Simple Summary
Immune checkpoint inhibitors (ICIs) are being increasingly used after primary treatment of early-stage tumors to treat any residual disease and prevent recurrence. Herein, we provide a comprehensive review of pivotal clinical studies demonstrating efficacy and safety outcomes when ICIs are utilized after surgery in patients with melanoma, urothelial cancer, renal cell carcinoma, lung cancer, gastroesophageal cancer, and hepatobiliary malignancies. In addition, we highlight the potential role of these agents within the emerging field of transplant oncology. To guide the selection of eligible patients for ICIs, we outline approved and emerging biomarkers that may predict benefit from use of these agents and help monitor response, especially in the absence of visible disease on imaging. Furthermore, we provide real-world considerations with regards to tolerability and cost-effectiveness of these agents and necessary future directions that should be explored to increase the survival outcomes associated with the use of ICIs after surgery.
Abstract
The rationale for administering immune checkpoint inhibitors (ICIs) in the adjuvant setting is to eradicate micro-metastases and, ultimately, prolong survival. Thus far, clinical trials have demonstrated that 1-year adjuvant courses of ICIs reduce the risk of recurrence in melanoma, urothelial cancer, renal cell carcinoma, non-small cell lung cancer, and esophageal and gastroesophageal junction cancers. Overall survival benefit has been shown in melanoma while survival data are still not mature in other malignancies. Emerging data also show the feasibility of utilizing ICIs in the peri-transplant setting for hepatobiliary malignancies. While ICIs are generally well-tolerated, the development of chronic immune-related adverse events, typically endocrinopathies or neurotoxicities, as well as delayed immune-related adverse events, warrants further scrutiny regarding the optimal duration of adjuvant therapy and requires a thorough risk–benefit determination. The advent of blood-based, dynamic biomarkers such as circulating tumor DNA (ctDNA) can help detect minimal residual disease and identify the subset of patients who would likely benefit from adjuvant treatment. In addition, the characterization of tumor-infiltrating lymphocytes, neutrophil-to-lymphocyte ratio, and ctDNA-adjusted blood tumor mutation burden (bTMB) has also shown promise in predicting response to immunotherapy. Until additional, prospective studies delineate the magnitude of overall survival benefit and validate the use of predictive biomarkers, a tailored, patient-centered approach to adjuvant ICIs that includes extensive patient counseling on potentially irreversible adverse effects should be routinely incorporated into clinical practice.
1. Introduction
Harnessing the immune system to recognize and attack cancer cells has demonstrated unprecedented, durable responses in various advanced malignancies. Genomic instability frequently arises in malignant cells and results in the creation of novel epitopes or “neoantigens” that can be targeted by the host immune system. Immune checkpoint inhibitors (ICIs) remove inhibitory signals of T-cell activation, which enables T cells to overcome regulatory mechanisms to mount an effective antitumor response [1,2]. Recently, there has been increased interest in utilizing immunotherapy in the early-stage setting for certain malignancies, following the theory that “treating earlier means treating better”. The goal of adjuvant treatment is to eradicate undetectable micro-metastases and prolong overall survival (OS). Disease-free survival (DFS) is, however, a surrogate endpoint for drug approval by both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA), as demonstrated with the approval of ICIs for adjuvant treatment of several solid tumors, including melanoma, renal cell carcinoma, and muscle-invasive bladder cancer [3]. This review focuses on the following areas of interest to oncology literature: current understanding of the utility of predictive biomarkers to ascertain patient response and survival benefit and streamline the allocation of resources with immunotherapy treatment, the clinical benefit of adjuvant ICIs reported in early-stage cancer trials, pertinent safety and economic considerations, and future areas of exploration that should guide the expanding utilization of adjuvant immunotherapy for other disease states.
2. Potential Predictive Biomarkers
2.1. Approved Biomarkers
A plethora of preclinical and clinical studies are being conducted to better understand the tumor genome and tumor microenvironment (TME) and identify predictive biomarkers of response. The advancement of multiplex immunohistochemical technology, high-throughput sequencing, and microarray technology has enabled the exploration of a variety of biomarker strategies to aid in clinical decision making [4]. Programmed death-ligand 1 (PD-L1), tumor mutation burden (TMB), microsatellite instability (MSI), and deficient mismatch repair system (dMMR) have emerged as the most studied biomarkers, but the predictive capabilities of each biomarker are different, and there are significant limitations to their use. Challenges with PD-L1 expression include assay variability, temporal and spatial heterogeneity of PD-L1 expression, and variable cut-off values and scoring strategies [4]. For example, the tumor proportion score (TPS) evaluates the percentage of viable tumor cells with partial or complete membrane PD-L1 staining, while the combined positive score (CPS) evaluates the number of PD-L1–staining cells (tumor cells, lymphocytes, macrophages) relative to all viable tumor cells. The CPS has been utilized in advanced hand and neck squamous cell carcinoma (HNSCC), esophageal or gastroesophageal junction (GEJ) carcinoma, cervical cancer, and triple-negative breast cancer, while TPS has been used in advanced non-small cell lung cancer (NSCLC) [5].
Significant correlations between high TMB and response to ICIs were reported in several cancer types, including urothelial carcinoma, small cell lung cancer (SCLC), NSCLC, melanoma, and human papilloma virus (HPV)-negative HNSCC. Nonetheless, different cut-off values across studies and a lack of standardization and interpretation of TMB complicate its use [6]. Some studies reported that “high TMB” is dependent on the tumor type; for example, melanoma has many more mutations, on average, than pancreatic cancer; therefore, “low TMB” in melanoma would be a “high TMB” in pancreatic cancer [7,8].
A large multicenter cohort study of 1552 patients with advanced NSCLC who received PD-1/PD-L1 inhibitors showed that patients with a high TMB (>19.0 mutations per megabase) were associated with improved objective response rate (ORR), progression-free survival (PFS), and OS across all levels of PD-L1 TPS subgroups. High TMB levels were associated with increased CD8+, PD-L1+ T-cell infiltration and increased PD-L1 expression on tumor and immune cells [9]. Other biomarkers that may predict ICI response, including CXCL9/CCR5/CXCL13 expression, TRAF2 loss, and CCND1 amplification, have been reported [10,11,12,13,14].
dMMR occurs due to inherited or acquired mutations in at least one of the genes that encodes proteins in the MMR system (MLH1, MSH2, MSH6, and PMS2) or through methylation of the MLH1 gene promoter. Germline mutations in MMR genes are referred to as Lynch syndrome. A deficient MMR system results in the accumulation of replication errors in DNA microsatellites. Tumors that have a dMMR system can develop MSI, which is the expansion or reduction in the length of repetitive sequences in tumor DNA compared with normal DNA [15]. dMMR is assayed by immunohistochemistry, while MSI is assayed by PCR. These are highly concordant (>90%) in most tumors, especially colorectal and endometrial cancers. MSI-high (MSH-H)/dMMR status occurs in different solid tumor types, including gastrointestinal, genitourinary, endometrial, lung, and thyroid cancers. Pembrolizumab garnered accelerated approval based on tumor response rate and durability of response for the treatment of patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors that have progressed following prior treatment. However, response rates were within the range of 30–50% in the relevant KEYNOTE studies (KEYNOTE-016, 164, 012, 028, 158) [16].
2.2. Emerging Biomarkers
Characterization of the density and phenotype of tumor-infiltrating lymphocytes (TILs) within the TME has attracted increasing attention over recent years. Galon et al. showed that a density of total lymphocytes (CD3+), CD8 effector T cells (CD8+), and memory T cells (CD45RO+) in the center of the tumor (CT) and at the invasive margin (IM) correlated with favorable DFS and OS in patients with colorectal cancer (CRC). In particular, the correlation of the density of CD3+ cells in the CT and IM with DFS was superior to the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) tumor, lymph nodes, and metastasis (TNM)-based classification of stage I–III CRC patients [17]. These results constituted the foundation for a digital pathology-based assay named Immunoscore that quantifies the two T cell subsets (CD3 and CD8) in the CT and IM and can help identify the subset of patients who would benefit from adjuvant therapy [18]. It was proposed to add this immune-based assay to the TNM classification system to better stratify patients according to prognosis and select effective treatment plans accordingly [18,19]. In the exploratory NICHE study, among patients with early-stage CRC and preserved mismatch repair system (pMMR), only those with high pre-treatment CD8+PD-1+ T cell infiltration responded to neoadjuvant immunotherapy, while all patients with dMMR tumors responded [20]. Therefore, Immunoscore might guide the selection of patients who are likely to benefit from neoadjuvant or adjuvant immunotherapy in patients with pMMR tumors.
A recent analysis of high-dimensional TILs flow cytometry data recognized that patients with NSCLC and high levels of CD8+ T cells expressing the inhibitory PD-1 receptor tend to have a poorer prognosis. Assessment of CD8+PD-1+ immune infiltration on surgical biopsies may help pinpoint this high-risk subset of patients that would derive the most benefit from adjuvant PD-1/PD-L1 inhibitors [21]. Similarly, pre-existing CD8+ T cells with expression of the PD-1/PD-L1 immune inhibitory axis can predict tumor regression and response to PD-1 inhibitors in patients with melanoma [22].
Research into the TME has also suggested that the neutrophil-to-lymphocyte ratio (NLR) can function as a new biomarker. A high NLR, reflecting a highly pro-inflammatory status, is related to worse survival outcomes, while high lymphocyte counts, reflecting an improved antigen-driven cytotoxic T cell response, confer a favorable prognosis [23,24]. Recent studies found that blood neutrophils were directly linked with the number of intra-tumoral neutrophils, while lower counts of blood lymphocytes usually reflect an impaired cell-mediated immunity [23,24]. Therefore, NLR is a suitable candidate for a cost-effective and widely accessible, non-invasive biomarker. It was shown that a high NLR that persists during treatment confers a poor prognosis in patients receiving PD-1/PD-L1 blockade. A mixed-effects regression analysis showed that responding patients have a consistent decrease in the NLR over time, whereas patients with stable disease or progression do not [25]. In a retrospective review of patients with advanced cancer treated with ICIs, patients with baseline and on-treatment NLR < 5 had significantly longer OS (p < 0.001). The study also suggested that NLR can function as a dynamic marker since the change in NLR during treatment was a predictor of OS and was observed to be non-linear in nature [26]. NLR can also be combined with other biomarkers, such as TMB, to aid in decision-making. In a retrospective cohort study of 1714 patients with 16 different cancer types treated with ICI, higher NLR was significantly associated with poorer OS and PFS after ICI therapy across all cancer types, except for endometrial and ovarian cancers. The probability of benefit from ICI was significantly higher in the NLR low/TMB high group (OR = 3.22; 95% CI, 2.26–4.58; p < 0.001) compared to the NLR high/TMB low group [27].
In the early-disease setting, NLR has been combined with PD-L1 to help guide treatment. In a prospective study of 60 patients with laryngeal carcinoma (LSCC), blood NLR, neutrophils, and lymphocytes counts were predictive of DFS. PD-L1 CPS ≥ 1 and TILs count rate ≥30% were associated with longer DFS. Increasing NLR was found to correlate with PD-L1 CPS < 1 and TILs count rates <30% [23]. In another retrospective study, the prognostic and predictive role of pre-treatment NLR was evaluated in a large cohort of stage III NSCLC patients treated with definitive chemoradiation and adjuvant immunotherapy with anti-PD-L1 agent durvalumab. A higher NLR ratio was found to be predictive of lower response to durvalumab [28]. Both studies lend credence to the suitability of pre-treatment NLR as a biomarker of immunotherapy benefit and provide the groundwork for future studies of NLR as a stratification factor to identify patients who would benefit from PD-1/PD-L1 inhibitors in the adjuvant and/or neoadjuvant setting.
Another emerging blood-based biomarker is circulating tumor DNA (ctDNA). ctDNA is a component of cell-free DNA (cfDNA) that is released from apoptotic or necrotic tumor cells. This biomarker has passed through several steps of improvement from 1948 until now [29,30,31,32,33,34,35,36,37]. In the last decade, it was found that ctDNA can be measured by polymerase chain reaction (PCR) and next-generation sequencing (NGS) technology [38]. Serial ctDNA assessments were used for the detection of minimal residual disease (MRD) and may identify patients with a high risk of recurrence who require active treatment rather than active surveillance [39]. In a retrospective study of patients with stage IB–IIIA NSCLC, ctDNA positivity at 3 months after surgery conferred a significantly inferior relapse free-survival (RFS), suggesting patients who are ctDNA-positive after surgery have a high risk of relapse and may need adjuvant therapy [40]. In the TRACERx study, serial ctDNA measurements in 78 patients with stage I–III NSCLC following surgical excision led to ctDNA detection suggesting minimal residual disease (MRD) at or before clinical relapse in 37 of 45 patients (82.2%); ctDNA detection preceded documented clinical relapse by a median of 151 days [41]. A similar study of ctDNA monitoring through next-generation sequencing (NGS) in 116 patients with resected NSCLC also demonstrated the utility of postsurgical ctDNA surveillance in detecting relapse prior to radiological imaging and guiding adjuvant treatment. Patients with detectable ctDNA who received adjuvant chemotherapy had a significantly prolonged RFS, and ctDNA status after treatment completion was found to be a predictor of RFS and, thereby, treatment effectiveness [42]. A similar benefit of baseline and longitudinal ctDNA assessment for early detection of relapse was shown in localized urothelial cancer [43], resected stage III melanoma [44], resected gastric cancer [45], and early-stage breast cancer [46].
Given the consistent correlation of ctDNA with the risk of recurrence, prospective studies will be crucial to test the clinical utility of ctDNA in guiding adjuvant treatment. In the DYNAMIC study, in which adjuvant chemotherapy was offered to ctDNA-positive patients at 4 or 7 weeks after surgery for stage II colon cancer, 2-year RFS was non-inferior to management per standard clinicopathological features [47]. So far, there is a study that has investigated the utility of ctDNA in guiding adjuvant immunotherapy. Although IMvigor010 failed to show that atezolizumab significantly improved DFS or OS in early-stage NSCLC, it did shed light on the use of ctDNA to guide therapy. A total of 581 patients were included in the ctDNA biomarker-evaluable population and followed for a median of 23.0 months. ctDNA positivity predicted a higher risk of recurrence, but adjuvant atezolizumab was able to significantly prolong DFS (HR = 0.58; 95% CI, 0.43–0.79) and OS (HR 0.59 95% CI, 0.41–0.86) in ctDNA positive patients. Adjuvant atezolizumab also led to increased ctDNA clearance by cycle 3 (18.2% vs. 3.8%, p = 0.0204), which translated to superior DFS (HR = 0.26; 95% CI: 0.12−0.56). Analyses of the secondary endpoint of OS yielded similar results. These findings suggest that atezolizumab may be beneficial in patients who are positive for ctDNA. Since no difference in clinical outcomes was observed between the atezolizumab and the observation arms in patients who were negative for ctDNA, this population may safely forgo adjuvant treatment. These findings are in favor of initiating adjuvant treatment based on the identification of MRD rather than treating unselected patients [48].
The clinical utility of blood TMB (bTMB) in predicting benefit to ICI is also under investigation. In exploratory analyses of the MYSTIC trial, a bTMB threshold of at least 20 mutations per megabase was identified for optimal clinical benefit with durvalumab plus tremelimumab in patients with metastatic NSCLC [49]. However, a systematic review and meta-analysis that included studies in patients with CRC, melanoma, NSCLC, and biliary tract cancers concluded that the level of bTMB after ICI treatment was not associated with OS. On the other hand, patients with ctDNA clearance during ICI treatment had better OS (HR = 4.94, 95%CI = 2.96–8.26, p < 0.00001) [50]. Another meta-analysis of seven trials in NSCLC also did not find a difference in OS between high and low bTMB; interestingly, ICIs were found to improve OS (HR = 0.74; 95% CI: 0.59–0.92, p = 0.006) compared to chemotherapy in patients with high TMB [51]. In a recent retrospective study of three different cohorts, including patients enrolled in OAK and POPLAR, patients with metastatic NSCLC and high ctDNA-adjusted bTMB had significantly higher ORR and durable clinical benefit (DCB), defined as PFS lasting 6 months or longer, than those with low ctDNA-adjusted bTMB [52]. Consequently, results suggest that ctDNA-adjusted bTMB may serve as a better predictor of clinical benefit to ICIs than bTMB. A retrospective study aimed to offer real-world data on the dynamic changes of ctDNA values assessed by sequential Guardant 360 liquid biopsies in patients with advanced solid cancer, as well as their relationship with clinical outcome. This study reported that there were 89 (95.70%) patient samples that were associated with clinical data and only 4 (4.30%) that were not. Follow-up time was 2.15 years (interquartile range: 1.10–4.12), the number of patients still alive was 24 (25.81%), and the median survival time was 2.4 (% CI: 1.78–2.76) years; thus, this study illustrated that molecular response evaluation utilizing ctDNA can be used as a noninvasive predictor of response to systemic therapy in several solid cancers [53].
A recent study was conducted to assess the potential roles of ctDNA in early relapse detection and disease status monitoring in early-stage pancreatic adenocarcinoma (PDAC) prediction, as well as evaluating other tumor markers such as carcinoembryonic antigen (CEA) and cancer antigen 19–9 (CA 19–9). The study reported that during follow-up, 44.4% of the patients relapsed, and of these patients, 100% had ctDNA detected before or at the time of the recurrence (100% sensitivity and specificity). According to this study, early-stage PDAC patients with positive ctDNA following surgery have a worse chance of remaining recurrence-free (log-rank p = 0.011); in addition, ctDNA was revealed to be a superior predictive factor during monitoring and a more accurate biomarker than CA-19-9 and CEA; furthermore, the study showed that ctDNA can be utilized to provide disease status information before imaging in patients with early-stage PDAC [54,55].
In the field of transplant oncology, ctDNA was proposed as a tool to monitor disease relapse in hepatocellular carcinoma (HCC) patients who have received liver transplantation. In a study that included 10 patients with HCC, stage I–IV, who underwent curative liver transplantation with long-term ctDNA surveillance, results showed that ctDNA was superior to AFP in detecting recurrence and was able to inform disease status ahead of imaging [56].
Undoubtedly, the availability and utilization of biomarkers for monitoring response during therapy will increase the clinician’s overall confidence in the efficacy of treatment in the adjuvant setting, especially since there is no visible disease to monitor on imaging [57].
3. Clinical Studies of Adjuvant Immunotherapy
3.1. Cutaneous Melanoma
Immunotherapy in the adjuvant setting was first evaluated in melanoma. Historically, interferon-α (IFN-α) was the only adjuvant therapy option, but it provided marginal efficacy and significant toxicity [58]. In 2015, the EORTC 18071 trial showed that high-dose ipilimumab (10 mg/kg) every 3 weeks for 4 doses, then every 3 months for up to 3 years led to significant improvement in the primary RFS endpoint and the secondary OS endpoint after a median follow-up of 7 years in patients with completely resected stage III melanoma at high risk of recurrence (Table 1). Survival benefits were unfortunately countered by significant toxicity, with 54.1% of patients receiving ipilimumab experiencing grade 3 or 4 adverse events, compared with 26.2% of patients in the placebo arm. In fact, 52% of patients who were in the ipilimumab arm discontinued treatment and 1% (five patients) of deaths were attributed to treatment [58,59,60]. The intergroup trial E1609 compared adjuvant ipilimumab at both 3 and 10 mg/kg doses every 3 weeks for 4 doses (induction), followed by the same dose every 12 weeks for up to 4 additional doses (maintenance) to high dose IFN-α (HDI) in patients with resected stage III or IV (M1a or M1b) melanoma to try to circumvent the high incidence of serious adverse events. The comparison of ipilimumab at 3 mg/kg vs. HDI revealed significantly improved OS (HR 0.78, p = 0.044) and a trend toward improved RFS (HR = 0.85, p = 0.065) (Table 1). The benefit of ipilimumab at 3 mg/kg may be understated due to the significant crossover toward ipilimumab and other salvage treatment in the HDI arm. On the other hand, ipilimumab at 10 mg/kg failed to show superiority over HDI and was highly toxic. Treatment-related grade ≥3 adverse events occurred in 37% of patients receiving 3 mg/kg of ipilimumab, 79% of those receiving HDI, and 58% of those receiving 10 mg/kg of ipilimumab [61].

Table 1.
Summary of clinical trials of ICIs in the adjuvant setting.
Given the favorable safety and efficacy profile seen with PD-1 inhibitors in unresectable metastatic melanoma, CheckMate 238 compared nivolumab at 3 mg/kg every 2 weeks vs. ipilimumab at 10 mg/kg every 3 weeks for 4 doses and then every 12 weeks in 906 patients with completely resected stage IIIB, IIIC, or IV cutaneous melanoma. CheckMate 238 was completed before the results of E1609 were published, supporting the use of ipilimumab at 3 mg/kg. One important distinction between CheckMate 238 and E1609 is that the patient population in the latter had a lower risk of recurrence (no stage IV M1c). The 12-month rate of RFS was 70.5% (95% confidence interval [CI] 66.1–74.5) in the nivolumab group and 60.8% (95% CI, 56.0–65.2) in the ipilimumab group (HR 0.65; 97.56% CI, 0.51–0.83; p < 0.001) (Table 1). Patients appeared to benefit more from nivolumab than from ipilimumab regardless of PD-L1 status. Treatment-related grade 3 or 4 adverse events were considerably fewer with nivolumab than ipilimumab (14.4% vs. 45.95%), which led to fewer treatment discontinuations (9.7% vs. 42.6%) [62]. Updated 4-year results confirmed sustained RFS benefit with nivolumab (51.7% vs. 41.2%, p = 0.0003), but fewer than anticipated deaths rendered OS similar between both groups [63]. The introduction of effective subsequent therapy options such as PD-1 inhibitors and BRAF-targeted treatments, as well as a higher percentage of patients in the ipilimumab group receiving PD-1 inhibitors as subsequent therapy, could have confounded OS results. Understandably, subsequent therapy given for progression or recurrent disease that is more effective in the standard arm than the investigational arm will tend to lead to no or minimal OS difference [64]. It was shown that retreatment with PD-1 inhibitors yields low response rates, likely due to the emergence of adaptive immune resistance [65]. Since the trial lacked a placebo arm, CheckMate 238 did not inform how adjuvant treatment compares with surveillance. With the substantial benefit observed with PD-1 inhibitors in patients with metastatic disease, it remained unclear whether adjuvant checkpoint blockade after resection is justified or should be reserved for relapse or progression in patients with stage III or IV melanoma. An indirect-treat-comparison (ITC) using the Bucher method sought to address this question by comparing nivolumab with placebo using intention-to-treat (ITT) population data from CheckMate 238 and EORTC18071 trials. The ITC accounted for differences in post-recurrence survival between the ipilimumab arms in EORTC 18071 and CheckMate 238 due to the availability of more efficacious subsequent therapies. The study indicated that adjuvant nivolumab provides RFS, distant metastasis-free survival (DMFS), and OS benefit over a watch-and-wait strategy [66].
Although the two arms were not designed to be compared statistically, CheckMate-067 showed that the combination of nivolumab and ipilimumab leads to numerically higher response rates, 5-year PFS, and 5-year OS over nivolumab alone in patients with untreated metastatic melanoma [67]. However, patients with resected stage IV melanoma and NED were excluded from the trial. Therefore, the phase II IMMUNED trial was designed to evaluate the combination of nivolumab and ipilimumab (1 mg/kg of nivolumab every 3 weeks plus 3 mg/kg of ipilimumab every 3 weeks for 4 doses, followed by 3 mg/kg of nivolumab every 2 weeks), nivolumab monotherapy (3 mg/kg of nivolumab every 2 weeks), or double-matching placebo group in patients with stage IV melanoma with NED after surgery or radiotherapy. Recurrence was lower in both the nivolumab plus ipilimumab group (HR 0.23; 97.5% CI 0.12–0.45; p < 0.0001) and the nivolumab group (HR 0.56; 97.5% CI 0.33–0.94; p = 0.011) vs. placebo; the combination group demonstrated the numerically largest RFS (Table 1). Treatment-related grade 3 or 4 adverse events occurred more frequently in the nivolumab plus ipilimumab group than in the nivolumab group (71% (95% CI 57–82) vs. 27% (16–40)) [68]. Final results at a median follow-up of 49.2 months confirmed the RFS benefit in the nivolumab plus ipilimumab group (HR 0.25 (97.5% CI 0.13–0.48; p < 0.0001) and nivolumab monotherapy group (HR 0.60; 95% CI 0.36–1.00; p = 0.024). On the other hand, OS was significantly prolonged exclusively in the combination arm (HR 0.41; 95% CI 0.17–0.99; p = 0.040), which supports the use of adjuvant combination nivolumab plus ipilimumab in patients with stage IV melanoma with NED after giving careful consideration to the increased risk of adverse events [69]. CheckMate 915 evaluated the combination of nivolumab (240 mg every 2 weeks) and ipilimumab (1 mg/kg every 6 weeks) or placebo-controlled nivolumab (480 mg every 4 weeks) for up to 1 year in completely resected stage IIIB–D or stage IV melanoma. The lower and less frequent dosing of ipilimumab sought to improve tolerability with its use. After a median follow-up of 28 months, the combination arm failed to improve RFS vs. nivolumab monotherapy. Compared with the aforementioned studies (EORTC 18071, IMMUNED), the lower ipilimumab dosing in CheckMate 915 may have been insufficient to provide a meaningful benefit; as such, the dosing scheme utilized in IMMUNED may be an option for patients with stage IV melanoma with NED, while further studies are needed to investigate the optimal dosing regimen and frequency of ipilimumab plus nivolumab in stage III melanoma [70].
The efficacy of pembrolizumab in the adjuvant setting was demonstrated in two phase III trials. EORTC 1325-MG/KEYNOTE-054 compared pembrolizumab 200 mg every 3 weeks to placebo for up to 18 cycles in 1019 patients with completely resected high-risk stage III melanoma (IIIA with at least one micrometastasis >1 mm, IIIB, IIIC). In the final updated analysis in the overall ITT population, the 3.5-year RFS was 59.8% in the pembrolizumab group and 41.4% in the placebo group (HR 0.59 (95% CI 0.49–0.70)) (Table 1). Similarly, the secondary endpoint of DMFS at a median follow up of 3.5 years was higher in the pembrolizumab group than in the placebo group (65.3% vs. 49.5%; HR 0.60 (95% CI 0.49–0.73); p < 0.0001). The benefit was consistent across all subgroups including PD-L1 status, AJCC-7 and -8 staging, and BRAF-V600E/K status. Patients with high-risk stage IIIA had an increased 3.5-year DMFS of approximately 10% and 3-year RFS of approximately 15%. The absolute benefit in low-risk stage IIIA is likely lower and must be balanced against the risk of chronic immune-related adverse events [71]. The trial also found the safety profile of pembrolizumab to be comparable to that of nivolumab as demonstrated in the Checkmate 238 trial, with rates of grade 3 or higher adverse events in each trial of 14.5% and 14.4%, respectively [72]. The phase III randomized intergroup S1404 trial compared pembrolizumab to either HDI or ipilimumab 10 mg/kg in patients with high-risk resected melanoma (Stages IIIA(N2), IIIB, IIIC, and IV (M1a, b, and c)). Pembrolizumab significantly improved RFS compared to the control group composed of HDI and ipilimumab (HR 0.740; 99.618% CI 0.571–0.958) (Table 1). There was no statistically significant improvement in OS. Expectedly, pembrolizumab was better tolerated than HDI and ipilimumab [73].
Based on the premise that neoadjuvant anti-PD-1 therapy expands more T-cell clones and would likely elicit a more robust immune response from TILs, SWOG S1801 compared 3 doses of preoperative pembrolizumab, followed by 15 doses of adjuvant pembrolizumab with upfront surgery, followed by 18 doses of pembrolizumab in 313 patients with stage III–IV cutaneous, acral, and mucosal melanomas without brain metastasis [74]. After a median follow-up of 14.7 months, event-free survival (EFS) (HR 0.58; 95% CI 0.4–0.86) and OS (HR 0.63; 95% CI 0.32–1.24) were significantly longer with the neoadjuvant approach. Following neoadjuvant treatment, 21% achieved complete pathologic response [75]. In order to better assess the relative benefits of neoadjuvant and adjuvant treatment, a well-designed, controlled trial that compares total neoadjuvant treatment with total adjuvant treatment is needed. The ongoing NADINA trial (NCT04949113), comparing neoadjuvant ipilimumab plus nivolumab with adjuvant nivolumab, will help shed light on the optimal approach for treatment [76].
Based on E1609, CheckMate 238, and KEYNOTE-054, PD-1 inhibitors have largely supplanted the use of ipilimumab and HDI in resected stage III or IV melanoma. For patients with resected high-risk node-positive melanoma and a BRAF V600 driver mutation, either adjuvant immunotherapy or targeted therapy with dabrafenib plus trametinib for one year may be selected [77]. A single-center retrospective analysis of 104 patients with resected stage III melanoma who received adjuvant immunotherapy or targeted treatment did not reveal any differences in RFS or DFMS between the two approaches [78]. Another study evaluated the use of BRAF inhibitors in V600 BRAF-mutated melanoma that recurs with the resectable disease on or after adjuvant immunotherapy for resected stage III/IV disease. The stage at the start of the second adjuvant BRAF/MEK inhibitors included IIIB (29%), IIIC (53%), IIID (4%), and IV (15%). Median RFS was 33.4 months (14.3.7-NR), and median DMFS was not reached. The authors concluded that while RFS appeared shorter compared to first-line trials, second adjuvant treatment with BRAF/MEK inhibitors was still active in preventing further recurrence [79]. Prospective, randomized control trials with longer follow-up and larger sample size are warranted to determine any difference in OS benefit between the two different treatments and guide optimal sequencing. A few considerations while selecting treatments include patient preference for parenteral vs. oral therapy and risk for potentially chronic immune-related adverse events with immunotherapy compared to typically manageable and/or reversible side effects with targeted treatments.
Since patients with stage IIB and IIC melanoma can have a similar risk of recurrence and melanoma-specific death to those with stage IIIB disease [80], KEYNOTE-716 assessed the effect of adjuvant pembrolizumab for up to 1 year as compared with placebo on RFS in patients with completely resected stage IIB or IIC melanoma. At the second interim analysis after a median follow-up of 20.9 months, 15% in the pembrolizumab group and 24% in the placebo group had a first recurrence or died (HR 0.61 [95% CI 0.45–0.82]) [81]. The fact that the most frequent type of first recurrence was distant metastases in the placebo group reinforces the need for effective adjuvant therapy in patients with high-risk stage II disease. The third interim analysis of the KEYNOTE-716 trial, which occurred after a median follow-up of 27.4 months, demonstrated that the secondary endpoint of DMFS was significantly improved with pembrolizumab (HR 0.64, 95% CI 0.47–0.88, p = 0.0029) (Table 1) [82]. Safety was consistent with previous adjuvant trials, with grade 3–4 treatment-related adverse events occurring in 17% in the pembrolizumab group and 5% in the placebo group [82]. Reassuringly, adjuvant pembrolizumab did not adversely affect health-related quality of life [83]. Mature OS data are still awaited; however, the cross-over design of this study may complicate the interpretation of OS. Based on these data, the FDA approved adjuvant pembrolizumab for adult patients with stage IIB and IIC melanoma following complete resection. Surveillance, particularly for patients with stage IIB who have a lower risk of disease recurrence, does remain a reasonable alternative until mature OS data are reported.
3.2. Urothelial Cancer
In the phase III open-label IMvigor010 study, 809 patients with muscle-invasive urothelial carcinoma (UC) following radical cystectomy or nephroureterectomy with lymph node dissection were randomized to receive atezolizumab 1200 mg every 3 weeks for up to 1 year or observation. Median DFS was not significantly improved with atezolizumab. While atezolizumab had an acceptable safety profile, higher frequencies of adverse events leading to discontinuation occurred compared to trials in the metastatic UC setting [84]. Interestingly, patients with detectable ctDNA that cleared with treatment had a superior DFS (HR = 0.26 (95% CI: 0.12−0.56), p = 0.0014) [32].
In the phase III CheckMate 274 trial, 709 patients with high-risk muscle-invasive UC following radical cystectomy were randomized to either 1 year of adjuvant nivolumab 240 mg every 2 weeks or placebo. Nivolumab significantly improved the percentage of patients who were alive and disease-free at 6 months, and the effect was more pronounced in patients with PD-L1 expression level of 1% or more. After a median follow-up of 20.9 months, the median DFS was 20.8 months with nivolumab compared with 10.8 months with placebo. The median RFS and DMFS were also longer with nivolumab. With additional 5 months of follow-up, DFS and DMFS benefit was maintained with a median DFS of 22 months with nivolumab compared with 10.9 months with placebo (Table 1) [85]. At the time of publication, OS data were still immature. Treatment-related adverse events of grade 3 or higher occurred more frequently in the nivolumab arm (17.9% vs. 7.2%) and included two treatment-related deaths due to pneumonitis and bowel perforation. However, the increased incidence of adverse events did not appear to have a detrimental effect on health-related quality of life [86]. These data led to FDA approval of adjuvant nivolumab in patients at high risk for recurrence after undergoing radical resection of UC. This is particularly relevant in patients not eligible for cisplatin or in those with pathological evidence of residual disease despite neoadjuvant cisplatin-based chemotherapy as no previous adjuvant systemic therapies were shown to improve outcomes. A higher probability of DFS with nivolumab than with placebo was observed regardless of nodal status, PD-L1 status, or use of previous neoadjuvant cisplatin-based chemotherapy; however, subgroup analyses do raise the question of whether beneficial results were mainly driven by patients who received cisplatin as neoadjuvant therapy, had a PD-L1 expression above 1%, and had primary tumors originating in the bladder. The predictive role of PD-L1 expression in patients deemed ineligible to receive cisplatin would require further exploration [87]. The causes for conflicting results between IMvigor010 and CheckMate214 remain speculative, but notable differences include considerably shorter DFS in the control group of CheckMate 274 than in the observation group of IMvigor010 (10.8 vs. 16.6 months), different percentages of upper-tract UC (21.1% in the CheckMate 274 trial vs. 6% in the IMvigor010 trial), and different methods used for the evaluation of PD-L1 expression. In addition, it is possible that PD-1 inhibitors such as nivolumab could be more potent than PD-L1 inhibitors such as atezolizumab. A systematic review and meta-analysis including studies in NSCLC, RCC, and UC showed that PD-1 inhibitors exhibited a superior OS and PFS compared with PD-L1 inhibitors [88]. Nivolumab binds to PD-1 and inhibits binding of both PD-L1 and PD-L2, whereas atezolizumab binds selectively to PD-L1, thereby allowing immune response escape through PD-1/PD-L2 interaction [62].
Encouraging results from CheckMate 274 trial led to the design of the randomized phase II CCTG BL13 trial assessing trimodality therapy consisting of transurethral resection of bladder tumor (TURBT), followed by chemoradiotherapy with or without adjuvant durvalumab to treat patients with muscle-invasive UC (NCT03768570) [89]. The principal investigators posit that chemoradiation may increase susceptibility of tumor cells to immune-mediated treatment in the adjuvant setting by triggering immunogenic cell death [90,91].
3.3. Renal Cell Carcinoma
Multiple approaches, including cytokine-based immunotherapy with interleukin-2 (IL-2) and/or IFN-α, were studied in the adjuvant setting of RCC but failed to show a significant improvement in DFS or OS [92]. Although the S-TRAC trial showed a significant DFS benefit of adjuvant sunitinib therapy over placebo, the DFS benefit did not translate to an OS advantage, and an increased incidence of adverse effects and lower quality-of-life scores were reported [93]. A meta-analysis of all studies of anti-angiogenics have further lowered confidence in the use of vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) as adjuvant treatment [94]. It was hypothesized that rapid revascularization of tumors after discontinuation of VEGFR-TKIs could explain discouraging results with their adjuvant use [95].
The phase III KEYNOTE-564 trial enrolled 994 patients with clear-cell RCC who were at high risk for recurrence after nephrectomy, defined as stage 2 with nuclear grade 4 or sarcomatoid differentiation, stage 3 or higher, regional lymph-node metastasis, or stage M1 following metastasectomy within 1 year of nephrectomy, and randomized them to receive either adjuvant pembrolizumab 200 mg or placebo every 3 weeks for up to 1 year. At the first interim analysis, approximately 40% of patients discontinued the trial regimen, with the most common reason being an adverse event (in 21.3%), occurring after a median of 7 cycles, followed by disease recurrence (in 10.5%). The risk of disease recurrence or death was 32% lower with adjuvant pembrolizumab therapy than with placebo (HR 0.68; 95% CI 0.53–0.87; p = 0.002) (Table 1) [96]. While 75% of the patient population had a positive PD-L1 score, the benefit of pembrolizumab was consistent across all prespecified subgroups and independent of PD-L1 status. Given the heterogeneous patient population enrolled, an important consideration will be to define which patient groups are most likely to derive benefit from therapy by exploring biomarkers. An exploratory analysis after 30.1 months of follow-up continued to show improved DFS with pembrolizumab (HR 0.63 [95% CI 0.50–0.80]); median DFS was not reached in either group. While there was a promising signal for improved OS, longer follow-up will be imperative to confirm OS benefit, especially since a trial-level meta-analysis of adjuvant systemic therapy for localized RCC studies did not show a strong correlation between 5-year DFS and 5-year OS rates [97]. In addition, since first-line ICIs are highly efficacious in metastatic RCC, OS will dictate whether early use of adjuvant pembrolizumab is warranted as compared with the delayed use of combination therapies in patients with metastatic disease [98]. Efficacy of first-line immunotherapy options in cases of progression or recurrent disease following adjuvant immunotherapy will also need to be investigated to elucidate the optimal front-line treatment strategy. Grade 3 or higher adverse events attributed to treatment occurred in 18.9% of the patients who received pembrolizumab and in 1.2% of those who received placebo. No new safety signals were observed with adjuvant therapy with pembrolizumab. Unlike studies with adjuvant VEGFR-TKIs, pembrolizumab did not negatively impact quality of life.
Despite pembrolizumab’s encouraging results, other immunotherapy agents have thus far failed to demonstrate utility in the adjuvant setting. In the phase III IMmotion010 trial, 778 patients with RCC with a clear cell or sarcomatoid component and increased risk of recurrence (T2 and grade 4, T3a and grade 3 or 4, T3b/T3c or T4 any grade, TxN+ and any grade, or M1 with no evidence of disease), 40% of whom were negative for PD-L1 expression, were randomized to receive atezolizumab 1200 mg or placebo every 3 weeks for 16 cycles or 1 year. After a median follow-up of 44.7 months, the primary endpoint of DFS was not significantly improved with atezolizumab (HR 0.93; 95% CI 0.75–1.15) [99]. Similarly, results from part A of CheckMate 914, which evaluated nivolumab 240 mg every 2 weeks (×12 doses) combined with ipilimumab 1 mg/kg every 6 weeks (×4 doses) vs. placebo in 816 patients with stage II–III, predominantly clear cell RCC at high risk of recurrence (T2a and grade 3 or 4, T2b/T3/T4 and any grade, and any T with N1M0) following partial or radical nephrectomy, showed no evidence of a positive clinical outcome. After a median follow-up of 37.0 months, the primary efficacy endpoint of DFS was not improved in the treatment arm (HR, 0.92; 95% CI 0.71–1.19; p = 0.53). The shorter duration of planned therapy in CheckMate 914 (6 months) compared to KEYNOTE-564 (1 year) could explain the lack of benefit with ipilimumab and nivolumab. Grade 3–4 treatment-related adverse events occurred in 28% of the investigation arm and 2% of patients in the placebo arm. Additionally, four treatment related deaths were reported in the nivolumab plus ipilimumab arm. Discontinuation attributed to any-grade adverse event occurred in 32% of patients treated with nivolumab plus ipilimumab and 2% of patients receiving placebo, although the authors suggest that travel constraints secondary to the COVID-19 pandemic may have affected the discontinuation rate [100]. PROSPER investigated perioperative nivolumab (1 preoperative dose followed by 9 adjuvant doses) in patients with localized RCC (≥T2 or T anyN+, M1 with no evidence of disease). The trial was stopped early for futility since the primary endpoint of RFS was not statistically different between the two arms (HR 0.97; 95% CI 0.74–1.28). OS and subset analyses including risk stratification by pathologic stage are yet to be reported [101]. As of now, pembrolizumab seems to be the only active agent in the adjuvant setting; however, updated results at longer follow-up, subset analyses, and additional ongoing studies of different ICIs (CheckMate 914 part B, RAMPART) may shed further light on the subset of patients that would derive the most benefit from adjuvant immunotherapy [102,103].
3.4. Non-Small Cell Lung Cancer
Based on the eighth edition of TNM staging of lung cancer, 5-year survival rates for patients with stage II–IIIA NSCLC range from 41% to 65%, which highlights the need for better treatment approaches [104]. So far, adjuvant therapy has not produced robust results. The International Adjuvant Lung Cancer Trial (IALT) showed that adjuvant cisplatin-based chemotherapy adds only a modest absolute benefit of 5% to DFS and OS, and that benefit decreased on longer follow-up [105]. The addition of bevacizumab to adjuvant chemotherapy failed to improve OS in patients with completely resected stage IB to IIIA NSCLC [106]. Recently, the ADAURA trial showed that epidermal growth factor receptor (EGFR) inhibitor osimertinib prolongs DFS in patients with stage IB to IIIA NSCLC harboring EGFR mutations [107]. Several limitations, such as inadequate workup and staging and substandard adjuvant chemotherapy, have led the study to be a topic of controversy [108]. More importantly, it remains to be seen whether the 2-year DFS superiority will translate into an OS benefit as data from the ADJUVANT/CTONG 1104 trial have shown that the substantial DFS benefit with gefitinib did not translate into an OS benefit [109]. In addition, patients with wild-type EGFR, which constitute 50–80% of the population, were not eligible to benefit from this new addition to the armamentarium of adjuvant therapies [110]. After the success of immunotherapy in prolonging survival in patients with advanced NSCLC with no targetable mutations as well as in patients unresectable, stage III NSCLC following chemoradiation, there was increased interest in introducing immunotherapy even earlier for resectable NSCLC [111,112]. The phase III IMpower010 compared adjuvant atezolizumab 1200 mg every 21 days for 16 cycles or 1 year vs. best supportive care (BSC) following up to 4 cycles of adjuvant platinum-based chemotherapy in 1005 patients with completely resected stage IB (tumor size ≥4 cm) to IIIA NSCLC (based on American Joint Committee on Cancer staging system 7th edition). In the ITT population, which included patients with stage IB, difference in DFS failed to reach statistical significance. On the other hand, atezolizumab significantly improved DFS in all stage II–IIIA patients (HR 0.79; 0.64–0.96; p = 0.020) (Table 1). The effect was particularly more pronounced in those with PD-L1 positive tumors (PD-L1 ≥ 1%) (HR 0.66; 95% CI 0.50–0.88; p = 0.0039), especially when PD-L1 was expressed on 50% or more of tumor cells (HR 0.43; 95% CI 0.27–0.68). The effect was negligible in patients in the stage II–IIIA population whose tumors expressed PD-L1 on less than 1% of tumor cells based on a post-hoc exploratory analysis (unstratified HR 0.97; 95% CI 0.72–1.31) [113]. The most common atezolizumab-related adverse events were hypothyroidism (11%), pruritis (9%), and rash (8%). Treatment-related serious adverse events occurred in 7% of the patients in the atezolizumab group. Ultimately, Impower010 suggests that atezolizumab offers a safe and promising adjuvant treatment for patients with stage II–IIIA whose tumors express PD-L1 on 1% or more of tumors cells, especially for patients with PD-L1 expression on 50% or more tumor cells. Results support PD-L1 testing in resectable NSCLC. Although preliminary, retrospective data predating the use of targeted therapy and immunotherapy have shown that recurrence after complete resection is associated with worse survival outcomes, a higher level of evidence is needed to establish DFS as a validated surrogate for OS benefit in patients with NSCLC receiving adjuvant cancer therapies [114]. As such, results upon longer follow-up are awaited and will reveal whether the observed DFS benefit will be consistent with an OS benefit. However, considering that only half of the patients in the BSC arm received standard-of-care ICI upon disease recurrence, there is concern this underutilization of ICI may skew OS data in favor of atezolizumab [3,115].
Data from the pre-specified second interim analysis of PEARLS/KEYNOTE-091 showed that adjuvant pembrolizumab for 1 year significantly improved DFS (median DFS 43.6 months vs. 42 months; HR 0.76; 95% CI 0.63–0.91; p = 0.0014) following complete resection and adjuvant chemotherapy in the overall population, which included patients with stage IB (T ≥ 4 cm)–IIIA NSCLC (based on American Joint Committee on Cancer staging system 7th edition) (Table 1) [116]. Interestingly, in subgroup analyses, DFS was not significantly improved in the PD-L1 TPS of 50% or greater population, and lack of between-group imbalances could not justify why patients with PD-L1 TPS of 50% or greater randomized to placebo performed better than patients with PD-L1 TPS < 50% randomized to placebo. A longer follow-up will determine if a significant benefit truly exists in this population. Moreover, given the signal of a lesser effect in subgroup analyses of patients that did not receive adjuvant chemotherapy and those with squamous cell histology, adequately powered, randomized, controlled trials will be needed to determine if those subgroups do benefit from adjuvant pembrolizumab. Future analyses will report OS. Grade 3 or worse treatment-related adverse events occurred in 15% of patients receiving pembrolizumab and 4% of patients receiving placebo. KEYNOTE-091 showed that pembrolizumab can be used in a broader patient population compared to atezolizumab, namely, in patients with stage IB (T2a ≥ 4 cm) and regardless of PD-L1 expression, and that distinction was reflected in pembrolizumab’s FDA approval [117].
Several other ongoing phase 3 adjuvant studies of PD-L1 and PD-1 inhibitors will clarify the benefit of ICI in the adjuvant setting. ANVIL, which is the arm of the larger ALCHEMIST platform trial, will assess adjuvant nivolumab in patients not eligible for the EGFR or ALK directed trials with co-primary endpoints of a 30% improvement in OS and/or a 33% improvement in DFS favoring nivolumab [118]. BR.31 will measure the effect of adjuvant durvalumab for 1 year on DFS in patients with stage IB–IIIA (NCT02273375). MERMAID-1 will evaluate the impact of adjuvant durvalumab for 1 year on DFS in patients with stage II–III NSCLC and MRD detected by ctDNA following surgery and thereby explore the utility of ctDNA in guiding adjuvant treatment [119]. MERMAID-2 will evaluate the benefit of adjuvant durvalumab for up to 24 months in patients with stage II–III NSCLC who become MRD+ on surveillance after surgery with or without adjuvant chemotherapy. The primary endpoint is DFS in patients with PD–L1 tumor cell expression ≥1% [120].
Another area of active investigation includes combining immunotherapy with locally ablative therapy (LAT) to all sites of disease in patients with oligometastatic disease. In a single-arm phase 2 trial, patients with oligometastatic NSCLC (≤4 metastases either at diagnosis or after initial local treatment), regardless of PD-L1 expression, who had completed LAT to all tumor sites and were immunotherapy-naïve, were treated with pembrolizumab for up to 8 cycles. Patients who did not progress after 8 cycles could receive an additional 8 cycles of therapy based on physician discretion, totaling 6–12 months of therapy. Patients received a median of 11 cycles. After a median follow-up of 25 months, the median PFS, measured from the start of LAT, was statistically significantly improved at 19.1 months compared to the historical estimate of 6.6 months (95% CI, 9.4–28.7 months; p = 0.005). Median OS was 41.6 months (95% CI, 27.0–56.2 months); a final analysis of OS after a longer-term follow-up is planned. No new safety issues were identified. Pneumonitis occurred in five patients (11%), all of whom had previously received thoracic radiation [121].
As use of ICIs gains momentum in early-stage NSCLC, the dilemma of ICI retreatment upon disease recurrence becomes accentuated. In advanced-stage NSCLC, reintroduction of ICI in patients whose disease progressed at least 6 months after their previous course of ICI treatment was shown to be a potentially effective strategy [122]. Conversely, it is difficult to determine the effectiveness of this approach in the adjuvant setting as few patients in the atezolizumab arm in IMpower010 (11%) were treated with ICIs following progression, and the time to retreatment was not specified [115]. Future real-world studies or ongoing phase III studies will hopefully provide a clearer answer regarding the benefit of retreatment upon progression.
3.5. Esophageal and Gastroesophageal Junction Cancer
The first-line treatment approach for resectable, locally advanced esophageal or gastroesophageal junction cancer is surgery followed by neoadjuvant chemotherapy [123]. Patients who do not achieve pathological complete response (pCR) following preoperative therapy are at a greater risk for recurrence and exhibit poorer OS rates [124]. Previous studies of targeted agents including the VEGF inhibitor bevacizumab, human epidermal growth factor receptor 2 (HER2) inhibitor trastuzumab, and EGFR inhibitor cetuximab, have not demonstrated increased survival when combined with chemoradiation or chemotherapy [125]. CheckMate 577 evaluated adjuvant nivolumab for 1 year in 794 patients with stage II or III esophageal or gastroesophageal junction (GEJ) cancer that had residual pathologic disease (at least ypT1 or ypN1) following neoadjuvant chemoradiation and achieved an R0 resection. The median DFS was significantly longer in the nivolumab arm compared to the placebo arm (HR 0.69; 96.4% CI, 0.56–0.86; p < 0.001) (Table 1) [126]. The benefit was consistent across prespecified subgroups such as histologic type (squamous-cell carcinoma and adenocarcinoma) and pathological lymph-node status (≥ypN1 and ypN0) and appeared to be independent of PD-L1 status. Distant recurrence was also lower in the atezolizumab arm (HR 0.74; 95% CI, 0.60–0.92). Treatment-related grade 3 or 4 adverse events occurred in 13% of patients in the nivolumab arm and 6% of patients in the placebo arm. No adverse effect was noted on patient-reported quality of life [126].
The phase III KEYNOTE-585 (NCT03221426) will evaluate the efficacy and safety of neoadjuvant pembrolizumab combined with chemotherapy for 3 cycles followed by adjuvant pembrolizumab for up to 11 additional cycles in patients with localized gastric or GEJ adenocarcinoma as defined by T3 or greater primary lesion or the presence of any positive clinical nodes. The primary end points are OS, EFS, and pCR. Results will clarify whether adjuvant immunotherapy can also play a role in gastric cancer [127].
3.6. Hepatobiliary Malignancies and Transplant Oncology
While surgical resection and liver transplantation remain the mainstay of curative therapy for local HCC, the recurrence rate following these treatments is elevated [128]. Unfortunately, the STORM trial failed to show the efficacy of adjuvant sorafenib in reducing recurrence following resection or ablation, and no effective adjuvant therapy has been established to date [129]. Since locoregional therapies, such as radiofrequency ablation (RFA), break down tumor cells to expose neoantigens that amplify host T-cell antitumor response, the use of immunotherapy to enhance that response is a reasonable approach [130]. In fact, it was shown that tremelimumab combined with ablation leads to proliferation of intratumoral CD8+ T cells [131]. The NIVOLVE trial was a single-arm multicenter trial that evaluated the use of nivolumab 240 mg every 2 weeks for 8 cycles, followed by 480 mg every 4 weeks for 8 cycles within 6 weeks after hepatectomy or RFA. The 1-year recurrence free survival rate (RFSR) and RFS were 78.6% and 26.3 months, respectively. More importantly, the trial delineated predictive biomarkers for recurrence with adjuvant nivolumab that included copy number gains (CNGs) in WNT/β-catenin-related genes, activation of the WNT/β-catenin pathway, and low numbers of CD8+ TILs [132]. Currently, there are two ongoing phase III studies of adjuvant ICI monotherapy: the CheckMate 9DX study with nivolumab and KEYNOTE-937 study with pembrolizumab [133,134]. Given that ICI monotherapy may be ineffective in preventing recurrence when CD8+ T cells are suppressed by activation of the β-catenin signaling pathway, combination therapy with the anti-VEGF antibody bevacizumab can counteract that by abrogating VEGF-mediated immunosuppression and increasing tumor infiltration of T cells [135]. Combination therapy with an anti-VEGF antibody is currently being investigated in two phase III studies, the EMERALD-2 study with durvalumab plus bevacizumab and the IMbrave050 study with atezolizumab plus bevacizumab [136,137].
Transplant oncology has transformed the treatment landscape for hepatobiliary malignancies by dramatically improving survival outcomes and quality of life metrics [37,55,56,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153]. In order to expand eligibility for liver transplant (LT), bridging therapies that can downstage disease or prolong PFS for patients on the waiting list have emerged [154]. Extrapolating from the demonstrated activity of ICI such as atezolizumab plus bevacizumab, tremelimumab plus durvalumab, nivolumab plus ipilimumab, pembrolizumab, and nivolumab and in the advanced setting, neoadjuvant immunotherapy was investigated as a potential down-staging therapy [155,156]. Of note, although immunotherapy in the peri-transplant period was initially discouraged due to the risk of allograft rejection, recent experience with ICI use has shown that LT recipients may be treated with immunotherapy in closely monitored and controlled settings [139,141]. Liver transplants are known to possess a more tolerogenic environment than other solid organ transplants, which theoretically would enable treatment with ICI [157]. For example, in a cohort study that enrolled 63 patients with initially unresectable HCC, a combination of tyrosine kinase inhibitor (TKI) and PD-1 inhibitor achieved a conversion resection rate of 15.9%. The 12-month survival rate of the 10 patients was 90.0%, and 12-month RFS rate after surgery was 80.0% [158]. Another retrospective study showed that this combination results in a better tumor response in macrovascular tumor thrombi than in intrahepatic tumor lesions [159]. Thus, combining systemic treatment with locoregional therapies presents a reasonable down-staging approach. In a single-center study investigating PD-1 inhibitor monotherapy, nine patients with HCC received a LT following nivolumab as bridging therapy, with the last dose administered within 4 weeks of LT in eight patients [160]. Interestingly, explant pathology showed near complete (>90%) tumor necrosis. The only case of acute tumor rejection was attributed to subtherapeutic tacrolimus levels and resolved quickly after dose optimization [160]. A case report of a 64-year old male with advanced HCC also described successful down-staging therapy with nivolumab. To decrease risk of disease recurrence, the last nivolumab dose was administered 16 days before LT. Following deceased donor LT, on day 9, the patient developed an early T-cell mediated rejection that was treated with high-dose solumedrol (a total of 1600 mg), followed by thymoglobulin 100 mg IV daily for 4 days. After being discharged on maintenance immunosuppression with mycophenolate mofetil, tacrolimus, and prednisone, the patient did not experience recurrence 16 months after liver transplant [161]. Based on prior case reports of acute rejection with a short period of time between ICI administration and LT, it was hypothesized that prolonging the period (8 weeks)between last ICI dose and LT, if feasible, can help mitigate risk for rejection [162,163,164].
The use of immunotherapy was also described in the post-transplant setting. A comprehensive review of 35 cases of immunotherapy in LT recipients is summarized in Table 2 and Figure 1. ICIs were used for recurrent HCC in 24 cases. In regard to the efficacy of ICI for recurrent HCC, the objective response rate (ORR) was 16.7%, which is consistent with that seen in the phase III KEYNOTE 240 trial [165]. Of note, 20 of 35 (57.1%) patients did not experience rejection after initiation of ICI. In contrast, 10 (28.5%) experienced rejection, 3 (8.6%) patients developed immune-mediated hepatitis, and 2 (5.7%) patients displayed nonspecific features of both rejection and immune hepatitis. Patients who developed rejection received immunotherapy at a median of 2 years (IQR 1.4–3.2) after transplant, whereas patients who had preserved graft function were initiated on immunotherapy at a median of 4.55 years (IQR 2.325–7.95) after transplant. While data are too scant to identify a safe interval of time for ICI use following LT, it appears that the risk of rejection may be highest immediately after LT and decreases with time. Rejection was mainly T-cell mediated in nature and occurred at a median of 2.95 (IQR, 1.25–7) weeks after initiation of ICI. Therefore, rejection appears to be an early adverse event and may have negatively skewed response rates due to early discontinuation. CTLA-4 inhibitors were used in 5 patients, 1 (20%) of which developed rejection, while PD-1 inhibitors were used in 31 patients, 11 (35.5%) of which developed rejection. Prior research has suggested that solid organ transplants treated with CTLA-4 inhibitors are less likely to experience rejection and graft loss [166]. The PD-1/PD-L1 pathway was implicated in both induction and maintenance of graft tolerance [167,168,169]. Conversely, CTLA-4 is essential in induction of tolerance, but its role in maintenance of tolerance is less clearly defined; however, a recent study reported that CTLA-4 agonists can be used as maintenance immunosuppression regimens [170,171,172]. In addition, allograft PD-L1 expression was positive in four patients who developed rejection. While this observation still needs to be validated in larger trials, allograft PD-L1 expression could become a predictive biomarker for graft rejection and inform which subset of patients may be eligible for PD-1 inhibitors in the post-transplant setting. Although high-dose steroids are typically effective for T-cell mediated rejections, no improvement was noted in seven cases, which was partly attributed to concurrent development of antibody-mediated rejection [173]. Still, no standard treatment for graft failure after ICI treatment can be recommended at this time. Interestingly, 4 of 5 patients who experienced graft rejection that did not resolve with treatment were all younger than 60 years of age, which could indicate that younger patients may mount a more robust immune response and, thus, are more susceptible to rejection [159]. Taken together, these case reports demonstrate that treatment with immunotherapy following LT can induce durable response in select patients. Randomized, controlled clinical trials are needed to identify predictive biomarkers and/or subpopulations that may safely receive ICI post LT without jeopardizing graft function. In the same vein, further research into the role of ICI in allograft tolerance, as well as the optimal immunosuppressive regimen while on ICI, is needed.

Table 2.
Summary of case reports of immunotherapy use following liver transplant.
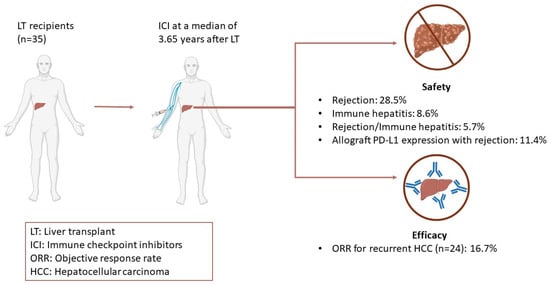
Figure 1.
Efficacy and Safety Outcomes of Adjuvant Immunotherapy after Liver Transplant.
3.7. Impact of Diet and Microbiome on Response to Immunotherapy
Given the ubiquitous use of ICIs across various malignancies, it is important to leverage molecular pathological epidemiology (MPE) research to identify environmental, dietary, and lifestyle factors that may be potentially associated with improved immunotherapy response [197].
In building on previous epidemiological studies that have linked diet, such as high consumption of red-meat, to increased incidence of malignancy and cancer-related mortality, Orillion et al. examined the impact of dietary protein restriction on the anti-tumor effects of immunotherapies in two animal models of prostate and renal cell carcinoma [198]. The results of this in vivo study demonstrated enhanced anti-tumor capacity of tumor-associated macrophages (TAMs) with dietary protein restriction and suggest that dietary protein restriction is a potential strategy that could be further investigated for oncology patients on ICI. Future ICI-related exploring parameters that would help to elucidate the translational significance of the effect of diet on circulating immune cells or the TME would be extremely valuable for consideration in clinical practice [199].
Inamura et al. provided a comprehensive review of preclinical and clinical studies examining the utilization of microbial interventions in response to immunotherapy for several solid tumors [200]. From a microbial perspective of the clinical studies utilizing antibiotics and fetal microbial transplantation, Vétizou et al. demonstrated that the immunostimulatory effects of ipilimumab (CTLA-4 blockade) were associated with T cell responses specific to the Bacteroides species such as B. thetaiotaomicron or B. fragilis. Microbial feces from melanoma patients who were treated with antibodies against CTLA-4 were transplanted into mice, resulting in an outgrowth of B. fragilis, thus, highlighting the potential role of the gut microbiome in modifying host immunity in cancer patients receiving ICI [201]. In another clinical study examining high fiber diet and probiotics as microbial interventions in melanoma patients, high fiber consumption with no probiotic use was associated with better response to (PD-1)-based therapy [202].
It is important to note that the translational significance of molecular pathological epidemiology research is critical to our understanding of the extent to which carcinogenic mechanisms and ICI-treatment-related outcomes may be impacted by environmental and lifestyle factors and is a promising future direction that should be evaluated to improve precision medicine.
3.8. Safety and Immune-Related Adverse Events
As the aim of adjuvant treatment is ideally to prolong OS, the severity and chronicity of adverse effects should be weighed against the alternative option of surveillance/observation. The characterization of immune-related adverse events (irAEs) in key clinical trials of adjuvant ICIs is detailed in Table 3. A meta-analysis that included a safety analysis of five randomized controlled trials (RCTs) of adjuvant PD1/PDL1 inhibitors comprising 3603 patients identified fatigue (risk ratio (RR) = 1.22; 95% CI 1.01–1.49, p = 0.04), nausea (RR = 1.47; 95% CI 1.11–1.94, p = 0.007), and pruritus (RR = 1.96; 95% CI 1.57–2.44, p < 0.00001) as the most common adverse events. Notably, the incidence of diarrhea was not found to be significantly higher with adjuvant ICI [203]. Another multicenter cohort study of 387 patients with stage III to IV melanomas sought to characterize chronic irAEs, defined as those that persisted beyond 12 weeks of anti–PD-1 discontinuation, following adjuvant treatment with anti–PD-1 for advanced melanoma.(101) In this cohort, chronic irAEs occurred in 167 (43.2%) patients, but the vast majority (96.4%) were grade 1 or 2. Only 24 (14.4%) of these resolved during the median 529-day follow-up. Certain side effects, such as endocrinopathies (73 of 88; 83.0%), arthritis (22 of 45; 48.9%), xerostomia (9 of 17; 52.9%), neurotoxicities (8 of 8; 100%), and ocular events (5 of 8; 62.5%) showed a proclivity to become chronic. On the other hand, irAEs involving visceral organs such as colitis, pneumonitis, and hepatitis had much lower rates of becoming chronic, which could potentially be explained by the tendency to discontinue treatment for these potentially life-threatening adverse effects as compared with endocrinopathies and xerostomia. Administration of glucocorticoids for an acute episode did not show an association with chronicity [204]. Studies have shown that irAEs predominantly occur within the first 3–6 months after the first dose of ICIs [205]. In this cohort, 59 of 167 (35.3%) irAEs developed more than 180 days after starting therapy [206]. Considering the short time to response with durable responses obtained after 2–4 months of treatment in the metastatic setting, exploring shorter durations of adjuvant therapy would be worthwhile [207]. Still, the risk of delayed irAEs despite discontinuation of ICI persists as development of irAEs has been reported up to 26 months after stopping PD-1 inhibitors [208].

Table 3.
Summary of immune-related adverse events of ICIs used in the adjuvant setting.
A retrospective chart review of 161 adult patients with melanoma treated with at least 1 cycle of ICI in the adjuvant or metastatic setting showed that 41% of patients developed permanent irAEs and 9.3% experienced long-term irAEs that resolved over a period longer than 6 months. Permanent irAEs occurred almost twice as much in patients treated with combination immunotherapy (65.6%) than in patients treated with monotherapy (34.9%). The most common permanent irAEs were endocrinopathies (35.5%) or cutaneous toxicities (32.7%) [209]. Fortuitously, endocrine and skin-related toxicities in NSCLC and melanoma are associated with better response rates, PFS, and OS, but this association needs additional validation in other disease states [210]. It is important to note that severity of the irAE does not necessarily correlate with the magnitude of response [210,211,212].
A cross-sectional study in which physicians and nurses were surveyed on factors that they take into consideration regarding selection of adjuvant immunotherapy for melanoma revealed that the patient’s age, performance status, and ability to promptly report toxicities were among the key factors. Indeed, a thorough discussion of the risks and uncertainty of benefits should precede treatment decision [57]. Given the potentially life-altering impact of irAEs, prevention of irAEs through identification of predictive biomarkers, such as HLA genes, autoimmune panels, and composition of the gut microbiome, has become a particularly attractive research topic that would allow risk-stratification and inform decision-making [213].
3.9. Economic Considerations
The bulk of adjuvant immunotherapy trials available in the literature have provided therapy for one year but have limited rationale for the duration specified in the design. However, the significant financial and economic impact associated with adjuvant immunotherapy in the real world warrants closer scrutiny of the optimal duration necessary for clinical benefit.
Patients’ preferences for adjuvant immunotherapy across varying levels of attributes, such as the chance of 3-year melanoma recurrence, mild, permanent, or fatal AE, drug regimen, and out-of-pocket costs were assessed in a discrete choice experiment (DCE), which was conducted in Australia. The results of this experiment showed that patients preferred adjuvant immunotherapy over surveillance in 70% of scenarios, including reduced probabilities of recurrence (OR 0.76, 95% CI 0.70–0.83, p < 0.001), fatal adverse events (AE) (OR 0.60, 95% CI 0.44–0.80, p = 0.006), permanent AE (OR 0.94, 95% CI 0.89–0.99, p = 0.046), and lowered out-of-pocket costs ((OR 0.63, 95% CI 0.47–0.85, p = 0.003. for those with lower incomes); (OR 0.84, 95% CI 0.15–4.86, p = 0.064, for those with higher incomes)). An increase in the risk of mild AE up to 37% was deemed an acceptable trade-off by the patients [214]. This study emphasizes the necessity to involve patients in decision-making by disclosing the risks and benefits; moreover, it highlights the need for non-inferiority clinical trials to explore shorter durations of adjuvant therapy and potential biomarkers to maximize benefit while minimizing risks. A 2018 budget impact analysis estimated that the cost per patient of 1 year of treatment with nivolumab for melanoma is $165,000. The authors estimated that the annual cost for adjuvant treatment with nivolumab for melanoma is approximately $1.15 billion for the entire eligible patient population in the United States [215]. For patients receiving adjuvant ICI for curative intent, ascertainment of OS benefit is essential to justify the substantial costs associated with extended therapy duration in lieu of surveillance.
4. Conclusions
A thorough review of the available literature on the utilization of immunotherapy in the adjuvant setting highlights significant DFS benefit in various disease states such as melanoma, UC, RCC, NSCLC, and esophageal and GEJ cancers. The clinical efficacy of adjuvant ICI in patients with these cancer types encourages research focused on utilization across different malignancies. Preliminary evidence also suggests immunotherapy may be used following liver transplant for HCC in highly controlled settings; however, larger studies are needed to identify the ideal conditions for ICI use to mitigate the risk of rejection. In addition, the utility of blood-based biomarkers, such as ctDNA and NLR, as predictive tools to identify the subset of patients who would derive the most benefit from adjuvant immunotherapy, is an area for future exploration. From a safety perspective, further assessment of irreversible irAEs will be important in the risk–benefit determination of adjuvant immunotherapy. Moreover, additional prospective studies aimed at elucidating the OS benefit of extended duration of adjuvant ICI therapy will enable clinicians strike a better balance of efficacy and toxicity. On the whole, the benefits of adjuvant immunotherapy, as well as the risks of chronic irAEs, should routinely be integrated into patient counseling and treatment decision-making.
Author Contributions
K.A. conceptualized and drafted the article. G.U., A.E. and M.A. reviewed and edited the content. K.A., G.U., A.E., A.A., N.M., H.O.A.-S., M.J., A.S., A.A.C., S.K., R.M.G. and M.A. did the critical review and approved the manuscript before submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to provide and express our deepest appreciation and gratitude to The Cockrell Center for Advanced Therapeutics, The William and Ella Owens Medical Research Foundation, and Houston Methodist Hospital Foundation for their support.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Yaghmour, G.; Pandey, M.; Ireland, C.; Patel, K.; Nunnery, S.; Powell, D.; Baum, S.; Wiedower, E.; Schwartzberg, L.S.; Martin, M.G. Role of Genomic Instability in Immunotherapy with Checkpoint Inhibitors. Anticancer Res. 2016, 36, 4033–4038. [Google Scholar] [CrossRef]
- Remon, J.; Besse, B. Adjuvant immunotherapy for NSCLC—Does treating earlier mean treating better? Nat. Rev. Clin. Oncol. 2022, 19, 7–8. [Google Scholar] [CrossRef]
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef]
- Sundar, R.; Smyth, E.C.; Peng, S.; Yeong, J.P.S.; Tan, P. Predictive Biomarkers of Immune Checkpoint Inhibition in Gastroesophageal Cancers. Front. Oncol. 2020, 10, 763. [Google Scholar] [CrossRef]
- Stenzinger, A.; Allen, J.D.; Maas, J.; Stewart, M.D.; Merino, D.M.; Wempe, M.M.; Dietel, M. Tumor mutational burden standardization initiatives: Recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer 2019, 58, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021, 184, 596–614.e514. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Wang, J.; Nadeem, Z.; Patel, N.; Postow, M.A.; Shoushtari, A.N.; Plitas, G.; Balachandran, V.P.; et al. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat. Genet. 2021, 53, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non–Small Cell Lung Cancers With Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef]
- Coleman, S.; Xie, M.; Tarhini, A.A.; Tan, A.C. Systematic evaluation of the predictive gene expression signatures of immune checkpoint inhibitors in metastatic melanoma. Mol. Carcinog. 2023, 62, 77–89. [Google Scholar] [CrossRef]
- Ferrándiz-Pulido, C.; Leiter, U.; Harwood, C.; Proby, C.M.; Guthoff, M.; Scheel, C.H.; Westhoff, T.H.; Bavinck, J.N.B.; Meyer, T.; Nägeli, M.C. Immune Checkpoint Inhibitors in Solid Organ Transplant Recipients With Advanced Skin Cancers—Emerging Strategies for Clinical Management. Transplantation 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Chen, Y.; Anandhan, S.; Szabo, P.M.; Basu, S.; Blando, J.M.; Liu, W.; Zhang, J.; Natarajan, S.; Xiong, L.; et al. Combinatorial biomarkers to predict responses to immune checkpoint therapy in metastatic urothelial cancer. J. Clin. Oncol. 2021, 39, 488. [Google Scholar] [CrossRef]
- Goswami, S.; Chen, Y.; Anandhan, S.; Szabo, P.M.; Basu, S.; Blando, J.M.; Liu, W.; Zhang, J.; Natarajan, S.M.; Xiong, L.; et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020, 12, eabc4220. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.A.; Hayward, N.K.; Pritchard, A.L. Immune signatures in cutaneous melanoma correlate with survival independently of immunotherapy treatment. Pigment. Cell Melanoma Res. 2023, 36, 246–251. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e2073. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.M.; Kirkwood, J.M.; Marshall, J.L.; Pritchard, C.C.; Azad, N.S.; Gulley, J.L. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother. Cancer 2018, 6, 35. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Pagès, F.; Marincola, F.M.; Thurin, M.; Trinchieri, G.; Fox, B.A.; Gajewski, T.F.; Ascierto, P.A. The immune score as a new possible approach for the classification of cancer. J. Transl. Med. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Velichinskii, R.; Lesh, R.W.; Ali, U.; Kubiak, M.; Bansal, P.; Borghaei, H.; Edelman, M.J.; Boumber, Y. Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors. Adv. Ther. 2019, 36, 2638–2678. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Federico, L.; McGrail, D.J.; Bentebibel, S.E.; Haymaker, C.; Ravelli, A.; Forget, M.A.; Karpinets, T.; Jiang, P.; Reuben, A.; Negrao, M.V.; et al. Distinct tumor-infiltrating lymphocyte landscapes are associated with clinical outcomes in localized non-small-cell lung cancer. Ann. Oncol. 2022, 33, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Franz, L.; Alessandrini, L.; Fasanaro, E.; Gaudioso, P.; Carli, A.; Nicolai, P.; Marioni, G. Prognostic impact of neutrophils-to-lymphocytes ratio (NLR), PD-L1 expression, and tumor immune microenvironment in laryngeal cancer. Ann. Diagn. Pathol. 2021, 50, 151657. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Ameratunga, M.; Chénard-Poirier, M.; Moreno Candilejo, I.; Pedregal, M.; Lui, A.; Dolling, D.; Aversa, C.; Ingles Garces, A.; Ang, J.E.; Banerji, U.; et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur. J. Cancer 2018, 89, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Spakowicz, D.; Burkart, J.; Patel, S.; Husain, M.; He, K.; Bertino, E.M.; Shields, P.G.; Carbone, D.P.; Verschraegen, C.F.; et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J. Cancer Res. Clin. Oncol. 2019, 145, 2541–2546. [Google Scholar] [CrossRef]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef]
- Bryant, A.K.; Sankar, K.; Strohbehn, G.W.; Zhao, L.; Elliott, D.; Qin, A.; Yentz, S.; Ramnath, N.; Green, M.D. Prognostic and predictive value of neutrophil-to-lymphocyte ratio with adjuvant immunotherapy in stage III non-small-cell lung cancer. Lung Cancer 2022, 163, 35–41. [Google Scholar] [CrossRef]
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current Status of Circulating Tumor DNA Liquid Biopsy in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 7651. [Google Scholar] [CrossRef]
- Lo, Y.M. Fetal DNA in maternal plasma: Biology and diagnostic applications. Clin. Chem. 2000, 46, 1903–1906. [Google Scholar] [CrossRef]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Mandel, P.; Metais, P. Nuclear Acids In Human Blood Plasma. C R Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar]
- Sorenson, G.D.; Pribish, D.M.; Valone, F.H.; Memoli, V.A.; Bzik, D.J.; Yao, S.L. Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol. Biomark. Prev. 1994, 3, 67–71. [Google Scholar]
- Tan, E.M.; Schur, P.H.; Carr, R.I.; Kunkel, H.G. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J. Clin. Investig. 1966, 45, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Vasioukhin, V.; Anker, P.; Maurice, P.; Lyautey, J.; Lederrey, C.; Stroun, M. Point mutations of the N-ras gene in the blood plasma DNA of patients with myelodysplastic syndrome or acute myelogenous leukaemia. Br. J. Haematol. 1994, 86, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C. Cell-free DNA (cfDNA): Clinical Significance and Utility in Cancer Shaped by Emerging Technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef]
- Reddy, T.; Esmail, A.; Chang, J.C.; Ghobrial, R.M.; Abdelrahim, M. Utility of Cell-Free DNA Detection in Transplant Oncology. Cancers 2022, 14, 743. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, P.A.; Wind, T.T.; van Kruchten, M.; Schuuring, E.; Hospers, G.A.P.; van der Wekken, A.J.; de Groot, D.J.; Schröder, C.P.; Fehrmann, R.S.N.; Reyners, A.K.L. Clinical utility of circulating tumor DNA as a response and follow-up marker in cancer therapy. Cancer Metastasis. Rev. 2020, 39, 999–1013. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Liu, W.; Chen, C.; Zhang, T.; Ma, Y.; Cui, L.; Gu, Y.; Bei, T.; Zhao, X.; Zhang, B.; et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl. Lung Cancer Res. 2022, 11, 263–276. [Google Scholar] [CrossRef]
- Abbosh, C.; Frankell, A.M.; Garnett, A.T.; Harrison, T.; Weichert, M.; Licon, A.; Veeriah, S.; Daber, B.; Moreau, M.; Chesh, A.S.; et al. Abstract CT023: Phylogenetic tracking and minimal residual disease detection using ctDNA in early-stage NSCLC: A lung TRACERx study. Cancer Res. 2020, 80, CT023. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Birkenkamp-Demtröder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.; Wu, H.T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V.; et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J. Clin. Oncol. 2019, 37, 1547–1557. [Google Scholar] [CrossRef]
- Tan, L.; Sandhu, S.; Lee, R.J.; Li, J.; Callahan, J.; Ftouni, S.; Dhomen, N.; Middlehurst, P.; Wallace, A.; Raleigh, J.; et al. Prediction and monitoring of relapse in stage III melanoma using circulating tumor DNA. Ann. Oncol. 2019, 30, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, Y.; Lam, V.K.; Shi, Y.; Guan, Y.; Zhang, Y.; Ji, L.; Chen, Y.; Zhao, Y.; Qian, F.; et al. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis. 2020, 11, 346. [Google Scholar] [CrossRef]
- Garcia-Murillas, I.; Schiavon, G.; Weigelt, B.; Ng, C.; Hrebien, S.; Cutts, R.J.; Cheang, M.; Osin, P.; Nerurkar, A.; Kozarewa, I.; et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 2015, 7, 302ra133. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E.; et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021, 595, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Feng, J.; Weng, Y.; Xu, Z.; Jin, Y.; Wang, P.; Cui, X.; Ruan, P.; Luo, R.; Li, N.; et al. The Prognostic Value of ctDNA and bTMB on Immune Checkpoint Inhibitors in Human Cancer. Front. Oncol. 2021, 11, 706910. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Wang, G.; He, X.; Mi, Y.; Cao, Y.; Yu, X. Predictive Efficacy of Blood-Based Tumor Mutation Burden Assay for Immune Checkpoint Inhibitors Therapy in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 795933. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, Z.-J.; Zhang, K.; Li, B.; Cai, Y.-R.; Wen, F.-C.; Zhang, D.; Bai, Y.-Z.; Zhang, X.-Y.; Wang, S.-Y.; et al. ctDNA-adjusted bTMB as a predictive biomarker for patients with NSCLC treated with PD-(L)1 inhibitors. BMC Med. 2022, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Guan, J.; Xu, J.; Al-Rawi, H.; Parks, B.; Saadi, N.A.; Khan, T.; Madoux, L.; Taha, M.; Abdelrahim, M. Prognostic value of molecular response via ctDNA measurement in predicating response of systemic therapy in patients with advanced solid cancer. J. Clin. Oncol. 2022, 40, e13001. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Katz, T.A.; Sharma, S.; Kalashnikova, E.; Malhotra, M.; Olshan, P.; Billings, P.R.; Aleshin, A. Early Relapse Detection and Monitoring Disease Status in Patients with Early-stage Pancreatic Adenocarcinoma using Circulating Tumor DNA. J. Surg. Res. 2021, 4, 602–615. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; McMillan, R.; He, A.R.; Starr, J.S.; Dhani, H.; Aushev, V.N.; Malashevich, A.K.; Rattigan, N.H.; et al. Feasibility of disease recurrence monitoring in liver post-transplantation for patients with hepatocellular carcinoma via personalized and tumor-informed ctDNA test. J. Clin. Oncol. 2022, 40, e16123. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; He, A.; Dhani, H.; Aushev, V.; Jurdi, A.; Starr, J.; Gauthier, P. P-165 The feasibility of tumor recurrence detection in liver post-transplantation for patients with hepatocellular carcinoma via personalized, tumor-informed ctDNA testing. Ann. Oncol. 2022, 33, S308–S309. [Google Scholar] [CrossRef]
- Livingstone, A.; Dempsey, K.; Stockler, M.R.; Howard, K.; Long, G.V.; Carlino, M.S.; Menzies, A.M.; Morton, R.L. Adjuvant immunotherapy recommendations for stage III melanoma: Physician and nurse interviews. BMC Cancer 2021, 21, 1014. [Google Scholar] [CrossRef]
- Thomas, D.; Bello, D.M. Adjuvant immunotherapy for melanoma. J. Surg. Oncol. 2021, 123, 789–797. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015, 16, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur. J. Cancer 2019, 119, 1–10. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Lee, S.J.; Hodi, F.S.; Rao, U.N.M.; Cohen, G.I.; Hamid, O.; Hutchins, L.F.; Sosman, J.A.; Kluger, H.M.; Eroglu, Z.; et al. Phase III Study of Adjuvant Ipilimumab (3 or 10 mg/kg) Versus High-Dose Interferon Alfa-2b for Resected High-Risk Melanoma: North American Intergroup E1609. J. Clin. Oncol. 2020, 38, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.L.; Freidlin, B.; Abrams, J.S. Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J. Clin. Oncol. 2011, 29, 2439–2442. [Google Scholar] [CrossRef]
- Betof Warner, A.; Palmer, J.S.; Shoushtari, A.N.; Goldman, D.A.; Panageas, K.S.; Hayes, S.A.; Bajwa, R.; Momtaz, P.; Callahan, M.K.; Wolchok, J.D.; et al. Long-Term Outcomes and Responses to Retreatment in Patients With Melanoma Treated With PD-1 Blockade. J. Clin. Oncol. 2020, 38, 1655–1663. [Google Scholar] [CrossRef]
- Weber, J.S.; Ascierto, P.A.; Middleton, M.R.; Hennicken, D.; Zoffoli, R.; Pieters, A.; Amadi, A.; Kupas, K.; Kotapati, S.; Moshyk, A.; et al. Indirect treatment comparison of nivolumab versus placebo as adjuvant treatment for resected melanoma. Eur. J. Cancer 2021, 158, 225–233. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, E.; Zimmer, L.; Hassel, J.C.; Fluck, M.; Eigentler, T.K.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): Final results of a randomised, double-blind, phase 2 trial. Lancet 2022, 400, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Schadendorf, D.; Del Vecchio, M.; Larkin, J.; Atkinson, V.; Schenker, M.; Pigozzo, J.; Gogas, H.; Dalle, S.; Meyer, N.; et al. Adjuvant Therapy of Nivolumab Combined With Ipilimumab Versus Nivolumab Alone in Patients With Resected Stage IIIB-D or Stage IV Melanoma (CheckMate 915). J. Clin. Oncol. 2023, 41, 517–527. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef]
- Grossmann, K.F.; Othus, M.; Patel, S.P.; Tarhini, A.A.; Sondak, V.K.; Petrella, T.M.; Truong, T.-G.; Khushalani, N.I.; Cohen, J.V.; Buchbinder, E.I.; et al. Final analysis of overall survival (OS) and relapse-free-survival (RFS) in the intergroup S1404 phase III randomized trial comparing either high-dose interferon (HDI) or ipilimumab to pembrolizumab in patients with high-risk resected melanoma. J. Clin. Oncol. 2021, 39, 9501. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Othus, M.; Prieto, V.; Lowe, M.; Buchbinder, E.; Chen, Y.M.; Hyngstrom, J.; Lao, C.D.; Truong, T.-G.; Chandra, S.; et al. LBA6—Neoadjvuant versus Adjuvant Pembrolizumab for Resected Stage III–IV Melanoma (SWOG S1801). Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress/neoadjvuant-versus-adjuvant-pembrolizumab-for-resected-stage-iii-iv-melanoma-swog-s1801 (accessed on 19 September 2022).
- Lucas, M.W.; Lijnsvelt, J.; Pulleman, S.; Scolyer, R.A.; Menzies, A.M.; Van Akkooi, A.C.J.; van Houdt, W.J.; Shannon, K.F.; Pennington, T.; Suijkerbuijk, K.; et al. The NADINA trial: A multicenter, randomised, phase 3 trial comparing the efficacy of neoadjuvant ipilimumab plus nivolumab with standard adjuvant nivolumab in macroscopic resectable stage III melanoma. J. Clin. Oncol. 2022, 40, TPS9605. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Dima, D.; Lopetegui-Lia, N.; Ogbue, O.; Osantowski, B.; Ahmed, R.; Basali, D.; Chamseddine, F.; Eicher, D.M.; Song, J.M.; Gastman, B.; et al. Immunotherapy versus targeted therapy in the adjuvant setting for resected stage III melanoma: A single institution study. J. Clin. Oncol. 2022, 40, e21578. [Google Scholar] [CrossRef]
- Taylor, A.M.; Galea, C.; Lo, S.N.; Dimitriou, F.; Jacques, S.; Allayous, C.; Yeoh, H.-L.; Ressler, J.M.; Kähler, K.C.; Festino, L.; et al. Efficacy and safety of “second adjuvant” therapy with BRAF/MEK inhibitors after resection of recurrent melanoma following adjuvant PD-1–based immunotherapy. J. Clin. Oncol. 2022, 40, 9575. [Google Scholar] [CrossRef]
- Yushak, M.; Mehnert, J.; Luke, J.; Poklepovic, A. Approaches to High-Risk Resected Stage II and III Melanoma. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e207–e211. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Long, G.V.; Luke, J.J.; Khattak, M.A.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): Distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Khattak, M.A.; Luke, J.J.; Long, G.V.; Ascierto, P.A.; Rutkowski, P.; Schadendorf, D.; Robert, C.; Grob, J.J.; de la Cruz Merino, L.; Del Vecchio, M.; et al. Adjuvant pembrolizumab versus placebo in resected high-risk stage II melanoma: Health-related quality of life from the randomized phase 3 KEYNOTE-716 study. Eur. J. Cancer 2022, 176, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H. PD10-01 disease-free survival with longer follow-up from the checkmate 274 trial of adjuvant nivolumab in patients after surgery for high-risk muscle-invasive urothelial carcinoma. J. Urol. 2022, 207, e183. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Mollica, V.; Rizzo, A.; Massari, F. Adjuvant Nivolumab in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 385, 956–957. [Google Scholar] [CrossRef]
- Duan, J.; Cui, L.; Zhao, X.; Bai, H.; Cai, S.; Wang, G.; Zhao, Z.; Zhao, J.; Chen, S.; Song, J.; et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kassouf, W.; Crabb, S.J.; Duran, I.; Brundage, M.D.; Reaume, M.N.; Dragomir, A.; Wyatt, A.W.; Badillo, F.E.V.; Lukka, H.; Niazi, T.; et al. CCTG BL13 a randomized phase II trial assessing trimodality therapy with or without adjuvant durvalumab to treat patients with muscle-invasive bladder cancer (NCT03768570). J. Clin. Oncol. 2022, 40, TPS4619. [Google Scholar] [CrossRef]
- Nardone, V.; Pastina, P.; Giannicola, R.; Agostino, R.; Croci, S.; Tini, P.; Pirtoli, L.; Giordano, A.; Tagliaferri, P.; Correale, P. How to Increase the Efficacy of Immunotherapy in NSCLC and HNSCC: Role of Radiation Therapy, Chemotherapy, and Other Strategies. Front. Immunol. 2018, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, J.; Kusaka, E.; Miyamoto, D.T. Immunotherapy Combined With Radiation Therapy for Genitourinary Malignancies. Front. Oncol. 2021, 11, 663852. [Google Scholar] [CrossRef]
- Janowitz, T.; Welsh, S.J.; Zaki, K.; Mulders, P.; Eisen, T. Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin. Oncol. 2013, 40, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Ravaud, A.; Patard, J.-J.; Pandha, H.S.; George, D.J.; Patel, A.; Chang, Y.-H.; Escudier, B.; Donskov, F.; Magheli, A. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: Subgroup analyses and updated overall survival results. Eur. Urol. 2018, 73, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Firwana, B.; Hilal, T.; Wang, Z.; Almader-Douglas, D.; Joseph, R.W.; Ho, T.H. Adjuvant Antiangiogenic Agents in Post-nephrectomy Renal Cell Carcinoma: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2018, 1, 101–108. [Google Scholar] [CrossRef]
- Porta, C.; Chiellino, S. ASSURE vs. S-TRAC: Conflicting results of adjuvant treatments for kidney cancer in the era of targeted agents and genomics. Ann. Transl. Med. 2016, 4, S14. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef]
- Harshman, L.C.; Xie, W.; Moreira, R.B.; Bossé, D.; Ruiz Ares, G.J.; Sweeney, C.J.; Choueiri, T.K. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: A trial-level meta-analysis. Cancer 2018, 124, 925–933. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R. The Promise of Adjuvant Immunotherapy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef]
- Motzer, R.J.; Russo, P.; Grünwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthélémy, P.; Goh, J.C.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Lancet 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.; Kim, S.E.; Harshman, L.C.; McDermott, D.; Master, V.A.; Signoretti, S.; Cole, S.; Moon, H.; Adra, N.; Singer, E.A.; et al. LBA67—Phase III Randomized Study Comparing Perioperative Nivolumab (Nivo) versus Observation in Patients (Pts) with Renal Cell Carcinoma (RCC) Undergoing Nephrectomy (PROSPER, ECOG-ACRIN EA8143), a National Clinical Trials Network trial. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress/phase-iii-randomized-study-comparing-perioperative-nivolumab-nivo-versus-observation-in-patients-pts-with-renal-cell-carcinoma-rcc-undergoing (accessed on 28 September 2022).
- Bex, A.; Russo, P.; Tomita, Y.; Grünwald, V.; Ramirez, L.-M.; McHenry, B.M.; Motzer, R.J. A phase III, randomized, placebo-controlled trial of nivolumab or nivolumab plus ipilimumab in patients with localized renal cell carcinoma at high-risk of relapse after radical or partial nephrectomy (CheckMate 914). J. Clin. Oncol. 2020, 38, TPS5099. [Google Scholar] [CrossRef]
- Oza, B.; Frangou, E.; Smith, B.; Bryant, H.; Kaplan, R.; Choodari-Oskooei, B.; Powles, T.; Stewart, G.D.; Albiges, L.; Bex, A.; et al. RAMPART: A phase III multi-arm multi-stage trial of adjuvant checkpoint inhibitors in patients with resected primary renal cell carcinoma (RCC) at high or intermediate risk of relapse. Contemp. Clin. Trials 2021, 108, 106482. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Dunant, A.; Pignon, J.P.; Bergman, B.; Chabowski, M.; Grunenwald, D.; Kozlowski, M.; Le Péchoux, C.; Pirker, R.; Pinel, M.I.; et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J. Clin. Oncol. 2010, 28, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.A.; Dahlberg, S.E.; Keller, S.M.; Tester, W.J.; Gandara, D.R.; Graziano, S.L.; Adjei, A.A.; Leighl, N.B.; Aisner, S.C.; Rothman, J.M. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; Naqash, A.R.; Puri, S.; Dienstmann, R. Is It Time to Implement Adjuvant Targeted Therapy in EGFR-Mutant Non-Small-Cell Lung Cancer? JCO Precis. Oncol. 2021, 5, 408–414. [Google Scholar] [CrossRef]
- Zhong, W.Z.; Wang, Q.; Mao, W.M.; Xu, S.T.; Wu, L.; Shen, Y.; Liu, Y.Y.; Chen, C.; Cheng, Y.; Xu, L.; et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open-label, phase 3 study. Lancet Oncol. 2018, 19, 139–148. [Google Scholar] [CrossRef]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst. 2005, 97, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- West, H.; Hu, X.; Burke, T.; Walker, M.; Wang, Y.; Samkari, A. 90P Disease-free survival (DFS) as a predictor of overall survival (OS) in completely resected early stage non-small cell lung cancer (NSCLC). Ann. Oncol. 2022, 33, S74–S75. [Google Scholar] [CrossRef]
- Felip, E.; Vallieres, E.; Zhou, C.; Wakelee, H.; Bondarenko, I.; Sakai, H.; Saito, H.; Ursol, G.; Kawaguchi, K.; Liu, Y. LBA9 IMpower010: Sites of relapse and subsequent therapy from a phase III study of atezolizumab vs best supportive care after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann. Oncol. 2021, 32, S1319. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Approves Pembrolizumab as Adjuvant Treatment for Non-Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-non-small-cell-lung-cancer#:~:text=On%20January%2026%2C%202023%2C%20the,full%20prescribing%20information%20for%20Keytruda (accessed on 15 February 2023).
- Chaft, J.E.; Dahlberg, S.E.; Khullar, O.V.; Edelman, M.J.; Simone, C.B.; Heymach, J.; Rudin, C.M.; Ramalingam, S.S. EA5142 adjuvant nivolumab in resected lung cancers (ANVIL). J. Clin. Oncol. 2018, 36, TPS8581. [Google Scholar] [CrossRef]
- Peters, S.; Spigel, D.; Ahn, M.; Tsuboi, M.; Chaft, J.; Harpole, D.; Goss, G.; Barlesi, F.; Abbosh, C.; Poole, L.; et al. P03.03 MERMAID-1: A Phase III Study of Adjuvant Durvalumab plus Chemotherapy in Resected NSCLC Patients with MRD+ Post-Surgery. J. Thorac. Oncol. 2021, 16, S258–S259. [Google Scholar] [CrossRef]
- Spigel, D.R.; Peters, S.A.E.; Ahn, M.-J.; Tsuboi, M.; Chaft, J.E.; Harpole, D.H.J.; Barlesi, F.; Abbosh, C.; Mann, H.; May, R.; et al. 93TiP MERMAID-2: Phase III study of durvalumab in patients with resected, stage II-III NSCLC who become MRD+ after curative-intent therapy. J. Thorac. Oncol. 2021, 16, S745–S746. [Google Scholar] [CrossRef]
- Bauml, J.M.; Mick, R.; Ciunci, C.; Aggarwal, C.; Davis, C.; Evans, T.; Deshpande, C.; Miller, L.; Patel, P.; Alley, E.; et al. Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non–Small Cell Lung Cancer. JAMA Oncol. 2019, 5, 1283. [Google Scholar] [CrossRef] [PubMed]
- Metro, G.; Signorelli, D. Immune checkpoints inhibitors rechallenge in non-small-cell lung cancer: Different scenarios with different solutions? Lung Cancer Manag. 2020, 8, Lmt18. [Google Scholar] [CrossRef]
- Shah, M.A.; Kennedy, E.B.; Catenacci, D.V.; Deighton, D.C.; Goodman, K.A.; Malhotra, N.K.; Willett, C.; Stiles, B.; Sharma, P.; Tang, L.; et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2677–2694. [Google Scholar] [CrossRef]
- Blum Murphy, M.; Xiao, L.; Patel, V.R.; Maru, D.M.; Correa, A.M.; Amlashi, F.G.; Liao, Z.; Komaki, R.; Lin, S.H.; Skinner, H.D.; et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 2017, 123, 4106–4113. [Google Scholar] [CrossRef] [PubMed]
- Ilson, D.H. Adjuvant Nivolumab in Esophageal Cancer—A New Standard of Care. N. Engl. J. Med. 2021, 384, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Fuchs, C.S.; Ohtsu, A.; Tabernero, J.; Ilson, D.H.; Hyung, W.J.; Strong, V.E.; Goetze, T.O.; Yoshikawa, T.; et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019, 15, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Adjuvant Immunotherapy after Curative Treatment for Hepatocellular Carcinoma. Liver Cancer 2021, 10, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Nakahira, S.; Nishida, N.; Ida, H.; Minami, Y.; Nakai, T.; Wada, H.; Kubo, S.; Ohkawa, K.; et al. Final results of adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): A phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. J. Clin. Oncol. 2022, 40, 416. [Google Scholar] [CrossRef]
- Jimenez Exposito, M.J.; Akce, M.; Alvarez, J.L.M.; Assenat, E.; Balart, L.A.; Baron, A.D.; Decaens, T.; Heurgue-Berlot, A.; Martin, A.O.; Paik, S.W.; et al. CA209-9DX: Phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann. Oncol. 2018, 29, ix65. [Google Scholar] [CrossRef]
- Vogel, A.; Zhu, A.X.; Cheng, A.L.; Yau, T.; Zhou, J.; Kim, E.; Malhotra, U.; Siegel, A.B.; Kudo, M. 1017TiP KEYNOTE-937 trial in progress: Adjuvant pembrolizumab in patients with hepatocellular carcinoma (HCC) and complete radiologic response after surgical resection or local ablation. Ann. Oncol. 2020, 31, S703. [Google Scholar] [CrossRef]
- Hack, S.P.; Zhu, A.X.; Wang, Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 2020, 11, 598877. [Google Scholar] [CrossRef]
- Knox, J.; Cheng, A.; Cleary, S.; Galle, P.; Kokudo, N.; Lencioni, R.; Park, J.; Zhou, J.; Mann, H.; Morgan, S.; et al. A phase 3 study of durvalumab with or without bevacizumab as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after curative hepatic resection or ablation: EMERALD-2. Ann. Oncol. 2019, 30, iv59–iv60. [Google Scholar] [CrossRef]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Hibi, T.; Toso, C.; Man, K.; Berenguer, M.; Heimbach, J.; Greten, T.F.; Pugh, T.J.; Dawson, L.A.; Mazzaferro, V. Transplant Oncology in Primary and Metastatic Liver Tumors: Principles, Evidence, and Opportunities. Ann. Surg. 2021, 273, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Abudayyeh, A.; Murakami, N.; Saharia, A.; McMillan, R.; Victor, D.; Kodali, S.; Shetty, A.; Nolte Fong, J.V.; et al. Transplant Oncology: An Evolving Field in Cancer Care. Cancers 2021, 13, 4911. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Al-Rawi, H.; Esmail, A.; Xu, J.; Umoru, G.; Ibnshamsah, F.; Abudayyeh, A.; Victor, D.; Saharia, A.; McMillan, R.; et al. Gemcitabine and Cisplatin as Neo-Adjuvant for Cholangiocarcinoma Patients Prior to Liver Transplantation: Case-Series. Curr. Oncol. 2022, 29, 3585–3594. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; Abudayyeh, A.; Abdel-Wahab, N.; Diab, A.; Murakami, N.; Kaseb, A.O.; Chang, J.C.; Gaber, A.O. Utilization of Immunotherapy for the Treatment of Hepatocellular Carcinoma in the Peri-Transplant Setting: Transplant Oncology View. Cancers 2022, 14, 1760. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; Kodali, S.; Victor, D.; Heyne, K.; Ghobrial, R. P-161 Trial in progress: Neoadjuvant combination therapy of lenvatinib plus transcatheter arterial chemoembolization (TACE) for transplant-eligible patients with large hepatocellular carcinoma. Ann. Oncol. 2022, 33, S307. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Saharia, A.; McMillan, R.; He, A.R.; Starr, J.; Dhani, H.; Aushev, V.; Malashevich, A.K.; Hook, N. Feasibility of personalized and tumor informed ctdna testing for recurrence monitoring in post-transplantation hepatocellular carcinoma. Transplantation 2022, 106, 161–162. [Google Scholar]
- Abdelrahim, M.; Esmail, A.; Umoru, G.; Westhart, K.; Abudayyeh, A.; Saharia, A.; Ghobrial, R.M. Immunotherapy as a Neoadjuvant Therapy for a Patient with Hepatocellular Carcinoma in the Pretransplant Setting: A Case Report. Curr. Oncol. 2022, 29, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A. P-168 Combination of gemcitabine plus cisplatin compared to non-gemcitabine and cisplatin regimens as neo-adjuvant treatment in liver transplant recipients with cholangiocarcinoma. Ann. Oncol. 2022, 33, S309–S310. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abudayyeh, A.; Victor, D.; McMillan, R.; Kodali, S.; et al. Gemcitabine Plus Cisplatin Versus Non-Gemcitabine and Cisplatin Regimens as Neoadjuvant Treatment for Cholangiocarcinoma Patients Prior to Liver Transplantation: An Institution Experience. Front. Oncol. 2022, 12, 908687. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.; Okane, G.; Esmail, A.; Saharia, A.; Kodali, S.; Victor, D. P-162 Atezolizumab and bevacizumab pre-liver transplantation for patients with hepatocellular carcinoma beyond Milan criteria. Ann. Oncol. 2022, 33, S308. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Victor, D.; Esmail, A.; Kodali, S.; Graviss, E.A.; Nguyen, D.T.; Moore, L.W.; Saharia, A.; McMillan, R.; Fong, J.N. Transarterial Chemoembolization (TACE) plus sorafenib compared to TACE alone in transplant recipients with hepatocellular carcinoma: An institution experience. Cancers 2022, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Kodali, S.; Graviss, E.; Nguyen, D.; Moore, L.; Saharia, A.; Uosef, A.; Victor, D.; Abdelrahim, M. P-163 Tyrosine kinase inhibitors (TKIs) plus transarterial chemoembolization (TACE) compared to TACE alone as downstaging therapy in transplant recipients with hepatocellular carcinoma. Ann. Oncol. 2022, 33, S308. [Google Scholar] [CrossRef]
- Esmail, A.; Victor, D.; Kodali, S.; Graviss, E.A.; Nguyen, D.T.; Moore, L.W.; Saharia, A.; McMillan, R.; Fong, J.N.; Uosef, A. Combination of transarterial chemoembolization (TACE) and tyrosine kinase inhibitors (TKIs) compared to TACE alone as bridging therapy transplant recipients with hepatocellular carcinoma: An update. J. Clin. Oncol. 2022, 40, e16201. [Google Scholar] [CrossRef]
- Esmail, A.; Xu, J.; Umoru, G.; Al-Rawi, H.; Saharia, A.; Abdelrahim, M. P-169 Feasibility of gemcitabine plus cisplatin as neo-adjuvant in cholangiocarcinoma patients prior to liver transplantation. Ann. Oncol. 2022, 33, S310. [Google Scholar] [CrossRef]
- Cho, S.M.; Esmail, A.; Raza, A.; Dacha, S.; Abdelrahim, M. Timeline of FDA-Approved Targeted Therapy for Cholangiocarcinoma. Cancers 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Esmail, A.; Mazzaferro, V.; Abdelrahim, M. Newest Therapies for Cholangiocarcinoma: An Updated Overview of Approved Treatments with Transplant Oncology Vision. Cancers 2022, 14, 5074. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): A randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- Ilyas, F.Z.; Beane, J.D.; Pawlik, T.M. The State of Immunotherapy in Hepatobiliary Cancers. Cells 2021, 10, 2096. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Dao, T.V.; Toni, E.N.D.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Sánchez-Fueyo, A.; Strom, T.B. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 2011, 140, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Huang, C.; Shen, Y.H.; Ji, Y.; Ge, N.L.; Qu, X.D.; Chen, L.; Shi, W.K.; Li, M.L.; Zhu, J.J.; et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer 2021, 10, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhu, X.D.; Shen, Y.H.; Wu, D.; Ji, Y.; Ge, N.L.; Chen, L.L.; Tan, C.J.; Zhou, J.; Fan, J.; et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: A retrospective analysis. Biomark. Res. 2021, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Florman, S.S.; Schwartz, M.E. PD-1 inhibitor as bridge therapy to liver transplantation? Am. J. Transplant. 2021, 21, 1979–1980. [Google Scholar] [CrossRef]
- Aby, E.S.; Lake, J.R. Immune Checkpoint Inhibitor Therapy Before Liver Transplantation-Case and Literature Review. Transplant. Direct. 2022, 8, e1304. [Google Scholar] [CrossRef] [PubMed]
- Schwacha-Eipper, B.; Minciuna, I.; Banz, V.; Dufour, J.F. Immunotherapy as a Downstaging Therapy for Liver Transplantation. Hepatology 2020, 72, 1488–1490. [Google Scholar] [CrossRef]
- Yin, C.; Baba, T.; He, A.R.; Smith, C. Immune checkpoint inhibitors in liver transplant recipients—A review of current literature. Hepatoma Res. 2021, 7, 52. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, X.; Wang, T.; Guo, Y.; Huang, C.; Li, M.; He, X.; Ju, W.; Chen, M. Prognosis after liver transplantation in patients treated with anti- PD-1 immunotherapy for advanced hepatocellular carcinoma: Case series. Ann. Palliat. Med. 2021, 10, 9354–9361. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wanchoo, R.; Riella, L.V.; Uppal, N.N.; Lopez, C.A.; Nair, V.; Devoe, C.; Jhaveri, K.D. Immune Checkpoint Inhibitors in the Cancer Patient with An Organ Transplant. J. Onco-Nephrol. 2017, 1, 42–48. [Google Scholar] [CrossRef]
- Riella, L.V.; Watanabe, T.; Sage, P.T.; Yang, J.; Yeung, M.; Azzi, J.; Vanguri, V.; Chandraker, A.; Sharpe, A.H.; Sayegh, M.H.; et al. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am. J. Transplant. 2011, 11, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Fujino, M.; Jiang, G.; Kitazawa, Y.; Xie, L.; Azuma, M.; Yagita, H.; Nagao, S.; Sugioka, A.; Kurosawa, Y.; et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am. J. Transplant. 2010, 10, 40–46. [Google Scholar] [CrossRef]
- Tanaka, K.; Albin, M.J.; Yuan, X.; Yamaura, K.; Habicht, A.; Murayama, T.; Grimm, M.; Waaga, A.M.; Ueno, T.; Padera, R.F.; et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J. Immunol. 2007, 179, 5204–5210. [Google Scholar] [CrossRef]
- Li, W.; Zheng, X.X.; Kuhr, C.S.; Perkins, J.D. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am. J. Transplant. 2005, 5, 978–986. [Google Scholar] [CrossRef]
- Judge, T.A.; Wu, Z.; Zheng, X.G.; Sharpe, A.H.; Sayegh, M.H.; Turka, L.A. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J. Immunol. 1999, 162, 1947–1951. [Google Scholar] [CrossRef]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef]
- Lee, B.T.; Horwich, B.H.; Chopra, S.; Ahearn, A.; Han, H.H. Checkpoint Inhibitor-Induced Rejection of a Liver Allograft: A Combination of Acute T Cell-Mediated and Antibody-Mediated Rejection. Liver Transpl. 2019, 25, 1845–1848. [Google Scholar] [CrossRef]
- Kumar, S. 2235 Nivolumab-Induced Severe Allograft Rejection in Recurrent Post-Transplant Hepatocellular Carcinoma. Off. J. Am. Coll. Gastroenterol. 2019, 114, S1251. [Google Scholar] [CrossRef]
- Gomez, P.; Naim, A.; Zucker, K.; Wong, M. A Case of Hepatocellular Carcinoma (HCC) Immunotherapy Inducing Liver Transplant Rejection: 2416. Off. J. Am. Coll. Gastroenterol. 2018, 113, S1347. [Google Scholar] [CrossRef]
- Anugwom, C.; Leventhal, T. Nivolumab-Induced Autoimmune-Like Cholestatic Hepatitis in a Liver Transplant Recipient. ACG Case Rep. J. 2020, 7, e00416. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Lewis, D.W.; Nugent, F.W. Preserved Liver Transplant After PD-1 Pathway Inhibitor for Hepatocellular Carcinoma. Am. J. Gastroenterol. 2017, 112, 1895–1896. [Google Scholar] [CrossRef] [PubMed]
- Friend, B.D.; Venick, R.S.; McDiarmid, S.V.; Zhou, X.; Naini, B.; Wang, H.; Farmer, D.G.; Busuttil, R.W.; Federman, N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, A.; Reddy, M.S.; Farouk, M.; Vargese, J.; Rela, M. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: The silver bullet? Hepatology 2018, 67, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Amjad, W.; Kotiah, S.; Gupta, A.; Morris, M.; Liu, L.; Thuluvath, P.J. Successful Treatment of Disseminated Hepatocellular Carcinoma After Liver Transplantation With Nivolumab. J. Clin. Exp. Hepatol. 2020, 10, 185–187. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, T.T.; Salomao, M.A.; Aqel, B.A.; Sonbol, M.B.; Yokoda, R.T.; Ali, A.H.; Moss, A.A.; Mathur, A.K.; Chascsa, D.M.; Rakela, J.; et al. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: The Mayo Clinic experience. J. Gastrointest. Oncol. 2018, 9, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, D.; Weiler, S.; Mertens, J.C.; Reiner, C.S.; Vrugt, B.; Nageli, M.; Mangana, J.; Mullhaupt, B.; Jenni, F.; Misselwitz, B. Liver Allograft Failure After Nivolumab Treatment-A Case Report With Systematic Literature Research. Transplant. Direct. 2018, 4, e376. [Google Scholar] [CrossRef]
- De Toni, E.N.; Gerbes, A.L. Tapering of Immunosuppression and Sustained Treatment With Nivolumab in a Liver Transplant Recipient. Gastroenterology 2017, 152, 1631–1633. [Google Scholar] [CrossRef]
- Al Jarroudi, O.; Ulusakarya, A.; Almohamad, W.; Afqir, S.; Morere, J.F. Anti-Programmed Cell Death Protein 1 (PD-1) Immunotherapy for Metastatic Hepatocellular Carcinoma After Liver Transplantation: A Report of Three Cases. Cureus 2020, 12, e11150. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.C.; Lilly, L.B.; Hogg, D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: A case report and literature review. Melanoma Res. 2018, 28, 61–64. [Google Scholar] [CrossRef]
- Wang, G.; Tang, h.; Zhang, Y.; Li, H.; Yi, S.; Jiang, N.; Wang, G.; Zhang, J.; Zhang, Q.; Yang, Y. Programmed death receptor (PD)-1 monoclonal antibody-induced acute immune hepatitis in the treatment; of recurrent hepatocellular carcinoma after liver transplantation: A case report. Organ Transplant. 2016, 7, 44–47. [Google Scholar]
- Nasr, F.; AlGhoche, A.; Diab, S.; Janah, M.; Layal, M.; Ali, K.; El Karim, G. Pembrolizumab Monother-Apy in Relapsed Hepatocellular Carcinoma Post Living Donor Liver Transplantation and Sorafenib. Int. J. Oncol. Res. 2018, 1, 009. [Google Scholar]
- Pandey, A.; Cohen, D.J. Ipilumumab for hepatocellular cancer in a liver transplant recipient, with durable response, tolerance and without allograft rejection. Immunotherapy 2020, 12, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Au, K.P.; Chok, K.S.H. Immunotherapy after liver transplantation: Where are we now? World J. Gastrointest. Surg. 2021, 13, 1267–1278. [Google Scholar] [CrossRef]
- Schvartsman, G.; Perez, K.; Sood, G.; Katkhuda, R.; Tawbi, H. Immune Checkpoint Inhibitor Therapy in a Liver Transplant Recipient With Melanoma. Ann. Intern. Med. 2017, 167, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, H.A.; Panella, T.J. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J. Immunother. 2015, 38, 211. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Guren, T.K.; Boberg, K.M.; Reims, H.M.; Grzyb, K.; Aamdal, S.; Julsrud, L.; Line, P.D. Acute liver graft rejection after ipilimumab therapy. Ann. Oncol. 2017, 28, 2619–2620. [Google Scholar] [CrossRef] [PubMed]
- Tio, M.; Rai, R.; Ezeoke, O.M.; McQuade, J.L.; Zimmer, L.; Khoo, C.; Park, J.J.; Spain, L.; Turajlic, S.; Ardolino, L.; et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur. J. Cancer 2018, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.E.; Shoushtari, A.N.; Walsh, M.M.; Grewal, P.; Lipson, E.J.; Carvajal, R.D. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J. Immunother. Cancer 2015, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A.; Esteghamat, N.; Kim, E.J.; Garcia, G.; Gong, J.; Fakih, M.G.; Bold, R.J.; Cho, M.T. PD-1 Blockade in a Liver Transplant Recipient With Microsatellite Unstable Metastatic Colorectal Cancer and Hepatic Impairment. J. Natl. Compr. Canc. Netw. 2019, 17, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Biondani, P.; De Martin, E.; Samuel, D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann. Oncol. 2018, 29, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Nowak, J.A.; Hamada, T.; Milner, D.A., Jr.; Nishihara, R. Insights into Pathogenic Interactions Among Environment, Host, and Tumor at the Crossroads of Molecular Pathology and Epidemiology. Annu. Rev. Pathol. 2019, 14, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Orillion, A.; Damayanti, N.P.; Shen, L.; Adelaiye-Ogala, R.; Affronti, H.; Elbanna, M.; Chintala, S.; Ciesielski, M.; Fontana, L.; Kao, C.; et al. Dietary Protein Restriction Reprograms Tumor-Associated Macrophages and Enhances Immunotherapy. Clin. Cancer Res. 2018, 24, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef]
- Inamura, K.; Hamada, T.; Bullman, S.; Ugai, T.; Yachida, S.; Ogino, S. Cancer as microenvironmental, systemic and environmental diseases: Opportunity for transdisciplinary microbiomics science. Gut 2022, 71, 2107. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Jin, Y.; Wei, J.; Weng, Y.; Feng, J.; Xu, Z.; Wang, P.; Cui, X.; Chen, X.; Wang, J.; Peng, M. Adjuvant Therapy With PD1/PDL1 Inhibitors for Human Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 732814. [Google Scholar] [CrossRef]
- Patrinely, J.R., Jr.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Patrinely, J.R., Jr.; Ye, F. Association of Adjuvant Immunotherapy Duration With Chronic Immune-Related Adverse Events—Reply. JAMA Oncol. 2021, 7, 1574–1575. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Owen, C.N.; Bai, X.; Quah, T.; Lo, S.N.; Allayous, C.; Callaghan, S.; Martínez-Vila, C.; Wallace, R.; Bhave, P.; Reijers, I.L.M.; et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann. Oncol. 2021, 32, 917–925. [Google Scholar] [CrossRef]
- Tong, J.; Kartolo, B.A.; Yeung, C.; Hopman, W.M.; Baetz, T.D. Long-term toxicities with immune checkpoint inhibitor (ICI) in melanoma patients. J. Clin. Oncol. 2022, 40, e21549. [Google Scholar] [CrossRef]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef] [PubMed]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Kang, N.; Lee, S.J.; Hodi, F.S.; Cohen, G.I.; Hamid, O.; Hutchins, L.F.; Sosman, J.A.; Kluger, H.M.; Eroglu, Z.; et al. Immune adverse events (irAEs) with adjuvant ipilimumab in melanoma, use of immunosuppressants and association with outcome: ECOG-ACRIN E1609 study analysis. J. Immunother. Cancer 2021, 9, e002535. [Google Scholar] [CrossRef] [PubMed]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Livingstone, A.; Menzies, A.M.; Howard, K.; Stockler, M.R.; Morton, R.L. Preferences for adjuvant immunotherapy in patients with resected stage III melanoma: A discrete choice experiment. J. Clin. Oncol. 2022, 40, 9558. [Google Scholar] [CrossRef]
- Stav, I.; Gyawali, B.; Goldstein, D.A. Duration of adjuvant immunotherapy-biologic, clinical and economic considerations. Med. Oncol. 2018, 35, 160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
