Sensitivity, Specificity, and Predictive Values of Tru-Cut® Biopsy in Grading Primary Localized Myxoid Liposarcomas of the Extremities
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Methods
2.2. Biopsy Procedure
2.3. Therapeutic Procedures
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Features
3.2. Determination of Histologic Accuracy: Cytohistologic Correlation of Grade Diagnosis
3.3. Risk Factors Associated with Downgrading
3.4. Impact of Misdiagnosis on Prognosis
3.5. Concordance Rate in the Group of Patients Not Treated
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dujardin, F.; Debled, M.; Guillemet, C.; Simonet, J.; Hamidou, H.; Cambon-Michot, C.; Dubray, B.; Vera, P. Diagnosis and treatment of soft-tissue tumors. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2006, 92, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Coffin, C.M.; Weiss, S.W.; Bridge, J.A.; Issakov, J.; Oliveira, A.M.; Folpe, A.L. Liposarcomas in Young Patients: A Study of 82 Cases Occurring in Patients Younger than 22 Years of Age. Am. J. Surg. Pathol. 2009, 33, 645–658. [Google Scholar] [CrossRef]
- Kilpatrick, S.E.; Doyon, J.; M Choong, P.F.; Sim, F.H.; Nascimento, A.G. The Clinicopathologic Spectrum of Myxoid and Round Cell Liposarcoma A Study of 95 Cases. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1996, 77, 1450–1458. [Google Scholar] [CrossRef]
- Tuzzato, G.; Laranga, R.; Ostetto, F.; Bubbico, E.; Vara, G.; Bianchi, G. Primary High-Grade Myxoid Liposarcoma of the Extremities: Prognostic Factors and Metastatic Pattern. Cancers 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Bettini, L.; Frenos, F.; Mondanelli, N.; Greto, D.; Livi, L.; Franchi, A.; Roselli, G.; Scorianz, M.; Capanna, R.; et al. Myxoid Liposarcoma: Prognostic Factors and Metastatic Pattern in a Series of 148 Patients Treated at a Single Institution. Int. J. Surg. Oncol. 2018, 2018, 8928706. [Google Scholar] [CrossRef] [PubMed]
- Lansu, J.; Van Houdt, W.J.; Schaapveld, M.; Walraven, I.; Van De Sande, M.A.J.; Ho, V.K.Y.; Haas, R.L. Time Trends and Prognostic Factors for Overall Survival in Myxoid Liposarcomas: A Population-Based Study. Sarcoma 2020, 437850. [Google Scholar] [CrossRef]
- Fiore, M.; Grosso, F.; Lo Vullo, S.; Pennacchioli, E.; Stacchiotti, S.; Ferrari, A.; Collini, P.; Lozza, L.; Mariani, L.; Casali, P.G.; et al. Myxoid/round cell and pleomorphic liposarcomas: Prognostic factors and survival in a series of patients treated at a single institution. Cancer 2007, 109, 2522–2531. [Google Scholar] [CrossRef]
- Hoffman, A.; Ghadimi, M.P.H.; Demicco, E.G.; Creighton, C.J.; Torres, K.; Colombo, C.; Peng, T.; Lusby, K.; Ingram, D.; Hornick, J.L.; et al. Localized and Metastatic Myxoid/Round Cell Liposarcoma Clinical and Molecular Observations. Cancer 2013, 119, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Losada, J.; Sánchez-Martín, M.; Rodríguez-García, M.A.; Pérez-Mancera, P.A.; Pintado, B.; Flores, T.; Battaner, E.; Sánchez-Garćia, I. Liposarcoma initiated by FUS/TLS-CHOP: The FUS/TLS domain plays a critical role in the pathogenesis of liposarcoma. Oncogene 2000, 19, 6015–6022. [Google Scholar] [CrossRef]
- Smith, T.A.; Easley, K.A.; Goldblum, J.R. Myxoid/round cell liposarcoma of the extremities. A clinicopathologic study of 29 cases with particular attention to extent of round cell liposarcoma. Am. J. Surg. Pathol. 1996, 20, 171–180. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Fletcher, C.D.M. Will We Ever Reliably Predict Prognosis in a Patient with Myxoid and Round Cell Liposarcoma? Adv. Anat. Pathol. 1997, 4, 108–113. [Google Scholar] [CrossRef]
- Nishida, Y.; Tsukushi, S.; Nakashima, H.; Ishiguro, N. Clinicopathologic prognostic factors of pure myxoid liposarcoma of the extremities and trunk wall. Clin. Orthop. Relat. Res. 2010, 468, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R.; Tschernyavsky, S.J.; Decuseara, R.; Woodruff, J.M.; Ladanyi, M.; Leung, D.H.; Brennan, M.F.; Bridge, J.A.; Neff, J.R.; Goldblum, J.R. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: A molecular and clinicopathologic study of 82 cases. Clin. Cancer Res. 2001, 7, 3977–3987. [Google Scholar]
- Siegal, G.P.; Bloem, J.L.; Cates, J.M.M. WHO Classification of Tumors 5th edition Soft Tissue and Bone Tumors. Adv. Anat. Pathol. 2020, 3, 472–474. [Google Scholar]
- Dürr, H.R.; Rauh, J.; Baur-Melnyk, A.; Knösel, T.; Lindner, L.; Roeder, F.; Jansson, V.; Klein, A. Myxoid liposarcoma: Local relapse and metastatic pattern in 43 patients. BMC Cancer 2018, 18, 304. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Bertuzzi, A.; Bielack, S.; Bjerkehagen, B.; Bonvalot, S.; Boukovinas, I.; Bruzzi, P.; Tos, A.P.D.; Dileo, P.; et al. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, iii113. [Google Scholar] [CrossRef]
- Tetikkurt, S.; Koy, Y.; Beytemur, O.; Albayrak, R.; Bakir, A. Diagnostic Accuracy of Tru-Cut Biopsy for Soft Tissue Tumors. Istanb. Med. J. 2018, 19, 295–299. [Google Scholar] [CrossRef]
- Adams, S.C.; Potter, B.K.; Pitcher, D.J.; Temple, H.T. Office-based core needle biopsy of bone and soft tissue malignancies: An accurate alternative to open biopsy with infrequent complications. Clin. Orthop. Relat. Res. 2010, 468, 2774–2780. [Google Scholar] [CrossRef]
- Mankin, H.J.; Mankin, C.J.; Simon, M.A. The hazards of the biopsy, revisited: For the members of the musculoskeletal tumor society. J. Bone Jt. Surg. 1996, 78, 656–663. [Google Scholar] [CrossRef]
- Skrzynski, M.C.; Biermann, J.S.; Montag, A.; Simon, M.A. Diagnostic Accuracy and Charge-Savings of Outpatient Core Needle Biopsy Compared with Open Biopsy of Musculoskeletal Tumors. J. Bone Jt. Surg. 1996, 78, 644–649. [Google Scholar] [CrossRef]
- Pohlig, F.; Kirchhoff, C.; Lenze, U.; Schauwecker, J.; Burgkart, R.; Rechl, H.; Von Eisenhart-Rothe, R. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: A retrospective study. Eur. J. Med. Res. 2012, 17, 29. [Google Scholar] [CrossRef]
- Seng, C.; Png, W.; Hong Tan, M. Accuracy of core needle biopsy for musculoskeletal tumours. J. Orthop. Surg. 2013, 21, 92–95. [Google Scholar] [CrossRef]
- Muratori, F.; Frenos, F.; Bettini, L.; Matera, D.; Mondanelli, N.; Scorianz, M.; Cuomo, P.; Scoccianti, G.; Beltrami, G.; Greto, D.; et al. Liposarcoma: Clinico-pathological analysis, prognostic factors and survival in a series of 307 patients treated at a single institution. J. Orthop. Sci. 2018, 23, 1038–1044. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 241–246. [Google Scholar] [CrossRef]
- Kasraeian, S.; Allison, D.C.; Ahlmann, E.R.; Fedenko, A.N.; Menendez, L.R. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin. Orthop. Relat. Res. 2010, 468, 2992–3002. [Google Scholar] [CrossRef]
- Kerm, S.A.Ê. Fine-needle aspiration cytology of soft tissue sarcom a: Bene® ts and limitations. Sarcoma 1998, 2, 155–161. [Google Scholar]
- Dei Tos, A.P. Liposarcoma: New entities and evolving concepts. Ann. Diagn. Pathol. 2000, 4, 252–266. [Google Scholar] [CrossRef]
- Hoeber, I.; Spillane, A.J.; Fisher, C.; Thomas, J.M. Accuracy of Biopsy Techniques for Limb and Limb Girdle Soft Tissue Tumors. Ann. Surg. Oncol. 2001, 8, 80–87. [Google Scholar] [CrossRef]
- Welker, J.A.; Henshaw, R.M.; Jelinek, J.; Shmookler, B.M.; Malawer, M.M. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer 2000, 89, 2677–2686. [Google Scholar] [CrossRef]
- Domanski, H.A.; Kerman, M.A.; Carlé, B.; Engellau, J.; Gustafson, P.; Jonsson, K.; Mertens, F.; Rydholm, A. Core-Needle Biopsy Performed by the Cytopathologist A Technique to Complement Fine-Needle Aspiration of Soft Tissue and Bone Lesions. Cancer 2005, 105, 229–239. [Google Scholar] [CrossRef]
- Bickels, J.; Jelinek, J.S.; Shmookler, B.M.; Neff, R.S.; Malawer, M.M. Biopsy of musculoskeletal tumors. Current concepts. Clin. Orthop. Relat. Res. 1999, 368, 212–219. [Google Scholar] [CrossRef][Green Version]
- Xu, W.; Hao, D.; Hou, F.; Zhang, D.; Wang, H. Soft Tissue Sarcoma: Preoperative MRI-Based Radiomics and Machine Learning May Be Accurate Predictors of Histopathologic Grade. Am. J. Roentgenol. 2020, 215, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.N.; Callstrom, M.R. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin. Interv. Radiol. 2010, 27, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Birgin, E.; Yang, C.; Hetjens, S.; Reissfelder, C.; Hohenberger, P.; Rahbari, N.N. Core Needle Biopsy Versus Incisional Biopsy for Differentiation of Soft-Tissue Sarcomas: A Systematic Review and Meta-Analysis. Cancer 2020, 126, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Mutschler, M.; Kurz, P.; Stark, G.B.; Bannasch, H.; Simunovic, F. Accuracy of core needle biopsy for histologic diagnosis of soft tissue sarcoma. Sci. Rep. 1886, 12, 1886. [Google Scholar] [CrossRef]
| Factor | Number of Patients | % |
|---|---|---|
| Patients | 144 | 100 |
| Gender | ||
| Male | 91 | 63 |
| Female | 53 | 37 |
| Location | ||
| Thigh | 100 | 69 |
| Lower limb | 33 | 21 |
| Buttock | 10 | 7 |
| Arm | 1 | 1 |
| Depth | ||
| Deep | 136 | 95 |
| Superficial | 8 | 5 |
| Tumor Size | ||
| >10 cm | 83 | 58 |
| 5–10 cm | 55 | 38 |
| <5 cm | 6 | 4 |
| Preoperative Grade | ||
| High | 87 | 60 |
| Low | 57 | 40 |
| Postoperative Grade | ||
| High | 59 | 41 |
| Low | 85 | 59 |
| Surgery | ||
| Excision | 142 | 99 |
| Amputation | 2 | 1 |
| Margin | ||
| Wide/Radical (R0) | 104 | 72 |
| Marginal (R1) | 35 | 25 |
| Intralesional (R2) | 5 | 3 |
| Radiotherapy | ||
| Preoperative | 92 | 64 |
| Postoperative | 31 | 21 |
| None | 21 | 15 |
| Chemotherapy | ||
| Preoperative | 46 | 32 |
| Postoperative | 20 | 14 |
| Pre- and postop. | 2 | 1 |
| None | 76 | 53 |
| Surgical Specimen | |||
|---|---|---|---|
| Core Needle Biopsy | Tot | High grade | Low grade |
| Group A (High Grade) | 87 | 47 (54%) | 40 (46%) |
| Group B (Low Grade) | 57 | 12 (21%) | 45 (79%) |
| Univariate Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Factor | N | Discordant (N) | Discordant (%) | OR | p-Value | 95% CI | |
| Age | <50 | 47 | 23 | 57 | 0.84 | 0.687 | 0.35–1.9 |
| >50 | 40 | 17 | 43 | ||||
| Location | Thigh (ref) | 55 | 24 | 60 | |||
| Lower limb | 25 | 13 | 33 | 1.39 | 0.488 | 0.54–3.61 | |
| Buttock Arm | 6 1 | 2 1 | 5 2 | 0.64 | 0.630 | 0.10–3.8 | |
| Size | >10 cm (ref) | 51 | 26 | 65 | |||
| 5 < to < 10 cm | 31 | 11 | 28 | 0.53 | 0.174 | 0.21–1.32 | |
| <5 cm | 5 | 3 | 7 | 1.44 | 0.701 | 0.22–9.37 | |
| Depth | Deep | 82 | 39 | 98 | 3.6 | 0.25 | 0.38–33.8 |
| Superficial | 5 | 1 | 2 | ||||
| Neoadjuvant therapy | ChT (ref) | 12 | 3 | 7.5 | |||
| RT | 23 | 14 | 35 | 4.66 | 0.052 | 0.9–21 | |
| ChT + RT | 36 | 23 | 57.5 | 5.30 | 0.026 | 1.3–24 | |
| None | 16 | 0 | |||||
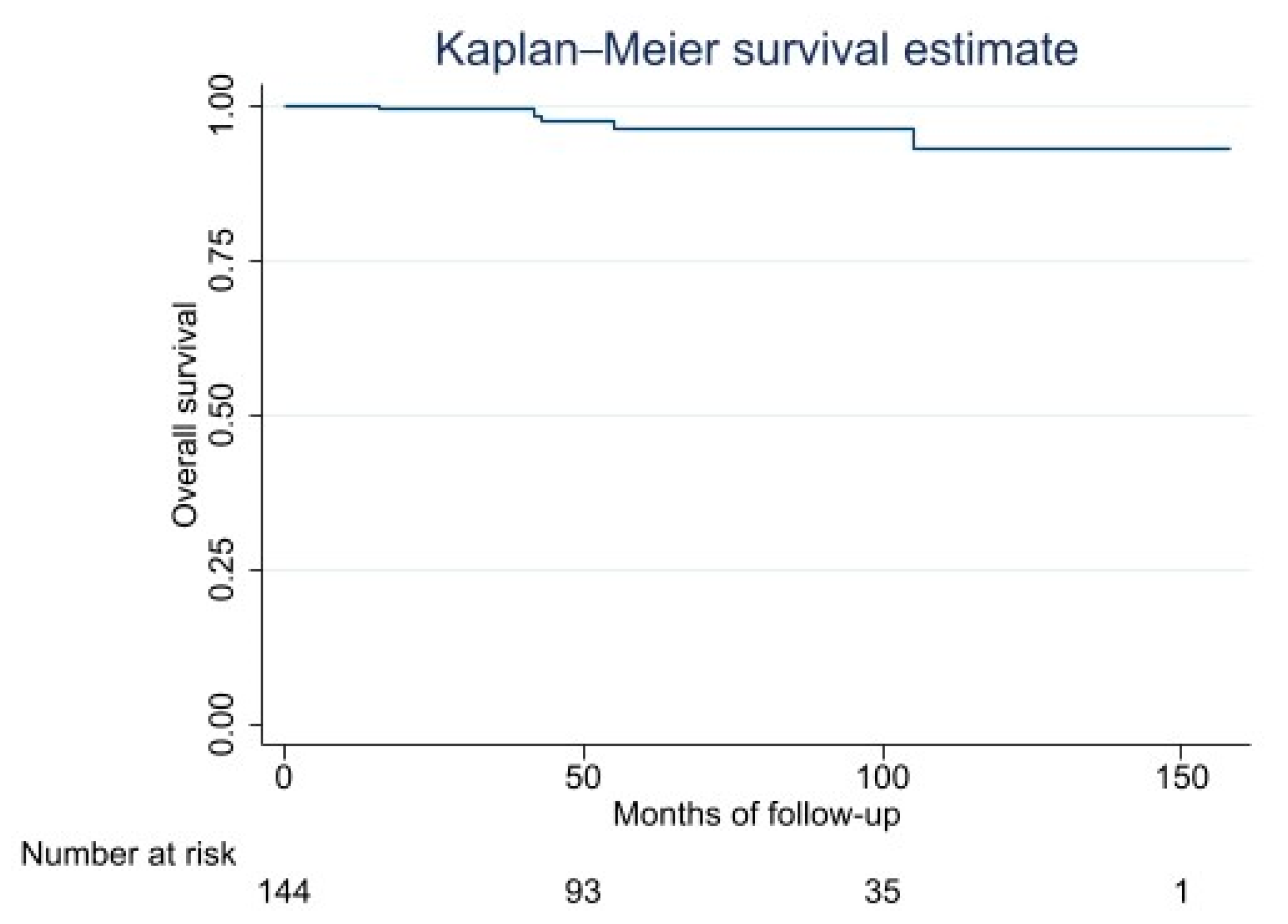
| Survival Function | 95% Confidence Interval | |
|---|---|---|
| 2 years | 99 | 94–99 |
| 5 years | 96 | 90–98 |
| 10 years | 93 | 81–97 |
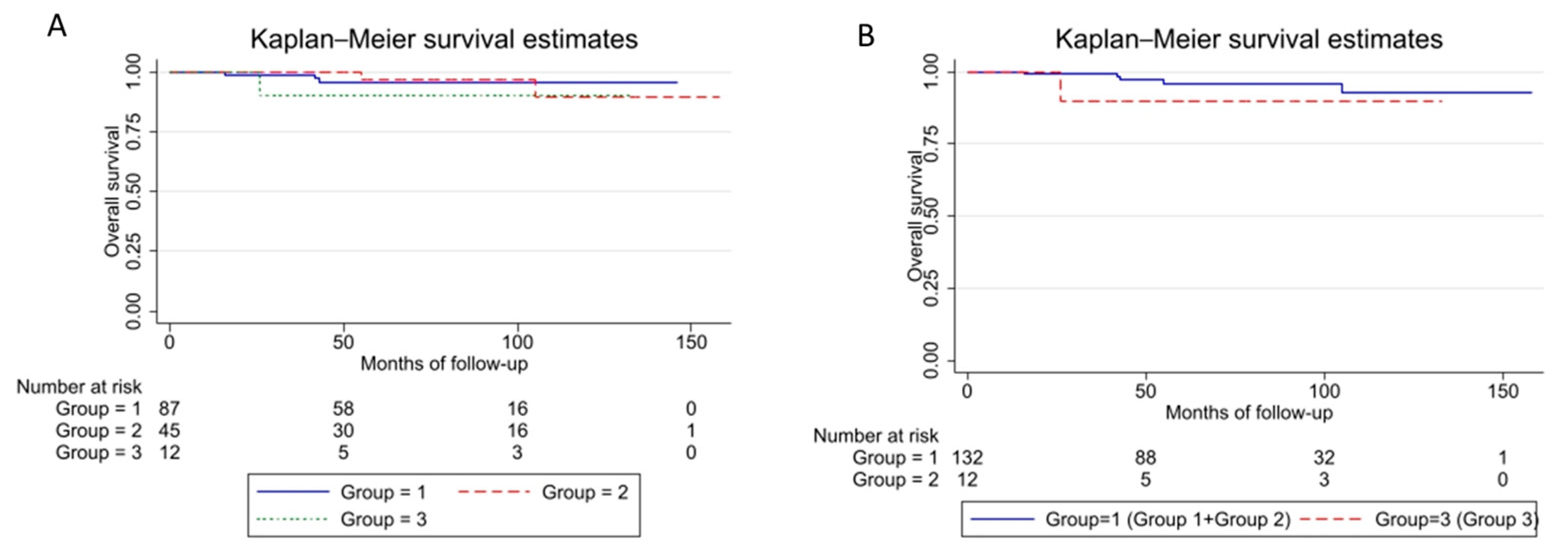
| Factor (N) | 5-Years OS (%) | 95% CI | 10-Years OS (%) | 95% CI | |
|---|---|---|---|---|---|
| Grade group | 1 (87) | 96 | 87–98 | 96 | 87–98 |
| 2 (45) | 97 | 78–99 | 90 | 62–97 | |
| 3 (12) | 90 | 47–98 | 90 | 47–98 | |
| Grade group | 1 + 2 (132) | 96 | 89–98 | 93 | 80–97 |
| 3 (12) | 90 | 47–99 | 90 | 47–99 |
| True Diagnosis (Surgical Specimen Histology) | |||
|---|---|---|---|
| High-Grade | Low-Grade | Tot | |
| TCB | |||
| High-grade | 16 (TP) | 0 (FP) | 16 |
| Low-grade | 12 (FN) | 12 (TN) | 24 |
| Tot | 28 | 12 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, G.; Laranga, R.; Spinnato, P.; Ostetto, F.; Bubbico, E.; Righi, A.; Donati, D.M. Sensitivity, Specificity, and Predictive Values of Tru-Cut® Biopsy in Grading Primary Localized Myxoid Liposarcomas of the Extremities. Cancers 2023, 15, 1391. https://doi.org/10.3390/cancers15051391
Bianchi G, Laranga R, Spinnato P, Ostetto F, Bubbico E, Righi A, Donati DM. Sensitivity, Specificity, and Predictive Values of Tru-Cut® Biopsy in Grading Primary Localized Myxoid Liposarcomas of the Extremities. Cancers. 2023; 15(5):1391. https://doi.org/10.3390/cancers15051391
Chicago/Turabian StyleBianchi, Giuseppe, Roberta Laranga, Paolo Spinnato, Federico Ostetto, Elisa Bubbico, Alberto Righi, and Davide Maria Donati. 2023. "Sensitivity, Specificity, and Predictive Values of Tru-Cut® Biopsy in Grading Primary Localized Myxoid Liposarcomas of the Extremities" Cancers 15, no. 5: 1391. https://doi.org/10.3390/cancers15051391
APA StyleBianchi, G., Laranga, R., Spinnato, P., Ostetto, F., Bubbico, E., Righi, A., & Donati, D. M. (2023). Sensitivity, Specificity, and Predictive Values of Tru-Cut® Biopsy in Grading Primary Localized Myxoid Liposarcomas of the Extremities. Cancers, 15(5), 1391. https://doi.org/10.3390/cancers15051391







