Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Preclinical Mechanisms of Rejection
3. Tregs, ICIs, and Transplantation
4. Tissue-Resident Memory T Cells, Exhausted T Cells, ICIs, and Transplantation
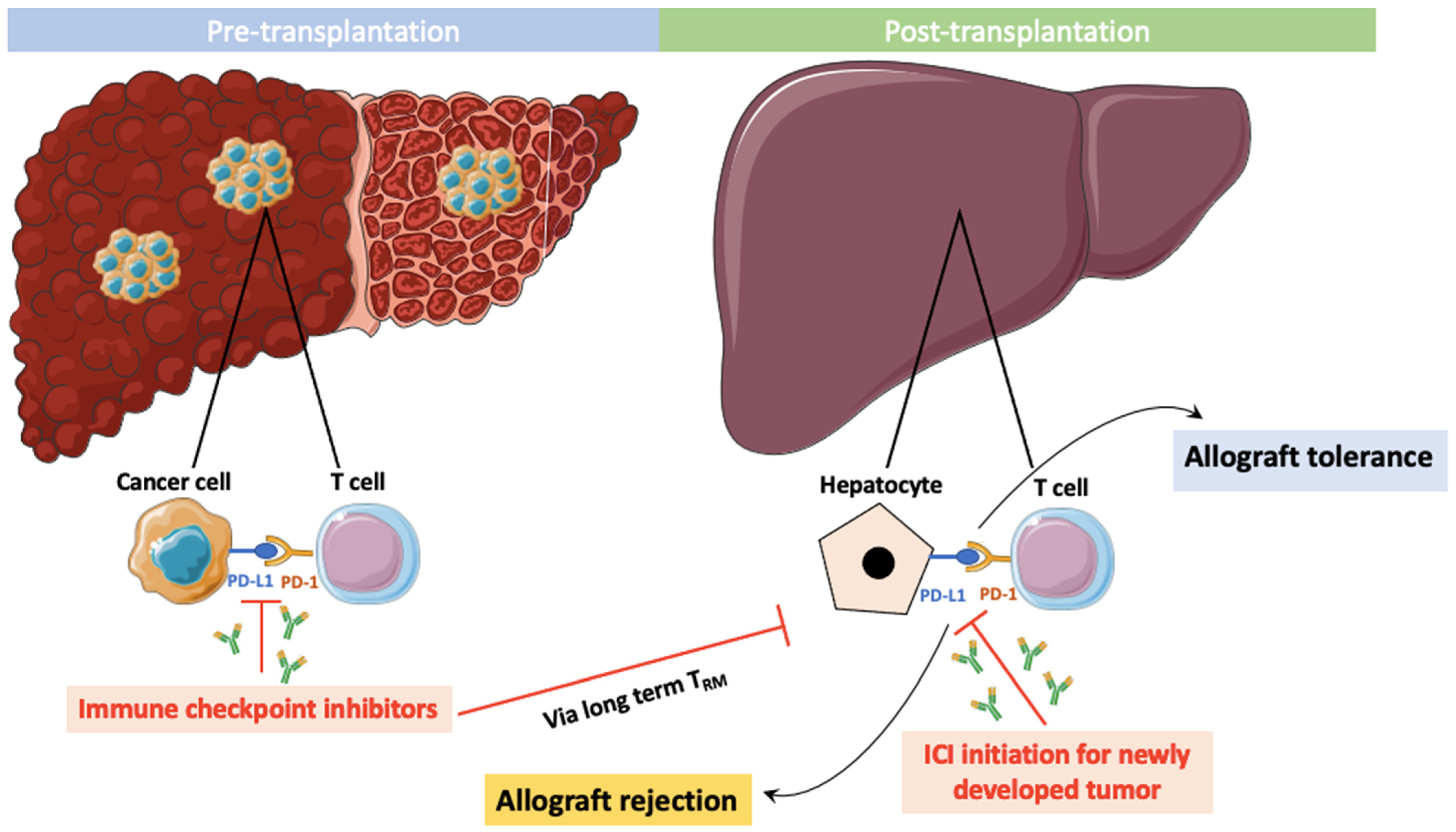
5. PD-1/PD-L1 Expression on Tumor, Immune, and Transplanted Liver Cells
6. Use of ICIs Prior to Liver Transplantation
7. ICIs after Liver Transplantation
8. Prevention and Management of ICI-Induced Liver Graft Rejection
9. Adjuvant ICIs after Curative HCC Treatment
10. Conclusions
11. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mehta, N. Liver Transplantation Criteria for Hepatocellular Carcinoma, Including Posttransplant Management. Clin. Liver Dis. 2021, 17, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021, 7, 6. [Google Scholar]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; HIMALAYA Investigators. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. Evid. 2022, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L. Changes in the Epidemiology of Hepatocellular Carcinoma in Asia. Cancers 2022, 14, 4473. [Google Scholar] [PubMed]
- Clavien, P.A.; Lesurtel, M.; Bossuyt, P.M.; Gores, G.J.; Langer, B.; Perrier, A. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [PubMed]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Validation of the UCSF-expanded criteria based on preoperative imaging. Am. J. Transplant. 2007, 7, 2587–2596. [Google Scholar]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.F.; Marotta, P.; Majno, P.; Kneteman, N.M. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015, 62, 158–165. [Google Scholar] [PubMed]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [PubMed]
- Abdelrahim, M.; Esmail, A.; Umoru, G.; Westhart, K.; Abudayyeh, A.; Saharia, A.; Ghobrial, R.M. Immunotherapy as a Neoadjuvant Therapy for a Patient with Hepatocellular Carcinoma in the Pretransplant Setting: A Case Report. Curr. Oncol. 2022, 29, 4267–4273. [Google Scholar] [CrossRef] [PubMed]
- Lizaola-Mayo, B.C.; Mathur, A.K.; Borad, M.J.; Jadlowiec, C.C.; Lam-Himlin, D.M.; Corey, R.L.; Iqbal, S.; Okubo, K.; Byrne, T.J.; Moss, A.A.; et al. Immunotherapy as a Downstaging Tool for Liver Transplantation in Hepatocellular Carcinoma. Am. J. Gastroenterol 2021, 116, 2478–2480. [Google Scholar]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar]
- Nordness, M.F.; Hamel, S.; Godfrey, C.M.; Shi, C.; Johnson, D.B.; Goff, L.W.; O’Dell, H.; Perri, R.E.; Alexopoulos, S.P. Fatal hepatic necrosis after nivolumab as a bridge to liver transplant for HCC: Are checkpoint inhibitors safe for the pretransplant patient? Am. J. Transplant. 2020, 20, 879–883. [Google Scholar]
- Chen, G.H.; Wang, G.B.; Huang, F.; Qin, R.; Yu, X.J.; Wu, R.L.; Hou, L.J.; Ye, Z.H.; Zhang, X.H.; Zhao, H.C. Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period. Transpl. Immunol. 2021, 66, 101386. [Google Scholar] [CrossRef]
- Qiao, Z.Y.; Zhang, Z.J.; Lv, Z.C.; Tong, H.; Xi, Z.F.; Wu, H.X.; Chen, X.S.; Xia, L.; Feng, H.; Zhang, J.J.; et al. Neoadjuvant Programmed Cell Death 1 (PD-1) Inhibitor Treatment in Patients with Hepatocellular Carcinoma Before Liver Transplant: A Cohort Study and Literature Review. Front. Immunol. 2021, 12, 653437. [Google Scholar] [CrossRef] [PubMed]
- Schwacha-Eipper, B.; Minciuna, I.; Banz, V.; Dufour, J.F. Immunotherapy as a Downstaging Therapy for Liver Transplantation. Hepatology 2020, 72, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, Y.; Schnickel, G.T.; Hosseini, M.; Burgoyne, A.M.; Ajmera, V.H.; Morris, G.P.; Mendler, M.H.; Parekh, J.R.; Abushamat, F.; Vodkin, I.; et al. Rescue liver re-transplantation after graft loss due to severe rejection in the setting of pre-transplant nivolumab therapy. Clin. J. Gastroenterol 2021, 14, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubata, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.C.; Latchman, Y.E.; Buhlmann, J.E.; Tomczak, M.F.; Horwitz, B.H.; Freeman, G.J.; Sharpe, A.H. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 2003, 33, 2706–2716. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef]
- Ozkaynak, E.; Wang, L.; Goodearl, A.; McDonald, K.; Qin, S.; O’Keefe, T.; Duong, T.; Smith, T.; Gutierrez-Ramos, J.C.; Rottman, J.B.; et al. Programmed death-1 targeting can promote allograft survival. J. Immunol. 2002, 169, 6546–6553. [Google Scholar] [CrossRef]
- Rickert, C.G.; Markmann, J.F. Current state of organ transplant tolerance. Curr. Opin. Organ. Transplant. 2019, 24, 441–450. [Google Scholar] [CrossRef]
- Wang, L.; Han, R.; Hancock, W.W. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur. J. Immunol. 2007, 37, 2983–2990. [Google Scholar] [CrossRef]
- Tanaka, K.; Albin, M.J.; Yuan, X.; Yamaura, K.; Habicht, A.; Murayama, T.; Grimm, M.; Waaga, A.M.; Ueno, T.; Padera, R.F.; et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J. Immunol. 2007, 179, 5204–5210. [Google Scholar] [CrossRef]
- Morita, M.; Fujino, M.; Jiang, G.; Kitazawa, Y.; Xie, L.; Azuma, M.; Yagita, H.; Nagao, S.; Sugioka, A.; Kurosawa, Y.; et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am. J. Transplant. 2010, 10, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, X.X.; Kuhr, C.S.; Perkins, J.D. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am. J. Transplant. 2005, 5, 978–986. [Google Scholar] [PubMed]
- Wanchoo, R.; Riella, L.; Uppal, N.N.; Lopez, C.A.; Nair, V.; Devoe, C.; Jhaveri, K.D. Immune checkpoint inhibitors in the cancer patient with an organ transplant. J. Onco-Nephrol. 2017, 1, 42–48. [Google Scholar] [CrossRef]
- Judge, T.A.; Wu, Z.; Zheng, X.G.; Sharpe, A.H.; Sayegh, M.H.; Turka, L.A. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J. Immunol. 1999, 162, 1947–1951. [Google Scholar]
- Blazar, B.R.; Carreno, B.M.; Panoskaltsis-Mortari, A.; Carter, L.; Iwai, Y.; Yagita, H.; Nishimura, H.; Taylor, P.A. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 2003, 171, 1272–1277. [Google Scholar] [PubMed]
- Kittai, A.S.; Oldham, H.; Cetnar, J.; Taylor, M. Immune Checkpoint Inhibitors in Organ Transplant Patients. J. Immunother. 2017, 40, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Herz, S.; Höfer, T.; Papapanagiotou, M.; Leyh, J.C.; Meyenburg, S.; Schadendorf, D.; Ugurel, S.; Roesch, A.; Livingstone, E.; Schilling, B.; et al. Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur. J. Cancer 2016, 67, 66–72. [Google Scholar]
- Spain, L.; Higgins, R.; Gopalakrishnan, K.; Turajlic, S.; Gore, M.; Larkin, J. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann. Oncol. 2016, 27, 1135–1137. [Google Scholar]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar]
- Salomon, B.; Lenschow, D.J.; Rhee, L.; Ashourian, N.; Singh, B.; Sharpe, A.; Bluestone, J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000, 12, 431–440. [Google Scholar]
- Kumar, P.; Saini, S.; Prabhakar, B.S. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin. Cancer Biol. 2020, 64, 29–35. [Google Scholar] [PubMed]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [PubMed]
- Zhang, Q.; Vignali, D.A. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity 2016, 44, 1034–1051. [Google Scholar] [PubMed]
- June, C.H.; Warshauer, J.T.; Bluestone, J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017, 23, 540–547. [Google Scholar]
- Ni, X.; Wang, Q.; Gu, J.; Lu, L. Clinical and Basic Research Progress on Treg-Induced Immune Tolerance in Liver Transplantation. Front. Immunol. 2021, 12, 535012. [Google Scholar]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T cell states in human cancer: Insights from single-cell analysis. Nat. Rev. Cancer 2020, 20, 218–232. [Google Scholar]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef]
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y.; et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927. [Google Scholar] [CrossRef]
- Okła, K.; Farber, D.L.; Zou, W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J. Exp. Med. 2021, 218, e20201605. [Google Scholar]
- Wan, S.; Zhao, E.; Weissinger, D.; Krantz, B.A.; Werba, G.; Freeman, D.; Khanna, L.G.; Siolas, D.; Oberstein, P.E.; Chattopadhyay, P.K.; et al. Tumor infiltrating T cell states and checkpoint inhibitor expression in hepatic and pancreatic malignancies. Front. Immunol. 2023, 14, 1067352. [Google Scholar] [PubMed]
- Edwards, J.; Wilmott, J.S.; Madore, J.; Gide, T.N.; Quek, C.; Tasker, A.; Ferguson, A.; Chen, J.; Hewavisenti, R.; Hersey, P.; et al. CD103(+) Tumor-Resident CD8(+) T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin. Cancer Res. 2018, 24, 3036–3045. [Google Scholar] [PubMed]
- Barsch, M.; Salié, H.; Schlaak, A.E.; Zhang, Z.; Hess, M.; Mayer, L.S.; Tauber, C.; Otto-Mora, P.; Ohtani, T.; Nilsson, T.; et al. T-cell exhaustion and residency dynamics inform clinical outcomes in hepatocellular carcinoma. J. Hepatol. 2022, 77, 397–409. [Google Scholar] [PubMed]
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.L.; Yau, T.; Furuse, J.; Park, J.W.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469. [Google Scholar] [PubMed]
- Lakkis, F.G.; Arakelov, A.; Konieczny, B.T.; Inoue, Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat. Med. 2000, 6, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, G.; Dai, Z.; Konieczny, B.T.; Baddoura, F.K.; Lakkis, F.G. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 2002, 99, 6175–6180. [Google Scholar]
- Zou, D.; Dai, Y.; Zhang, X.; Wang, G.; Xiao, X.; Jia, P.; Li, X.C.; Guo, Z.; Chen, W. T cell exhaustion is associated with antigen abundance and promotes transplant acceptance. Am. J. Transplant. 2020, 20, 2540–2550. [Google Scholar] [CrossRef]
- Abou-Daya, K.I.; Tieu, R.; Zhao, D.; Rammal, R.; Sacirbegovic, F.; Williams, A.L.; Shlomchik, W.D.; Oberbarnscheidt, M.H.; Lakkis, F.G. Resident memory T cells form during persistent antigen exposure leading to allograft rejection. Sci. Immunol. 2021, 6, eabc8122. [Google Scholar]
- Kim, H.; Kim, H.; Lee, S.K.; Jin, X.L.; Kim, T.J.; Park, C.; Lee, J.I.; Kim, H.S.; Hong, S.K.; Yoon, K.C.; et al. Memory T cells are significantly increased in rejected liver allografts of rhesus monkeys. Liver Transpl. 2018, 24, 256–268. [Google Scholar]
- Feng, Y.; Wang, D.; Yuan, R.; Parker, C.M.; Farber, D.L.; Hadley, G.A. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J. Exp. Med. 2002, 196, 877–886. [Google Scholar]
- Yuan, R.; El-Asady, R.; Liu, K.; Wang, D.; Drachenberg, C.B.; Hadley, G.A. Critical role for CD103+CD8+ effectors in promoting tubular injury following allogeneic renal transplantation. J. Immunol. 2005, 175, 2868–2879. [Google Scholar] [CrossRef] [PubMed]
- Pallett, L.J.; Burton, A.R.; Amin, O.E.; Rodriguez-Tajes, S.; Patel, A.A.; Zakeri, N.; Jeffery-Smith, A.; Swadling, L.; Schmidt, N.M.; Baiges, A.; et al. Longevity and replenishment of human liver-resident memory T cells and mononuclear phagocytes. J. Exp. Med. 2020, 217, e20200050. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018, 33, 843–852.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet. Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Song, G.W.; Park, S.; Jung, M.K.; Kim, M.H.; Kang, H.J.; Yoo, C.; Yi, K.; Kim, K.H.; Eo, S.; et al. Association Between Expression Level of PD1 by Tumor-Infiltrating CD8(+) T Cells and Features of Hepatocellular Carcinoma. Gastroenterology 2018, 155, 1936–1950.e17. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, F.; Zhou, L.; Zhang, M.N.; Chen, Y.; Chang, X.J.; Lu, Y.Y.; Bai, W.L.; Qu, J.H.; Wang, C.P.; et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS ONE 2011, 6, e23621. [Google Scholar] [CrossRef]
- Shi, X.L.; Mancham, S.; Hansen, B.E.; de Knegt, R.J.; de Jonge, J.; van der Laan, L.J.; Rivadeneira, F.; Metselaar, H.J.; Kwekkeboom, J. Counter-regulation of rejection activity against human liver grafts by donor PD-L1 and recipient PD-1 interaction. J. Hepatol. 2016, 64, 1274–1282. [Google Scholar] [CrossRef]
- Lei, Q.; Yan, X.; Zou, H.; Jiang, Y.; Lai, Y.; Ung, C.O.L.; Hu, H. Efficacy and safety of monotherapy and combination therapy of immune checkpoint inhibitors as first-line treatment for unresectable hepatocellular carcinoma: A systematic review, meta-analysis and network meta-analysis. Discov. Oncol. 2022, 13, 95. [Google Scholar] [CrossRef]
- Plaz Torres, M.C.; Lai, Q.; Piscaglia, F.; Caturelli, E.; Cabibbo, G.; Biasini, E.; Pelizzaro, F.; Marra, F.; Trevisani, F.; Giannini, E.G. Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors and Applicability of First-Line Atezolizumab/Bevacizumab in a Real-Life Setting. J. Clin. Med. 2021, 10, 3201. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Aby, E.S.; Lake, J.R. Immune Checkpoint Inhibitor Therapy Before Liver Transplantation-Case and Literature Review. Transplant. Direct 2022, 8, e1304. [Google Scholar] [CrossRef] [PubMed]
- Sogbe, M.; López-Guerra, D.; Blanco-Fernández, G.; Sangro, B.; Narváez-Rodriguez, I. Durvalumab as a Successful Downstaging Therapy for Liver Transplantation in Hepatocellular Carcinoma: The Importance of a Washout Period. Transplantation 2021, 105, e398–e400. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Martinez, M.; Moisander-Joyce, H.; Saenger, Y.M.; Griesemer, A.D.; Kato, T.; Yamashiro, D.J.; Remotti, H.; Gartrell, R.D. Stable liver graft post anti-PD1 therapy as a bridge to transplantation in an adolescent with hepatocellular carcinoma. Pediatr. Transplant. 2022, 26, e14209. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Florman, S.S.; Schwartz, M.E. PD-1 inhibitor as bridge therapy to liver transplantation? Am. J. Transplant. 2021, 21, 1979–1980. [Google Scholar] [CrossRef]
- Schnickel, G.T.; Fabbri, K.; Hosseini, M.; Misel, M.; Berumen, J.; Parekh, J.; Mekeel, K.; Dehghan, Y.; Kono, Y.; Ajmera, V. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am. J. Transplant. 2022, 22, 1699–1704. [Google Scholar] [CrossRef]
- Dave, S.; Yang, K.; Schnickel, G.T.; Kono, Y.; Delebecque, F.; Arellano, D.; Liu, A.; Zhang, X.; Tu, X.M.; Ajmera, V. The Impact of Treatment of Hepatocellular Carcinoma with Immune Checkpoint Inhibitors on Pre- and Post-liver Transplant Outcomes. Transplantation 2022, 106, e308–e309. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Liu, Y.; Jia, Y.; Ju, W.; Chen, M.; Zhao, Q.; Wang, D.; Guo, Z.; Tang, Y.; et al. Neoadjuvant programmed cell death 1 inhibitor before liver transplantation for HCC is not associated with increased graft loss. Liver Transpl. 2023, 29, 598–606. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, X.; Wang, T.; Guo, Y.; Huang, C.; Li, M.; He, X.; Ju, W.; Chen, M. Prognosis after liver transplantation in patients treated with anti-PD-1 immunotherapy for advanced hepatocellular carcinoma: Case series. Ann. Palliat. Med. 2021, 10, 9354–9361. [Google Scholar] [CrossRef]
- Tabrizian, P.; Ajmera, V.; Kim, A.; Zhou, K.; Schnickel, G.; Torosian, K.; Hoteit, M.; Yao, F.; Florman, S.; Schwartz, M.; et al. Impact of immune checkpoint inhibitors pre-transplantation: Intention to treat outcomes from a multi-center study. In Proceedings of the ILTS Annual Congress 2023, Rotterdam, The Netherlands, 3–6 May 2023. [Google Scholar]
- Gao, Q.; Anwar, I.J.; Abraham, N.; Barbas, A.S. Liver Transplantation for Hepatocellular Carcinoma after Downstaging or Bridging Therapy with Immune Checkpoint Inhibitors. Cancers 2021, 13, 6307. [Google Scholar] [CrossRef]
- Available online: https://ansm.sante.fr/uploads/2021/04/02/20210402-atuc-opdivo-rcp-v4-mars-2021.pdf (accessed on 14 June 2023).
- Available online: https://www.bms.com/assets/bms/ca/documents/productmonograph_fr/OPDIVO_FR_PM.pdf (accessed on 14 June 2023).
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, J.; Tabrizian, P.; Bhangui, P.; Pinato, D.J.; Rodríguez-Perálvarez, M.L.; Sapisochin, G.; Bhoori, S.; Pascual, S.; Senzolo, M.; Al-Adra, D.; et al. De Novo Malignancy After Liver Transplantation: Risk Assessment, Prevention, and Management-Guidelines From the ILTS-SETH Consensus Conference. Transplantation 2022, 106, e30–e45. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAZMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Lee, P.H.; Ho, C.M.; Wu, Y.M.; Ho, M.C.; Hu, R.H. Post-transplant malignancy in liver transplantation: A single center experience. Medicine (Baltimore) 2014, 93, e310. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, P.; Van Gestel, D.; Ost, P.; Kruse, V.; Brochez, L.; Van Vlierberghe, H.; Devresse, A.; Del Marmol, V.; Le Moine, A.; Aspeslagh, S. Immune checkpoint blockade for organ transplant patients with advanced cancer: How far can we go? Curr. Opin. Oncol. 2019, 31, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.; Shah, S.A.; Quillin, R.; Lemon, K.; Olowokure, O.; Latif, T.; Sohal, D. Immune checkpoint inhibitors in liver transplant: A case series. J. Gastrointest. Oncol. 2023, 14, 1141–1148. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, J.; Zhuang, L.; Mou, H.; Zheng, S. Preliminary Evaluation of Atezolizumab Plus Bevacizumab as Salvage Treatment for Recurrent Hepatocellular Carcinoma After Liver Transplantation. Liver Transpl. 2022, 28, 895–896. [Google Scholar] [CrossRef]
- Ben Khaled, N.; Roessler, D.; Reiter, F.P.; Seidensticker, M.; Guba, M.; De Toni, E.N. Extending the Use of Atezolizumab and Bevacizumab to a Liver Transplant Recipient: Need for a Posttransplant Registry. Liver Transpl. 2021, 27, 928–929. [Google Scholar] [CrossRef]
- De Toni, E.N.; Gerbes, A.L. Tapering of Immunosuppression and Sustained Treatment with Nivolumab in a Liver Transplant Recipient. Gastroenterology 2017, 152, 1631–1633. [Google Scholar] [CrossRef]
- Brumfiel, C.M.; Patel, M.H.; Aqel, B.; Lehrer, M.; Patel, S.H.; Seetharam, M. Immune checkpoint inhibitor therapy in a liver transplant recipient with autoimmune disease and metastatic cutaneous squamous cell carcinoma. JAAD Case Rep. 2021, 14, 78–81. [Google Scholar] [CrossRef]
- Bittner, A.; Radke, J.; Eurich, D.; Wiener, E.; Denker, S.; Anagnostopoulos, I.; Na, I.K.; Heppner, F.L.; Bullinger, L.; Schmitt, C.A. Cerebral EBV-positive PTLD controlled by PD-1 checkpoint blockade in a liver transplant patient. Leuk. Lymphoma 2021, 62, 2026–2029. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kawachi, S.; Nakatsugawa, M.; Takeda, A.; Kikawada, N.; Aihara, Y.; Okimura, A.; Hirano, H.; Ogawa, Y.; Tsukahara, K. Nivolumab for recurrent/metastatic hypopharyngeal squamous cell carcinoma in a liver transplant recipient. Auris. Nasus. Larynx. 2022, 49, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Tsung, I.; Worden, F.P.; Fontana, R.J. A Pilot Study of Checkpoint Inhibitors in Solid Organ Transplant Recipients with Metastatic Cutaneous Squamous Cell Carcinoma. Oncologist 2021, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Owoyemi, I.; Vaughan, L.E.; Costello, C.M.; Thongprayoon, C.; Markovic, S.N.; Herrmann, J.; Otley, C.C.; Taner, T.; Mangold, A.R.; Leung, N.; et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: A single-center analysis. Cancer 2020, 126, 4780–4787. [Google Scholar] [CrossRef]
- Al Jarroudi, O.; Ulusakarya, A.; Almohamad, W.; Afqir, S.; Morere, J.F. Anti-Programmed Cell Death Protein 1 (PD-1) Immunotherapy for Metastatic Hepatocellular Carcinoma After Liver Transplantation: A Report of Three Cases. Cureus. 2020, 12, e11150. [Google Scholar] [CrossRef]
- Braun, M.; Fuchs, V.; Kian, W.; Roisman, L.; Peled, N.; Rosenberg, E.; Friedel, L. Nivolumab Induced Hepatocanalicular Cholestasis and Liver Rejection in a Patient with Lung Cancer and Liver Transplant. J. Thorac. Oncol. 2020, 15, e149–e150. [Google Scholar] [CrossRef]
- Anugwom, C.; Leventhal, T. Nivolumab-Induced Autoimmune-Like Cholestatic Hepatitis in a Liver Transplant Recipient. ACG Case Rep. J. 2020, 7, e00416. [Google Scholar] [CrossRef]
- Pandey, A.; Cohen, D.J. Ipilumumab for hepatocellular cancer in a liver transplant recipient, with durable response, tolerance and without allograft rejection. Immunotherapy 2020, 12, 287–292. [Google Scholar] [CrossRef]
- Amjad, W.; Kotiah, S.; Gupta, A.; Morris, M.; Liu, L.; Thuluvath, P.J. Successful Treatment of Disseminated Hepatocellular Carcinoma After Liver Transplantation with Nivolumab. J. Clin. Exp. Hepatol. 2020, 10, 185–187. [Google Scholar] [CrossRef]
- Zhuang, L.; Mou, H.B.; Yu, L.F.; Zhu, H.K.; Yang, Z.; Liao, Q.; Zheng, S.S. Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation. Hepatobiliary Pancreat. Dis. In.t 2020, 19, 91–93. [Google Scholar] [CrossRef]
- Lee, B.T.; Horwich, B.H.; Chopra, S.; Ahearn, A.; Han, H.H. Checkpoint Inhibitor-Induced Rejection of a Liver Allograft: A Combination of Acute T Cell-Mediated and Antibody-Mediated Rejection. Liver Transpl. 2019, 25, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.A.; Esteghamat, N.; Kim, E.J.; Garcia, G.; Gong, J.; Fakih, M.G.; Bold, R.J.; Cho, M.T. PD-1 Blockade in a Liver Transplant Recipient with Microsatellite Unstable Metastatic Colorectal Cancer and Hepatic Impairment. J. Natl. Compr. Canc. Netw. 2019, 17, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.; Safa, H.; Abudayyeh, A.; Johnson, D.H.; Trinh, V.A.; Zobniw, C.M.; Lin, H.; Wong, M.K.; Abdelrahim, M.; Gaber, A.O.; et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: An institutional experience and a systematic review of the literature. J. Immunother. Cancer 2019, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, T.T.; Salomao, M.A.; Aqel, B.A.; Sonbol, M.B.; Yokoda, R.T.; Ali, A.H.; Moss, A.A.; Mathur, A.K.; Chascsa, D.M.; Rakela, J.; et al. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: The Mayo Clinic experience. J. Gastrointest. Oncol 2018, 9, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Tio, M.; Rai, R.; Ezeoke, O.M.; McQuade, J.L.; Zimmer, L.; Khoo, C.; Park, J.J.; Spain, L.; Turajlic, S.; Ardolino, L.; et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur. J. Cancer 2018, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Fadi Nasr, A.A.G.; Diab, S.; Maddah, J.; Mansour, L.; Kassem, A.; El Karim Gheith, A. Pembrolizumab Monotherapy in Relapsed Hepatocellular Carcinoma Post Living Donor Liver Transplantation and Sorafenib. Int. J. oncol. Res. 2017, 1, 9. [Google Scholar]
- Wang, G.; Tang, H.; Zhang, Y.; Li, H.; Yi, S.; Jiang, N.; Wang, G.; Zhang, J.; Zhang, Q.; Yang, Y.; et al. Programmed death receptor(PD)-1 monoclonal antibody-induced acute immune hepatitis in the treatment of recurrent hepatocellular carcinoma after liver transplantation: A case report. Organ Transplantation 2016, 7, 44–47. [Google Scholar]
- Gassmann, D.; Weiler, S.; Mertens, J.C.; Reiner, C.S.; Vrugt, B.; Nägeli, M.; Mangana, J.; Müllhaupt, B.; Jenni, F.; Misselwitz, B. Liver Allograft Failure After Nivolumab Treatment-A Case Report with Systematic Literature Research. Transplant. Direct 2018, 4, e376. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, M.S.; Farouk, M.; Vargese, J.; Rela, M. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: The silver bullet? Hepatology 2018, 67, 1166–1168. [Google Scholar] [CrossRef]
- Kuo, J.C.; Lilly, L.B.; Hogg, D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: A case report and literature review. Melanoma Res. 2018, 28, 61–64. [Google Scholar] [CrossRef]
- Biondani, P.; De Martin, E.; Samuel, D. Safety of an anti-PD-1 immune checkpoint inhibitor in a liver transplant recipient. Ann. Oncol. 2018, 29, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Lewis, D.W.; Nugent, F.W. Preserved Liver Transplant After PD-1 Pathway Inhibitor for Hepatocellular Carcinoma. Am. J. Gastroenterol. 2017, 112, 1895–1896. [Google Scholar] [CrossRef] [PubMed]
- Friend, B.D.; Venick, R.S.; McDiarmid, S.V.; Zhou, X.; Naini, B.; Wang, H.; Farmer, D.G.; Busuttil, R.W.; Federman, N. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr. Blood Cancer 2017, 64, e26682. [Google Scholar] [CrossRef] [PubMed]
- Dueland, S.; Guren, T.K.; Boberg, K.M.; Reims, H.M.; Grzyb, K.; Aamdal, S.; Julsrud, L.; Line, P.D. Acute liver graft rejection after ipilimumab therapy. Ann. Oncol. 2017, 28, 2619–2620. [Google Scholar] [CrossRef]
- Schvartsman, G.; Perez, K.; Sood, G.; Katkhuda, R.; Tawbi, H. Immune Checkpoint Inhibitor Therapy in a Liver Transplant Recipient with Melanoma. Ann. Intern. Med. 2017, 167, 361–362. [Google Scholar] [CrossRef]
- Morales, R.E.; Shoushtari, A.N.; Walsh, M.M.; Grewal, P.; Lipson, E.J.; Carvajal, R.D. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J. Immunother. Cancer 2015, 3, 22. [Google Scholar] [CrossRef]
- Ranganath, H.A.; Panella, T.J. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J. Immunother. 2015, 38, 211. [Google Scholar] [CrossRef]
- Au, K.P.; Chok, K.S.H. Immunotherapy after liver transplantation: Where are we now? World J. Gastrointest. Surg. 2021, 13, 1267–1278. [Google Scholar] [CrossRef]
- Kayali, S.; Pasta, A.; Plaz Torres, M.C.; Jaffe, A.; Strazzabosco, M.; Marenco, S.; Giannini, E.G. Immune checkpoint inhibitors in malignancies after liver transplantation: A systematic review and pooled analysis. Liver Int. 2023, 43, 8–17. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, G.; Li, L.; Lai, G.; Wang, Z.; Sun, C.; Xia, W.; Wu, L. Immune checkpoint inhibitor therapy for malignant tumors in liver transplantation recipients: A systematic review of the literature. Transplant. Rev. 2022, 36, 100712. [Google Scholar] [CrossRef]
- Munker, S.; De Toni, E.N. Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterol. J. 2018, 6, 970–973. [Google Scholar] [CrossRef]
- Neil, D.A.; Hübscher, S.G. Current views on rejection pathology in liver transplantation. Transpl. Int. 2010, 23, 971–983. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, J.; Lee, J.J.; Song, S.H.; Ju, M.K.; Choi, G.H.; Kim, M.S.; Choi, J.S.; Kim, S.I.; Joo, D.J. Efficacy of rabbit anti-thymocyte globulin for steroid-resistant acute rejection after liver transplantation. Medicine 2016, 95, e3711. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.M.; Phillips, M.; Sawyer, R.G.; Northup, P.; Hagspiel, K.D.; Pruett, T.L.; Bonatti, H.J. Anti-thymocyte globulin for the treatment of acute cellular rejection following liver transplantation. Dig. Dis. Sci. 2010, 55, 3224–3234. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, C.; Sevmis, S.; Aktas, S.; Karakayali, H.; Demirhan, B.; Haberal, M. Steroid-resistant acute rejections after liver transplant. Exp. Clin. Transplant. 2010, 8, 172–177. [Google Scholar] [PubMed]
- Hu, B.; Yang, X.B.; Sang, X.T. Liver graft rejection following immune checkpoint inhibitors treatment: A review. Med. Oncol. 2019, 36, 94. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Adjuvant Immunotherapy after Curative Treatment for Hepatocellular Carcinoma. Liver Cancer 2021, 10, 399–403. [Google Scholar] [CrossRef]
- Nakashima, O.; Sugihara, S.; Kage, M.; Kojiro, M. Pathomorphologic characteristics of small hepatocellular carcinoma: A special reference to small hepatocellular carcinoma with indistinct margins. Hepatology 1995, 22, 101–105. [Google Scholar]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]
- Chow, P.; Chen, M.; Cheng, A.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.; Zhou, J.; Wang, L.; Wen, X.; et al. IMbrave050: Phase 3 study of adjuvant atezolizumab + bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation. In Proceedings of the American Association for Cancer Research, AACR Annual Meeting 2023, Orlando, FL, USA, 14–19 April 2023; p. 83. [Google Scholar]
| Cell Type | Implication | ||
|---|---|---|---|
| HCC | ICI | Transplantation | |
| Regulatory T cells (Treg) | - Promote tumor cell evasion  - Function and expansion of: - Function and expansion of: - T cells (CD4, CD8) - B cells - NK cells - APCs - FoxP3 + CTLA-4 + CD4 + enriched in viral-related HCC correlate with poor prognoses | - Treg depletion - Suppressive activity on PD-1/PD-L1 blockade - Suppressive activity on PD-1/PD-L1 blockade | - Participate in allograft tolerance |
| Resident memory T (TRM) cells | - Participate in TME homeostasis - Expression of ICOS, PD-1, TIGIT, and Tim-3 - Expression of ICOS, PD-1, TIGIT, and Tim-3- Association of TRM cell presence in the TME and better oncological outcomes - TRM cell dysfunction participates in tumor development | - High level of CD103 + TRM cells in the tumor correlates with responses to ICIs and prognoses - High expression of ICs in CD8 subgroup | - Nonexhausted TRM cells participate in chronic rejection - Suspected to be responsible for acute rejection after ICI therapy (long-term memory) |
| Exhausted T (TEX) cells | - High fraction of CD8 + PD-1 + expressing CTLA-4, CD39, and LAG-3 correlate with poor overall survival - High fraction in advanced HCC | - Their presence is correlated with poor responses to ICIs | - Suspected to become exhausted after chronic exposition to alloantigens |
 = decreased,
= decreased,  = increased.
= increased.| Study | Study Type | Number of Patients Receiving ICIs Pre-Transplantation (Rejections) | Age/Sex | Underlying Liver Disease | ICI | Duration | Washout Period (Days) | Rejection Proved by Biopsy | Retransplantation | Postoperative Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Schwacha-Eipper, 2020 [22] | Case report | 1 (0) | 62/M | ALD | Nivolumab | 34 cycles | 105 | No rejection | - | 12 months |
| Nordness, 2019 [19] | Case report | 1 (1) | 65/M | HCV | Nivolumab | 24 months | 8 | POD 6 | No, deceased at POD 10 | - |
| Chen G, 2021 [20] | Case report | 1 (1) | 39/M | HBV | Toripalimab | 10 months | 93 | POD 2 | No, deceased at POD 3 | - |
| Dehghan, 2021 [23] | Case report | 1 (1) | 65/F | HCV | Nivolumab | 15 months | 35 | POD 10 | Yes, POD 34 | 18 |
| Aby, 2021 [72] | Case report | 1 (1) | 64/M | HCV | Nivolumab | 23 months | 16 | POD 9 | No, high-dose corticosteroids | 16 months |
| Sogbe, 2021 [73] | Case report | 1 (0) | 61/M | HBV | Durvalumab | 18 months | 92 | No rejection | - | 24 months |
| Tabrizian, 2021 [75] | Case series | 9 (2 *) | N/D | HBV | Nivolumab | N/D | 1–253 | N/D | N/D | N/D |
| Qiao Z, 2021 [21] | Case series | 7 (1) | Mean age 53 +/− 12.1/M | N/D | Pembrolizumab or camrelizumab | N/D | 40 on average | POD 11 | No, corticosteroids | N/D |
| Schnickel, 2022 [76] | Case series | 5 (2) | 60/F 65/M | HCV HCV | Nivolumab Nivolumab | 18 months 8 months | 35 10 | POD 14 <POD 14 | No, corticosteroids No, rATG, rituximab, or IVIGs | 38 months 3 months |
| Dave, 2022 [77] | Case series | 5 (2) | Mean age 61 +/−6.52/N/D | N/D N/D | Nivolumab Nivolumab | N/D N/D | <90 days <90 days | Yes Yes | Yes, successful No, death 2 months after transplantation | N/D N/D |
| Kang, 2022 [74] | Case report | 1 (0) | 14 | None | Pembrolizumab | 3 | 138 | No | No | 96 months |
| Chen, 2021 [79] | Case series | 5 (0) | Mean age 53.2 +/− 5.4/4M, 1F | N/D | Nivolumab | N/D | 63.80 ± 18.3 | No | No | 12 months |
| Wang, 2023 [78] | Case series | 16 (9) | 37–67/14 M–2 F | 14 HBV 2 ALD | 2 nivolumab, 7 pembrolizumab, 4 sintilimab, 2 camrelizumab, and 1 multiple | 1–27 cycles | 7–184 | 352.5 (median) |
| Author | Year of Publication | n | Acute Graft Rejection Rate | Death Due to Rejection | OS (Months) | Most Commonly Used ICIs | IS While on ICI | Tumor PD-L1 Staining | Indication | Time from Transplant to ICI Initiation (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| De Toni [92] | 2017 | 1 | No | No | 7 | Nivolumab | Tacrolimus | / | HCC recurrence | 11 |
| Brumfiel [93] | 2021 | 1 | No | No | 15 | Nivolumab | MMF + prednisone + tacrolimus | / | Cutaneous SCC | >21 |
| Bittner [94] | 2021 | 1 | Yes | No | >14 | Nivolumab | MMF relayed by tacrolimus and everolimus due to rejection | Positive | PTLD | 11 |
| Ben Khaled [91] | 2021 | 1 | No | No (POD) | / | Atezolizumab/bevacizumab | - | / | HCC recurrence | 4 |
| Kondo [95] | 2022 | 1 | No | No (POD) | / | Nivolumab | Cyclosporine + MMF | Positive | Hypopharyngeal SCC | >3 |
| Tsung [96] | 2021 | 2 | No | No | / | Cemiplimab | Tacrolimus | / | Cutaneous SCC | / |
| Owoyemi [97] | 2020 | 8 | 1/4 | No (POD) | / | Nivolumab 75% Pembrolizumab 25% | Calcineurin inhibitors alone 65%, tacrolimus + prednisone 13%, MMF and pred 13%, other | / | 1/8 SSC, 5/8 HCC, 2/8 melanoma | 3 |
| Al Jarroudi [98] | 2020 | 3 | No | No | >4 months | Nivolumab | Tacrolimus | / | HCC recurrence | 1 to 3 |
| Braun [99] | 2020 | 1 | Accelerated chronic rejection | Yes | 2 | Nivolumab | Tacrolimus | / | Lung NSCLC | 3 |
| Anugwom [100] | 2020 | 1 | Hepatitis linked to ICIs | No | 2 | Nivolumab | Tacrolimus | Negative | Metastatic HCC + NSCLC | 1 |
| Pandey [101] | 2020 | 1 | No | No | >27 | Ipilimumab | Tacrolimus | / | HCC recurrence | 7.5 |
| Amjad [102] | 2020 | 1 | No | No | >24 | Nivolumab + prednisone | Tacrolimus + MMF | Positive | HCC recurrence | 2 |
| Zhuang [103] | 2020 | 1 | No | No | 20 | Nivolumab | Tacrolimus | / | HCC recurrence | 2 |
| Lee [104] | 2019 | 1 | Yes | Yes, delayed | / | Nivolumab | Everolimus | / | SCC | 1 |
| Chen [105] | 2019 | 1 | No | No | / | Pembrolizumab + prednisone | Tacrolimus | / | Metastatic CRC | 4 |
| Deleon [107] | 2018 | 5 | 1/5 | / | / | Nivolumab | Sirolimus or tacrolimus or MMF + sirolimus | Positive 1/5 | HCC | 3.92 (mean) |
| 2 | 1/2 | / | Pembrolizumab | Sirolimus or tacrolimus or MMF + sirolimus | Positive 1/2 | Melanoma | 4.3 (mean) | |||
| Tio [108] | 2018 | 1 | Yes | Yes | / | Pembrolizumab | Cyclosporine | / | Melanoma | / |
| Nasr [109] | 2017 | 1 | No | No | >12 | Pembrolizumab | Tacrolimus + MMF | / | HCC recurrence | 4 |
| Guoying [110] | 2016 | 1 | Hepatitis linked to ICIs | No | / | Pembrolizumab | Tacrolimus + sirolimus | / | HCC recurrence | 1 |
| Gassmann [111] | 2018 | 1 | Yes | Yes | / | Nivolumab | MMF + everolimus | / | HCC recurrence | 2 |
| Rammohan [112] | 2018 | 1 | No | No | > 10 | Pembrolizumab | Rapamycine + tacrolimus | / | HCC recurrence | 3 |
| Kuo [113] | 2018 | 1 | No | No | / | Ipilimumab, followed with pembrolizumab | Sirolimus | / | Melanoma | 1 |
| Biondani [114] | 2018 | 1 | No | No (POD) | / | Nivolumab + prednisone | Tacrolimus + everolimus | / | Lung NSCLC | 13 |
| Varkaris [115] | 2017 | 1 | No | No (POD) | / | Pembrolizumab | Tacrolimus | / | HCC | 8 |
| Friend [116] | 2017 | 2 | Yes | Yes | / | Nivolumab | Sirolimus or tacrolimus | Positive | HCC | 3 and 4 |
| Dueland [117] | 2017 | 1 | Yes | Yes | / | Ipilimumab | Prednisolone | / | Ocular melanoma | 1.5 |
| Schvartsman [118] | 2017 | 1 | Hepatitis linked to ICIs | No | >6 | Pembrolizumab | MMF | / | Melanoma | >20 |
| Morales [119] | 2015 | 1 | No | No | >4 | Ipilimumab | Tacrolimus | / | Melanoma | 8 |
| Ranganath [120] | 2015 | 1 | No | No | >5 | Ipilimumab | Tacrolimus | / | Melanoma | 8 |
| Abdel-Wahab [106] | 2019 | 11 | 4/11 | 1/11 | / | Ipilimumab/nivolumab/pembrolizumab | / | / | 6/11 melanoma 4/11 HCC recurrence | 6.87 (mean) |
| Name | Number of the Study | Study Start | Phase | Main Outcome | Expected Study Termination | Location | |
|---|---|---|---|---|---|---|---|
| Atezolizumab and Bevacizumab Pre-Liver Transplantation for Patients with Hepatocellular Carcinoma Beyond Milan Criteria | NCT05185505 | 30.01.23 | 4 | Proportion of patients receiving liver transplant experiencing acute rejection | 31.10.27 | Houston, USA | |
| Atezolizumab and Bevacizumab before surgery for the treatment of resectable liver cancer | NCT04721132 | 10.02.21 | 2 | Pathologic complete response rate | 31.12.27 | Houston, USA | |
| Neoadjuvant combination of atezolizumab/bevacizumab versus Neoadjuvant radiation therapy | ADVANCE HCC | NCT05137899 | 18.10.22 | 2 | Proportion of patients who undergo hepatectomy in each arm | 30.06.26 | Canada |
| A study of atezolizumab plus bevacizumab versus active surveillance as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after surgical resection or ablation | IMbrave050 | NCT04102098 | 31.12.19 | 3 | Recurrence-free survival | 16.07.27 | International (USA, Canada, Australia, New Zealand, Austria, Belgium, France, Spain, Germany, Italy, Czechia, Netherlands, Poland, Turkey, Peru, Brazil, Costa Rica, Mexico, China, Hong Kong, Japan, Korea, Thailand, Taiwan, Singapore, Russia) |
| A study evaluating the efficacy and safety of neoadjuvant immunotherapy combinations in patients with surgically resectable hepatocellular carcinoma | MORPHEUS-NEO HCC | NCT05908786 | 01.09.23 | Ib/2 | Major pathologic response rate | 31.03.25 | USA, Canada |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wassmer, C.-H.; El Hajji, S.; Papazarkadas, X.; Compagnon, P.; Tabrizian, P.; Lacotte, S.; Toso, C. Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data. Cancers 2023, 15, 4574. https://doi.org/10.3390/cancers15184574
Wassmer C-H, El Hajji S, Papazarkadas X, Compagnon P, Tabrizian P, Lacotte S, Toso C. Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data. Cancers. 2023; 15(18):4574. https://doi.org/10.3390/cancers15184574
Chicago/Turabian StyleWassmer, Charles-Henri, Sofia El Hajji, Xenofon Papazarkadas, Philippe Compagnon, Parissa Tabrizian, Stéphanie Lacotte, and Christian Toso. 2023. "Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data" Cancers 15, no. 18: 4574. https://doi.org/10.3390/cancers15184574
APA StyleWassmer, C.-H., El Hajji, S., Papazarkadas, X., Compagnon, P., Tabrizian, P., Lacotte, S., & Toso, C. (2023). Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data. Cancers, 15(18), 4574. https://doi.org/10.3390/cancers15184574







