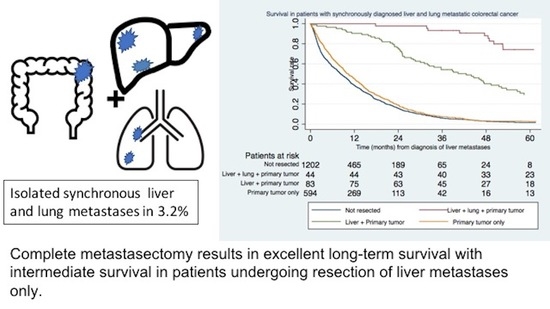
The Resection Rate of Synchronously Detected Liver and Lung Metastasis from Colorectal Cancer Is Low—A National Registry-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
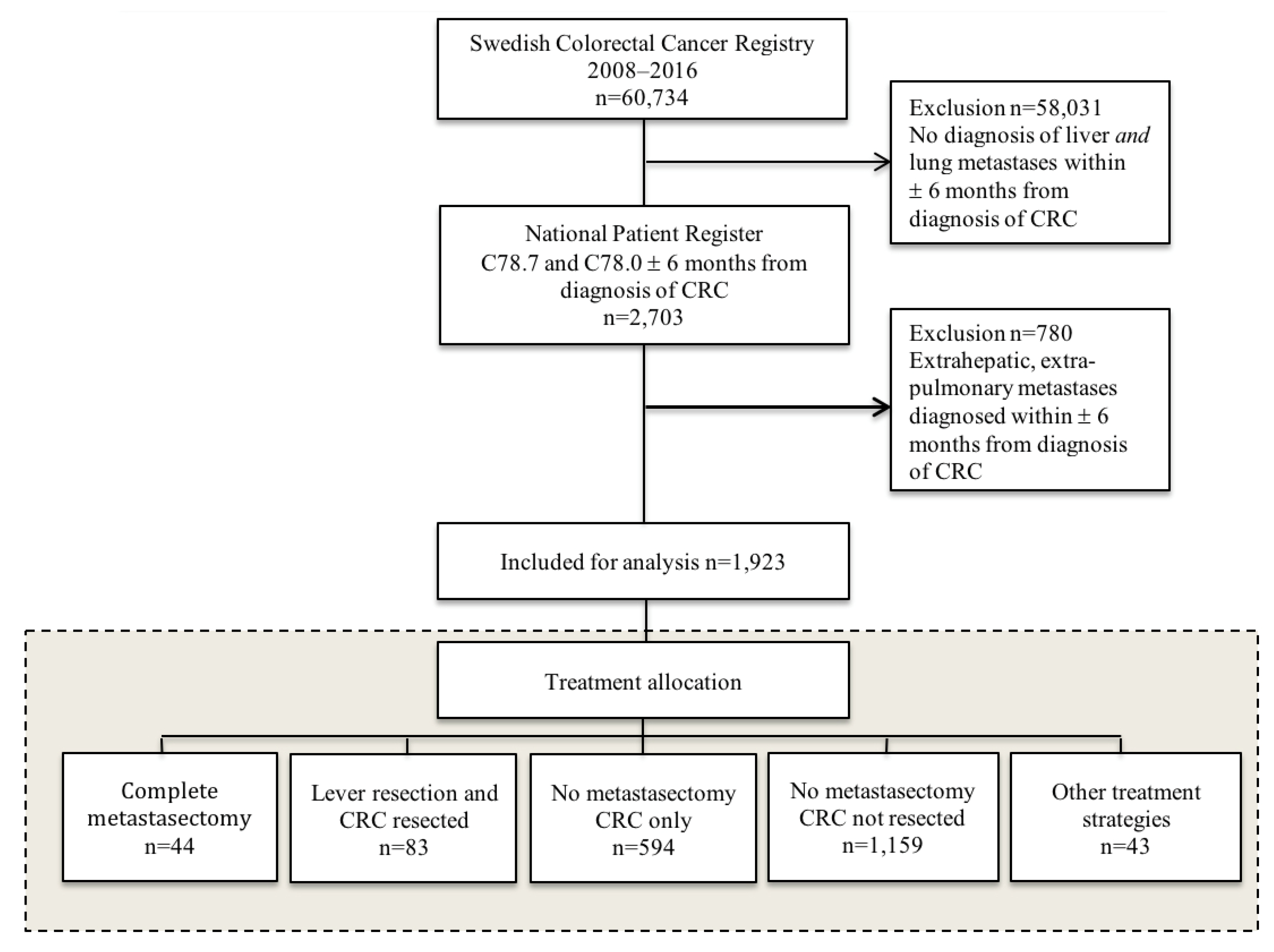
2. Materials and Methods
3. Results
3.1. Participants and Baseline Characteristics
3.2. Treatment of Liver and Lung Metastases
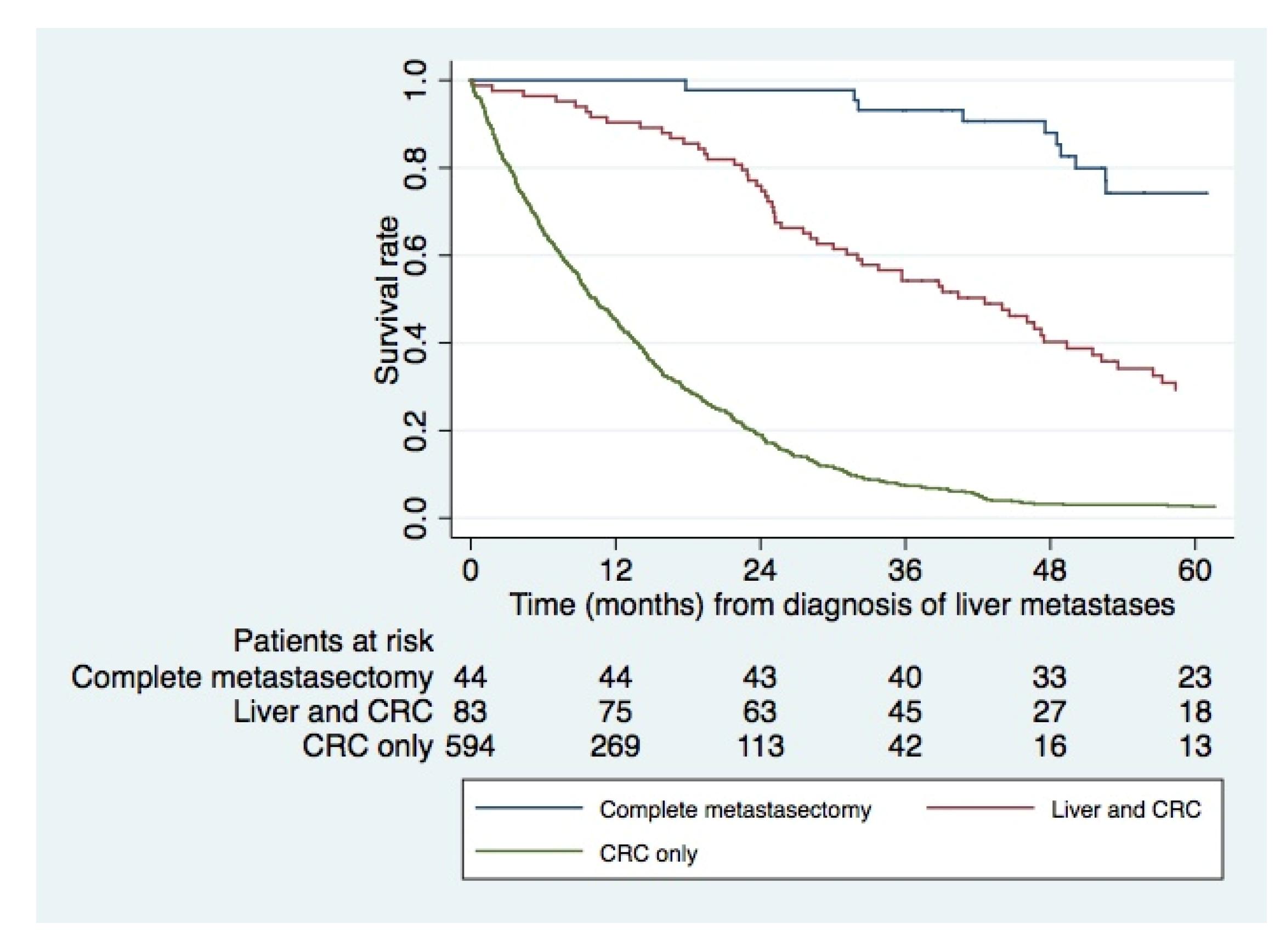
3.3. Survival
3.4. Trend over Time
3.5. Regional Differences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistics on Cancer Incidence. 2017. Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2018-12-51.pdf (accessed on 21 December 2022).
- Van der Geest, L.G.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.; de Wilt, J.H. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases-a population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Handy, J.R.; Bremner, R.M.; Crocenzi, T.S.; Detterbeck, F.C.; Fernando, H.C.; Fidias, P.M.; Firestone, S.; Johnstone, C.A.; Lanuti, M.; Litle, V.R.; et al. Expert consensus document on pulmonary metastasectomy. Ann. Thorac. Surg. 2019, 107, 631–649. [Google Scholar] [CrossRef]
- Dave, R.V.; Pathak, S.; White, A.D.; Hidalgo, E.; Prasad, K.R.; Lodge, J.P.; Milton, R.; Toogood, G.J. Outcome after liver resection in patients presenting with simultaneous hepatopulmonary colorectal metastases. Br. J. Surg. 2015, 102, 261–268. [Google Scholar] [CrossRef]
- Andres, A.; Mentha, G.; Adam, R.; Gerstel, E.; Skipenko, O.G.; Barroso, E.; Lopez-Ben, S.; Hubert, C.; Majno, P.E.; Toso, C. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br. J. Surg. 2015, 102, 691–699. [Google Scholar] [CrossRef]
- Matsumura, M.; Yamashita, S.; Ishizawa, T.; Akamatsu, N.; Kaneko, J.; Arita, J.; Nakajima, J.; Kokudo, N.; Hasegawa, K. Oncological benefit of complete metastasectomy for simultaneous colorectal liver and lung metastases. Am. J. Surg. 2020, 219, 80–87. [Google Scholar] [CrossRef]
- Engstrand, J.; Sterner, J.; Hasselgren, K.; Stromberg, C.; Sturesson, C. Treatment intention and outcome in patients with simultaneously diagnosed liver and lung metastases from colorectal cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2022, 48, 1799–1806. [Google Scholar] [CrossRef]
- Petersson, J.; Bock, D.; Martling, A.; Smedby, K.E.; Angenete, E.; Saraste, D. Increasing incidence of colorectal cancer among the younger population in Sweden. BJS Open 2020, 4, 645–658. [Google Scholar] [CrossRef]
- Moberger, P.; Sköldberg, F.; Birgisson, H. Evaluation of the Swedish colorectal cancer registry: An overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018, 57, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Osterman, E.; Hammarström, K.; Imam, I.; Osterlund, E.; Sjöblom, T.; Glimelius, B. Completeness and accuracy of the registration of recurrences in the Swedish colorectal cancer registry (SCRCR) and an update of recurrence risk in colon cancer. Acta Oncol. 2021, 60, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Cancer i Lever Och Gallvägar, SweLiv. Available online: https://cancercentrum.se/globalassets/cancerdiagnoser/lever-och-galla/kvalitetsregister/sweliv_arsrapport2021.pdf (accessed on 21 December 2022).
- Täckningsgrader för Nationella Kvalitetsregister. 2020. Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2020-12-7049.pdf (accessed on 21 December 2022).
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Abu Hilal, M.; Berardi, G.; Ciria, R.; Abe, Y.; Aoki, T.; Asbun, H.J.; Chan, A.C.Y.; et al. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J. Hepato-Biliary-Pancreat. Sci. 2022, 29, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, V.; Wirta, E.V.; Seppälä, T.; Sihvo, E.; Mecklin, J.P.; Vasala, K.; Kellokumpu, I. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: A population-based study. BJS Open 2020, 4, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Adair, R.; Culverwell, A.; Guthrie, J.A.; Botterill, I.D.; Toogood, G.J.; Lodge, J.P.; Prasad, K.R. Variation in referral practice for patients with colorectal cancer liver metastases. Br. J. Surg. 2013, 100, 1627–1632. [Google Scholar] [CrossRef]
- Wei, A.C.; Sandhu, L.; Devitt, K.S.; Gagliardi, A.R.; Kennedy, E.D.; Urbach, D.R.; Gallinger, S.; Baxter, N.N. Practice patterns for the management of hepatic metastases from colorectal cancer: A mixed methods analysis. Ann. Surg. Oncol. 2013, 20, 1567–1574. [Google Scholar] [CrossRef]
- Homayounfar, K.; Bleckmann, A.; Helms, H.J.; Lordick, F.; Ruschoff, J.; Conradi, L.C.; Sprenger, T.; Ghadimi, M.; Liersch, T. Discrepancies between medical oncologists and surgeons in assessment of resectability and indication for chemotherapy in patients with colorectal liver metastases. Br. J. Surg. 2014, 101, 550–557. [Google Scholar] [CrossRef]
- Krell, R.W.; Reames, B.N.; Hendren, S.; Frankel, T.L.; Pawlik, T.M.; Chung, M.; Kwon, D.; Wong, S.L. Surgical referral for colorectal liver metastases: A population-based survey. Ann. Surg. Oncol. 2015, 22, 2179–2194. [Google Scholar] [CrossRef]
- Isoniemi, H.; Uutela, A.; Nordin, A.; Lantto, E.; Kellokumpu, I.; Ovissi, A.; Kosunen, J.; Kallio, R.; Soveri, L.M.; Salminen, T.; et al. Centralized repeated resectability assessment of patients with colorectal liver metastases during first-line treatment: Prospective study. Br. J. Surg. 2021, 108, 817–825. [Google Scholar] [CrossRef]
- Engstrand, J.; Kartalis, N.; Stromberg, C.; Broberg, M.; Stillstrom, A.; Lekberg, T.; Jonas, E.; Freedman, J.; Nilsson, H. The Impact of a hepatobiliary multidisciplinary team assessment in patients with colorectal cancer liver metastases: A population-based study. Oncol. 2017, 22, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.P.; Vauthey, J.N.; Adam, R.; Rees, M.; Berry, D.; Jackson, R.; Grimes, N.; Fenwick, S.W.; Poston, G.J.; Malik, H.Z. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br. J. Surg. 2012, 99, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.; Galeone, C.; Braglia, L.; Ugoletti, L.; Siciliani, A.; Nachira, D.; Margaritora, S.; Pedrazzoli, C.; Paci, M.; Lococo, F. Long-term outcomes after surgical resection for synchronous or metachronous hepatic and pulmonary colorectal cancer metastases. Digestion 2020, 101, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Heo, J.S.; Park, J.Y.; Choi, D.W.; Choi, S.H. Surgical resection of synchronous and metachronous lung and liver metastases of colorectal cancers. Ann. Surg. Treat. Res. 2017, 92, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Edwards, J.; Tsang, D.; Dunning, J.; Shackcloth, M.; Batchelor, T.; Coonar, A.; Hasan, J.; Davidson, B.; Marchbank, A.; et al. Pulmonary metastasectomy in colorectal cancer: Updated analysis of 93 randomized patients-control survival is much better than previously assumed. Color. Dis. Off. J. Assoc. Coloproctol. 2020, 22, 1314–1324. [Google Scholar] [CrossRef]
- Mise, Y.; Kopetz, S.; Mehran, R.J.; Aloia, T.A.; Conrad, C.; Brudvik, K.W.; Taggart, M.W.; Vauthey, J.N. Is complete liver resection without resection of synchronous lung metastases justified? Ann. Surg. Oncol. 2015, 22, 1585–1592. [Google Scholar] [CrossRef]
- Siebenhüner, A.R.; Güller, U.; Warschkow, R. Population-based SEER analysis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer 2020, 20, 246. [Google Scholar] [CrossRef]
- Hu, C.Y.; Bailey, C.E.; You, Y.N.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Feig, B.W.; Chang, G.J. Time trend analysis of primary tumor resection for stage IV colorectal cancer: Less surgery, improved survival. JAMA Surg. 2015, 150, 245–251. [Google Scholar] [CrossRef]
- Tarantino, I.; Warschkow, R.; Worni, M.; Cerny, T.; Ulrich, A.; Schmied, B.M.; Güller, U. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: A population-based, propensity score-adjusted trend analysis. Ann. Surg. 2015, 262, 112–120. [Google Scholar] [CrossRef]
- Okuno, M.; Kawaguchi, Y.; De Bellis, M.; Vega, E.A.; Huang, S.Y.; Ahrar, K.; Gupta, S.; Vauthey, J.N.; Odisio, B.C. A new sequential treatment strategy for multiple colorectal liver metastases: Planned incomplete resection and postoperative completion ablation for intentionally-untreated tumors under guidance of cross-sectional imaging. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 311–316. [Google Scholar] [CrossRef]
- Takahashi, H.; Berber, E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg. Nutr. 2020, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, P.; Dal, G.; Bottai, M.; Nilsson, H.; Freedman, J.; Engstrand, J. Microwave ablation versus resection for colorectal cancer liver metastases-A propensity score analysis from a population-based nationwide registry. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Nieuwenhuizen, S.; Puijk, R.S.; van den Bemd, B.; Aldrighetti, L.; Arntz, M.; van den Boezem, P.B.; Bruynzeel, A.M.E.; Burgmans, M.C.; de Cobelli, F.; Coolsen, M.M.E.; et al. Resectability and ablatability criteria for the treatment of liver only colorectal metastases: Multidisciplinary consensus document from the COLLISION trial group. Cancers 2020, 12, 1779. [Google Scholar] [CrossRef]
- Fenton, H.M.; Finan, P.J.; Milton, R.; Shackcloth, M.; Taylor, J.C.; Treasure, T.; Morris, E.J.A. National variation in pulmonary metastasectomy for colorectal cancer. Color. Dis. Off. J. Assoc. Coloproctol. 2021, 23, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Noren, A.; Sandstrom, P.; Gunnarsdottir, K.; Ardnor, B.; Isaksson, B.; Lindell, G.; Rizell, M. Identification of inequalities in the selection of liver surgery for colorectal liver metastases in Sweden. Scand. J. Surg. SJS Off. Organ Finn. Surg. Soc. Scand. Surg. Soc. 2018, 107, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Nationellt Vårdprogram Tjock-Och Ändtarmscancer. Available online: https://kunskapsbanken.cancercentrum.se/diagnoser/tjock-och-andtarmscancer/vardprogram/ (accessed on 21 December 2022).

| n = 1923 (%) | |
|---|---|
| Patient characteristics | |
| Gender, n = 1818 | |
| Male/Female | 1024 (56)/794 (44) |
| Age (years), median (IQR) | 70 (14) |
| ASA, n = 671 | |
| 1 | 68 (10) |
| 2 | 323 (48) |
| 3 | 232 (35) |
| 4 | 48 (7) |
| Primary tumor characteristics | |
| Primary tumor location, n = 1912 | |
| Caecum | 206 (11) |
| Ascending colon | 151 (8) |
| Hepatic flexure | 73 (4) |
| Transverse colon | 69 (4) |
| Splenic flexure | 31 (2) |
| Descending colon | 59 (3) |
| Sigmoid colon | 515 (27) |
| Rectum | 808 (42) |
| Resection of primary tumor | 734 (38) |
| Pathological tumor stage, n = 527 | |
| pT0 | 5 (1) |
| pT1 | 4 (1) |
| pT2 | 15 (3) |
| pT3 | 290 (55) |
| pT4 | 213 (40) |
| Pathological nodal stage, n = 506 | |
| pN0 | 95 (19) |
| pN1 | 166 (33) |
| pN2 | 245 (48) |
| Referred for metastasectomy 1 | 275 (14) |
| Liver and Lung Metastasectomy and Resection of Primary, n = 44 | Liver Resection and Resection of Primary Only, n = 83 | Resection of Primary Only, n = 594 | p *,† | |
|---|---|---|---|---|
| Age (IQR) | 62 (13) | 68 (13) | 71 (55) | <0.001 ‡ |
| Gender, female | 21 (48) | 33 (40) | 272 (47) | 0.477 |
| ASA 1 | ||||
| 1 | 11 (25) | 17 (21) | 39 (7) | <0.001 |
| 2 | 22 (50) | 39 (49) | 255 (48) | |
| 3 | 11 (25) | 24 (30) | 193 (36) | |
| 4 | 0 (0) | 0 (0) | 47 (9) | |
| Missing | 0 | 3 | 60 | |
| Primary tumor location 2 | ||||
| Right-sided colon | 4 (9) | 27 (33) | 186 (31) | 0.032 |
| Left-sided | 21 (49) | 28 (34) | 190 (32) | |
| Rectum | 18 (42) | 28 (34) | 217 (37) | |
| Missing | 1 | 0 | 1 | |
| Tumor stage of primary 2 | ||||
| T1–T2 | 4 (9) | 5 (7) | 8 (2) | <0.001 |
| T3 | 30 (70) | 51 (66) | 201 (52) | |
| T4 | 9 (21) | 21 (27) | 181 (46) | |
| Missing | 1 | 6 | 204 | |
| Referral for metastatic surgery 3 | 37 (84) | 53 (64) | 58 (10) | <0.001 |
| Number of liver metastases 1 | N/A | |||
| 1 | 10 (29) | 17 (36) | 0.646 ** | |
| 2–5 | 18 (51) | 24 (50) | ||
| 6–10 | 5 (14) | 3 (6) | ||
| ≥11 | 2 (6) | 4 (8) | ||
| Missing | 9 | 35 | ||
| Liver resection 1 | N/A | |||
| Major hepatectomy | 13 (32) | 28 (37) | 0.303 ** | |
| Minor hepatectomy | 28 (68) | 44 (58) | ||
| Ablation only | 0 | 4 (5) | ||
| Missing | 3 | 7 | ||
| Size of largest liver metastasis, mm (IQR) 1 | 20 (18) | 25 (21) | N/A | 0.016 ‡ |
| Missing | 9 | 12 | ||
| Number of lung metastases (min, max) 4 | 1 (1, 9) | N/A | N/A | |
| Missing | 12 | |||
| Unilateral lung metastases 4 | 32 (100) | N/A | N/A | |
| Missing | 12 |
| Treatment Allocation | n = 1923 |
|---|---|
| Liver + Lung + CRC | 44 (2.3) |
| Liver + CRC | 83 (4.3) |
| CRC only | 594 (30.9) |
| No metastasectomy, no resection of CRC | 1159 (60.3) |
| Liver + Lung | 3 (0.2) |
| Liver only | 26 (1.4) |
| Lung only | 1 (0.05) |
| Lung + CRC | 13 (0.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engstrand, J.; Taflin, H.; Rystedt, J.L.; Hemmingsson, O.; Urdzik, J.; Sandström, P.; Björnsson, B.; Hasselgren, K. The Resection Rate of Synchronously Detected Liver and Lung Metastasis from Colorectal Cancer Is Low—A National Registry-Based Study. Cancers 2023, 15, 1434. https://doi.org/10.3390/cancers15051434
Engstrand J, Taflin H, Rystedt JL, Hemmingsson O, Urdzik J, Sandström P, Björnsson B, Hasselgren K. The Resection Rate of Synchronously Detected Liver and Lung Metastasis from Colorectal Cancer Is Low—A National Registry-Based Study. Cancers. 2023; 15(5):1434. https://doi.org/10.3390/cancers15051434
Chicago/Turabian StyleEngstrand, Jennie, Helena Taflin, Jenny Lundmark Rystedt, Oskar Hemmingsson, Jozef Urdzik, Per Sandström, Bergthor Björnsson, and Kristina Hasselgren. 2023. "The Resection Rate of Synchronously Detected Liver and Lung Metastasis from Colorectal Cancer Is Low—A National Registry-Based Study" Cancers 15, no. 5: 1434. https://doi.org/10.3390/cancers15051434
APA StyleEngstrand, J., Taflin, H., Rystedt, J. L., Hemmingsson, O., Urdzik, J., Sandström, P., Björnsson, B., & Hasselgren, K. (2023). The Resection Rate of Synchronously Detected Liver and Lung Metastasis from Colorectal Cancer Is Low—A National Registry-Based Study. Cancers, 15(5), 1434. https://doi.org/10.3390/cancers15051434