Utilization of a Machine Learning Algorithm for the Application of Ancillary Features to LI-RADS Categories LR3 and LR4 on Gadoxetate Disodium-Enhanced MRI
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. MRI Techniques
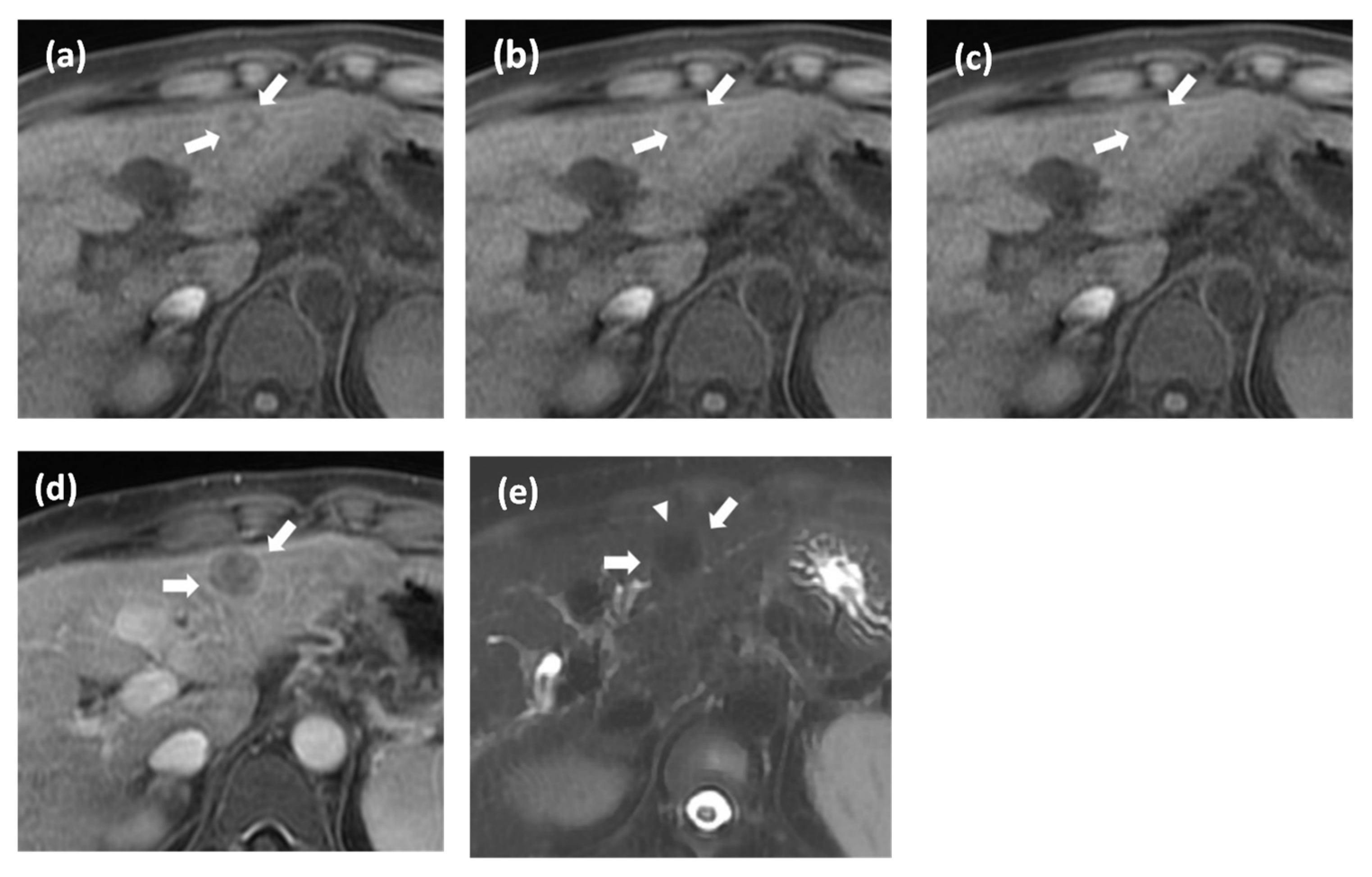
2.3. Image Analysis
2.4. Reference Standard
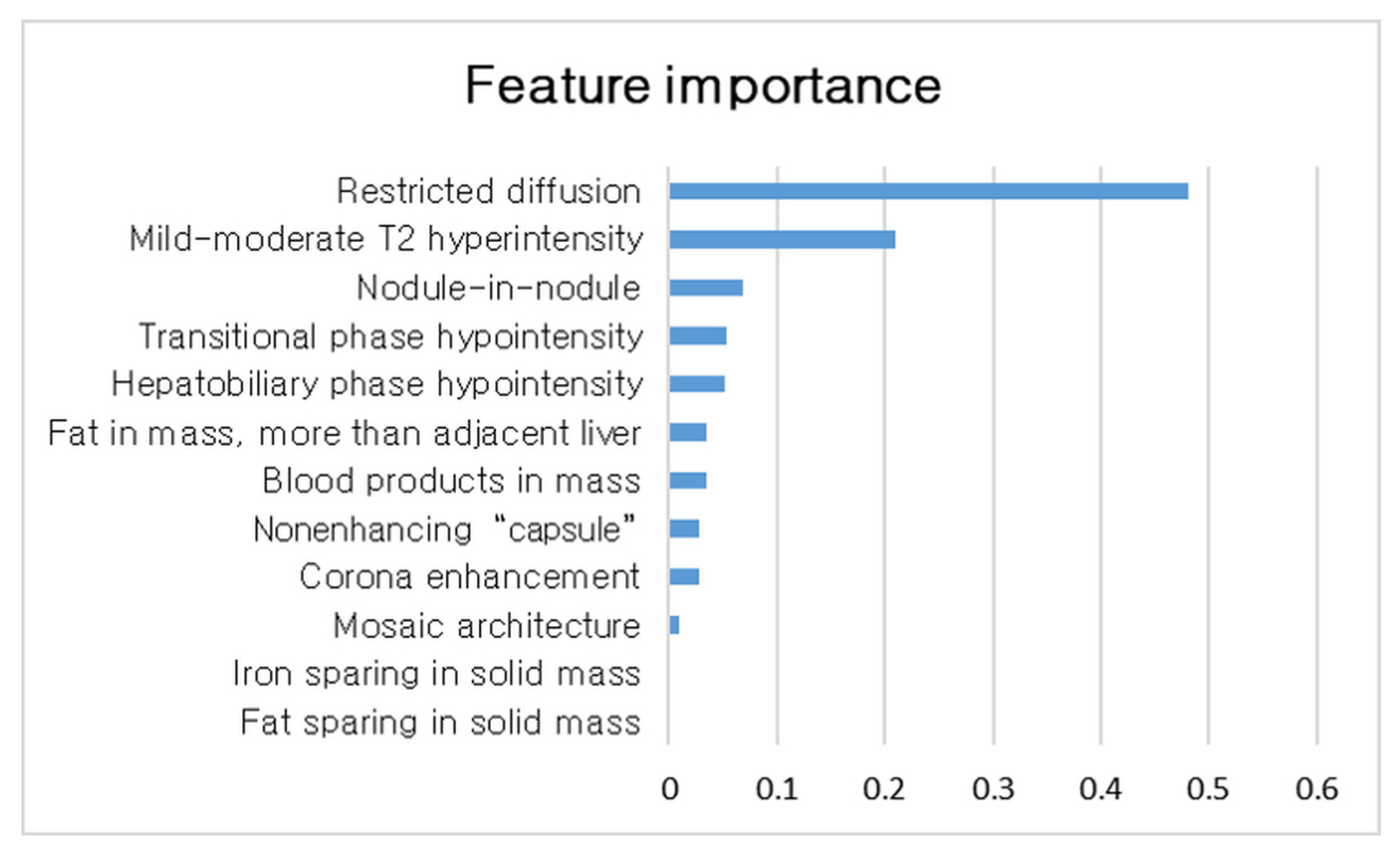
2.5. Extracting Important Features and Constructing a Machine-Learning-Based Algorithm for Applying AFs
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients and Observations
3.2. Comparison of Imaging Features between HCCs and Non-Malignant Nodules and Important Features for Diagnosis HCC in LR3 and LR4 Observation
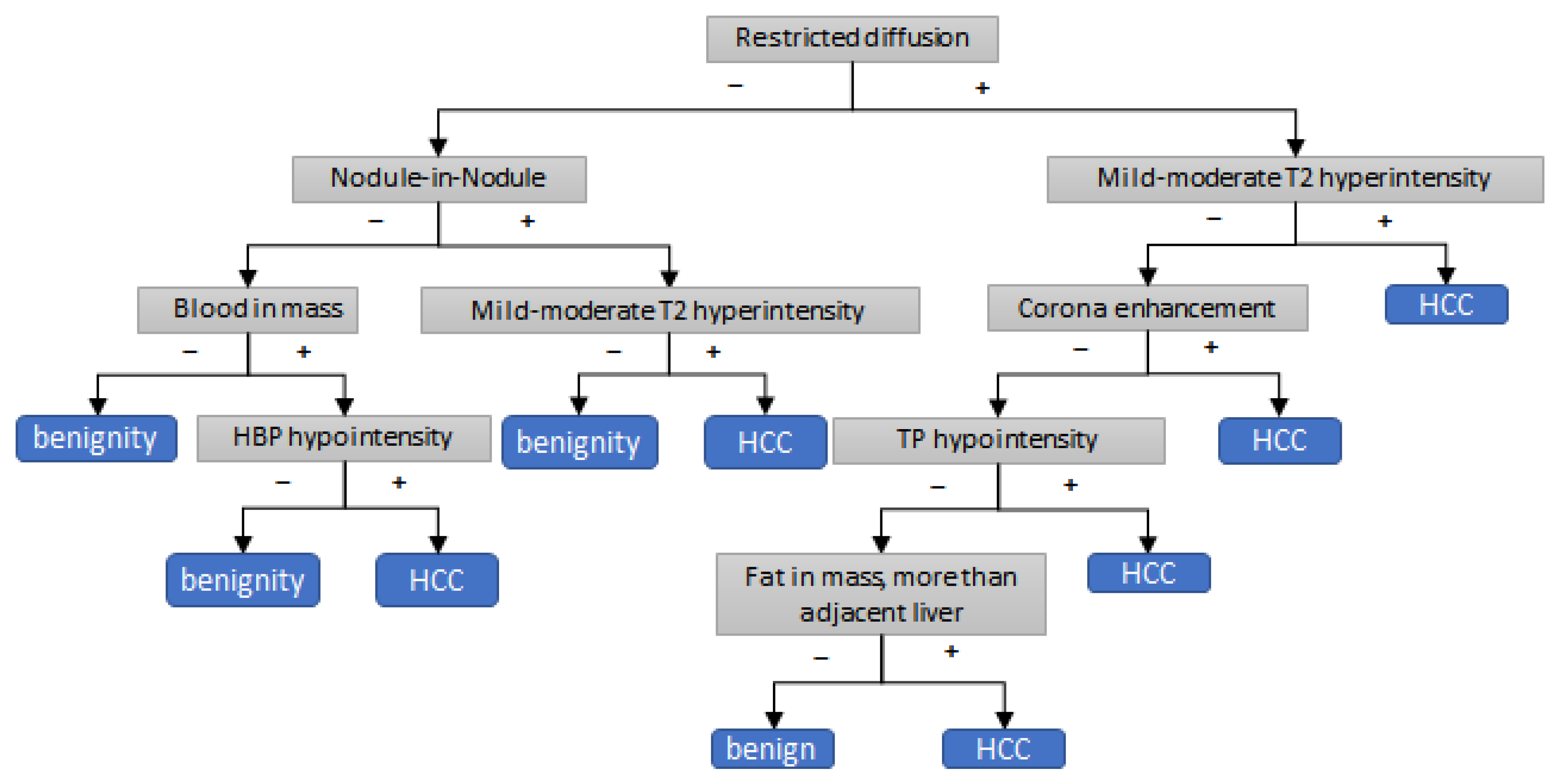
3.3. Development of Decision Tree Algorithm for Application of AFs to LR3 and LR4 Observation
3.4. Comparison of Diagnostic Performance of Decision Tree Algorithm with Alternative Criteria of Applying Afs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sequence | ||||||||
|---|---|---|---|---|---|---|---|---|
| T1 LAVA/VIBE | Dual-echo T1 GRE | Navigator-triggered TSE T2 | DWI † | |||||
| SIGNA Architect | MAGNETOM Vida | SIGNA Architect | MAGNETOM Vida | SIGNA Architect | MAGNETOM Vida | SIGNA Architect | MAGNETOM Vida | |
| Repetition time (ms) | 43.42 | 3.2 | 125 | 164 | 2100 | 1300 | 2600 | 2300 |
| Echo time (ms) | 1 | 1 | 1, OP; 3, IP | 1, OP; 3, IP | 96 | 83 | 67 | 60 |
| Flip angle (°) | 10 | 11 | 60 | 57 | 90 | 120 | 90 | 90 |
| Matrix | 260 × 220 | 352 × 209 | 260 × 220 | 320 × 216 | 260 × 260 | 256 × 216 | 260 × 260 | 130 × 106 |
| Field of view | 360 × 360 | 400 × 338 | 360 × 360 | 400 × 338 | 360 × 360 | 400 × 338 | 360 × 360 | 400 × 326 |
| Section thickness (mm) | 4 | 3 | 5 | 5 | 5 | 5 | 5 | 5 |
| No. of signal acquisitions | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 2 |
| Favoring Malignancy in General, Not HCC in Particular | Agreement Proportion | Kappa Value |
|---|---|---|
| Corona enhancement | 98.1 (269) | 0.66 |
| Restricted diffusion | 78.8 (216) | 0.55 |
| Mild–moderate T2 hyperintensity | 78.8 (216) | 0.55 |
| ron sparing in solid mass | 100 (274) | 0.05 |
| Fat sparing in solid mass | 100 (274) | 0.50 |
| Transitional-phase hypointensity | 85.4 (234) | 0.47 |
| Hepatobiliary-phase hypointensity | 94.2 (258) | 0.74 |
| Favoring HCC in particular | ||
| Nonenhancing capsule | 96.2 (264) | 0.65 |
| Nodule-in-nodule architecture | 82.7 (227) | 0.21 |
| Mosaic architecture | 96.2 (264) | 0.48 |
| Fat in mass, more than adjacent liver | 92.3 (253) | 0.73 |
| Blood products in mass | 96.2 (264) | 0.49 |
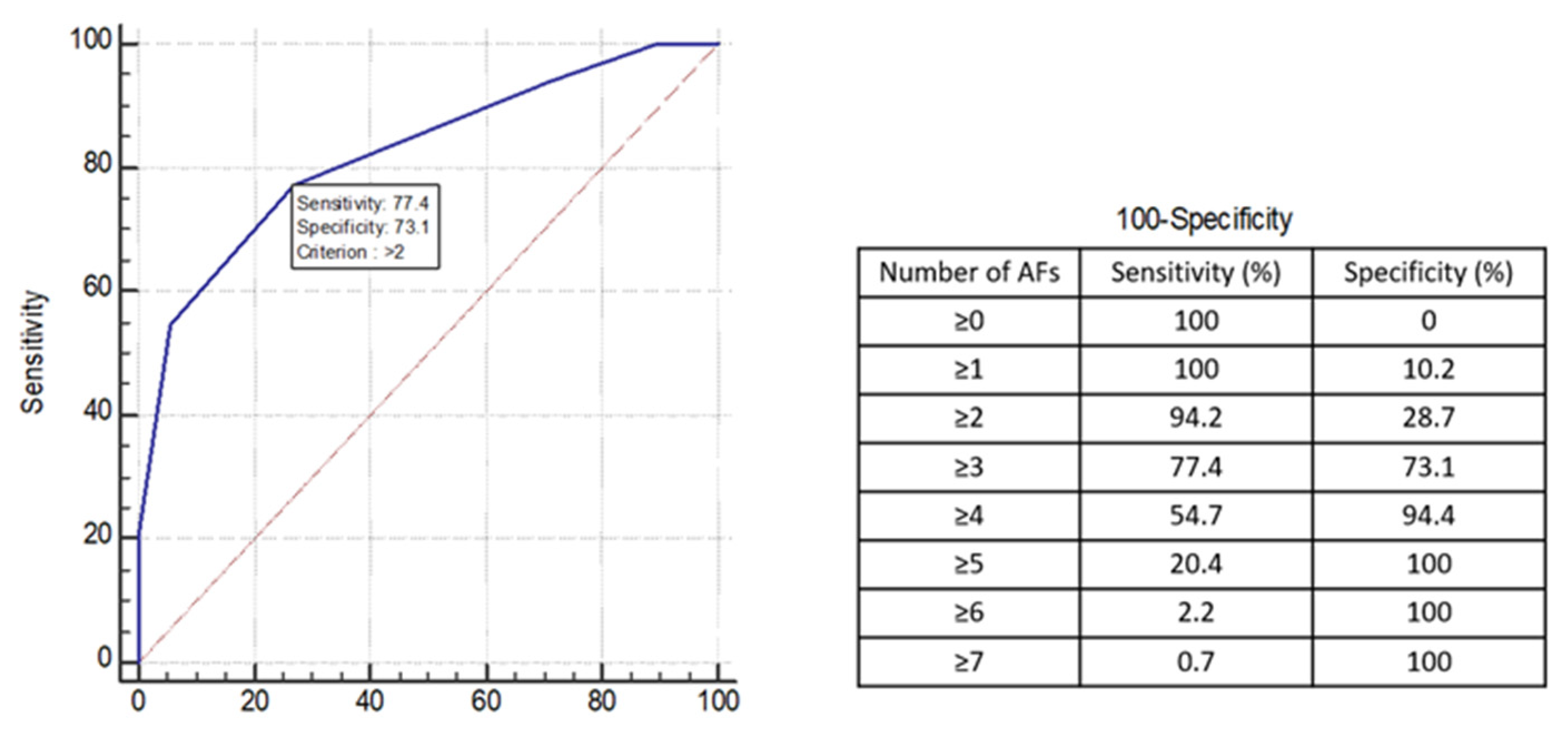
| AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | PPV (95% CI) | NPV 95% CI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | 0.78 (0.77, 0.78) | 64.5% (63.6, 65.4) | 91.3% (90.8, 91.7) | 76.4% (75.7, 77.0) | 90.3% (89.9, 90.7) | 67.2% (66.1, 68.2) | ||||||||||||
| (2) | 0.73 (0.72, 0.73) | 63.1% (61.7, 64.5) | 82.3% (81.4, 83.1) | 71.6% (70.9, 72.3) | 81.7% (80.8, 82.6) | 64.0% (62.8, 65.1) | ||||||||||||
| (3) | 0.76 (0.75, 0.76) | 72.7% (71.8, 73.7) | 78.3% (77.5, 79.0) | 75.2% (74.8, 75.6) | 80.8% (80.0, 81.5) | 69.6% (68.5, 70.6) | ||||||||||||
| (4) | 0.75 (0.74, 0.76) | 54.9% (53.6, 56.1) | 95.3% (94.9, 95.7) | 72.8% (71.9, 73.6) | 93.6% (93.1, 94.1) | 62.7% (61.6, 63.8) | ||||||||||||
| Comparisons of each criterion, p-value | ||||||||||||||||||
| AUC | sensitivity | specificity | accuracy | PPV | NPV | |||||||||||||
| (2) | (3) | (4) | (2) | (3) | (4) | (2) | (3) | (4) | (2) | (3) | (4) | (2) | (3) | (4) | (2) | (3) | (4) | |
| (1) | <0.01 | 0.03 | 0.01 | 0.91 | 0.18 | 0.13 | 0.08 | 0.01 | 0.37 | 0.27 | 0.84 | 0.42 | 0.12 | 0.08 | 0.6 | 0.66 | 0.77 | 0.48 |
| (2) | <0.01 | 0.12 | 0.12 | 0.209 | 0.57 | 0.01 | 0.42 | 0.85 | 0.99 | 0.03 | 0.41 | 0.91 | ||||||
| (3) | 0.35 | 0.003 | <0.01 | 0.62 | 0.02 | 0.27 | ||||||||||||
| True Prediction (n = 200) | False Prediction (n = 45) | p-Value | |
|---|---|---|---|
| Favoring malignancy in general | |||
| Corona enhancement | 3 (1.5) | 1 (2.2) | 0.56 |
| Fat sparing in solid mass | 0 (0.0) | 0 (0.0) | |
| Restricted diffusion | 89 (44.5) | 8 (17.8) | 0.01 |
| Mild–moderate T2 hyperintensity | 93 (46.5) | 12 (26.7) | 0.02 |
| Iron sparing in solid mass | 0 (0.0) | 0 (0.0) | |
| Transitional phase hypointensity | 154 (77.0) | 32 (71.1) | 0.4 |
| Hepatobiliary phase hypointensity | 180 (90.0) | 45 (100.0) | 0.03 |
| Favoring HCC in particular | |||
| Nonenhancing “capsule” | 12 (6.0) | 0 (0.0) | 0.13 |
| Nodule-in-nodule appearance | 12 (6.0) | 1 (2.2) | 0.472 |
| Mosaic architecture | 5 (2.5) | 0 (0.0) | 0.59 |
| Fat in mass, more than adjacent liver | 28 (14.0) | 8 (17.8) | 0.52 |
| Blood products in mass | 5 (2.5) | 0 (0.0) | 0.59 |
References
- American College of Radiology. Liver Imaging Reporting and Data System Version. 2018. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS (accessed on 1 November 2022).
- Cerny, M.; Chernyak, V.; Olivié, D.; Billiard, J.S.; Murphy-Lavallée, J.; Kielar, A.Z.; Elsayes, K.M.; Bourque, L.; Hooker, J.C.; Sirlin, C.B.; et al. LI-RADS Version 2018 Ancillary Features at MRI. Radiographics 2018, 38, 1973–2001. [Google Scholar] [CrossRef]
- van der Pol, C.B.; Lim, C.S.; Sirlin, C.B.; McGrath, T.A.; Salameh, J.P.; Bashir, M.R.; Tang, A.; Singal, A.G.; Costa, A.F.; Fowler, K.; et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy—A Systematic Review. Gastroenterology 2019, 156, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Boatright, C.; Peterson, J.; Williams, V.L.; Best, S.; Ash, R. LI-RADS v2018: Utilizing ancillary features on gadoxetate-enhanced MRI to modify final LI-RADS category. Abdom. Radiol. 2020, 45, 3136–3143. [Google Scholar] [CrossRef] [PubMed]
- Cerny, M.; Bergeron, C.; Billiard, J.S.; Murphy-Lavallée, J.; Olivié, D.; Bérubé, J.; Fan, B.; Castel, H.; Turcotte, S.; Perreault, P.; et al. LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features. Radiology 2018, 288, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Lee, J.M.; Lee, D.H.; Ahn, S.J.; Lee, E.S.; Han, J.K. Liver imaging reporting and data system v2014 categorization of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: Comparison with multiphasic multidetector computed tomography. J. Magn. Reson. Imaging 2017, 45, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, A.; Cerny, M.; Olivié, D.; Billiard, J.-S.; Bergeron, C.; Brown, K.; Bodson-Clermont, P.; Castel, H.; Turcotte, S.; Perreault, P. LI-RADS for CT diagnosis of hepatocellular carcinoma: Performance of major and ancillary features. Abdom. Radiol. 2019, 44, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Kim, J.M.; Kim, Y.K.; Kang, T.W.; Lee, S.J.; Choi, G.S.; Choi, S.Y.; Ahn, S. Prospective intraindividual comparison of magnetic resonance imaging with gadoxetic acid and extracellular contrast for diagnosis of hepatocellular carcinomas using the Liver Imaging Reporting and Data System. Hepatology 2018, 68, 2254–2266. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.S.; Bae, H.; Shin, J.; Yoon, J.K.; Kim, M.J. Application of Liver Imaging Reporting and Data System version 2018 ancillary features to upgrade from LR-4 to LR-5 on gadoxetic acid-enhanced MRI. Eur. Radiol. 2021, 31, 855–863. [Google Scholar] [CrossRef]
- Cannella, R.; Vernuccio, F.; Sagreiya, H.; Choudhury, K.R.; Iranpour, N.; Marin, D.; Furlan, A. Liver Imaging Reporting and Data System (LI-RADS) v2018: Diagnostic value of ancillary features favoring malignancy in hypervascular observations ≥ 10 mm at intermediate (LR-3) and high probability (LR-4) for hepatocellular carcinoma. Eur. Radiol. 2020, 30, 3770–3781. [Google Scholar] [CrossRef]
- Kang, J.H.; Choi, S.H.; Byun, J.H.; Kim, D.H.; Lee, S.J.; Kim, S.Y.; Won, H.J.; Shin, Y.M.; Kim, P.N. Ancillary features in the Liver Imaging Reporting and Data System: How to improve diagnosis of hepatocellular carcinoma ≤ 3 cm on magnetic resonance imaging. Eur. Radiol. 2020, 30, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Nevin, L. Advancing the beneficial use of machine learning in health care and medicine: Toward a community understanding. PLoS Med. 2018, 15, e1002708. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, Z.; Emanuel, E.J. Predicting the Future—Big Data, Machine Learning, and Clinical Medicine. N. Engl. J. Med. 2016, 375, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Song, J.S.; Choi, E.J.; Choi, H.; Yang, J.D.; Moon, W.S. Hypovascular hypointense nodules in hepatobiliary phase without T2 hyperintensity: Long-term outcomes and added value of DWI in predicting hypervascular transformation. Clin. Imaging 2018, 50, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; An, C.; Kim, S.; Kim, M.J. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. Eur. Radiol. 2018, 28, 2038–2046. [Google Scholar] [CrossRef]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular carcinoma tumour volume doubling time: A systematic review and meta-analysis. Gut 2021, 70, 401–407. [Google Scholar] [CrossRef]
- Géron, A. Hands-On Machine Learning with Scikit-Learn, Keras, and TensorFlow; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2022. [Google Scholar]
- Gael, V.A.; Vincent, M.G. Scikit-Learn Machine Learning in Python. Available online: https://scikit-learn.org/stable/ (accessed on 1 November 2022).
- Shankar, S.; Kalra, N.; Bhatia, A.; Srinivasan, R.; Singh, P.; Dhiman, R.K.; Khandelwal, N.; Chawla, Y. Role of Diffusion Weighted Imaging (DWI) for Hepatocellular Carcinoma (HCC) Detection and its Grading on 3T MRI: A Prospective Study. J. Clin. Exp. Hepatol. 2016, 6, 303–310. [Google Scholar] [CrossRef]
- Cha, D.I.; Jang, K.M.; Kim, S.H.; Kang, T.W.; Song, K.D. Liver Imaging Reporting and Data System on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur. Radiol. 2017, 27, 4394–4405. [Google Scholar] [CrossRef]
- Basha, M.A.A.; Refaat, R.; Mohammad, F.F.; Khamis, M.E.M.; El-Maghraby, A.M.; El Sammak, A.A.; Al-Molla, R.M.; Mohamed, H.A.E.; Alnaggar, A.A.; Hassan, H.A.; et al. The utility of diffusion-weighted imaging in improving the sensitivity of LI-RADS classification of small hepatic observations suspected of malignancy. Abdom. Radiol. 2019, 44, 1773–1784. [Google Scholar] [CrossRef]
- Bruegel, M.; Holzapfel, K.; Gaa, J.; Woertler, K.; Waldt, S.; Kiefer, B.; Stemmer, A.; Ganter, C.; Rummeny, E.J. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur. Radiol. 2008, 18, 477–485. [Google Scholar] [CrossRef]
- Parikh, T.; Drew, S.J.; Lee, V.S.; Wong, S.; Hecht, E.M.; Babb, J.S.; Taouli, B. Focal liver lesion detection and characterization with diffusion-weighted MR imaging: Comparison with standard breath-hold T2-weighted imaging. Radiology 2008, 246, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Ichikawa, T.; Motosugi, U.; Sano, K.; Morisaka, H.; Enomoto, N.; Matsuda, M.; Fujii, H. Was Hypervascular Hepatocellular Carcinoma Visible on Previous Gadoxetic Acid-Enhanced Magnetic Resonance Images? Liver Cancer 2015, 4, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kim, Y.K.; Jeong, W.K.; Choi, D.; Rhim, H.; Lee, W.J. Nonhypervascular Hypointense Nodules at Gadoxetic Acid-enhanced MR Imaging in Chronic Liver Disease: Diffusion-weighted Imaging for Characterization. Radiology 2015, 276, 137–146. [Google Scholar] [CrossRef]
- Matsui, O.; Kadoya, M.; Kameyama, T.; Yoshikawa, J.; Arai, K.; Gabata, T.; Takashima, T.; Nakanuma, Y.; Terada, T.; Ida, M. Adenomatous hyperplastic nodules in the cirrhotic liver: Differentiation from hepatocellular carcinoma with MR imaging. Radiology 1989, 173, 123–126. [Google Scholar] [CrossRef]
- Joo, I.; Kim, S.Y.; Kang, T.W.; Kim, Y.K.; Park, B.J.; Lee, Y.J.; Choi, J.I.; Lee, C.H.; Park, H.S.; Lee, K.; et al. Radiologic-Pathologic Correlation of Hepatobiliary Phase Hypointense Nodules without Arterial Phase Hyperenhancement at Gadoxetic Acid-enhanced MRI: A Multicenter Study. Radiology 2020, 296, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Vernuccio, F.; Cannella, R.; Meyer, M.; Choudhoury, K.R.; Gonzáles, F.; Schwartz, F.R.; Gupta, R.T.; Bashir, M.R.; Furlan, A.; Marin, D. LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10–19 mm with Arterial Phase Hyperenhancement. AJR Am. J. Roentgenol. 2019, 213, W57–W65. [Google Scholar] [CrossRef]
- Renzulli, M.; Biselli, M.; Brocchi, S.; Granito, A.; Vasuri, F.; Tovoli, F.; Sessagesimi, E.; Piscaglia, F.; D’Errico, A.; Bolondi, L.; et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: A new diagnostic algorithm. Gut 2018, 67, 1674–1682. [Google Scholar] [CrossRef]
- Kim, B.R.; Lee, J.M.; Lee, D.H.; Yoon, J.H.; Hur, B.Y.; Suh, K.S.; Yi, N.J.; Lee, K.B.; Han, J.K. Diagnostic Performance of Gadoxetic Acid-enhanced Liver MR Imaging versus Multidetector CT in the Detection of Dysplastic Nodules and Early Hepatocellular Carcinoma. Radiology 2017, 285, 134–146. [Google Scholar] [CrossRef]
- Jeon, S.K.; Joo, I.; Bae, J.S.; Park, S.J.; Lee, J.M. LI-RADS v2018: How to appropriately use ancillary features in category adjustment from intermediate probability of malignancy (LR-3) to probably HCC (LR-4) on gadoxetic acid-enhanced MRI. Eur. Radiol. 2022, 32, 46–55. [Google Scholar] [CrossRef]
- Tang, A.; Cruite, I.; Mitchell, D.G.; Sirlin, C.B. Hepatocellular carcinoma imaging systems: Why they exist, how they have evolved, and how they differ. Abdom. Radiol. 2018, 43, 3–12. [Google Scholar] [CrossRef]
- Fowler, K.J.; Sirlin, C.B. Is It Time to Expand the Definition of Washout Appearance in LI-RADS? Radiology 2019, 291, 658–659. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Development Group | Test Group | |
|---|---|---|---|
| Age * | 62.8 ± 8.4 | 64.4 ± 10.6 | |
| Sex | Male | 132 (79.0) | 17 (85.0) |
| Female | 35 (23.) | 3 (15.0) | |
| Underlying disease | Hepatitis B with/without cirrhosis | 117 (77.0) | 13 (65.0) |
| Hepatitis C with cirrhosis | 10 (6.6) | 2 (10.0) | |
| Alcoholic liver disease with cirrhosis | 30 (19.7) | 4 (20.0) | |
| NASH with cirrhosis | 3 (2.0) | 0 (0.0) | |
| Cryptogenic cirrhosis | 7 (4.6) | 1 (5.0) | |
| Laboratory † | AST (IU/L) | 36 (27.0, 52.5) | 46 (25, 96) |
| ALT (IU/L) | 28 (19.0, 41.8) | 28 (6, 148) | |
| Total bilirubin (mg/dL) | 0.8 (0.5, 1.2) | 1.2 (0.4, 5.7) | |
| Prothrombin time (s) | 12.7 (12.0, 13.6) | 13.3 (12, 22) | |
| Platelet (×1000/μL) | 123 (82.5, 177.5) | 116 (37, 299) | |
| AFP (ng/mL) | 7.1 (3.5, 26.7) | 10.4 (1.8, 239) | |
| No. of nodules included in the analysis | 1 | 112 (73.7) | 13 (65.0) |
| 2 | 36 (23.7) | 4 (20.0) | |
| 3 | 16 (10.5) | 3 (15.0) | |
| 4 | 2 (1.3) | ||
| 5 | 1 (0.7) | ||
| LiRADS category | LR3 | 199 (81.2) | 20 (66.7) |
| LR4 | 46 (18.8) | 10 (33.3) | |
| Major feature | Size † | 13 (10.0, 17.8) | 11 (6, 23) |
| APHE | 96 (39.2) | 20 (66.7) | |
| Non-peripheral washout | 74 (30.2) | 9 (30.0) | |
| Enhancing capsule | 15 (6.1) | 6 (20.0) | |
| Reference standard | Pathologic diagnosis | 41 (16.7) | 11 (36.7) |
| Imaging follow-up | 204 (83.3) | 19 (63.3) | |
| Final diagnosis | HCC | 137 (55.9) | 16 (53.3) |
| Non-malignant nodule | 108 (44.1) | 14 (46.7) | |
| Development Group | Test Group | |||||
|---|---|---|---|---|---|---|
| HCC (n = 137) | Non-Malignant Nodules (n = 108) | p-Value | HCC (n = 16) | Non-Malignant Nodules (n = 14) | p-Value | |
| Favoring malignancy in general | ||||||
| Corona enhancement | 3 (2.2) | 1 (0.9) | 0.633 | 1 (6.3) | 0 (0.0) | 1.0 |
| Fat sparing in solid mass | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Restricted diffusion | 88 (64.2) | 9 (8.3) | <0.001 | 13 (81.3) | 0 (0) | <0.001 |
| Mild–moderate T2 hyperintensity | 86 (62.8) | 19 (17.6) | <0.001 | 14 (87.5) | 1 (7.1) | <0.001 |
| Iron sparing in solid mass | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Transitional phase hypointensity | 113 (82.5) | 73 (67.6) | 0.007 | 9 (56.3) | 11 (78.6) | 0.20 |
| Hepatobiliary phase hypointensity | 131 (95.6) | 94 (87.0) | 0.015 | 12 (75.0) | 12 (85.7) | 0.46 |
| Favoring HCC in particular | ||||||
| Nonenhancing “capsule” | 11 (8.0) | 1 (0.9) | 0.011 | 0 (0.0) | 0 (0.0) | NA |
| Nodule-in-nodule appearance | 12 (8.8) | 1 (0.9) | 0.007 | 1 (6.3) | 0 (0.0) | 1.0 |
| Mosaic architecture | 5 (3.6) | 0 (0.0) | 0.069 | 2 (12.5) | 0 (0.0) | 0.53 |
| Fat in mass, more than adjacent liver | 25 (18.2) | 11 (10.2) | 0.077 | 1 (6.3) | 3 (21.4) | 0.50 |
| Blood products in mass | 5 (3.6) | 0 (0.0) | 0.069 | 1 (6.3) | 0 (0.0) | 1.0 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Favoring malignancy in general | ||||
| Corona enhancement | 2.4 (0.2, 23.4) | 0.452 | ||
| Fat sparing in solid mass | NA | |||
| Restricted diffusion | 19.8 (9.2, 42.5) | <0.001 | 12.4 (5.1, 30.3) | <0.001 |
| Mild–moderate T2 hyperintensity | 7.9 (4.3, 14.5) | <0.001 | 2.5 (1.1, 5.3) | 0.022 |
| Iron sparing in solid mass | NA | |||
| Transitional phase hypointensity | 2.3 (1.2, 4.1) | 0.008 | 0.9 (0.4, 2.1) | 0.883 |
| Hepatobiliary phase hypointensity | 3.3 (1.2, 8.8) | 0.020 | 5.5 (0.9, 32.1) | 0.057 |
| Favoring HCC in particular | ||||
| Nonenhancing “capsule” | 9.3 (1.2, 73.5) | 0.034 | 15.8 (0.8, 318.2) | 0.072 |
| Nodule-in-nodule appearance | 10.3 (1.3, 80.3) | 0.026 | 16.5 (0.9, 142.1) | 0.051 |
| Mosaic architecture | 1,321,752,144.2 (0.0) | 0.999 | ||
| Fat in mass, more than adjacent liver | 2.0 (0.9, 4.2) | 0.081 | ||
| Blood products in mass | 1,321,752,144.2 (0.0) | 0.999 | ||
| AUC (95% CI) | Sensitivity (9% CI) | Specificity (95% CI) | Accuracy (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| I. Decision tree algorithm | ||||||
| Development cohort | 0.84 (0.84, 0.85) | 92.0% (91.6, 92.4) | 71.1% (70.9, 71.4) | 84.5% (84.1, 84.8) | 70.5% (70.2, 70.9) | 92.2% (91.8, 92.7) |
| Test cohort | 0.82 (0.76–0.88), | 94.0% (89.3–98.2) | 68.7% (58.0–79.4) | 81.9% (75.8–87.9) | 70.7% (63.0–78.5) | 92.3% (87.5–97.2) |
| II. Number of AFs ≥ 3 | 0.75 (0.75, 0.76) | 77.6% (76.4, 78.8) | 72.9% (72.2, 73.7) | 75.5% (75.0, 76.1) | 78.3% (77.4, 79.1) | 72.2% (70.9, 73.6) |
| III. Restricted DWI | 0.78 (0.77, 0.78) | 64.5% (63.6, 65.4) | 91.3% (90.8, 91.7) | 76.4% (75.7, 77.0) | 90.3% (89.9, 90.7) | 67.2% (66.1, 68.2) |
| Comparison of each approach for applying AFs | ||||||
| I vs. II | p < 0.001 | p = 0.002 | p = 0.886 | p = 0.017 | p = 0.216 | p < 0.001 |
| I vs. III | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.032 | p < 0.001 | p < 0.001 |
| II vs. III | p = 0.011 | p = 0.024 | p < 0.001 | p = 0.899 | p = 0.025 | p = 0.469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Byun, J.; Hwang, S.M. Utilization of a Machine Learning Algorithm for the Application of Ancillary Features to LI-RADS Categories LR3 and LR4 on Gadoxetate Disodium-Enhanced MRI. Cancers 2023, 15, 1361. https://doi.org/10.3390/cancers15051361
Park S, Byun J, Hwang SM. Utilization of a Machine Learning Algorithm for the Application of Ancillary Features to LI-RADS Categories LR3 and LR4 on Gadoxetate Disodium-Enhanced MRI. Cancers. 2023; 15(5):1361. https://doi.org/10.3390/cancers15051361
Chicago/Turabian StylePark, Seongkeun, Jieun Byun, and Sook Min Hwang. 2023. "Utilization of a Machine Learning Algorithm for the Application of Ancillary Features to LI-RADS Categories LR3 and LR4 on Gadoxetate Disodium-Enhanced MRI" Cancers 15, no. 5: 1361. https://doi.org/10.3390/cancers15051361
APA StylePark, S., Byun, J., & Hwang, S. M. (2023). Utilization of a Machine Learning Algorithm for the Application of Ancillary Features to LI-RADS Categories LR3 and LR4 on Gadoxetate Disodium-Enhanced MRI. Cancers, 15(5), 1361. https://doi.org/10.3390/cancers15051361






