Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethical Approvals
2.2. Recruitment of Study Participants
2.3. Saliva Sample Collection and Processing
2.4. Interleukin Quantification
2.5. Statistical Analysis
3. Results
3.1. Study Cohort
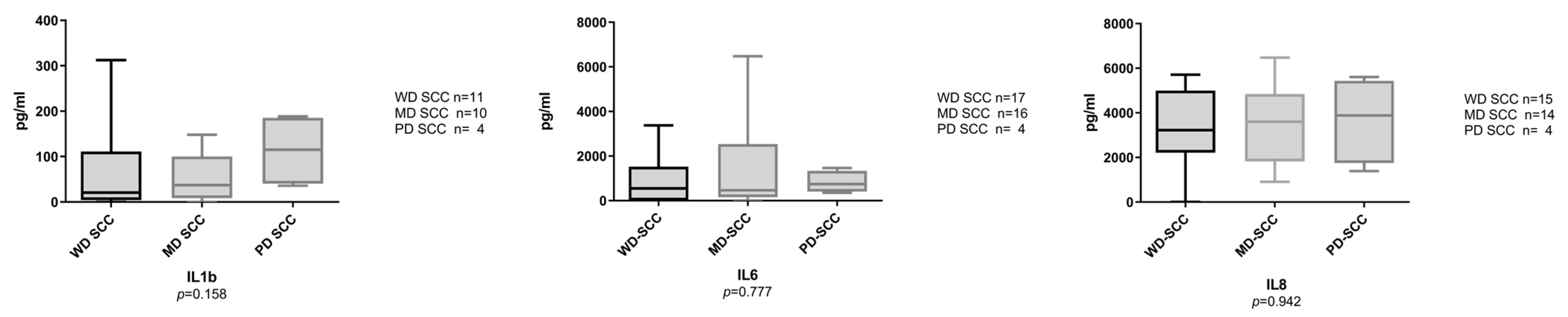
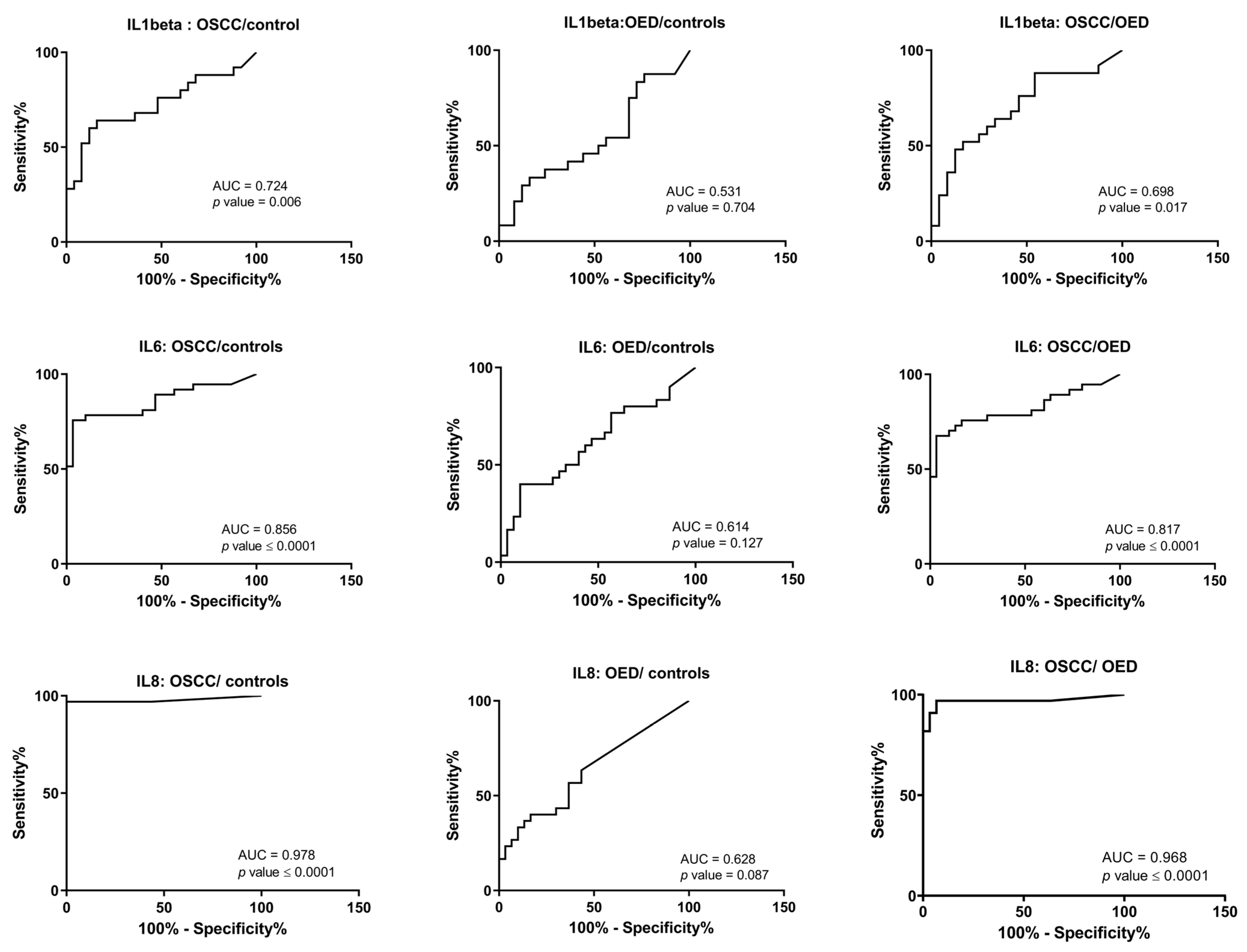
3.2. Salivary Interleukins at the Protein Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization GLOBOCAN 2020. Cancer Today 2020. Available online: https://gco.iarc.fr/today/home (accessed on 19 September 2022).
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef] [PubMed]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. A Mon. 2020, 66, 100988. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Risk Assessment in Oral Health Cancer. In Risk Assessment in Oral Health; Springer: Berlin/Heidelberg, Germany, 2020; pp. 119–132. [Google Scholar] [CrossRef]
- National Cancer Control Program. Sri Lanka Cancer Incidence Data Book. 2019. Available online: https://www.nccp.health.gov.lk/en/incedenceData (accessed on 25 September 2022).
- Edirisinghe, S.T.; Weerasekera, M.; De Silva, D.K.; Liyanage, I.; Niluka, M.; Madushika, K.; Deegodagamage, S.; Wijesundara, C.; Rich, A.M.; De Silva, H.; et al. The Risk of Oral Cancer among Different Categories of Exposure to Tobacco Smoking in Sri Lanka. Asian Pac. J. Cancer Prev. 2022, 23, 2929–2935. [Google Scholar] [CrossRef]
- Ariyawardana, A.; Warnakulasuriya, S. Declining oral cancer rates in Sri Lanka: Are we winning the war after being at the top of the cancer league table? Oral Dis. 2011, 17, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care 2020, 29, e13207. [Google Scholar] [CrossRef]
- Jayasooriya, P.R.; Pitakotuwage, T.N.; Mendis, B.R.R.N.; Lombardi, T. Descriptive study of 896 Oral squamous cell carcinomas from the only University based Oral Pathology Diagnostic Service in Sri Lanka. BMC Oral Health 2016, 16, 1. [Google Scholar] [CrossRef]
- Blanchard, P.; Belkhir, F.; Temam, S.; El Khoury, C.; De Felice, F.; Casiraghi, O.; Patrikidou, A.; Mirghani, H.; Levy, A.; Even, C.; et al. Outcomes and prognostic factors for squamous cell carcinoma of the oral tongue in young adults: A single-institution case-matched analysis. Eur. Arch. Oto Rhino Laryngol. 2016, 274, 1683–1690. [Google Scholar] [CrossRef]
- Orlandi, E.; Iacovelli, N.A.; Tombolini, V.; Rancati, T.; Polimeni, A.; De Cecco, L.; Valdagni, R.; De Felice, F. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019, 99, 104453. [Google Scholar] [CrossRef]
- DE Sanctis, V.; Belgioia, L.; Cante, D.; LA Porta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; DE Felice, F.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients With Head and Neck Tumors: A Multicentric Randomized Study. Anticancer Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef]
- Capote-Moreno, A.; Brabyn, P.; Muñoz-Guerra, M.; Sastre-Pérez, J.; Escorial-Hernandez, V.; Rodríguez-Campo, F.; García, T.; Naval-Gías, L. Oral squamous cell carcinoma: Epidemiological study and risk factor assessment based on a 39-year series. Int. J. Oral Maxillofac. Surg. 2020, 49, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, H.; Jayasinghe, R.D.; Dharmagunawardene, D.; Attygalla, M.; A Scuffham, P.; Johnson, N.; Kularatna, S. Economic burden of managing oral cancer patients in Sri Lanka: A cross-sectional hospital -based costing study. BMJ Open 2019, 9, e027661. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, A.; Jahangiri, R.; Olyaeemanesh, A.; Kalaghchi, B.; Nouhi, M.; Nahvijou, A. The economic burden of oral cancer in Iran. PLoS ONE 2018, 13, e0203059. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Warnakulasuriya, S. Screening for oral cancer: Future prospects, research and policy development for Asia. Oral Oncol. 2020, 105, 104632. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, W.M.; Jayasooriya, P.R.; Jayasuriya, N.S.; De Silva, R.K. Oral epithelial dysplasia: Causes, quantification, prognosis, and management challenges. Periodontol. 2000 2019, 80, 126–147. [Google Scholar] [CrossRef]
- Nikitakis, N.G.; Pentenero, M.; Georgaki, M.; Poh, C.F.; Peterson, D.E.; Edwards, P.; Lingen, M.; Sauk, J.J.; Nikitakis, N.G.; Pentenero, M.; et al. Molecular markers associated with development and progression of potentially premalignant oral epithelial lesions: Current knowledge and future implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 650–669. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pezzi, M.; Cassi, D.; Pertinhez, T.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Al Rawi, N.; Elmabrouk, N.; Abu Kou, R.; Mkadmi, S.; Rizvi, Z.; Hamdoon, Z. The role of differentially expressed salivary microRNA in oral squamous cell carcinoma. A systematic review. Arch. Oral Biol. 2021, 125, 105108. [Google Scholar] [CrossRef]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef]
- Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites 2021, 11, 587. [Google Scholar] [CrossRef]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Dikova, V.; Principe, S.; Bagan, J. Salivary inflammatory proteins in patients with oral potentially malignant disorders. J. Clin. Exp. Dent. 2019, 11, e659–e664. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Márton, I.J.; Horváth, J.; Lábiscsák, P.; Márkus, B.; Dezső, B.; Szabó, A.; Tar, I.; Piffkó, J.; Jakus, P.; Barabás, J.; et al. Salivary IL-6 mRNA is a Robust Biomarker in Oral Squamous Cell Carcinoma. J. Clin. Med. 2019, 8, 1958. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Singh, P.; Verma, J.K.; Singh, J.K. Validation of Salivary Markers, IL-1β, IL-8 and Lgals3bp for Detection of Oral Squamous Cell Carcinoma in an Indian Population. Sci. Rep. 2020, 10, 7365. [Google Scholar] [CrossRef]
- John, M.A.R.S.; Li, Y.; Zhou, X.; Denny, P.; Ho, C.-M.; Montemagno, C.; Shi, W.; Qi, F.; Wu, B.; Sinha, U.; et al. Interleukin 6 and Interleukin 8 as Potential Biomarkers for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. Arch. Otolaryngol. Neck Surg. 2004, 130, 929–935. [Google Scholar] [CrossRef]
- Cheng, Y.-S.L.; Jordan, L.; Gorugantula, L.M.; Schneiderman, E.; Chen, H.-S.; Rees, T. Salivary Interleukin-6 and -8 in Patients with Oral Cancer and Patients With Chronic Oral Inflammatory Diseases. J. Periodontol. 2014, 85, 956–965. [Google Scholar] [CrossRef]
- Gleber-Netto, F.O.; Yakob, M.; Li, F.; Feng, Z.; Dai, J.; Kao, H.-K.; Chang, Y.-L.; Chang, K.-P.; Wong, D.T. Salivary Biomarkers for Detection of Oral Squamous Cell Carcinoma in a Taiwanese Population. Clin. Cancer Res. 2016, 22, 3340–3347. [Google Scholar] [CrossRef]
- Rezaei, F.; Mozaffari, H.R.; Tavasoli, J.; Zavattaro, E.; Imani, M.M.; Sadeghi, M. Evaluation of Serum and Salivary Interleukin-6 and Interleukin-8 Levels in Oral Squamous Cell Carcinoma Patients: Systematic Review and Meta-Analysis. J. Interf. Cytokine Res. 2019, 39, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Chiamulera, M.M.A.; Zancan, C.B.; Remor, A.P.; Cordeiro, M.F.; Gleber-Netto, F.O.; Baptistella, A.R. Salivary cytokines as biomarkers of oral cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Piyarathne, N.S.; Rasnayake, R.; Angammana, R.; Chandrasekera, P.; Ramachandra, S.; Weerasekera, M.; Yasawardene, S.; Abu-Eid, R.; Jayasinghe, J.A.P.; Gupta, E. Diagnostic salivary biomarkers in oral cancer and oral potentially malignant disorders and their relationships to risk factors—A systematic review. Expert Rev. Mol. Diagn. 2021, 21, 789–807. [Google Scholar] [CrossRef] [PubMed]
- Csősz, É.; Lábiscsák, P.; Kalló, G.; Márkus, B.; Emri, M.; Szabó, A.; Tar, I.; Tőzsér, J.; Kiss, C.; Márton, I. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS ONE 2017, 12, e0177282. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Saad, M.; Grogan, T.R.; Li, F.; Heo, Y.J.; Elashoff, D.; Bresalier, R.S.; Wong, D.T.W.; Kim, Y. Performance of Salivary Extracellular RNA Biomarker Panels for Gastric Cancer Differs between Distinct Populations. Cancers 2022, 14, 3632. [Google Scholar] [CrossRef]
- Pepe, M.S. The Statistical Evaluation of Medical Tests for Classification and Prediction; UOP OXFORD: Oxford, UK, 2004. [Google Scholar]
- Rai, V.; Mukherjee, R.; Ghosh, A.K.; Routray, A.; Chakraborty, C. “Omics” in oral cancer: New approaches for biomarker discovery. Arch. Oral Biol. 2018, 87, 15–34. [Google Scholar] [CrossRef]
- Rodríguez-Molinero, J.; Migueláñez-Medrán, B.D.C.; Delgado-Somolinos, E.; Carreras-Presas, C.M.; López-Sánchez, A.F. Advances in the Diagnosis, Monitoring, and Progression of Oral Cancer through Saliva: An Update. BioMed Res. Int. 2022, 2022, 2739869. [Google Scholar] [CrossRef]
- González-Moles, M.; Warnakulasuriya, S.; López-Ansio, M.; Ramos-García, P. Hallmarks of Cancer Applied to Oral and Oropharyngeal Carcinogenesis: A Scoping Review of the Evidence Gaps Found in Published Systematic Reviews. Cancers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Lee, L.; Wong, Y.; Hsiao, H.; Wang, Y.; Chan, M.; Chang, K. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef]
- Selvam, N.P.; Sadaksharam, J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia-Pacific. J. Clin. Oncol. 2015, 11, 236–241. [Google Scholar] [CrossRef]
- Punyani, S.R.; Sathawane, R.S. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin. Oral Investig. 2013, 17, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, K.; Nandhini, G.; Ramya, R.; Rajashree, P.; Kumar, A.R.; Anandan, S.N. Validation of the diagnostic utility of salivary interleukin 8 in the differentiation of potentially malignant oral lesions and oral squamous cell carcinoma in a region with high endemicity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Korostoff, A.; Reder, L.; Masood, R.; Sinha, U.K. The role of salivary cytokine biomarkers in tongue cancer invasion and mortality. Oral Oncol. 2011, 47, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L.; Cheng, B.; Myers, S.; Miller, L.; Ho, V.; Ondrey, F. The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol. Carcinog. 2005, 44, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Khyani, I.A.M.; Qureshi, M.; Mirza, T.; Farooq, M.U. Detection of interleukins-6 and 8 in saliva as potential biomarkers of oral pre-malignant lesion and oral carcinoma: A breakthrough in salivary diagnostics in Pakistan. Pak. J. Pharm. Sci. 2017, 30, 817–823. [Google Scholar]
- Arduino, P.G.; Menegatti, E.; Cappello, N.; Martina, E.; Gardino, N.; Tanteri, C.; Cavallo, F.; Scully, C.; Broccoletti, R. Possible Role for Interleukins as Biomarkers for Mortality and Recurrence in Oral Cancer. Int. J. Biol. Markers 2015, 30, e262–e266. [Google Scholar] [CrossRef]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Y Cir. Bucal 2012, 17, e10–e15. [Google Scholar] [CrossRef]
- Arellano-Garcia, M.; Hu, S.; Wang, J.; Henson, B.; Zhou, H.; Chia, D.; Wong, D. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008, 14, 705–712. [Google Scholar] [CrossRef]
- Wei, L.-Y.; Lee, J.-J.; Yeh, C.-Y.; Yang, C.-J.; Kok, S.-H.; Ko, J.-Y.; Tsai, F.-C.; Chia, J.-S. Reciprocal activation of cancer-associated fibroblasts and oral squamous carcinoma cells through CXCL1. Oral Oncol. 2019, 88, 115–123. [Google Scholar] [CrossRef]
- Kayamori, K.; Sakamoto, K.; Nakashima, T.; Takayanagi, H.; Morita, K.-I.; Omura, K.; Nguyen, S.T.; Miki, Y.; Iimura, T.; Himeno, A.; et al. Roles of Interleukin-6 and Parathyroid Hormone-Related Peptide in Osteoclast Formation Associated with Oral Cancers: Significance of Interleukin-6 Synthesized by Stromal Cells in Response to Cancer Cells. Am. J. Pathol. 2010, 176, 968–980. [Google Scholar] [CrossRef]
- Garon, E.B.; Yang, J.C.-H.; Dubinett, S.M. The Role of Interleukin 1β in the Pathogenesis of Lung Cancer. JTO Clin. Res. Rep. 2020, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Raković, N.; Milovanović, J. Interleukin-8 in Breast Cancer Progression. J. Interf. Cytokine Res. 2013, 33, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Najdaghi, S.; Razi, S.; Rezaei, N. An overview of the role of interleukin-8 in colorectal cancer. Cytokine 2020, 135, 155205. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kimura, T.; Ueta, E.; Tatemoto, Y.; Osaki, T. Characteristic Cytokine Generation Patterns in Cancer Cells and Infiltrating Lymphocytes in Oral Squamous Cell Carcinomas and the Influence of Chemoradiation Combined with Immunotherapy on These Patterns. Oncology 2003, 64, 407–415. [Google Scholar] [CrossRef]
- Shahzad, A.; Knapp, M.; Lang, I.; Köhler, G. Interleukin 8 (IL-8)—A universal biomarker? Int. Arch. Med. 2010, 3, 11. [Google Scholar] [CrossRef]
- Taylor, J.J. Protein Biomarkers of Periodontitis in Saliva. ISRN Inflamm. 2014, 2014, 593151. [Google Scholar] [CrossRef] [PubMed]
- Batool, H.; Nadeem, A.; Kashif, M.; Shahzad, F.; Tahir, R.; Afzal, N. Salivary Levels of IL-6 and IL-17 Could Be an Indicator of Disease Severity in Patients with Calculus Associated Chronic Periodontitis. BioMed Res. Int. 2018, 2018, 8531961. [Google Scholar] [CrossRef]


| OSCC (n = 37) | OED (n = 30) | Controls (n = 30) | ||
|---|---|---|---|---|
| Age (years) | Mean Range | 60.0 ± 11.5 33–78 | 56.2 ± 12.3 29–80 | 62.2 ± 10.2 36–79 |
| Income (LKR) | Mean Range | 24,875 ± 19,766 1000–70,000 | 26,782 ± 17,474 5000–60,000 | 40,533 ± 10,953 25,000–60,000 |
| Sex | Male (%) Female | 31 (84%) 6 (16%) | 24 (80%) 6 (20%) | 24 (80%) 6 (20%) |
| Ethnicity | Sinhala (%) Other | 30 (81%) 7 (9%) | 28 (93%) 2 (7%) | 30 (100%) - |
| Smoking | Daily Never Ex-user | 12 (32%) 20 (54%) 5 (14%) | 10 (33%) 16 (54%) 4 (13%) | 6 (20%) 15 (50%) 9 (30%) |
| Alcohol | Daily Never Ex-user | 23 (62%) 12 (33%) 2 (5%) | 13 (44%) 15 (50%) 2 (6%) | 11 (37%) 16 (53%) 3 (10%) |
| Betel quid | Daily Never Ex-user | 25 (67%) 11 (30%) 1 (3%) | 17 (57%) 6 (20%) 7 (23%) | 10 (34%) 18 (60%) 2 (6%) |
| Family history of any cancer type | Yes No | 9 (24%) 28 (76%) | 6 (20%) 24 (80%) | 5 (17%) 83 (83%) |
| Mouthwash use | Yes No | 3 (8%) 34 (92%) | 9 (30%) 21 (70%) | 1 (4%) 29 (96%) |
| Co-morbidity | Yes No | 8 (22%) 29 (78%) | 16 (53%) 14 (47%) | 15 (50%) 15 (50%) |
| OSCC (n = 37) | OED (n = 30) | |
|---|---|---|
| Lips/labial mucosa | 1 (2.8%) | 2 (6.6%) |
| Buccal mucosa | 14 (37.8%) | 24 (80.2%) |
| Tongue | 14 (37.8%) | 2 (6.6%) |
| Gingiva | 1 (2.8%) | - |
| Floor of the mouth | 3 (8.4%) | - |
| Palate | 2 (5.6%) | 2 (6.6%) |
| Alveolus/bone | 2 (5.6%) | - |
| OSCC/Controls | OED/Controls | OSCC/OED | ||
|---|---|---|---|---|
| IL1β | AUC | 0.724 | 0.531 | 0.698 |
| 95% CI for AUC | 0.579–0.870 | 0.366–0.697 | 0.549–0.847 | |
| p-value | 0.006 | 0.704 | 0.017 | |
| Cut-off value | >20.49 pg/mL | - | >33.4 pg/mL | |
| Sensitivity at COV | 64% | - | 52% | |
| Specificity at COV | 84% | - | 83.3% | |
| IL6 | AUC | 0.856 | 0.614 | 0.817 |
| 95% CI for AUC | 0.763–0.950 | 0.470–0.758 | 0.712–0.922 | |
| p-value | <0.0001 | 0.127 | <0.0001 | |
| Cut-off value | >95.9 pg/mL | - | >169.7 pg/mL | |
| Sensitivity at COV | 75.6% | - | 70.2% | |
| Specificity at COV | 96.6% | - | 90% | |
| IL8 | AUC | 0.978 | 0.628 | 0.968 |
| 95% CI for AUC | 0.934–1.02 | 0.486–0.769 | 0.918–1.01 | |
| p-value | <0.0001 | 0.087 | <0.0001 | |
| Cut-off value | >394.3 pg/mL | - | >1067 pg/mL | |
| Sensitivity at COV | 96.9% | - | 90.9% | |
| Specificity at COV | 96.7% | - | 96.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piyarathne, N.S.; Weerasekera, M.M.; Fonseka, P.F.D.; Karunatilleke, A.H.T.S.; Liyanage, R.L.P.R.; Jayasinghe, R.D.; De Silva, K.; Yasawardene, S.; Gupta, E.; Jayasinghe, J.A.P.; et al. Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study. Cancers 2023, 15, 1510. https://doi.org/10.3390/cancers15051510
Piyarathne NS, Weerasekera MM, Fonseka PFD, Karunatilleke AHTS, Liyanage RLPR, Jayasinghe RD, De Silva K, Yasawardene S, Gupta E, Jayasinghe JAP, et al. Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study. Cancers. 2023; 15(5):1510. https://doi.org/10.3390/cancers15051510
Chicago/Turabian StylePiyarathne, Nadisha S., Manjula M. Weerasekera, Pasquel Fonsekalage Damith Fonseka, Appu Hennedi Thotahewage Sunil Karunatilleke, Rubasinha Liyanage Pemith Ranura Liyanage, Ruwan Duminda Jayasinghe, Kanishka De Silva, Surangi Yasawardene, Ekta Gupta, Jayasinghe Arachchilage Premasiri Jayasinghe, and et al. 2023. "Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study" Cancers 15, no. 5: 1510. https://doi.org/10.3390/cancers15051510
APA StylePiyarathne, N. S., Weerasekera, M. M., Fonseka, P. F. D., Karunatilleke, A. H. T. S., Liyanage, R. L. P. R., Jayasinghe, R. D., De Silva, K., Yasawardene, S., Gupta, E., Jayasinghe, J. A. P., & Abu-Eid, R. (2023). Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study. Cancers, 15(5), 1510. https://doi.org/10.3390/cancers15051510









