Impact of Infections in Patients Receiving Pembrolizumab-Based Therapies for Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Background
2. Materials/Methods
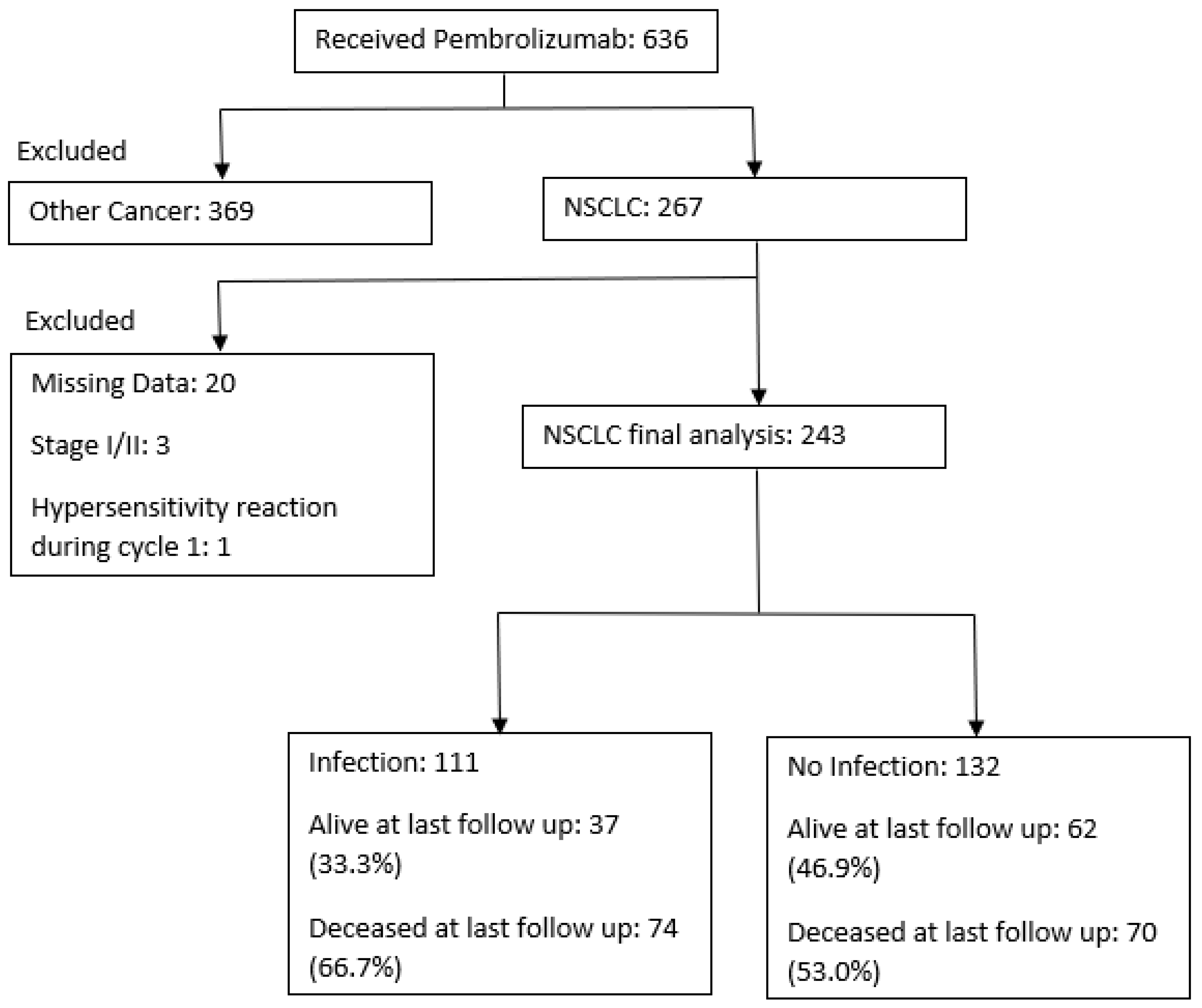
2.1. Study Design
2.2. Data Collection and Outcomes
3. Statistical Analysis and Outcomes
4. Results
4.1. Baseline Factors
4.2. Infections
4.3. Outcomes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSCLC | Non-small cell lung cancer |
| ICI | Immune checkpoint inhibitor |
| irAE | Immune related adverse event |
| ED | Emergency Department |
| IMU | Intermediate Care Unit |
| ICU | Intensive Care Unit |
| OS | Overall survival |
| PFS | Progression free survival |
| OR | Odds ratio |
| CI | Confidence interval |
| ECOG | Eastern Cooperative Oncology Group |
| PCR | Polymerase Chain Reaction |
| SSTI | soft tissue/skin infection |
| UTI | Urinary Tract Infection |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HIV | Human Immunodeficiency |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| ESCMID | European Society of Clinical Microbiology and Infectious Diseases |
| RR | Relative Risk |
| PD-1 | Programmed Death-1 |
References
- Awad, M.M.; Gadgeel, S.M.; Borghaei, H.; Patnaik, A.; Yang, J.C.-H.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Altan, M.; et al. Long-Term Overall Survival From KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin With or Without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Nosaki, K.; Saka, H.; Hosomi, Y.; Baas, P.; de Castro, G., Jr.; Reck, M.; Wu, Y.L.; Brahmer, J.R.; Felip, E.; Sawada, T.; et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Sae-Tia, S.; Naidoo, J.; Mehta, S. Infections in patients receiving immune checkpoint inhibitors. J. Clin. Oncol. 2019, 37 (Suppl. 8), 155. [Google Scholar] [CrossRef]
- Ross, J.A.; Komoda, K.; Pal, S.; Dickter, J.; Salgia, R.; Dadwal, S. Infectious complications of immune checkpoint inhibitors in solid organ malignancies. Cancer Med. 2022, 11, 21–27. [Google Scholar] [CrossRef]
- del Castillo, M.; Romero, F.A.; Argüello, E.; Kyi, C.; Postow, M.A.; Redelman-Sidi, G. The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma. Clin. Infect. Dis. 2016, 63, 1490–1493. [Google Scholar] [CrossRef]
- Petrelli, F.; Morelli, A.M.; Luciani, A.; Ghidini, A.; Solinas, C. Risk of Infection with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. Target Oncol. 2021, 16, 553–568. [Google Scholar] [CrossRef]
- Karam, J.D.; Noel, N.; Voisin, A.L.; Lanoy, E.; Michot, J.M.; Lambotte, O. Infectious complications in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2020, 141, 137–142. [Google Scholar] [CrossRef]
- Kieser, R.B.; Xu, J.; Burns, E.; Muhsen, I.; Shah, S.M.; Umoru, G.; Mylavarapu, C.; Sun, K.; Zhang, Y.; Crenshaw, A.; et al. Outcomes of patients with advanced urothelial cancer who develop infection while on treatment with pembrolizumab. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4573. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Muhsen, I.; Burns, E.; Shah, S.M.; Umoru, G.; Mylavarapu, C.; Sun, K.; Crenshaw, A.; Esmail, A.; et al. Infections and their impact on patients on pembrolizumab-based therapies for head and neck cancer. J. Clin. Oncol. 2022, 40, 6035. [Google Scholar]
- Redelman-Sidi, G.; Michielin, O.; Cervera, C.; Ribi, C.; Aguado, J.M.; Fernández-Ruiz, M.; Manuel, O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors). Clin. Microbiol. Infect. 2018, 24 (Suppl. 2), S95–S107. [Google Scholar] [PubMed]
- Keytruda [Package Insert]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125514s110lbl.pdf (accessed on 27 October 2022).
- Picchi, H.; Mateus, C.; Chouaid, C.; Besse, B.; Marabelle, A.; Michot, J.-M.; Champiat, S.; Voisin, A.; Lambotte, O. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: Reactivation of tuberculosis after anti PD-1 treatment. Clin. Microbiol. Infect. 2018, 24, 216–218. [Google Scholar] [CrossRef]
- Thye, T.; Scarisbrick, G.; Browne, E.N.L.; Chinbuah, M.A.; Gyapong, J.; Osei, I.; Owusu-Dabo, E.; Niemann, S.; Rüsch-Gerdes, S.; Meyer, C.G.; et al. CTLA4 autoimmunity-associated genotype contributes to severe pulmonary tuberculosis in an African population. PLoS ONE 2009, 4, e6307. [Google Scholar] [CrossRef]
- Gámez-Díaz, L.; August, D.; Stepensky, P.; Revel-Vilk, S.; Seidel, M.G.; Noriko, M.; Morio, T.; Worth, A.J.; Blessing, J.; Van de Veerdonk, F.; et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J. Allergy Clin. Immunol. 2016, 137, 223–230. [Google Scholar] [CrossRef]
- Lo, B.; Fritz, J.M.; Su, H.C.; Uzel, G.; Jordan, M.B.; Lenardo, M.J. CHAI and LATAIE: New genetic diseases of CTLA-4 checkpoint insufficiency. Blood 2016, 128, 1037–1042. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Botticelli, A.; Zizzari, I.; Mazzuca, F.; Ascierto, P.A.; Putignani, L.; Marchetti, L.; Napoletano, C.; Nuti, M.; Marchetti, P. Cross-talk between microbiota and immune fitness to steer and control response to anti PD-1/PDL-1 treatment. Oncotarget 2017, 8, 8890–8899. [Google Scholar] [CrossRef]
- Tsikala-Vafea, M.; Belani, N.; Vieira, K.; Khan, H.; Farmakiotis, D. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 106, 142–154. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, N.; Zhou, C.; Tan, G.; Rack, S.; Lorigan, P.; Blackhall, F.; Krebs, M.; Carter, L.; Thistlethwaite, F.; Graham, D.; et al. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Oncologist 2020, 25, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Patel, P.; Poudel, A.; Kafle, S.; Thapa Magar, M.; Cancarevic, I. Influence of Microbiome and Antibiotics on the Efficacy of Immune Checkpoint Inhibitors. Cureus 2021, 13, e16829. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef]
- Giordan, Q.; Salleron, J.; Vallance, C.; Moriana, C.; Clement-Duchene, C. Impact of Antibiotics and Proton Pump Inhibitors on Efficacy and Tolerance of Anti-PD-1 Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 716317. [Google Scholar] [CrossRef]
- Schett, A.; Rothschild, S.I.; Curioni-Fontecedro, A.; Krähenbühl, S.; Früh, M.; Schmid, S.; Driessen, C.; Joerger, M. Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors: Antibiotics immune checkpoint inhibitors in advanced NSCLC. Cancer Chemother. Pharmacol. 2020, 85, 121–131. [Google Scholar] [CrossRef]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Dennehy, C.; Mullally, W.; Goggin, C.; Gleeson, J.P.; Greene, J.P.; Power, D.G.; O’Reilly, S.; Collins, D.C.; O’Mahony, D.; Bambury, R.M. Real world experience with pembrolizumab and nivolumab for treatment of non-small cell lung cancer (NSCLC) in an Irish Regional Cancer Center. J. Clin. Oncol. 2018, 36 (Suppl. 15), e21196. [Google Scholar] [CrossRef]
- Bagley, S.J.; Dhopeshwarkar, N.; Narayan, V.; Meropol, N.J.; Mamtani, R.; Boursi, B. Impact of antibiotics (ABX) on overall survival (OS) in patients (pts) with advanced non-small-cell lung cancer (aNSCLC) and melanoma (aMel) treated with first-line immune checkpoint inhibition (ICI). J. Clin. Oncol. 2019, 37 (Suppl. 15), e20643. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumar, A.; Parikh, K.; Anwar, A.; Knoll, B.M.; Puccio, C.; Chun, H.; Fanucchi, M.; Lim, S.H. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology 2018, 7, e1507670. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wu, C.; Wu, Q.; Luo, S.; Liu, J.; Xie, X. Impact of probiotics use on clinical outcomes of immune checkpoint inhibitors therapy in cancer patients. Cancer Med. 2022. [Google Scholar] [CrossRef] [PubMed]

| Infection | ||||
|---|---|---|---|---|
| Yes | No | Total | p-Value | |
| N = 111 | N = 132 | N = 243 | ||
| Age | 69.12 ± 11.00 | 67.68 ± 11.28 | 68.34 ± 11.15 | 0.32 |
| Female | 59 (53.15%) | 56 (42.42%) | 115 (47.33%) | 0.095 |
| Race | 0.85 | |||
| White | 83 (74.77%) | 97 (73.48%) | 180 (74.07%) | |
| Black | 18 (16.22%) | 20 (15.15%) | 38 (15.64%) | |
| Other Comorbidities | 10 (9.01%) | 15 (11.36%) | 25 (10.29%) | |
| T2DM | 26 (23.42%) | 34 (25.76%) | 60 (24.69%) | 0.67 |
| CAD | 33 (29.73%) | 27 (20.45%) | 60 (24.69%) | 0.095 |
| ESRD | 1 (0.90%) | 0 (0.00%) | 1 (0.41%) | 0.27 |
| COPD | 49 (44.14%) | 38 (28.79%) | 87 (35.80%) | 0.013 |
| IV Drug Use | 0 (0.00%) | 1 (0.76%) | 1 (0.41%) | 0.36 |
| Alcoholism | 5 (4.50%) | 10 (7.58%) | 15 (6.17%) | 0.32 |
| Chronic Indwelling Catheter | 3 (2.70%) | 1 (0.76%) | 4 (1.65%) | 0.24 |
| Smoking Status | 0.30 | |||
| Never | 13 (11.71%) | 21 (15.91%) | 34 (13.99%) | |
| Previous | 81 (72.97%) | 84 (63.64%) | 165 (67.90%) | |
| Current | 17 (15.32%) | 27 (20.45%) | 44 (18.11%) | |
| ECOG | 0.001 | |||
| 0 | 5 (4.50%) | 23 (17.42%) | 28 (11.52%) | |
| 1 | 47 (42.34%) | 67 (50.76%) | 114 (46.91%) | |
| 2 | 23 (20.72%) | 13 (9.85%) | 36 (14.81%) | |
| 3 | 3 (2.70%) | 3 (2.27%) | 6 (2.47%) | |
| 4 | 0 (0.00%) | 1 (0.76%) | 1 (0.41%) | |
| Unknown | 33 (29.73%) | 25 (18.94%) | 58 (23.87%) | |
| Previous ICI therapy | 6 (5.41%) | 8 (6.06%) | 14 (5.76%) | 0.83 |
| History of Chronic Infections | 15 (13.51%) | 7 (5.30%) | 22 (9.05%) | 0.026 |
| NSCLC Subtype | ||||
| Adenocarcinoma | 93 (83.8%) | 101 (76.5%) | 194 (79.8%) | 0.366 |
| Squamous Cell | 15 (13.5%) | 24 (18.2%) | 39 (16.0%) | |
| Other | 3 (2.7%) | 7 (5.3%) | 10 (4.1%) | |
| PD-L1 Activity | 0.78 | |||
| Absent | 39 (35.14%) | 48 (36.36%) | 87 (35.80%) | |
| 1–10% | 13 (11.71%) | 22 (16.67%) | 35 (14.40%) | |
| 11–20% | 6 (5.41%) | 5 (3.79%) | 11 (4.53%) | |
| >20% | 37 (33.33%) | 38 (28.79%) | 75 (30.86%) | |
| Unknown | 16 (14.41%) | 19 (14.39%) | 35 (14.40%) | |
| Therapy | 0.27 | |||
| Monotherapy | 41 (36.94%) | 39 (29.55%) | 80 (32.92%) | |
| Combination with Chemotherapy | 70 (63.06%) | 93 (70.45%) | 163 (67.08%) | |
| Radiation Therapy | 27 (24.3%) | 38 (28.8%) | 65 (26.7%) | 0.469 |
| Surgery | 0.097 | |||
| No Surgery | 90 (81.08%) | 95 (71.97%) | 185 (76.13%) | |
| Primary Surgery | 9 (8.11%) | 23 (17.42%) | 32 (13.17%) | |
| Secondary Surgery | 12 (10.81%) | 14 (10.61%) | 26 (10.70%) | |
| Number of Cycles | ||||
| Total (median) | 5.00 (2.00-13.00) | 8.00(4.00–12.00) | 7.00 (3.00–12.00) | 0.057 |
| Distribution of cycles | 0.067 | |||
| 1 | 20 (18.02%) | 11 (8.33%) | 31 (12.76%) | |
| 2 | 10 (9.01%) | 11 (8.33%) | 21 (8.64%) | |
| ≥3 | 81 (72.97%) | 110 (83.33%) | 191 (78.60%) | |
| Line of Therapy | 0.60 | |||
| 1L | 85 (76.58%) | 93 (70.45%) | 178 (73.25%) | |
| 2L | 21 (18.92%) | 31 (23.48%) | 52 (21.40%) | |
| >2L | 5 (4.50%) | 8 (6.06%) | 13 (5.35%) | |
| Antibiotics at time of ICI initiation | 21 (18.92%) | 8 (6.06%) | 29 (11.93%) | 0.002 |
| GCSF Use | 12 (10.81%) | 16 (12.12%) | 28 (11.52%) | 0.75 |
| 1st Infection | 2nd Infection | 3rd Infection | |
|---|---|---|---|
| Total | 111 | 55 | 18 |
| Bacteremia | 8 (7.2%) | 3 (5.4%) | 0 (0.0%) |
| COVID-19 | 3 (2.7%) | 0 (0.0%) | 0 (0.0%) |
| Head and Neck | 5 (4.5%) | 3 (5.4%) | 0 (0.0%) |
| Intra-abdominal | 6 (5.4%) | 3 (5.4%) | 1 (5.6%) |
| Intracranial | 0 (0.0%) | 1 (1.8%) | 0 (0.0%) |
| Neutropenic Fever | 3 (2.7%) | 0 (0.0%) | 2 (11.1%) |
| Presumed Sepsis | 3 (2.7%) | 3 (5.4%) | 1 (5.6%) |
| Pulmonary | 48 (43.2%) | 28 (50.9%) | 9 (50.0%) |
| SSTI | 15 (13.5%) | 5 (9.1%) | 4 (22.2%) |
| Urinary | 20 (18.0%) | 9 (16.4%) | 1 (5.6%) |
| Type of Pathogen | |||
| Bacterial | 90 (81.1%) | 50 (90.9%) | 15 (83.3%) |
| Virus | 18 (16.2%) | 2 (3.6%) | 2 (11.1%) |
| Fungal | 3 (2.7%) | 3 (5.4%) | 1 (5.6%) |
| Culture Data Available | 51 (45.9%) | 21 (38.2%) | 6 (33.3%) |
| Result of Infection | |||
| ED Visits | 6 (5.4%) | 3 (5.4%) | 0 (0.0%) |
| Inpatient Admission | 78 (70.3%) | 36 (65.4%) | 11 (61.1%) |
| IMU/ICU Admission | 13 (11.7%) | 14 (25.4%) | 6 (33.3%) |
| Outpatient | 14 (12.6%) | 3 (5.4%) | 1 (5.6%) |
| Median time to infection (days) | 58 (0–1465) | 124 (6–844) | 213.5 (25–1414) |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 1.01 (0.99, 1.04) | 0.319 | ||
| Female | 1.54 (0.93, 2.56) | 0.096 | 1.52 (0.86, 2.70) | 0.153 |
| T2DM | 0.88 (0.49, 1.59) | 0.674 | ||
| CAD | 1.65 (0.91, 2.96) | 0.096 | 1.53 (0.79, 2.95) | 0.204 |
| COPD | 1.96 (1.15, 3.33) | 0.013 | 1.59 (0.88, 2.85) | 0.121 |
| Alcoholism | 0.58 (0.19, 1.74) | 0.327 | ||
| Chronic indwelling catheter1y | 3.64 (0.37, 35.49) | 0.266 | ||
| Smoking Status | ||||
| Never | Reference | |||
| Previous | 1.56 (0.73,3.32) | 0.251 | ||
| Current | 1.02 (0.41,2.55) | 0.971 | ||
| ECOG | ||||
| 0 | Reference | Reference | ||
| 1 | 3.23 (1.14, 9.10) | 0.027 | 2.49 (0.86, 7.25) | 0.094 |
| 2 | 8.14 (2.50, 26.55) | 0.001 | 4.85 (1.41, 16.70) | 0.012 |
| 3 | 4.6 (0.71, 29.84) | 0.11 | 1.80 (0.22, 14.79) | 0.583 |
| 4 | Empty | Empty | ||
| Unknown | 6.07 (2.03, 18.20) | 0.001 | 4.52 (1.44, 14.13) | 0.010 |
| Previous ICI therapy | 0.89 (0.3, 2.63) | 0.827 | ||
| Surgery | ||||
| No Surgery | Reference | Reference | ||
| Primary Surgery | 0.41 (0.18, 0.94) | 0.035 | 0.42 (0.17, 1.04) | 0.060 |
| Secondary Surgery | 0.90 (0.40, 2.06) | 0.812 | 1.02 (0.43, 2.43) | 0.966 |
| Immunosuppressive therapy for irAE | 1.87 (1.0–3.51) | 0.057 | ||
| History of Chronic Infections | 2.79 (1.09, 7.11) | 0.032 | 1.74 (0.61, 4.97) | 0.304 |
| Antibiotics/antivirals | 3.62 (1.53, 8.53) | 0.003 | 3.17 (1.16, 8.66) | 0.024 |
| GCSF use | 0.88 (0.4, 1.95) | 0.75 | ||
| Line of therapy | ||||
| 1L | Reference | |||
| 2L | 0.74 (0.40, 1.39) | 0.349 | ||
| >2L | 0.68 (0.22, 2.17) | 0.519 | ||
| Therapy | ||||
| Monotherapy | Reference | |||
| Combination | 0.72 (0.42, 1.22) | 0.223 | ||
| Number of cycles | ||||
| 1 | Reference | Reference | ||
| 2 | 0.5 (0.16–1.55) | 0.23 | 0.44 (0.12, 1.54) | |
| 3 or more | 0.41 (0.18–0.89) | 0.025 | 0.42 (0.17, 1.02) | |
| Infection | ||||
|---|---|---|---|---|
| Yes | No | Total | p-Value | |
| N = 111 | N = 132 | N = 243 | ||
| Immune Related Adverse Events | 33 (29.73%) | 33 (25.00%) | 66 (27.16%) | 0.41 |
| Colitis | 4 (3.60%) | 7 (5.30%) | 11 (4.53%) | 0.53 |
| Adrenalitis | 0 (0.00%) | 1 (0.76%) | 1 (0.41%) | 0.36 |
| Thyroiditis | 8 (7.21%) | 9 (6.82%) | 17 (7.00%) | 0.91 |
| Pneumonitis | 17 (15.32%) | 7 (5.30%) | 24 (9.88%) | 0.009 |
| Dermatitis | 9 (8.11%) | 13 (9.85%) | 22 (9.05%) | 0.64 |
| Hepatitis | 3 (2.70%) | 0 (0.00%) | 3 (1.23%) | 0.057 |
| Other | 8 (7.21%) | 5 (3.79%) | 13 (5.35%) | 0.24 |
| ED Visit | 37 (33.33%) | 26 (19.70%) | 63 (25.93%) | 0.016 |
| Inpatient | 87 (78.38%) | 53 (40.15%) | 140 (57.61%) | <0.001 |
| IMU/ICU admissions | 26 (23.42%) | 5 (3.79%) | 31 (12.76%) | <0.001 |
| Death | 74 (66.67%) | 70 (53.03%) | 144 (59.26%) | 0.031 |
| Cause of Death | 0.041 | |||
| Primary Disease | 49 (66.22%) | 61 (87.14%) | 110 (76.39%) | |
| Infection | 11 (14.86%) | 4 (5.71%) | 15 (10.42%) | |
| Multiorgan Failure | 9 (12.16%) | 3 (4.29%) | 12 (8.33%) | |
| Second Cancer | 1 (1.35%) | 0 (0.00%) | 1 (0.69%) | |
| Other | 4 (5.41%) | 2 (2.86%) | 6 (4.17%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, E.A.; Gee, K.; Kieser, R.B.; Xu, J.; Zhang, Y.; Crenshaw, A.; Muhsen, I.N.; Mylavarapu, C.; Esmail, A.; Shah, S.; et al. Impact of Infections in Patients Receiving Pembrolizumab-Based Therapies for Non-Small Cell Lung Cancer. Cancers 2023, 15, 81. https://doi.org/10.3390/cancers15010081
Burns EA, Gee K, Kieser RB, Xu J, Zhang Y, Crenshaw A, Muhsen IN, Mylavarapu C, Esmail A, Shah S, et al. Impact of Infections in Patients Receiving Pembrolizumab-Based Therapies for Non-Small Cell Lung Cancer. Cancers. 2023; 15(1):81. https://doi.org/10.3390/cancers15010081
Chicago/Turabian StyleBurns, Ethan A., Kelly Gee, Ryan B. Kieser, Jiaqiong Xu, Yuqi Zhang, Aubrey Crenshaw, Ibrahim N. Muhsen, Charisma Mylavarapu, Abdullah Esmail, Shivan Shah, and et al. 2023. "Impact of Infections in Patients Receiving Pembrolizumab-Based Therapies for Non-Small Cell Lung Cancer" Cancers 15, no. 1: 81. https://doi.org/10.3390/cancers15010081
APA StyleBurns, E. A., Gee, K., Kieser, R. B., Xu, J., Zhang, Y., Crenshaw, A., Muhsen, I. N., Mylavarapu, C., Esmail, A., Shah, S., Umoru, G., Sun, K., Guerrero, C., Gong, Z., Heyne, K., Singh, M., Zhang, J., Bernicker, E. H., & Abdelrahim, M. (2023). Impact of Infections in Patients Receiving Pembrolizumab-Based Therapies for Non-Small Cell Lung Cancer. Cancers, 15(1), 81. https://doi.org/10.3390/cancers15010081








