Body Composition in Patients with Follicular Lymphoma: Asso-Ciations between Changes in Radiomic Parameters in Patients Treated with R-CHOP-like and R-B Regimens: LyRa 01F
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Radiomics Parameters
2.3. Statistical Analysis
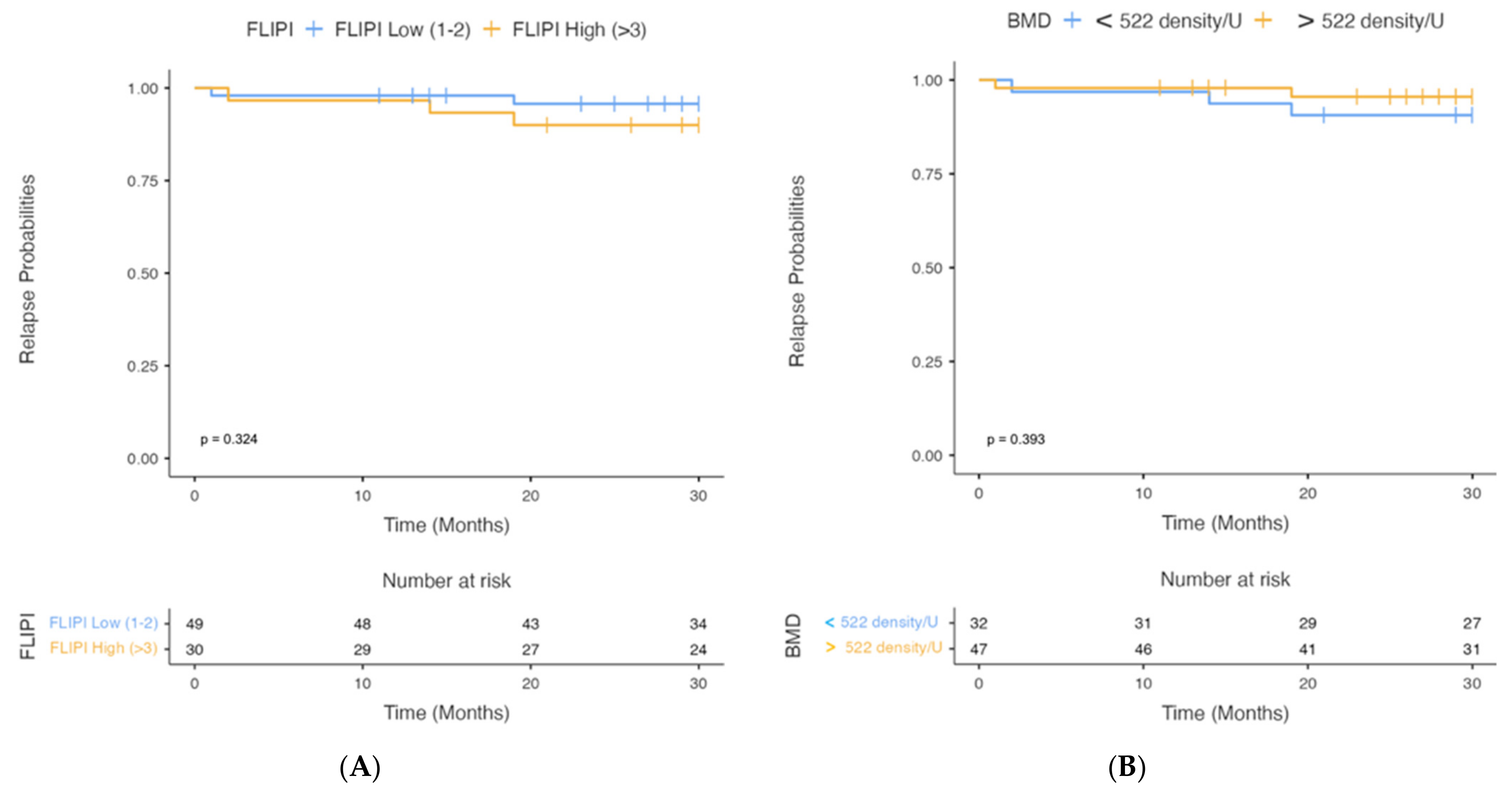
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polyatskin, I.L.; Artemyeva, A.S.; Krivolapov, Y.A. Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017 (4th edition): Lymphoid tumors. Arkhiv Patol. 2019, 81, 59–65. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The updated WHO Classification of hematological malignancies. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Dreyling, M.; Ghielmini, M.; Rule, S.; Salles, G.; Ladetto, M.; Tonino, S.H.; Herfarth, K.; Seymour, J.; Jerkeman, M. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 298–308. [Google Scholar] [CrossRef]
- Freedman, A.; Jacobsen, E. Follicular lymphoma: 2020 update on diagnosis and management. Am. J. Hematol. 2020, 95, 316–327. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.D.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Solal-Céligny, P.; Roy, P.; Colombat, P.; White, J.; Armitage, J.O.; Arranz-Saez, R.; Au, W.Y.; Bellei, M.; Brice, P.; Caballero, D.; et al. Follicular lymphoma international prognostic index. Blood 2004, 104, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Maurer, M.J.; Habermann, T.M.; Gelas-Dore, B.; Maucort-Boulch, D.; Estell, J.A.; Neste, E.V.D.; Bouabdallah, R.; Gyan, E.; Feldman, A.L.; et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood 2018, 132, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hiddemann, W.; Kneba, M.; Dreyling, M.; Schmitz, N.; Lengfelder, E.; Schmits, R.; Reiser, M.; Metzner, B.; Harder, H.; Hegewisch-Becker, S.; et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005, 106, 3725–3732. [Google Scholar]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grünhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210. [Google Scholar] [CrossRef]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.S.; Wood, P.; Hawkins, T.E.; Macdonald, D.; Hertzberg, M.; Kwan, Y.-L.; Simpson, D.M.; Craig, M.; et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014, 123, 2944–2952. [Google Scholar] [CrossRef]
- Barrington, S.; Barrington, S.F.; Trotman, J. The role of PET in the first-line treatment of the most common subtypes of non-Hodgkin lymphoma. Lancet Haematol. 2021, 8, e80–e93. [Google Scholar] [CrossRef]
- Jang, S.; Graffy, P.M.; Ziemlewicz, T.J.; Lee, S.J.; Summers, R.M.; Pickhardt, P.J. Opportunistic osteoporosis screening at routine abdominal and Thoracic CT: Normative L1 trabecular attenuation values in more than 20,000 adults. Radiology 2019, 291, 360–367. [Google Scholar] [CrossRef]
- Halpenny, D.; Goncalves, M.; Schwitzer, E.; Golia Pernicka, J.; Jackson, J.; Gandelman, S.; Moskowitz, C.S.; Postow, M.; Mourtzakis, M.; Caan, B.; et al. Computed tomography-derived assessments of regional muscle volume: Validating their use as predictors of whole body muscle volume in cancer patients. Br. J. Radiol. 2018, 91, 20180451. [Google Scholar] [CrossRef]
- Daly, L.E.; Prado, C.M.; Ryan, A.M. A window beneath the skin: How computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc. Nutr. Soc. 2018, 77, 135–151. [Google Scholar] [CrossRef]
- Mamot, C.; Klingbiel, D.; Hitz, F.; Renner, C.; Pabst, T.; Driessen, C.; Mey, U.; Pless, M.; Bargetzi, M.; Krasniqi, F.; et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J. Clin. Oncol. 2015, 33, 2523–2529. [Google Scholar] [CrossRef]
- Pregno, P.; Chiappella, A.; Bellò, M.; Botto, B.; Ferrero, S.; Franceschetti, S.; Giunta, F.; Ladetto, M.; Limerutti, G.; Menga, M.; et al. Interim 18-FDG-PET/CT failed to predict the outcome in diffuse large B-cell lymphoma patients treated at the diagnosis with rituximab-CHOP. Blood 2012, 119, 2066–2073. [Google Scholar] [CrossRef]
- Verhoef, G.; Spaepen, K.; Stroobants, S.; Dupont, P.; Vandenberghe, P.; Thomas, J.; de Groot, T.; Balzarini, J.; De Wolf-Peeters, C.; Mortelmans, L.; et al. Early restaging positron emission tomography with 18F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann. Oncol. 2002, 13, 1356–1363. [Google Scholar]
- Cottereau, A.S.; Hapdey, S.; Chartier, L.; Modzelewski, R.; Casasnovas, O.; Itti, E.; Tilly, H.; Vera, P.; Meignan, M.A.; Becker, S. Baseline total metabolic tumor volume measured with fixed or different adaptive thresholding methods equally predicts outcome in peripheral T cell lymphoma. J. Nucl. Med. 2017, 58, 276–281. [Google Scholar] [CrossRef]
- Dupuis, J.; Berriolo-Riedinger, A.; Julian, A.; Brice, P.; Tychyj-Pinel, C.; Tilly, H.; Mounier, N.; Gallamini, A.; Feugier, P.; Soubeyran, P.; et al. Impact of [18F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: A prospective study from the Groupe d’Etudes des Lymphomes de l’Adulte and GOELAMS. J. Clin. Oncol. 2012, 30, 4317–4322. [Google Scholar] [CrossRef]
- Bodden, J.; Sun, D.; Joseph, G.B.; Huang, L.W.; Andreadis, C.; Hughes-Fulford, M.; Lang, T.F.; Link, T.M. Identification of non-Hodgkin lymphoma patients at risk for treatment-related vertebral density loss and fractures. Osteoporos. Int. 2021, 32, 281–291. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Lu, H.; Fang, S.; Du, X.L. Elderly patients with non-Hodgkin lymphoma who receive chemotherapy are at higher risk for osteoporosis and fractures. Leuk. Lymphoma 2007, 48, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Merlusca, L.; Henry-Desailly, I.; Parcelier, A.; Gruson, B.; Royer, B.; Charbonnier, A.; Ursu, D.; Desailloud, R.; Garidi, R.; et al. Alterations in bone mineral density and bone turnover markers in newly diagnosed adults with lymphoma receiving chemotherapy: A 1-year prospective pilot study. Ann. Oncol. 2014, 25, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, P.; Shekhrajka, N.; Nielsen, K.L.; Vestergaard, P.; Poulsen, M.Ø.; Vistisen, A.K.; Munksgaard, P.S.; Severinsen, M.T.; Jensen, P.; Johnsen, H.E.; et al. R-CHOP(-like) treatment of diffuse large B-cell lymphoma significantly reduces CT-assessed vertebral bone density: A single center study of 111 patients. Leuk. Lymphoma 2017, 58, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Anargyrou, K.; Fotiou, D.; Vassilakopoulos, T.P.; Christoulas, D.; Makras, P.; Dimou, M.; Ntanasis-Stathopoulos, I.; Masouridou, S.; Angelopoulou, M.K.; Papatheodorou, A.; et al. Low Bone Mineral Density and High Bone Turnover in Patients With Non-Hodgkin’s Lymphoma (NHL) Who Receive Frontline Therapy: Results of a Multicenter Prospective Study. Hemasphere 2019, 3, e303. [Google Scholar] [CrossRef]
- Xiao, D.Y.; Luo, S.; O’Brian, K.; Ganti, A.; Riedell, P.; Sanfilippo, K.M.; Lynch, R.C.; Liu, W.; Carson, K.R. Impact of sarcopenia on treatment tolerance in United States veterans with diffuse large B-cell lymphoma treated with CHOP-based chemotherapy. Am. J. Hematol. 2016, 91, 1002–1007. [Google Scholar] [CrossRef]
- Montoto, S.; Fitzgibbon, J. Transformation of indolent B-cell lymphomas. J. Clin. Oncol. 2011, 29, 1827–1834. [Google Scholar] [CrossRef]
- Chihara, D.; Oki, Y.; Onoda, H.; Taji, H.; Yamamoto, K.; Tamaki, T.; Morishima, Y. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int. J. Hematol. 2011, 93, 502–508. [Google Scholar] [CrossRef]
- Ceriani, L.; Milan, L.; Cascione, L.; Gritti, G.; Dalmasso, F.; Esposito, F.; Pirosa, M.C.; Schär, S.; Bruno, A.; Dirnhofer, S.; et al. Generation and validation of a PET radiomics model that predicts survival in diffuse large B cell lymphoma treated with R-CHOP14: A SAKK 38/07 trial post-hoc analysis. Hematol. Oncol. 2022, 40, 11–21. [Google Scholar] [CrossRef]
- Kim, C.Y.; Hong, C.M.; Kim, D.H.; Son, S.H.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.-C. Prognostic value of whole-body metabolic tumour volume and total lesion glycolysis measured on 18F-FDG PET/CT in patients with extranodal NK/T-cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1321–1329. [Google Scholar] [CrossRef]
- Ceriani, L.; Martelli, M.; Zinzani, P.L.; Andr’, A.; Ferreri, A.J.M.; Botto, B.; Stelitano, C.; Gotti, M.; Cabras, M.G.; Rigacci, L.; et al. Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 2015, 126, 950–956. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Peng, X.; Chen, B.; Pentassuglia, L.; Lim, C.C. Mechanisms of anthracycline cardiac injury: Can we identify strategies for cardioprotection? Prog. Cardiovasc. Dis. 2010, 53, 105–113. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Chotiyarnwong, P.; McCloskey, E.v. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 2020, 16, 437–447. [Google Scholar] [CrossRef]
- Haugeberg, G.; Uhlig, T.; Falch, J.A.; Halse, J.I.; Kvien, T.K. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis Results from 394 Patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheumatol. 2000, 43, 522–530. [Google Scholar] [CrossRef]
- Sinigaglia, L.; Nervetti, A.; Mela, Q.; Bianchi, G.; del Puente, A.; di Munno, O.; Frediani, B.; Cantatore, F.; Pellerito, R.; Bartolone, S.; et al. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J. Rheumatol. 2000, 27, 2582–2589. [Google Scholar]
- Gravallese, E.M.; Goldring, S.R. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheumatol. 2000, 43, 2143–2151. [Google Scholar] [CrossRef]
- Kong, Y.; Feige, U.; Sarosi, I.; Bolon, B.; Tafuri, A.; Morony, S.; Capparelli, C.; Li, J.; Elliott, R.; McCabe, S.; et al. Activated T cells regulatebone loss and joint destructionin adjuvant arthritis throughosteoprotegerin ligand. Nature 1999, 402, 304–309. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Taxel, P.; Kaneko, H.; Lee, S.K.; Aguila, H.L.; Raisz, L.G.; Lorenzo, J.A. Estradiol rapidly inhibits osteoclastogenesis and RANKL expression in bone marrow cultures in postmenopausal women: A pilot study. Osteoporos. Int. 2008, 19, 193–199. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc. Natl. Acad. Sci. USA 2004, 101, 16711–16712. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Heulsmann, A.; Tondravi, M.M.; Mukherjee, A.; Abu-Amer, Y. Tumor necrosis factor-α (TNF) stimulates RANKL-induced osteoclastogenesis via coupling of TNF type 1 receptor and RANK signaling pathways. J. Biol. Chem. 2001, 276, 563–568. [Google Scholar] [CrossRef] [PubMed]
- O’Gradaigh, D.; Ireland, D.; Bord, S.; Compston, J.E. Joint erosion in rheumatoid arthritis: Interactions between tumour necrosis factor α, interleukin 1, and receptor activator of nuclear factor κB ligand (RANKL) regulate osteoclasts. Ann. Rheum. Dis. 2004, 63, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, X.; He, C. Pro-inflammatory Cytokines: Cellular and Molecular Drug Targets for Glucocorticoid-induced-osteoporosis via Osteocyte. Curr. Drug Targets 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, B.; Maurer, M.J.; Ansell, S.M.; Slager, S.L.; Fredericksen, Z.S.; Ziesmer, S.C.; Macon, W.R.; Habermann, T.M.; Witzig, T.E.; Link, B.K.; et al. Pretreatment circulating serum cytokines associated with follicular and diffuse large B-cell lymphoma: A clinic-based case-control study. Cytokine 2012, 60, 882–889. [Google Scholar] [CrossRef]
- Duletić-Načinović, A.; Sever-Prebelić, M.; Štifter, S.; Jonjić, N.; Hasan, M.; Labar, B. Interleukin-6 in Patients with Aggressive and Indolent Non-Hodgkin’s Lymphoma: A Predictor of Prognosis? Clin. Oncol. 2006, 18, 367–368. [Google Scholar] [CrossRef]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar] [CrossRef]
- Khalifa, K.A.; Alkilani, A.A.; Ismail, H.; Soliman, M.A. Evaluation of some biochemical markers as prognostic factors in malignant lymphoma. J. Egypt. Natl. Cancer Inst. 2008, 20, 47–54. [Google Scholar]
- Aschebrook-Kilfoy, B.; Zheng, T.; Foss, F.; Ma, S.; Han, X.; Lan, Q.; Holford, T.; Chen, Y.; Leaderer, B.; Rothman, N.; et al. Polymorphisms in immune function genes and non-Hodgkin lymphoma survival. J. Cancer Surviv. 2012, 6, 102–114. [Google Scholar] [CrossRef]
- Mozas, P.; Rivas-Delgado, A.; Rivero, A.; Dlouhy, I.; Nadeu, F.; Balagué, O.; González-Farré, B.; Baumann, T.; Giné, E.; Delgado, J.; et al. High serum levels of IL-2R, IL-6, and TNF-α are associated with higher tumor burden and poorer outcome of follicular lymphoma patients in the rituximab era. Leuk. Res. 2020, 94, 106371. [Google Scholar] [CrossRef]
- Fayad, L.; Cabanillas, F.; Talpaz, M.; Mclaughlin, P.; Kurzrock, R. High serum interleukin-6 levels correlate with a shorter failure-free survival in indolent lymphoma. Leuk. Lymphoma 1998, 30, 563–571. [Google Scholar] [CrossRef]
- Niederer, D.; Schmidt, K.; Vogt, L.; Egen, J.; Klingler, J.; Hübscher, M.; Thiel, C.; Bernhörster, M.; Banzer, W. Functional capacity and fear of falling in cancer patients undergoing chemotherapy. Gait Posture 2014, 39, 865–869. [Google Scholar] [CrossRef]
- Richardson, J.K.; Hurvitz, E.A. Peripheral Neuropathy: A True Risk Factor for Falls. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, M211–M215. [Google Scholar] [CrossRef]
- Anagnostis, P.; Athyros, V.G.; Tziomalos, K.; Karagiannis, A.; Mikhailidis, D.P. The pathogenetic role of cortisol in the metabolic syndrome: A hypothesis. J. Clin. Endocrinol. Metab. 2009, 94, 2692–2701. [Google Scholar] [CrossRef]
- Collins, L.; Ann Zarzabal, L.; Nayiager, T.; Pollock, B.H.; Barr, R.D. Growth in Children with Acute Lymphoblastic Leukemia during Treatment. J. Pediatr. Hematol. Oncol. 2010, 32, e304–e307. [Google Scholar] [CrossRef]
- Mckay, L.I.; Cidlowski, J.A. Molecular Control of Immune/Inflammatory Responses: Interactions between Nuclear Factor-B and Steroid Receptor-Signaling Pathways. Endocr. Rev. 1999, 20, 435–459. [Google Scholar]
- Oakley, R.H.; Sar, M.; Cidlowski, J.A. The human glucocorticoid receptor β isoform: Expression, biochemical properties, and putative function. J. Biol. Chem. 1996, 271, 9550–9559. [Google Scholar] [CrossRef]
- Starkie, R.L.; Arkinstall, M.J.; Koukoulas, I.; Hawley, J.A.; Febbraio, M.A. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 2001, 533 Pt 2, 585–591. [Google Scholar] [CrossRef]
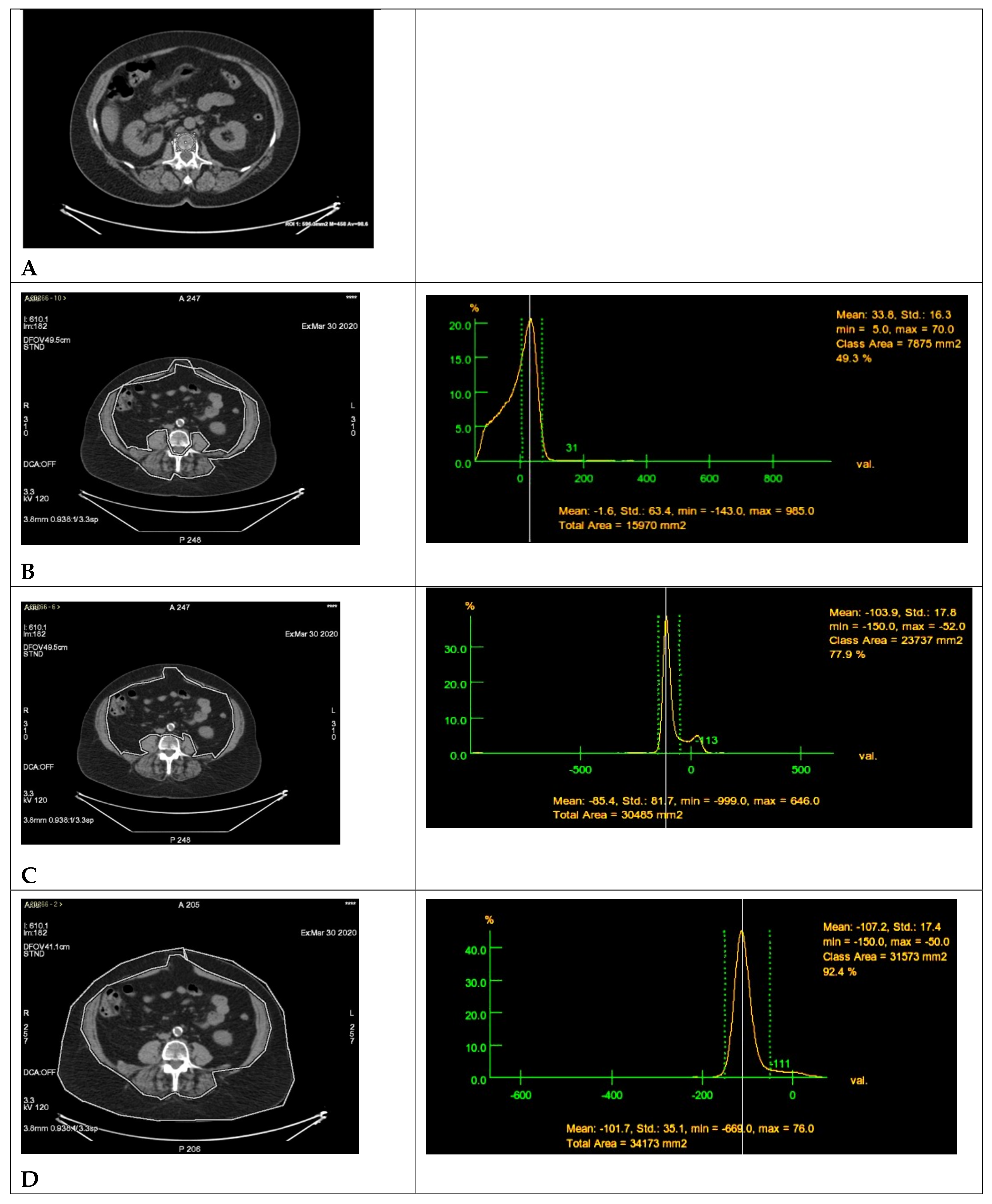
| Population (n = 79) | R-CHOP (n = 48) | R-Bendamustine (n = 31) | p-Value |
|---|---|---|---|
| Median age, years (range) | 66 (52–77) | 70 (59–81) | 0.058 |
| Male:Female ratio | 0.7 (20:28) | 1.4 (18:13) | 0.154 |
| Median height, cm± SD (range) | 166.3 ± 9.1 (150.0–189.0) | 167.5 ± 7.9 (150.0–184.0) | 0.556 |
| Median height, cm ± SD (range)-Male | 170.5 ± 7.5 (162.0–189.0) | 172.0 ± 5.7 (160.0–184.0) | 0.652 |
| Median height, cm ± SD (range)-Female | 162.0 ± 6.8 (150.0–173.0) | 160.0 ± 6.4 (150.0–172.0) | 0.988 |
| Median weight, Kg± SD (range) | 75.8 ± 21.2 (48.0–187.0) | 72.0 ± 14.2 (48.0–97.0) | 0.380 |
| Median weight, Kg ± SD (range)-Male | 79.5 ± 13.0 (65.0–121.0) | 77.5 ± 12.5 (60.0–97.0) | 0.810 |
| Median weight, Kg ± SD (range)-Female | 66.5 ± 25.3 (48.0–187.0) | 63.0 ± 10.4 (48.0–80.0) | 0.133 |
| Median BMI± SD (range) | 22.8 ± 6.3 (16.0–56.7) | 21.4 ± 3.8 (14.8–28.8) | 0.510 |
| Median BMI ± SD (range)-Male | 22.6 ± 3.4 (19.7–34.0) | 22.7 ± 3.7 (17.1–28.8) | 0.915 |
| Median BMI ± SD (range)-Female | 20.0 ± 7.8 (16.0–56.7) | 19.9 ± 2.8 (14.8–24.4) | 0.161 |
| Grading | |||
| G1 | 4.2% (2/47) | 16.1% (5/31) | 0.241 |
| G1-G2 | 14.9% (7/47) | 16.1% (5/31) | |
| G2 | 49.0% (23/47) | 42.0% (13/31) | |
| G2-G3A | 6.4% (3/47) | 12.9% (4/31) | |
| G3A | 25.5% (12/47) | 12.9% (4/31) | |
| FLIPI Score | |||
| Low | 2.1% (1/48) | 9.7% (3/31) | 0.322 |
| Intermediate | 58.3% (28/48) | 54.8% (17/31) | |
| High | 39.6% (19/48) | 35.5% (11/31) |
| Population (n = 79) | R-CHOP | R-Bendamustine | ||||
|---|---|---|---|---|---|---|
| Primary Endpoint | Pre-Therapy | Post-Therapy | p-Value | Pre-Therapy | Post-Therapy | p-Value |
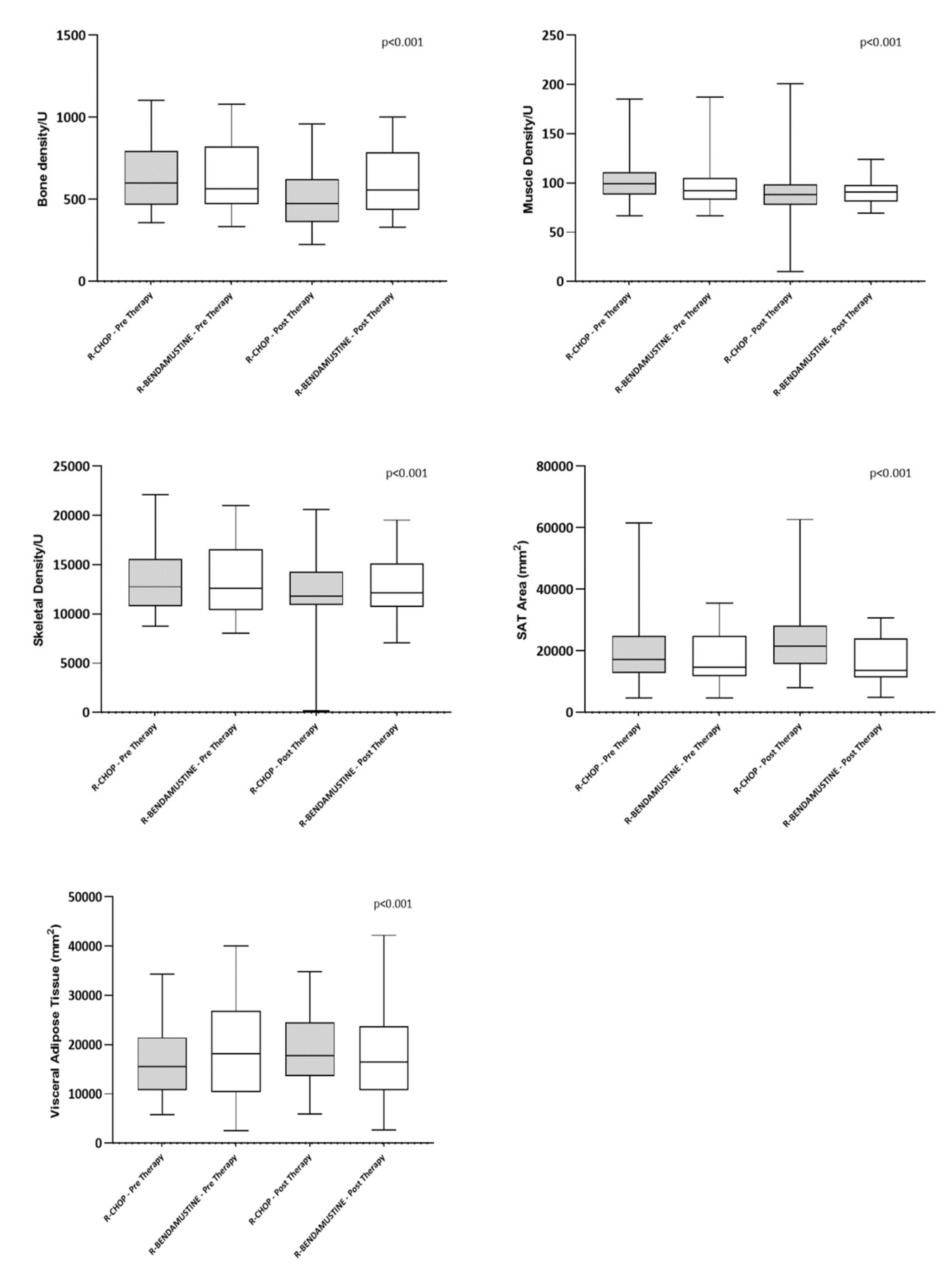
| Mean ± SD Bone Mineral Density L1 (density/U) | 632.79 ± 194.99 | 506.94 ± 180.25 | <0.001 | 632.58 ± 205.80 | 608.39 ± 193.82 | 0.060 |
| Mean ± SD Skeletal Muscle Density L3 (density/U) | 101.38 ± 22.57 | 91.19 ± 25.13 | 0.017 | 95.55 ± 22.20 | 92.03 ± 14.35 | 0.432 |
| Mean ± SD Skeletal Muscle Area L3 (mm2) | 13,283.23 ± 3015.18 | 12,200.69 ± 3622.12 | 0.005 | 13,501.45 ± 3387.46 | 12,842.10 ± 3001.80 | 0.042 |
| Mean ± SD Subcutaneous Adipose Tissue L3 (mm2) | 19,404.23 ± 10,027.24 | 22,731.52 ± 9839.26 | <0.001 | 17,935.26 ± 7897.96 | 16,569.84 ± 7601.68 | 0.006 |
| Mean ± SD Visceral Adipose Tissue L3 (mm2) | 16,445.08 ± 7067.21 | 19,388.00 ± 7229.37 | <0.001 | 18,591.36 ± 9731.36 | 17,557.81 ± 9150.47 | 0.053 |
| Population (n = 79) | R-CHOP (n = 48) | R-Bendamustine (n = 31) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | p-Value | |||||||||
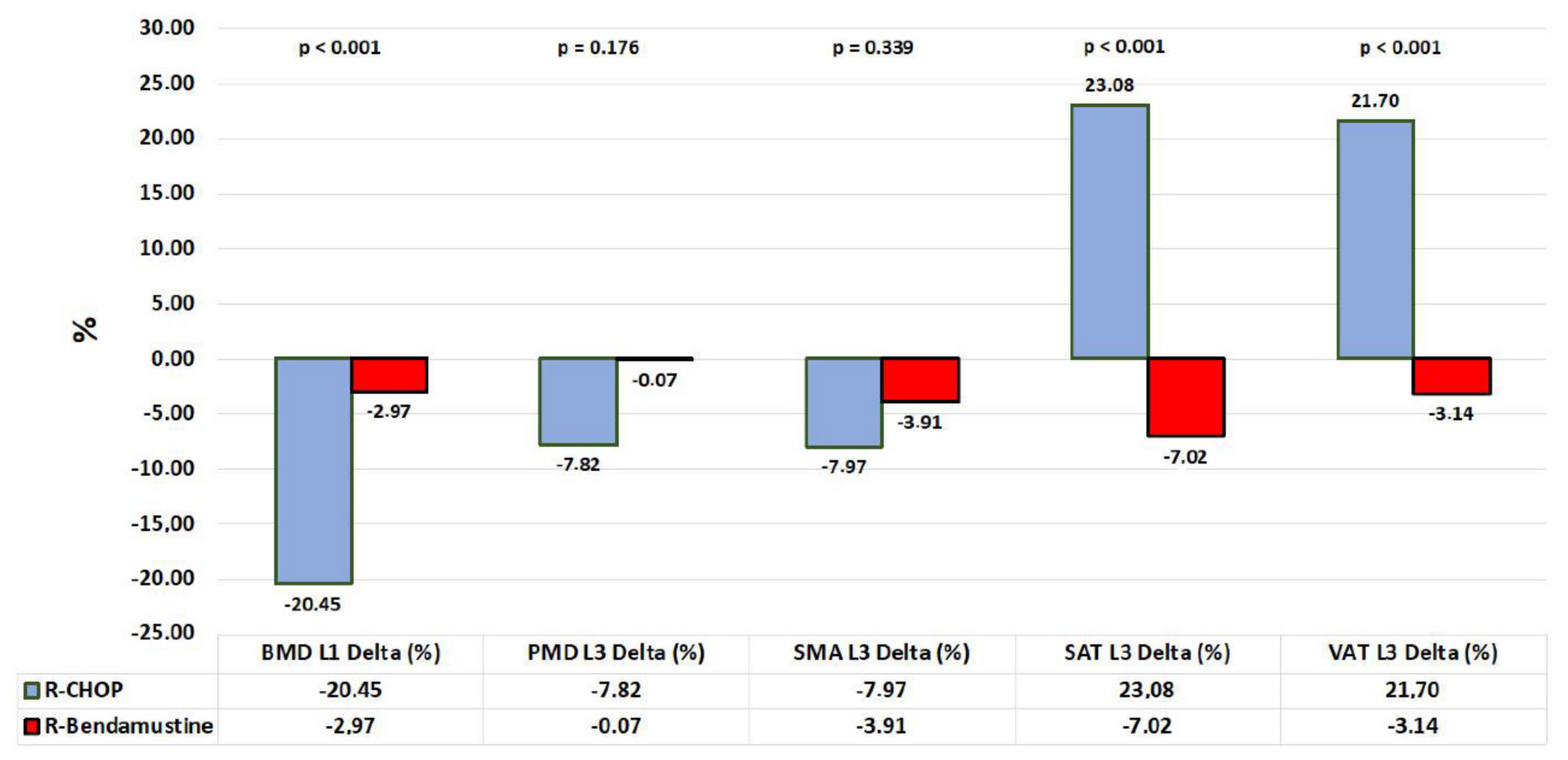
| BMD L1 Delta | −125.85 | ± | 72.64 | (−146.40 | – | −105.30) | −24.19 | ± | 68.84 | (−48.42 | – | 0.04) | <0.001 |
| BMD L1 Delta (%) | −20.45 | ± | 11.05 | (−23.58 | – | −17.32) | −2.97 | ± | 11.51 | (−7.02 | – | 1.08) | <0.001 |
| SMD L3 Delta | −10.19 | ± | 28.48 | (−18.25 | – | −2.13) | −3.52 | ± | 24.56 | (−12.17 | – | 5.13) | 0.232 |
| SMD L3 Delta (%) | −7.82 | ± | 24.96 | (−14.88 | – | −0.76) | −0.07 | ± | 24.14 | (−8.57 | – | 8.43) | 0.176 |
| SMA L3 Delta (mm2) | −1082.54 | ± | 2541.00 | (−1801.38 | – | −363.70) | −659.36 | ± | 1724.97 | (−1266.58 | – | −52.14) | 0.641 |
| SMA L3 Delta (%) | −7.97 | ± | 21.70 | (−14.11 | – | −1.83) | −3.91 | ± | 11.15 | (−7.84 | – | 0.02) | 0.339 |
| SAT L3 Delta (mm2) | 3327.29 | ± | 2792.39 | (2537.33 | – | 4117.25) | −1365.42 | ± | 2596.55 | (−2279.46 | – | −451.38) | <0.001 |
| SAT L3 Delta (%) | 23.08 | ± | 24.71 | (16.09 | – | 30.07) | −7.02 | ± | 16.28 | (−12.75 | – | −1.29) | <0.001 |
| VAT L3 Delta (mm2) | 2942.92 | ± | 2692.75 | (2181.15 | – | 3704.69) | −1033.55 | ± | 2851.65 | (−2037.39 | – | −29.71) | <0.001 |
| VAT L3 Delta (%) | 21.70 | ± | 21.36 | (15.66 | – | 27.74) | −3.14 | ± | 11.20 | (−7.08 | – | 0.80) | <0.001 |
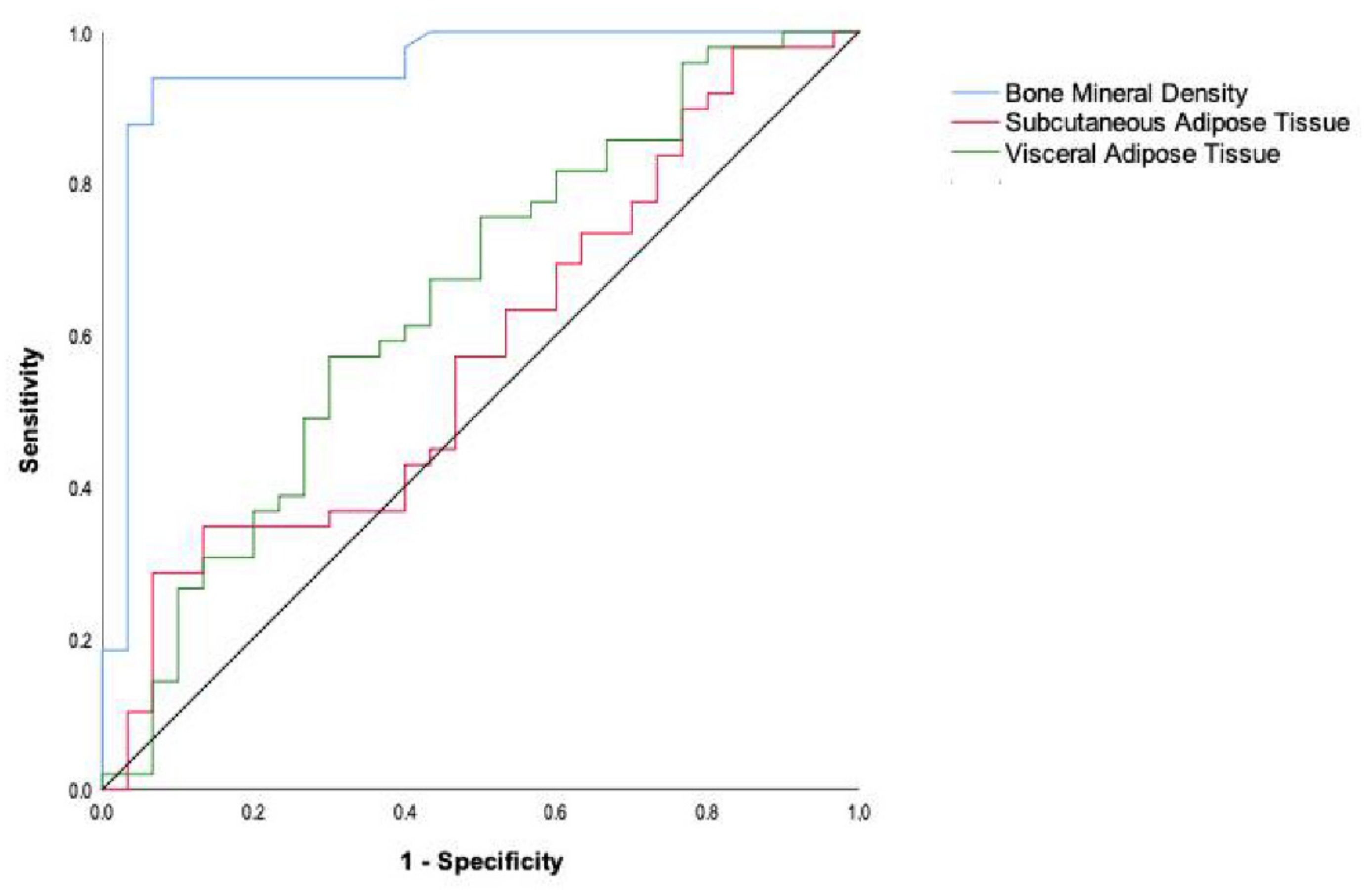
| Variable | AUC | 95% CI | Cut-Off Point | Sensitivity (%) | Specificity (%) | Youden’s Index | p Value |
|---|---|---|---|---|---|---|---|
| BMD | 0.948 | 0.889–0.996 | 522 | 93.9 | 93.3 | 0.872 | <0.001 |
| SAT | 0.577 | 0.446–0.707 | 25,976 | 93.3 | 87.6 | 0.219 | 0.537 |
| VAT | 0.646 | 0.518–0.774 | 16,693 | 70.0 | 75.7 | 0.646 | 0.042 |
| Population (n = 79) | R-CHOP | R-Bendamustine | ||||
|---|---|---|---|---|---|---|
| Primary Endpoint | BMD < 522 | BMD > 522 | p-Value | BMD < 522 | BMD > 522 | p-Value |
| FLIPI Score Low (1–2) | 3.5% (1/29) | 96.5% (28/29) | <0.001 | 15.0% (3/20) | 85% (17/20) | <0.001 |
| FLIPI Score High (≥3) | 94.7% (18/19) | 5.3% (1/19) | 90.9% (10/11) | 9.1% (1/11) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, F.; Pascale, M.R.; Tesei, C.; Gigliotti, P.E.; Luciano, A.; Angeloni, C.; Marinoni, M.; Meconi, F.; Secchi, R.; Patanè, A.; et al. Body Composition in Patients with Follicular Lymphoma: Asso-Ciations between Changes in Radiomic Parameters in Patients Treated with R-CHOP-like and R-B Regimens: LyRa 01F. Cancers 2023, 15, 999. https://doi.org/10.3390/cancers15040999
Esposito F, Pascale MR, Tesei C, Gigliotti PE, Luciano A, Angeloni C, Marinoni M, Meconi F, Secchi R, Patanè A, et al. Body Composition in Patients with Follicular Lymphoma: Asso-Ciations between Changes in Radiomic Parameters in Patients Treated with R-CHOP-like and R-B Regimens: LyRa 01F. Cancers. 2023; 15(4):999. https://doi.org/10.3390/cancers15040999
Chicago/Turabian StyleEsposito, Fabiana, Maria Rosaria Pascale, Cristiano Tesei, Paola Elda Gigliotti, Alessandra Luciano, Cecilia Angeloni, Massimiliano Marinoni, Federico Meconi, Roberto Secchi, Alberto Patanè, and et al. 2023. "Body Composition in Patients with Follicular Lymphoma: Asso-Ciations between Changes in Radiomic Parameters in Patients Treated with R-CHOP-like and R-B Regimens: LyRa 01F" Cancers 15, no. 4: 999. https://doi.org/10.3390/cancers15040999
APA StyleEsposito, F., Pascale, M. R., Tesei, C., Gigliotti, P. E., Luciano, A., Angeloni, C., Marinoni, M., Meconi, F., Secchi, R., Patanè, A., Postorino, M., Cantonetti, M., & Manenti, G. (2023). Body Composition in Patients with Follicular Lymphoma: Asso-Ciations between Changes in Radiomic Parameters in Patients Treated with R-CHOP-like and R-B Regimens: LyRa 01F. Cancers, 15(4), 999. https://doi.org/10.3390/cancers15040999








