, A.; Asbun, H.J.; Bassi, C.; Bockhorn, M.; et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: Definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 2014, 156, 1–14. [Google Scholar] [CrossRef]
- Hartwig, W.; Gluth, A.; Hinz, U.; Koliogiannis, D.; Strobel, O.; Hackert, T.; Werner, J.; Büchler, M.W. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br. J. Surg. 2016, 103, 1683–1694. [Google Scholar] [CrossRef]
- Raptis, D.A.; Sánchez-Velázquez, P.; Machairas, N.; Sauvanet, A.; Rueda de Leon, A.; Oba, A.; Groot Koerkamp, B.; Lovasik, B.; Chan, C.; Yeo, C.J.; et al. Defining Benchmark Outcomes for Pancreatoduodenectomy with Portomesenteric Venous Resection. Ann. Surg. 2020, 272, 731–737. [Google Scholar] [CrossRef]
- Groen, J.V.; Michiels, N.; van Roessel, S.; Besselink, M.G.; Bosscha, K.; Busch, O.R.; van Dam, R.; van Eijck, C.H.J.; Koerkamp, B.G.; van der Harst, E.; et al. Venous wedge and segment resection during pancreatoduodenectomy for pancreatic cancer: Impact on short- and long-term outcomes in a nationwide cohort analysis. Br. J. Surg. 2021, 109, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Dokmak, S.; Aussilhou, B.; Sauvanet, A.; Nagarajan, G.; Farges, O.; Belghiti, J. Parietal Peritoneum as an Autologous Substitute for Venous Reconstruction in Hepatopancreatobiliary Surgery. Ann. Surg. 2015, 262, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Strobel, O.; Schneider, M.; Diener, M.K.; Berchtold, C.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.P.; Hackert, T.; Büchler, M.W. Cavernous transformation of the portal vein in pancreatic cancer surgery-venous bypass graft first. Langenbecks Arch. Surg. 2020, 405, 1045–1050. [Google Scholar] [CrossRef]
- Oehme, F.; Distler, M.; Müssle, B.; Kahlert, C.; Weitz, J.; Welsch, T. Results of portosystemic shunts during extended pancreatic resections. Langenbecks Arch. Surg. 2019, 404, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Bachellier, P.; Rosso, E.; Fuchshuber, P.; Addeo, P.; David, P.; Oussoultzoglou, E.; Lucescu, I. Use of a temporary intraoperative mesentericoportal shunt for pancreatic resection for locally advanced pancreatic cancer with portal vein occlusion and portal hypertension. Surgery 2014, 155, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, A.; Büdeyri, I.; Heckler, M.; Partsakhashvili, J.; Ukkat, J.; Ronellenfitsch, U.; Michalski, C.W.; Kleeff, J. Systematic review and meta-analysis of contemporary pancreas surgery with arterial resection. Langenbeck’s Arch. Surg. 2020, 405, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Małczak, P.; Sierżęga, M.; Stefura, T.; Kacprzyk, A.; Dros’, J.; Skomarovska, O.; Krzysztofik, M.; Major, P.; Pędziwiatr, M. Arterial resections in pancreatic cancer—Systematic review and meta-analysis. HPB 2020, 22, 961–968. [Google Scholar] [CrossRef]
- Inoue, Y.; Oba, A.; Ono, Y.; Sato, T.; Ito, H.; Takahashi, Y. Radical Resection for Locally Advanced Pancreatic Cancers in the Era of New Neoadjuvant Therapy-Arterial Resection, Arterial Divestment and Total Pancreatectomy. Cancers 2021, 13, 1818. [Google Scholar] [CrossRef]
- Tee, M.C.; Krajewski, A.C.; Groeschl, R.T.; Farnell, M.B.; Nagorney, D.M.; Kendrick, M.L.; Cleary, S.P.; Smoot, R.L.; Croome, K.P.; Truty, M.J. Indications and Perioperative Outcomes for Pancreatectomy with Arterial Resection. J. Am. Coll. Surg. 2018, 227, 255–269. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Rangelova, E.; Halimi, A.; Ateeb, Z.; Scandavini, C.; Valente, R.; Segersvärd, R.; Arnelo, U.; Verbeke, C.S. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB 2019, 21, 219–225. [Google Scholar] [CrossRef]
- Loos, M.; Kester, T.; Klaiber, U.; Mihaljevic, A.L.; Mehrabi, A.; Müller-Stich, B.M.; Diener, M.K.; Schneider, M.A.; Berchtold, C.; Hinz, U.; et al. Arterial Resection in Pancreatic Cancer Surgery: Effective After a Learning Curve. Ann. Surg. 2022, 275, 759–768. [Google Scholar] [CrossRef] [PubMed]
- De Santibañes, M.; Alvarez, F.A.; Mazza, O.M.; Claria, R.S.; Santos, F.R.; Brandi, C.; de Santibañes, E.; Pekolj, J. Surgical strategies for restoring liver arterial perfusion in pancreatic resections. Langenbecks Arch. Surg. 2016, 401, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.K.; Mihaljevic, A.L.; Strobel, O.; Loos, M.; Schmidt, T.; Schneider, M.; Berchtold, C.; Mehrabi, A.; Müller-Stich, B.P.; Jiang, K.; et al. Periarterial divestment in pancreatic cancer surgery. Surgery 2021, 169, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Santibañes, M.; Pekolj, J.; Sanchez Claria, R.; de Santibañes, E.; Mazza, O.M. Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer. Cancers 2023, 15, 1509. https://doi.org/10.3390/cancers15051509
de Santibañes M, Pekolj J, Sanchez Claria R, de Santibañes E, Mazza OM. Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer. Cancers. 2023; 15(5):1509. https://doi.org/10.3390/cancers15051509
Chicago/Turabian Stylede Santibañes, Martín, Juan Pekolj, Rodrigo Sanchez Claria, Eduardo de Santibañes, and Oscar Maria Mazza. 2023. "Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer" Cancers 15, no. 5: 1509. https://doi.org/10.3390/cancers15051509
APA Stylede Santibañes, M., Pekolj, J., Sanchez Claria, R., de Santibañes, E., & Mazza, O. M. (2023). Technical Implications for Surgical Resection in Locally Advanced Pancreatic Cancer. Cancers, 15(5), 1509. https://doi.org/10.3390/cancers15051509



