Breast Cancer: How Hippotherapy Bridges the Gap between Healing and Recovery—A Randomized Controlled Clinical Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Selection
2.2. Statistics and Reproducibility
- The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30 and EORTC QLQ-BR23)
- The Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog V3)
- The Multidimensional Fatigue Inventory (MFI-20)
- The Hospital Anxiety and Depression (HADS)
- The Body Image Scale (BIS)
2.3. Treatment
2.4. Questionnaires
2.4.1. Primary End Point
2.4.2. Secondary End Points
3. Results
3.1. Both Groups Are Demographically and Clinically Equivalent
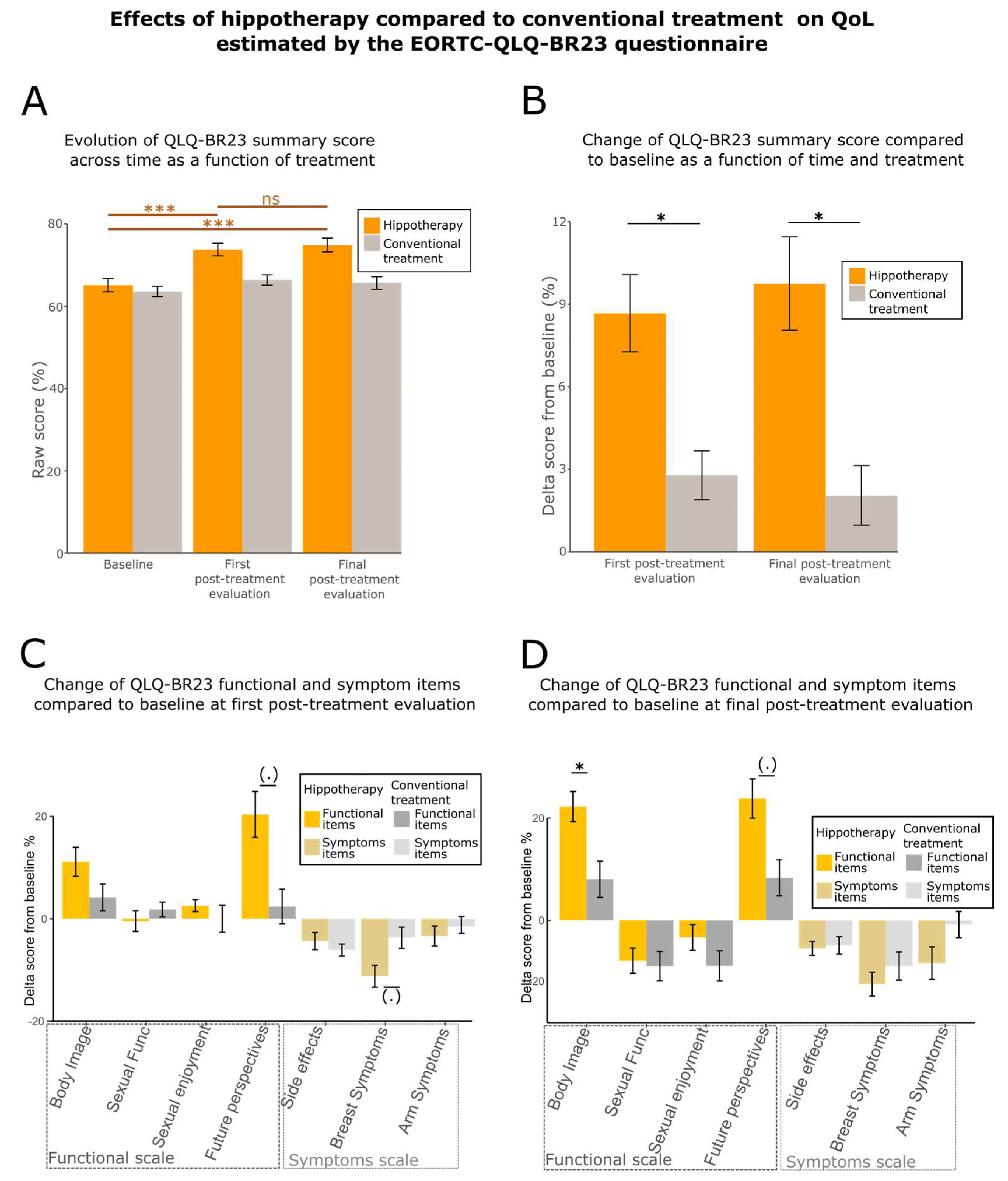
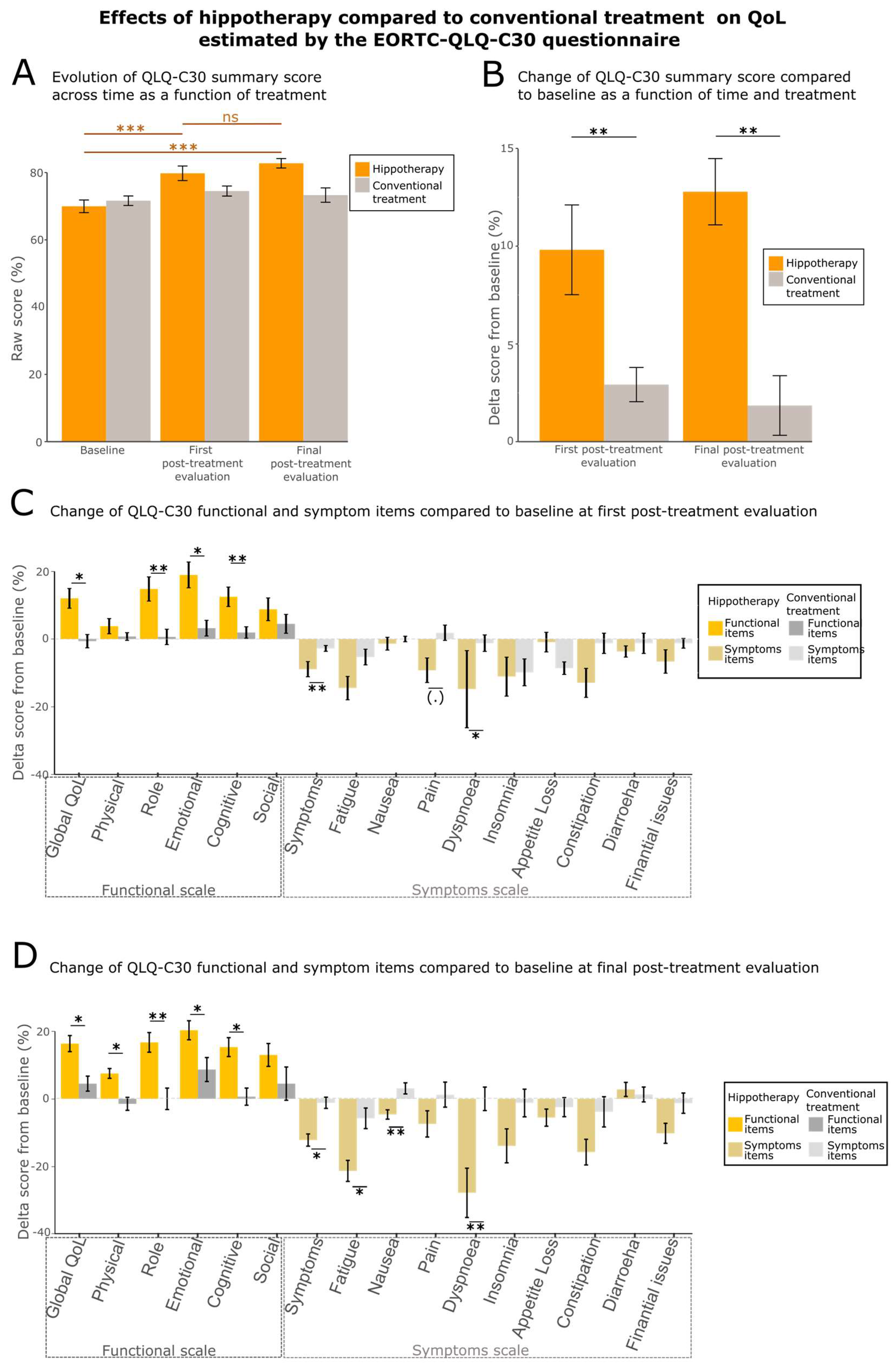
3.2. Quality of Life (QoL) Is Enhanced by Hippotherapy
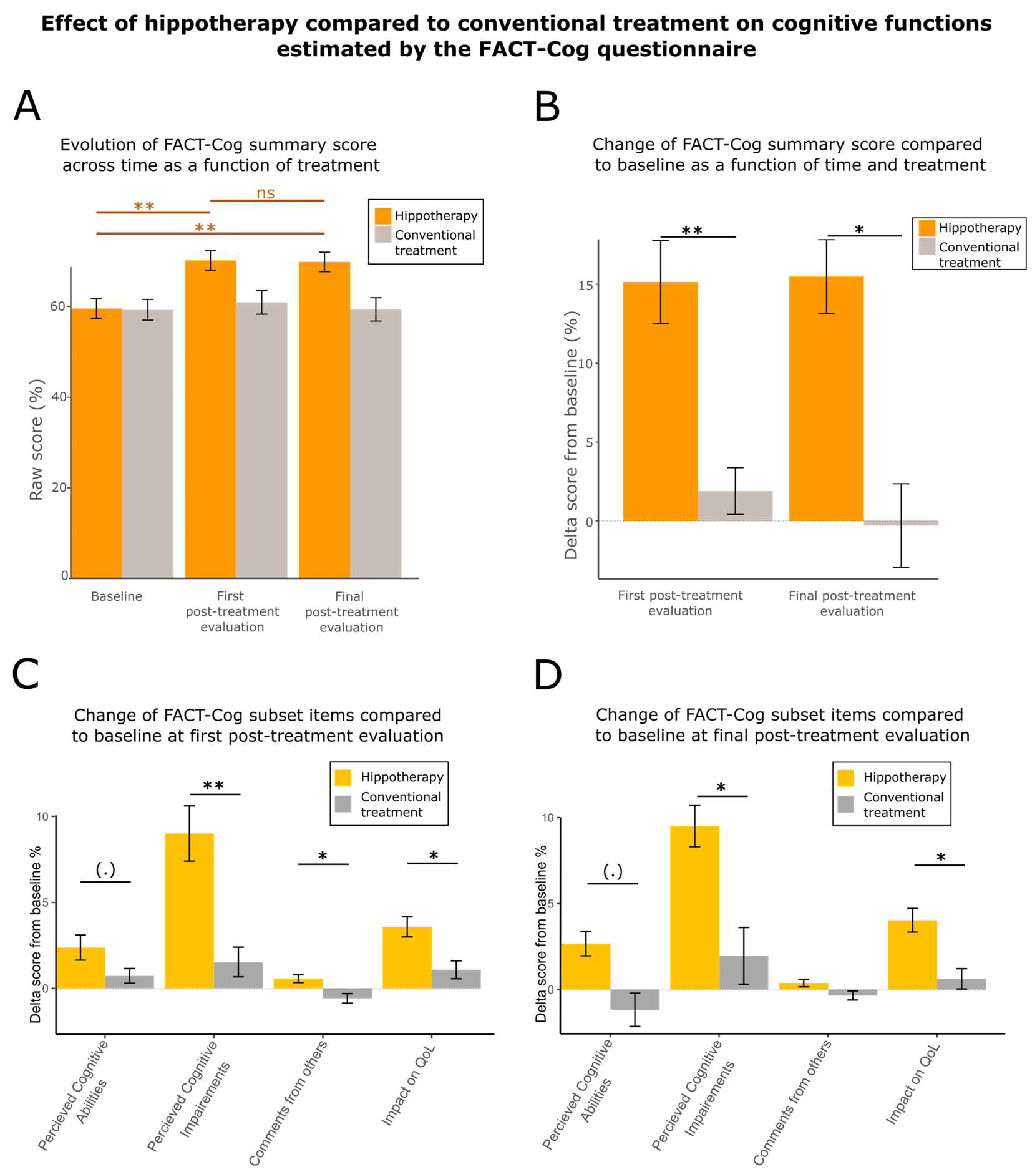
3.3. Hippotherapy Speeds Up Cognitive Recovery
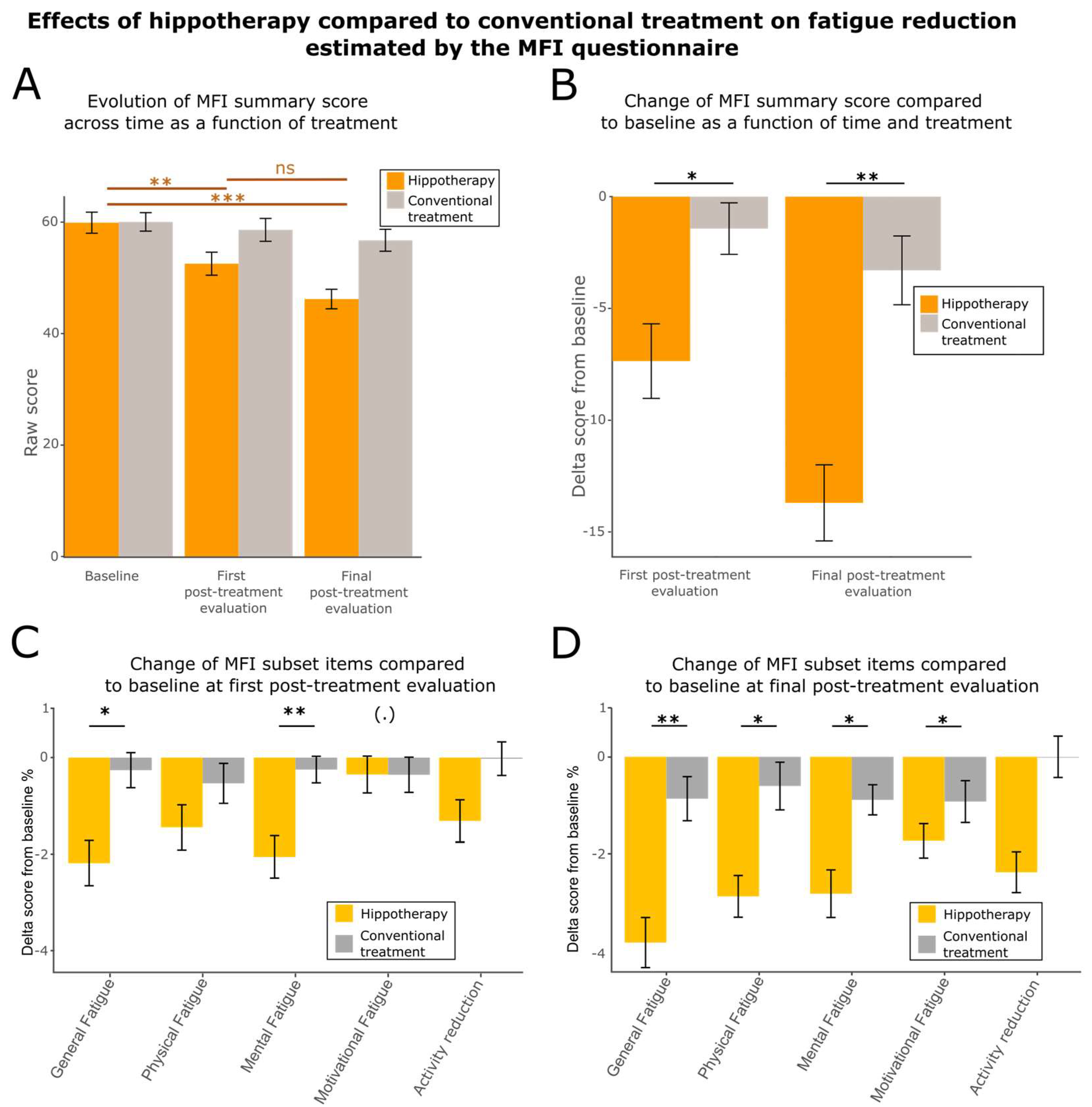
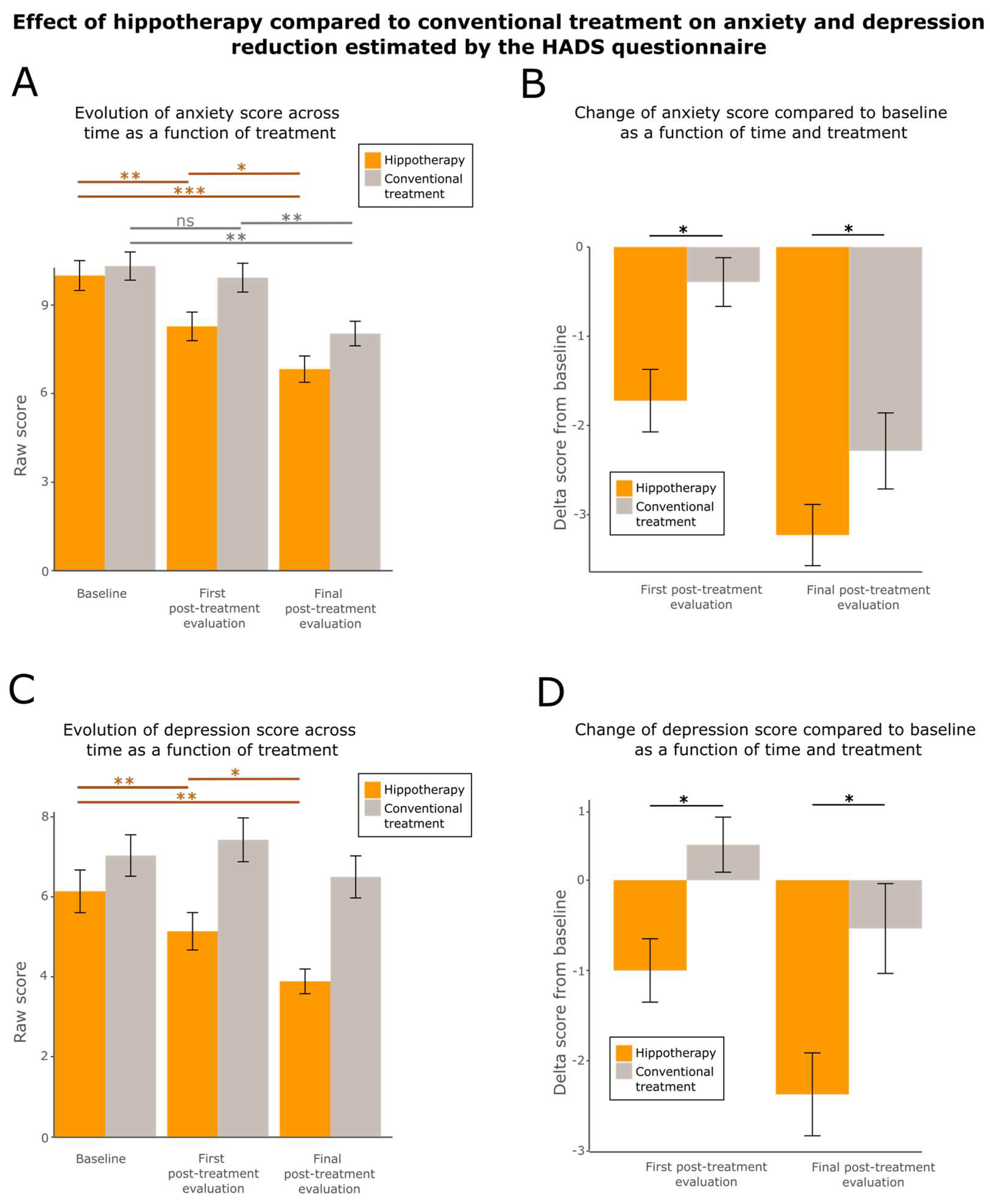
3.4. Hippotherapy Reduces Fatigue, Anxiety, and Depression
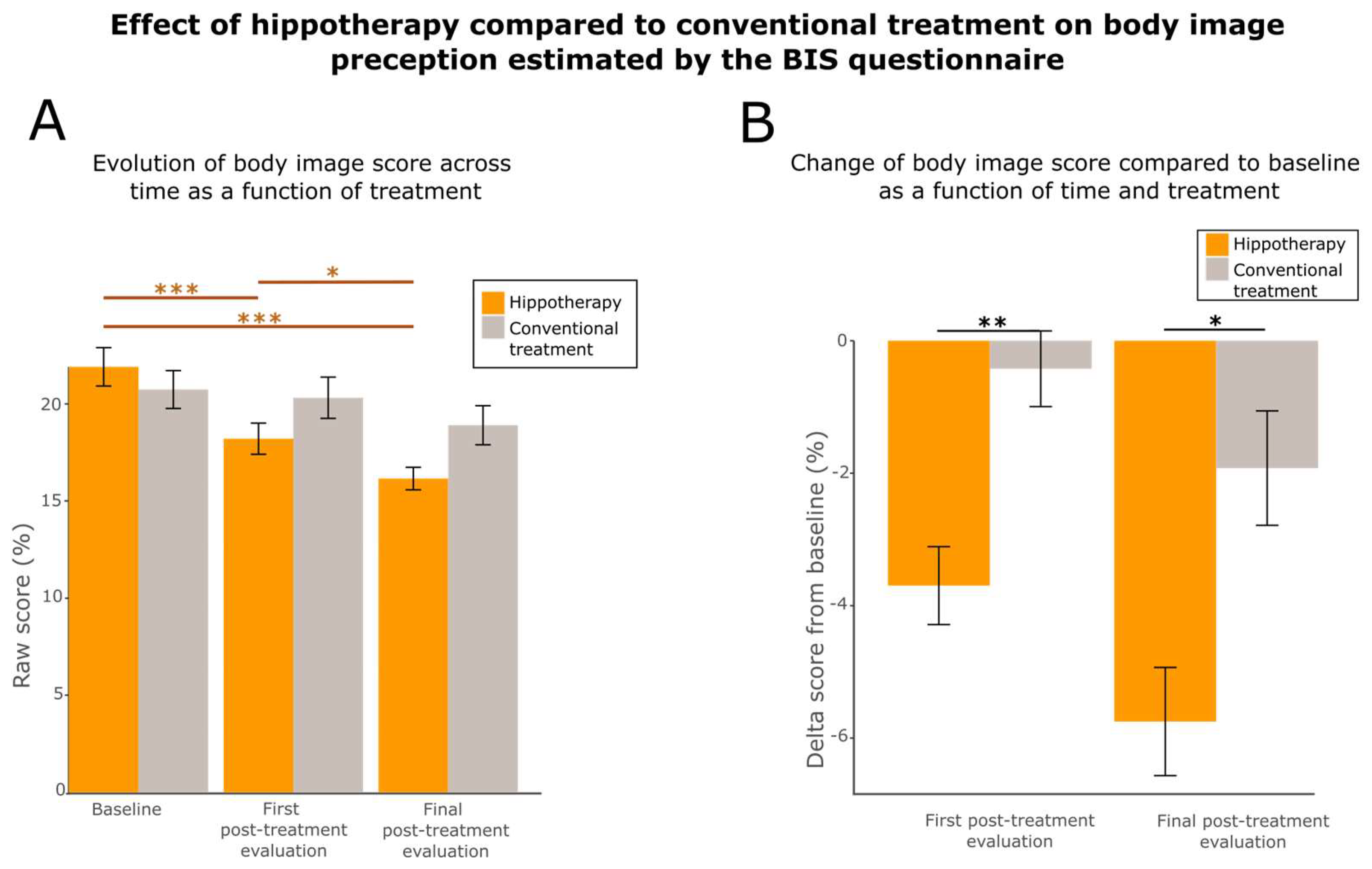
3.5. Hippotherapy Improves Body Image
4. Discussion
5. Conclusions
“Defects, disorders, diseases, in this sense, can play a paradoxical role, by bringing out latent powers, developments, evolutions, forms of life, that might never be seen, or even be imaginable, in their absence”[134]
6. Study Limitations and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Hippotherapy Group | Control Group | p-Value | |
|---|---|---|---|
| EORTC QLQ-C30 (summary score) | 79.8 ± 17.3 | 74.5 ± 11.9 | ** (0.001826) |
| 69.3 ± 21.5 | 57.1 ± 19.8 | * (0.01198) |
| 83.8 ± 18.6 | 86.2 ± 13.8 | ns (0.2731) |
| 81.9 ± 26.5 | 72.8 ± 29.7 | ** (0.008633) |
| 74.5 ± 24.8 | 59.0 ± 29.2 | * (0.0136) |
| 74.5 ± 24.4 | 66.0 ± 29.4 | ** (0.002765) |
| 84.7 ± 25.6 | 74.7 ± 24.6 | ns (0.3099) |
| 22.0 ± 17.2 | 25.5 ± 11.6 | ** (0.006804) |
| 35.5 ± 26.9 | 45.7 ± 24.9 | ns (0.1263) |
| 5.1 ± 11.8 | 2.5 ± 6.0 | ns (0.3584) |
| 24.5 ± 26.0 | 30.9 ± 23.0 | (.) (0.0534) |
| 31.5 ± 69.0 | 22.2 ± 26.1 | * (0.03973) |
| 40.7 ± 36.6 | 42.0 ± 34.1 | ns (0.8851) |
| 8.3 ± 21.6 | 6.2 ± 22.7 | ns (0.1946) |
| 15.7 ± 27.0 | 30.9 ± 29.1 | ns (0.2218) |
| 0.9 ± 5.6 | 9.9 ±18.1 | ns (0.6014) |
| 22.9 ± 32.1 | 17.3 ± 29.8 | ns (0.3597) |
| EORTC QLQ-BR23 (summary score) | 71.8 ± 12.4 | 66.4 ± 10.2 | * (0.0234) |
| 72.5 ± 25.7 | 64.0 ± 31.9 | ns (0.3642) |
| 76.9 ± 26.2 | 79.2 ± 20.6 | ns (0.2385) |
| 56.2 ± 29.1 | 50.0 ± 29.8 | ns (0.9708) |
| 62.0 ± 31.0 | 40.5 ± 31.9 | (.) (0.05385) |
| 15.2 ± 13.2 | 16.7 ± 12.8 | ns (0.8489) |
| 24.2 ± 13.4 | 37.8 ± 21.0 | (.) (0.07437) |
| 22.5 ± 22.3 | 21.4 ± 18.1 | ns (0.753) |
| FACT-Cog (summary score) | 105.0 ± 25.9 | 91.3 ± 31.2 | ** (0.00123) |
| 21.5 ± 7.3 | 18.8 ± 8.0 | (.) (0.07068) |
| 56.0 ± 15.5 | 48.5 ± 16.7 | ** (0.00145) |
| 15.0 ± 2.2 | 13.9 ± 3.7 | * (0.02248) |
| 11.4 ± 5.0 | 10.1 ± 5.3 | * (0.02203) |
| MFI (summary score) | 52.5 ± 16.5 | 58.6 ± 16.4 | * (0.03185) |
| 11.9 ± 4.0 | 14.1 ± 3.6 | * (0.01711) |
| 11.0 ± 4.4 | 11.9 ± 4.0 | ns (0.2017) |
| 10.1 ± 3.8 | 11.7 ± 4.9 | ** (0.007088) |
| 10.8 ± 4.2 | 10.2 ± 3.8 | (.) (0.07136) |
| 8.7 ± 3.7 | 10.7 ± 3.9 | ns (0.9557) |
| HADS | |||
| 8.3 ± 3.9 | 9.9 ± 3.9 | * (0.02569) |
| 5.1 ± 3.7 | 7.4 ± 4.4 | * (0.0239) |
| BIS | |||
| 18.2 ± 6.4 | 20.3 ± 8.5 | ** (0.00405) |
| Hippotherapy Group | Control Group | p-Value | |
|---|---|---|---|
| EORTC QLQ-C30 (summary score) | 82.8 ± 11.2 | 73.3 ± 17.1 | ** (0.008539) |
| 73.7 ± 7.1 | 60.7 ± 21.6 | * (0.01414) |
| 87.5 ± 15.0 | 84.3 ± 15.9 | * (0.02294) |
| 83.8 ± 19.7 | 72.6 ± 23.7 | ** (0.006906) |
| 75.8 ± 19.1 | 63.1 ± 26.4 | * (0.0259) |
| 77.3 ± 21.9 | 65.5 ± 28.7 | * (0.04366) |
| 88.9 ± 17.8 | 75.0 ± 36.1 | ns (0.3801) |
| 18.7 ± 11.4 | 26.8 ± 16.5 | * (0.01212) |
| 28.7 ± 19.9 | 44.4 ± 24.0 | * (0.0257) |
| 1.9 ± 6.6 | 5.4 ± 12.0 | ** (0.009145) |
| 26.4 ± 25 | 30.4 ± 26.1 | ns (0.2942) |
| 18.5 ± 25.8 | 22.6 ± 32.8 | ** (0.009792) |
| 38.0 ± 32.0 | 51.2 ± 36.8 | ns (0.1209) |
| 3.7 ± 10.6 | 11.9 ± 27.5 | ns (0.9926) |
| 38.0 ± 32.0 | 51.2 ± 36.8 | ns (0.2829) |
| 7.4 ± 16.2 | 11.9 ± 24.4 | ns (0.7873) |
| 20.4 ± 30.1 | 16.7 ± 24.8 | ns (0.1587) |
| EORTC QLQ-BR23 (summary score) | 74.9 ± 13.3 | 65.7 ± 12.2 | * (0.01707) |
| 83.6 ± 20.3 | 67.9 ± 28.3 | * (0.01201) |
| 69.4 ± 28.3 | 68.5 ± 25.4 | ns (0.8962) |
| 47.7 ± 32.6 | 41.7 ± 26.2 | ns (0.7359) |
| 65.7 ± 32.8 | 46.4 ± 31.9 | (.) (0.06796) |
| 14.1 ± 11.4 | 17.9 ± 16.6 | ns (0.7963) |
| 22.9 ± 19.0 | 33.6 ± 24.4 | ns (0.6398) |
| 17.6 ± 19.9 | 22.6 ± 19.9 | ns (0.4898) |
| FACT-Cog (summary score) | 105.0 ± 25.8 | 89.0 ± 30.9 | * (0.02425) |
| 21.7 ± 6.4 | 17.6 ± 8.6 | (.) (0.06037) |
| 56.7 ± 14.5 | 47.7 ± 16.1 | * (0.01185) |
| 14.7 ± 2.5 | 13.5 ± 4.2 | ns (0.1307) |
| 11.9 ± 4.3 | 9.9 ± 5.4 | * (0.04445) |
| MFI (summary score) | 46.2 ± 14.0 | 56.8 ± 15.7 | ** (0.003591) |
| 10.4 ± 3.7 | 13.5 ± 3.4 | ** (0.004449) |
| 9.6 ± 3.9 | 11.9 ± 4.0 | * (0.0246) |
| 9.3 ± 3.7 | 11.0 ± 4.2 | * (0.03953) |
| 9.3 ± 3.8 | 9.6 ± 3.9 | * (0.01256) |
| 7.6 ± 3.0 | 10.8 ± 3.6 | ns (0.3907) |
| HADS | |||
| 6.8 ± 3.6 | 8.0 ± 3.3 | * (0.0419) |
| 3.9 ± 2.5 | 6.5 ± 4.2 | * (0.03755) |
| BIS | |||
| 16.1 ± 4.7 | 18.9 ± 8.0 | * (0.0107) |
| Questionnaire | Test | Global QLQ | Physical | Role | Cognitive | Emotional | Social | Symptoms | Fatigue | Nausea | Pain | Dyspnea | Insomnia | Appetite Loss | Constipation | Diarrhea | Financial Issues |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First post-treatment evaluation | ANOVA | p-value = 0.0023 statistics value = 4.3862 df = 2 ** | |||||||||||||||
| Post hoc tests | p-value = 0.01198 effect size = 0.320 s * | p-value = 0.2731 effect size = 0.139 | p-value = 0.008633 effect size = 0.332 ** | p-value = 0.002765 effect size = 0.381 ** | p-value = 0.0136 effect size = 0.314 * | p-value = 0.3099 effect size = 0.130 | p-value = 0.006804 effect size = 0.342 ** | p-value = 0.1263 effect size = 0.194 | p-value = 0.3584 effect size = 0.117 | p-value = 0.0534 effect size = 0.244 (.) | p-value = 0.03973 effect size = 0.260 * | p-value = 0.8851 effect size = 0.0191 | p-value = 0.1946 effect size = 0.165 | p-value = 0.2218 effect size = 0.156 | p-value = 0.6014 effect size = 0.0676 | p-value = 0.3597 effect size = 0.118 | |
| Final post-treatment evaluation | ANOVA | p-value = 0.0012 statistics value = 6.4769 df = 2 ** | |||||||||||||||
| Post hoc tests | p-value = 0.01414 effect size = 0.330 * | p-value = 0.02294 effect size = 0.289 * | p-value = 0.006906 effect size = 0.344 ** | p-value = 0.04366 effect size = 0.277 * | p-value = 0.0259 effect size = 0.266 * | p-value = 0.3801 effect size = 0.110 | p-value = 0.01212 effect size = 0.309 * | p-value = 0.0257 effect size = 0.266 * | p-value = 0.009145 effect size = 0.335 ** | p-value = 0.2942 effect size = 0.158 | p-value = 0.009792 effect size = 0.305 ** | p-value = 0.1209 effect size = 0.155 | p-value = 0.9926 effect size = 0.0381 | p-value = 0.2829 effect size = 0.139 | p-value = 0.7873 effect size = 0.00128 | p-value = 0.1587 effect size = 0.183 | |
| Questionnaire | Test | Body Image | Sexual Functioning | Sexual Enjoyment | Future Perspectives | Side Effects | Breast Symptoms | Arm Symptoms |
|---|---|---|---|---|---|---|---|---|
| First post-treatment evaluation | ANOVA | p-value = 0.0184 statistics value = 4.1610 df = 2 * | ||||||
| Post hoc tests | p-value = 0.3642 effect size = 0.114 | p-value = 0.2385 effect size = 0.148 | p-value = 0.9708 effect size = 0.0136 | p-value = 0.05385 effect size = 0.242 (.) | p-value = 0.8489 effect size = 0.0247 | p-value = 0.07437 effect size = 0.226 (.) | p-value = 0.753 effect size = 0.0405 | |
| Final post-treatment evaluation | ANOVA | p-value = 0.023 statistics value = 3.2578 df = 2 * | ||||||
| Post hoc tests | p-value = 0.01201 effect size = 0.323 * | p-value = 0.8962 effect size = 0.0512 | p-value = 0.7359 effect size = 0.0693 | p-value = 0.06796 effect size = 0.218 (.) | p-value = 0.7963 effect size = 0.0262 | p-value = 0.6398 effect size = 0.608 | p-value = 0.4898 effect size = 0.104 | |
| Questionnaire | Perceived Cognitive Abilities | Perceived Cognitive Impairments | Impact on Quality of Life | Comments from Others | |
|---|---|---|---|---|---|
| First post-treatment evaluation | ANOVA | p-value = 0.0019 statistics value = 12.219 df = 2 | |||
| Post hoc tests | p-value = 0.07068 effect size = 0.196 (.) | p-value = 0.00145 effect size = 0.396 ** | p-value = 0.02203 effect size = 0.268 * | p-value = 0.02248 effect size = 0.2 * | |
| Final post-treatment evaluation | ANOVA | p-value = 0.0150 statistics value = 6.6277 df = 2 | |||
| Post hoc tests | p-value = 0.06037 effect size = 0.198 (.) | p-value = 0.01185 effect size = 0.328 ** | p-value = 0.04445 effect size = 0.233 * | p-value = 0.1307 effect size = 0.153 | |
| Questionnaire | Test | General Fatigue | Physical Fatigue | Mental Fatigue | Activity Reduction | Motivation |
|---|---|---|---|---|---|---|
| First post-treatment evaluation | ANOVA | p-value = 0.0124 statistics value = 6.8586 df = 2 * | ||||
| Post hoc tests | p-value = 0.01711 effect size = 0.301 * | p-value = 0.2017 effect size = 0.162 | p-value = 0.007088 effect size = 0.340 ** | p-value = 0.9557 effect size = 0.00788 | p-value = 0.07136 effect size = 0.228 (.) | |
| Final post-treatment evaluation | ANOVA | p-value = 0.0164 statistics value = 6.2769 df = 2 * | ||||
| Post hoc tests | p-value = 0.004449 effect size = 0.334 ** | p-value = 0.0246 effect size = 0.289 * | p-value = 0.03953 effect size = 0.235 * | p-value = 0.3907 effect size = 0.109 | p-value = 0.01256 effect size = 0.296 * | |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Veronesi, U.; Boyle, P.; Goldhirsch, A.; Orecchia, R.; Viale, G. Breast cancer. Lancet 2005, 365, 1727–1741. [Google Scholar] [CrossRef]
- Warrier, S.; Tapia, G.; Goltsman, D.; Beith, J. An update in breast cancer screening and management. Womens Health 2016, 12, 229–239. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Stan, D.; Loprinzi, C.L.; Ruddy, K.J. Breast cancer survivorship issues. Hematol. Oncol. Clin. N. Am. 2013, 27, 805–827. [Google Scholar] [CrossRef]
- Gegechkori, N.; Haines, L.; Lin, J.J. Long Term and Latent Side Effects of Specific Cancer Types. Med. Clin. N. Am. 2017, 101, 1053–1073. [Google Scholar] [CrossRef]
- Pinto, M.; Calafiore, D.; Piccirillo, M.C.; Costa, M.; Taskiran, O.O.; de Sire, A. Breast Cancer Survivorship: The Role of Rehabilitation According to the International Classification of Functioning Disability and Health-a Scoping Review. Curr. Oncol. Rep. 2022, 24, 1163–1175. [Google Scholar] [CrossRef]
- Romaniuk, M.; Evans, J.; Kidd, C. Evaluation of an equine-assisted therapy program for veterans who identify as “wounded, injured or ill” and their partners. PLoS ONE 2018, 13, e0203943. [Google Scholar] [CrossRef]
- Mutoh, T.; Mutoh, T.; Tsubone, H.; Takada, M.; Doumura, M.; Ihara, M.; Shimomura, H.; Taki, Y.; Ihara, M. Impact of Long-Term Hippotherapy on the Walking Ability of Children With Cerebral Palsy and Quality of Life of Their Caregivers. Front. Neurol. 2019, 10, 834. [Google Scholar] [CrossRef]
- Viruega, H.; Gaillard, I.; Carr, J.; Greenwood, B.; Gaviria, M. Short- and Mid-Term Improvement of Postural Balance after a Neurorehabilitation Program via Hippotherapy in Patients with Sensorimotor Impairment after Cerebral Palsy: A Preliminary Kinetic Approach. Brain Sci. 2019, 9, 261. [Google Scholar] [CrossRef]
- Moraes, A.G.; Copetti, F.; Ângelo, V.R.; Chiavoloni, L.; de David, A.C. Hippotherapy on postural balance in the sitting position of children with cerebral palsy—Longitudinal study. Physiother. Theory Pract. 2020, 36, 259–266. [Google Scholar] [CrossRef]
- Viruega, H.; Gaillard, I.; Briatte, L.; Gaviria, M. Inter-Day Reliability and Changes of Surface Electromyography on Two Postural Muscles Throughout 12 Weeks of Hippotherapy on Patients with Cerebral Palsy: A Pilot Study. Brain Sci. 2020, 10, 281. [Google Scholar] [CrossRef]
- Santos de Assis, G.; Schlichting, T.; Rodrigues Mateus, B.; Gomes Lemos, A.; Dos Santos, A.N. Physical therapy with hippotherapy compared to physical therapy alone in children with cerebral palsy: Systematic review and meta-analysis. Dev. Med. Child Neurol. 2022, 64, 156–161. [Google Scholar] [CrossRef]
- Viruega, H.; Imbernon, C.; Chausson, N.; Altarcha, T.; Aghasaryan, M.; Soumah, D.; Lescieux, E.; Flamand-Roze, C.; Simon, O.; Bedin, A.; et al. Neurorehabilitation through Hippotherapy on Neurofunctional Sequels of Stroke: Effect on Patients’ Functional Independence, Sensorimotor/Cognitive Capacities and Quality of Life, and the Quality of Life of Their Caregivers—A Study Protocol. Brain Sci. 2022, 12, 619. [Google Scholar] [CrossRef]
- Buetow, S.A.; Martínez-Martín, P.; McCormack, B. Ultrabilitation: Beyond recovery-oriented rehabilitation. Disabil. Rehabil. 2019, 41, 740–745. [Google Scholar] [CrossRef]
- Kapur, N. Paradoxes in rehabilitation. Disabil. Rehabil. 2020, 42, 1495–1502. [Google Scholar] [CrossRef]
- Viruega, H.; Gaviria, M. After 55 Years of Neurorehabilitation, What Is the Plan? Brain Sci. 2022, 12, 982. [Google Scholar] [CrossRef]
- Oh, Y.; Joung, Y.-S.; Jang, B.; Yoo, J.H.; Song, J.; Kim, J.; Kim, K.; Kim, S.; Lee, J.; Shin, H.-Y.; et al. Efficacy of Hippotherapy Versus Pharmacotherapy in Attention-Deficit/Hyperactivity Disorder: A Randomized Clinical Trial. J. Altern. Complement. Med. 2018, 24, 463–471. [Google Scholar] [CrossRef]
- Guindos-Sanchez, L.D.; Lucena-Anton, D.; Moral-Munoz, J.A.; Salazar, A.; Carmona-Barrientos, I. The Effectiveness of Hippotherapy to Recover Gross Motor Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Children 2020, 7, 106. [Google Scholar] [CrossRef]
- Marquez, J.; Weerasekara, I.; Chambers, L. Hippotherapy in adults with acquired brain injury: A systematic review. Physiother. Theory Pract. 2020, 36, 779–790. [Google Scholar] [CrossRef]
- Wood, W.H.; Fields, B.E. Hippotherapy: A systematic mapping review of peer-reviewed research, 1980 to 2018. Disabil. Rehabil. 2021, 43, 1463–1487. [Google Scholar] [CrossRef]
- Maresca, G.; Portaro, S.; Naro, A.; Crisafulli, R.; Raffa, A.; Scarcella, I.; Aliberti, B.; Gemelli, G.; Calabrò, R.S. Hippotherapy in neurodevelopmental disorders: A narrative review focusing on cognitive and behavioral outcomes. Appl. Neuropsychol. Child 2022, 11, 553–560. [Google Scholar] [CrossRef]
- Potvin-Bélanger, A.; Vincent, C.; Freeman, A.; Flamand, V.H. Impact of hippotherapy on the life habits of children with disabilities: A systematic review. Disabil. Rehabil. 2022, 44, 8161–8175. [Google Scholar] [CrossRef]
- Uchiyama, H.; Ohtani, N.; Ohta, M. Three-dimensional analysis of horse and human gaits in therapeutic riding. Appl. Anim. Behav. Sci. 2011, 135, 271–276. [Google Scholar] [CrossRef]
- Gabriels, R.L.; Pan, Z.; Dechant, B.; Agnew, J.A.; Brim, N.; Mesibov, G. Randomized Controlled Trial of Therapeutic Horseback Riding in Children and Adolescents With Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 541–549. [Google Scholar] [CrossRef]
- Fisher, P.W.; Lazarov, A.; Lowell, A.; Arnon, S.; Turner, J.B.; Bergman, M.; Ryba, M.; Such, S.; Marohasy, C.; Zhu, X.; et al. Equine-Assisted Therapy for Posttraumatic Stress Disorder Among Military Veterans: An Open Trial. J. Clin. Psychiatry 2021, 82, 21m14005. [Google Scholar] [CrossRef]
- Menor-Rodríguez, M.J.; Sevilla Martín, M.; Sánchez-García, J.C.; Montiel-Troya, M.; Cortés-Martín, J.; Rodríguez-Blanque, R. Role and Effects of Hippotherapy in the Treatment of Children with Cerebral Palsy: A Systematic Review of the Literature. J. Clin. Med. 2021, 10, 2589. [Google Scholar] [CrossRef]
- Moraes, A.G.; Neri, S.G.R.; Motl, R.W.; Tauil, C.B.; von Glehn, F.; Corrêa, É.C.; de David, A.C. Effects of hippotherapy on postural balance, functional mobility, self-perceived fatigue, and quality of life in people with relapsing-remitting multiple sclerosis: Secondary results of an exploratory clinical trial. Mult. Scler. Relat. Disord. 2021, 52, 102948. [Google Scholar] [CrossRef]
- Lee, N.; Park, S.; Kim, J. Hippotherapy and neurofeedback training effect on the brain function and serum brain-derived neurotrophic factor level changes in children with attention-deficit or/and hyperactivity disorder. J. Exerc. Nutr. Biochem. 2017, 21, 35–42. [Google Scholar] [CrossRef]
- Silkwood-Sherer, D.J.; McGibbon, N.H. Can hippotherapy make a difference in the quality of life of children with cerebral palsy? A pragmatic study. Physiother. Theory Pract. 2022, 38, 390–400. [Google Scholar] [CrossRef]
- Bunketorp-Käll, L.; Lundgren-Nilsson, Å.; Samuelsson, H.; Pekny, T.; Blomvé, K.; Pekna, M.; Pekny, M.; Blomstrand, C.; Nilsson, M. Long-Term Improvements After Multimodal Rehabilitation in Late Phase After Stroke: A Randomized Controlled Trial. Stroke 2017, 48, 1916–1924. [Google Scholar] [CrossRef]
- Bunketorp-Käll, L.; Pekna, M.; Pekny, M.; Blomstrand, C.; Nilsson, M. Effects of horse-riding therapy and rhythm and music-based therapy on functional mobility in late phase after stroke. NeuroRehabilitation 2019, 45, 483–492. [Google Scholar] [CrossRef]
- Cerulli, C.; Minganti, C.; De Santis, C.; Tranchita, E.; Quaranta, F.; Parisi, A. Therapeutic horseback riding in breast cancer survivors: A pilot study. J. Altern. Complement. Med. 2014, 20, 623–629. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2022. Available online: https://www.R-project.org/ (accessed on 1 October 2022).
- Klein, D.; Mercier, M.; Abeilard, E.; Puyraveau, M.; Danzon, A.; Dalstein, V.; Pozet, A.; Guizard, A.-V.; Henry-Amar, M.; Velten, M. Long-term quality of life after breast cancer: A French registry-based controlled study. Breast Cancer Res. Treat. 2011, 129, 125–134. [Google Scholar] [CrossRef]
- Klafke, N.; Mahler, C.; von Hagens, C.; Rochon, J.; Schneeweiss, A.; Müller, A.; Salize, H.-J.; Joos, S. A complex nursing intervention of complementary and alternative medicine (CAM) to increase quality of life in patients with breast and gynecologic cancer undergoing chemotherapy: Study protocol for a partially randomized patient preference trial. Trials 2015, 16, 51. [Google Scholar] [CrossRef]
- Lua, P.L.; Salihah, N.; Mazlan, N. Effects of inhaled ginger aromatherapy on chemotherapy-induced nausea and vomiting and health-related quality of life in women with breast cancer. Complement. Ther. Med. 2015, 23, 396–404. [Google Scholar] [CrossRef]
- Mohammadi, S.; Sulaiman, S.; Koon, P.B.; Amani, R.; Hosseini, S.M. Impact of healthy eating practices and physical activity on quality of life among breast cancer survivors. Asian Pac. J. Cancer Prev. APJCP 2013, 14, 481–487. [Google Scholar] [CrossRef]
- Vardar Yağlı, N.; Şener, G.; Arıkan, H.; Sağlam, M.; İnal İnce, D.; Savcı, S.; Çalık Kutukcu, E.; Altundağ, K.; Kaya, E.B.; Kutluk, T.; et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr. Cancer Ther. 2015, 14, 125–132. [Google Scholar] [CrossRef]
- Cousson-Gélie, F.; Bruchon-Schweitzer, M.; Atzeni, T.; Houede, N. Evaluation of a psychosocial intervention on social support, perceived control, coping strategies, emotional distress, and quality of life of breast cancer patients. Psychol. Rep. 2011, 108, 923–942. [Google Scholar] [CrossRef]
- Field, A.P.; Wilcox, R.R. Robust statistical methods: A primer for clinical psychology and experimental psychopathology researchers. Behav. Res. Ther. 2017, 98, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Pitron, V.; Alsmith, A.; de Vignemont, F. How do the body schema and the body image interact? Conscious. Cogn. 2018, 65, 352–358. [Google Scholar] [CrossRef]
- Bromberg, P. Standing in the Spaces: Essays on Clinical Process, Trauma, and Dissociation. Psychoanal. Psychol. 2000, 17, 432–436. [Google Scholar] [CrossRef]
- Gaviria, M.; Celeghin, A.; Michael-Titus, A.T.; Pallier, P.N. Editorial: Brain Plasticity and Contribution of the Emotional Brain to Neural Remodelling After Injury. Front. Neurol. 2020, 11, 606271. [Google Scholar] [CrossRef]
- Viruega, H.; Gaviria, M. Functional Weight of Somatic and Cognitive Networks and Asymmetry of Compensatory Mechanisms: Collaboration or Divergency among Hemispheres after Cerebrovascular Accident? Life 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Diaz Heijtz, R.; Kolb, B.; Forssberg, H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur. J. Neurosci. 2003, 18, 3394–3399. [Google Scholar] [CrossRef] [PubMed]
- Horn, G. Pathways of the past: The imprint of memory. Nat. Rev. Neurosci. 2004, 5, 108–120. [Google Scholar] [CrossRef]
- Kolb, B.; Muhammad, A. Harnessing the power of neuroplasticity for intervention. Front. Hum. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001; ISBN 978-2-930064-16-1. [Google Scholar]
- Costa, D.S.J.; Aaronson, N.K.; Fayers, P.M.; Pallant, J.F.; Velikova, G.; King, M.T. Testing the measurement invariance of the EORTC QLQ-C30 across primary cancer sites using multi-group confirmatory factor analysis. Qual. Life Res. 2015, 24, 125–133. [Google Scholar] [CrossRef]
- Sprangers, M.A.; Groenvold, M.; Arraras, J.I.; Franklin, J.; te Velde, A.; Muller, M.; Franzini, L.; Williams, A.; de Haes, H.C.; Hopwood, P.; et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J. Clin. Oncol. 1996, 14, 2756–2768. [Google Scholar] [CrossRef]
- Nguyen, J.; Popovic, M.; Chow, E.; Cella, D.; Beaumont, J.L.; Chu, D.; DiGiovanni, J.; Lam, H.; Pulenzas, N.; Bottomley, A. EORTC QLQ-BR23 and FACT-B for the assessment of quality of life in patients with breast cancer: A literature review. J. Comp. Eff. Res. 2015, 4, 157–166. [Google Scholar] [CrossRef]
- McLachlan, S.A.; Devins, G.M.; Goodwin, P.J. Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) as a measure of psychosocial function in breast cancer patients. Eur. J. Cancer 1998, 34, 510–517. [Google Scholar] [CrossRef]
- Joly, F.; Lange, M.; Rigal, O.; Correia, H.; Giffard, B.; Beaumont, J.L.; Clisant, S.; Wagner, L. French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) version 3. Support. Care Cancer 2012, 20, 3297–3305. [Google Scholar] [CrossRef]
- Wagner, L.I.; Sweet, J.; Butt, Z.; Lai, J.; Cella, D. Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument. J. Support. Oncol. 2009, 7, W32–W39. [Google Scholar]
- Lange, M.; Heutte, N.; Morel, N.; Eustache, F.; Joly, F.; Giffard, B. Cognitive complaints in cancer: The French version of the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog), normative data from a healthy population. Neuropsychol. Rehabil. 2016, 26, 392–409. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Smets, E.M.; Garssen, B.; Cull, A.; de Haes, J.C. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br. J. Cancer 1996, 73, 241–245. [Google Scholar] [CrossRef]
- Gentile, S.; Delarozière, J.C.; Favre, F.; Sambuc, R.; San Marco, J.L. Validation of the French “multidimensional fatigue inventory” (MFI 20). Eur. J. Cancer Care 2003, 12, 58–64. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Razavi, D.; Delvaux, N.; Farvacques, C.; Robaye, E. Validation de la version française du HADS dans une population de patients cancéreux hospitalisés. [Validation of the French version of the Hospital Anxiety and Depression Scale (HADS) in a population of hospitalized cancer patients.]. Rev. Psychol. Appliquée 1989, 39, 295–307. [Google Scholar]
- Annunziata, M.A.; Muzzatti, B.; Bidoli, E.; Flaiban, C.; Bomben, F.; Piccinin, M.; Gipponi, K.M.; Mariutti, G.; Busato, S.; Mella, S. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support. Care Cancer 2020, 28, 3921–3926. [Google Scholar] [CrossRef]
- Hopwood, P.; Fletcher, I.; Lee, A.; Al Ghazal, S. A body image scale for use with cancer patients. Eur. J. Cancer 2001, 37, 189–197. [Google Scholar] [CrossRef]
- Brédart, A.; Swaine Verdier, A.; Dolbeault, S. Traduction/adaptation française de l’échelle “Body Image Scale” (BIS) évaluant la perception de l’image du corps chez des femmes atteintes de cancer du sein. Psycho-Oncol. 2007, 1, 24–30. [Google Scholar] [CrossRef]
- WHOQOL—Measuring Quality of Life|The World Health Organization. Available online: https://www.who.int/tools/whoqol (accessed on 20 September 2022).
- Karsten, M.M.; Roehle, R.; Albers, S.; Pross, T.; Hage, A.M.; Weiler, K.; Fischer, F.; Rose, M.; Kühn, F.; Blohmer, J.-U. Real-world reference scores for EORTC QLQ-C30 and EORTC QLQ-BR23 in early breast cancer patients. Eur. J. Cancer 2022, 163, 128–139. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Foo, Y.L.; Shwe, M.; Tan, Y.P.; Fan, G.; Yong, W.S.; Madhukumar, P.; Ooi, W.S.; Chay, W.Y.; Dent, R.A.; et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J. Clin. Epidemiol. 2014, 67, 811–820. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Kamen, C.; Mustian, K.M.; Mohile, S.G.; Magnuson, A.; Kleckner, I.R.; Guido, J.J.; Young, K.L.; et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J. Clin. Oncol. 2017, 35, 506–514. [Google Scholar] [CrossRef]
- Maass, S.W.M.C.; Brandenbarg, D.; Boerman, L.M.; Verhaak, P.F.M.; de Bock, G.H.; Berendsen, A.J. Fatigue among Long-Term Breast Cancer Survivors: A Controlled Cross-Sectional Study. Cancers 2021, 13, 1301. [Google Scholar] [CrossRef]
- Perez-Tejada, J.; Labaka, A.; Vegas, O.; Larraioz, A.; Pescador, A.; Arregi, A. Anxiety and depression after breast cancer: The predictive role of monoamine levels. Eur. J. Oncol. Nurs. 2021, 52, 101953. [Google Scholar] [CrossRef]
- Mallet, J.; Huillard, O.; Goldwasser, F.; Dubertret, C.; Strat, Y.L. Mental disorders associated with recent cancer diagnosis: Results from a nationally representative survey. Eur. J. Cancer 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Cheng, K.K.F.; Lim, Y.T.E.; Koh, Z.M.; Tam, W.W.S. Home-based multidimensional survivorship programmes for breast cancer survivors. Cochrane Database Syst. Rev. 2017, 8, CD011152. [Google Scholar] [CrossRef]
- Park, E.M.; Gelber, S.; Rosenberg, S.M.; Seah, D.S.E.; Schapira, L.; Come, S.E.; Partridge, A.H. Anxiety and Depression in Young Women With Metastatic Breast Cancer: A Cross-Sectional Study. Psychosomatics 2018, 59, 251–258. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.; Pawłowski, T. Negative body image in breast cancer patients. Adv. Clin. Exp. Med. 2019, 28, 1137–1142. [Google Scholar] [CrossRef]
- Brunet, J.; Price, J. A scoping review of measures used to assess body image in women with breast cancer. Psychooncology 2021, 30, 669–680. [Google Scholar] [CrossRef]
- Morales-Sánchez, L.; Luque-Ribelles, V.; Gil-Olarte, P.; Ruiz-González, P.; Guil, R. Enhancing Self-Esteem and Body Image of Breast Cancer Women through Interventions: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1640. [Google Scholar] [CrossRef]
- Greenlee, H.; DuPont-Reyes, M.J.; Balneaves, L.G.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef]
- Latte-Naor, S.; Mao, J.J. Putting Integrative Oncology Into Practice: Concepts and Approaches. J. Oncol. Pract. 2019, 15, 7–14. [Google Scholar] [CrossRef]
- Jeibouei, S.; Akbari, M.E.; Kalbasi, A.; Aref, A.R.; Ajoudanian, M.; Rezvani, A.; Zali, H. Personalized medicine in breast cancer: Pharmacogenomics approaches. Pharm. Pers. Med. 2019, 12, 59–73. [Google Scholar] [CrossRef]
- Parsons, J.; Francavilla, C. ’Omics Approaches to Explore the Breast Cancer Landscape. Front. Cell Dev. Biol. 2019, 7, 395. [Google Scholar] [CrossRef]
- de Freitas, A.J.A.; Causin, R.L.; Varuzza, M.B.; Hidalgo Filho, C.M.T.; da Silva, V.D.; Souza, C.d.P.; Marques, M.M.C. Molecular Biomarkers Predict Pathological Complete Response of Neoadjuvant Chemotherapy in Breast Cancer Patients: Review. Cancers 2021, 13, 5477. [Google Scholar] [CrossRef]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Consalvo, A.; Upadhyaya, P.; Sala, G.; Antonucci, I.; Del Boccio, P.; Stuppia, L.; De Laurenzi, V. Breast cancer in the era of integrating “Omics” approaches. Oncogenesis 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Skuse, A. Constructions of Cancer in Early Modern England: Ravenous Natures; Wellcome Trust–Funded Monographs and Book Chapters; Palgrave Macmillan: London, UK, 2015; ISBN 978-1-137-56919-6. [Google Scholar]
- Greenlee, H.; Balneaves, L.G.; Carlson, L.E.; Cohen, M.; Deng, G.; Hershman, D.; Mumber, M.; Perlmutter, J.; Seely, D.; Sen, A.; et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J. Natl. Cancer Inst. Monogr. 2014, 2014, 346–358. [Google Scholar] [CrossRef]
- Lyman, G.H.; Greenlee, H.; Bohlke, K.; Bao, T.; DeMichele, A.M.; Deng, G.E.; Fouladbakhsh, J.M.; Gil, B.; Hershman, D.L.; Mansfield, S.; et al. Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 2647–2655. [Google Scholar] [CrossRef]
- Wirtshafter, H.S.; Wilson, M.A. Locomotor and Hippocampal Processing Converge in the Lateral Septum. Curr. Biol. CB 2019, 29, 3177–3192.e3. [Google Scholar] [CrossRef]
- Wirtshafter, H.S.; Wilson, M.A. Differences in reward biased spatial representations in the lateral septum and hippocampus. eLife 2020, 9, e55252. [Google Scholar] [CrossRef]
- Wirtshafter, H.S.; Wilson, M.A. Lateral septum as a nexus for mood, motivation, and movement. Neurosci. Biobehav. Rev. 2021, 126, 544–559. [Google Scholar] [CrossRef]
- Maravita, A.; Spence, C.; Driver, J. Multisensory integration and the body schema: Close to hand and within reach. Curr. Biol. CB 2003, 13, R531–R539. [Google Scholar] [CrossRef]
- Crochet, S.; Lee, S.-H.; Petersen, C.C.H. Neural Circuits for Goal-Directed Sensorimotor Transformations. Trends Neurosci. 2019, 42, 66–77. [Google Scholar] [CrossRef]
- Buch, E.R.; Liew, S.-L.; Cohen, L.G. Plasticity of Sensorimotor Networks: Multiple Overlapping Mechanisms. Neuroscientist 2017, 23, 185–196. [Google Scholar] [CrossRef]
- Manière, G.; Coureaud, G. Editorial: From Stimulus to Behavioral Decision-Making. Front. Behav. Neurosci. 2019, 13, 274. [Google Scholar] [CrossRef]
- Le Merre, P.; Esmaeili, V.; Charrière, E.; Galan, K.; Salin, P.-A.; Petersen, C.C.H.; Crochet, S. Reward-Based Learning Drives Rapid Sensory Signals in Medial Prefrontal Cortex and Dorsal Hippocampus Necessary for Goal-Directed Behavior. Neuron 2018, 97, 83–91.e5. [Google Scholar] [CrossRef]
- Muller, R.U.; Kubie, J.L. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 1987, 7, 1951–1968. [Google Scholar] [CrossRef]
- Aronov, D.; Nevers, R.; Tank, D.W. Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 2017, 543, 719–722. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Liu, Y.; Zhu, J.; Liu, N.; Zeng, W.; Huang, N.; Rasch, M.J.; Jiang, H.; Gu, X.; et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 2017, 20, 559–570. [Google Scholar] [CrossRef]
- Kassab, R.; Alexandre, F. Integration of exteroceptive and interoceptive information within the hippocampus: A computational study. Front. Syst. Neurosci. 2015, 9, 87. [Google Scholar] [CrossRef]
- Maguire, E.A.; Mullally, S.L. The hippocampus: A manifesto for change. J. Exp. Psychol. Gen. 2013, 142, 1180–1189. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Ray Chaudhuri, K.; Reynolds, R.; Tan, E.-K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain J. Neurol. 2021, 144, 2571–2593. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Stefano, G.B.; Pilonis, N.; Ptacek, R.; Raboch, J.; Vnukova, M.; Kream, R.M. Gut, Microbiome, and Brain Regulatory Axis: Relevance to Neurodegenerative and Psychiatric Disorders. Cell. Mol. Neurobiol. 2018, 38, 1197–1206. [Google Scholar] [CrossRef]
- Evrensel, A.; Ceylan, M.E. The Gut-Brain Axis: The Missing Link in Depression. Clin. Psychopharmacol. Neurosci. 2015, 13, 239–244. [Google Scholar] [CrossRef]
- Leclercq, S.; Forsythe, P.; Bienenstock, J. Posttraumatic Stress Disorder: Does the Gut Microbiome Hold the Key? Can. J. Psychiatry Rev. Can. Psychiatr. 2016, 61, 204–213. [Google Scholar] [CrossRef]
- Consorti, A.; Di Marco, I.; Sansevero, G. Physical Exercise Modulates Brain Physiology Through a Network of Long- and Short-Range Cellular Interactions. Front. Mol. Neurosci. 2021, 14, 710303. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- Ehlers, D.K.; DuBois, K.; Salerno, E.A. The effects of exercise on cancer-related fatigue in breast cancer patients during primary treatment: A meta-analysis and systematic review. Expert Rev. Anticancer Ther. 2020, 20, 865–877. [Google Scholar] [CrossRef]
- Thomas, R.J.; Kenfield, S.A.; Jimenez, A. Exercise-induced biochemical changes and their potential influence on cancer: A scientific review. Br. J. Sports Med. 2017, 51, 640–644. [Google Scholar] [CrossRef]
- Wagoner, C.W.; Capozzi, L.C.; Culos-Reed, S.N. Tailoring the Evidence for Exercise Oncology within Breast Cancer Care. Curr. Oncol. 2022, 29, 4827–4841. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Wang, L.; Zhang, C.; Ma, J.; Zhao, Q. Does aquatic physical therapy affect the rehabilitation of breast cancer in women? A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0272337. [Google Scholar] [CrossRef]
- Pudkasam, S.; Polman, R.; Pitcher, M.; Fisher, M.; Chinlumprasert, N.; Stojanovska, L.; Apostolopoulos, V. Physical activity and breast cancer survivors: Importance of adherence, motivational interviewing and psychological health. Maturitas 2018, 116, 66–72. [Google Scholar] [CrossRef]
- Sale, A.; Berardi, N.; Maffei, L. Environment and brain plasticity: Towards an endogenous pharmacotherapy. Physiol. Rev. 2014, 94, 189–234. [Google Scholar] [CrossRef]
- Rodenkirch, C.; Carmel, J.B.; Wang, Q. Rapid Effects of Vagus Nerve Stimulation on Sensory Processing Through Activation of Neuromodulatory Systems. Front. Neurosci. 2022, 16, 922424. [Google Scholar] [CrossRef]
- Collins, L.; Boddington, L.; Steffan, P.J.; McCormick, D. Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr. Biol. CB 2021, 31, 2088–2098.e3. [Google Scholar] [CrossRef]
- Qin, L.; Jing, D.; Parauda, S.; Carmel, J.; Ratan, R.R.; Lee, F.S.; Cho, S. An Adaptive Role for BDNF Val66Met Polymorphism in Motor Recovery in Chronic Stroke. J. Neurosci. 2014, 34, 2493–2502. [Google Scholar] [CrossRef]
- Koroleva, E.S.; Tolmachev, I.V.; Alifirova, V.M.; Boiko, A.S.; Levchuk, L.A.; Loonen, A.J.M.; Ivanova, S.A. Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke. Brain Sci. 2020, 10, 623. [Google Scholar] [CrossRef]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef]
- Welsh, D.K.; Logothetis, D.E.; Meister, M.; Reppert, S.M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995, 14, 697–706. [Google Scholar] [CrossRef]
- Cunha-Reis, D.; Caulino-Rocha, A. VIP Modulation of Hippocampal Synaptic Plasticity: A Role for VIP Receptors as Therapeutic Targets in Cognitive Decline and Mesial Temporal Lobe Epilepsy. Front. Cell. Neurosci. 2020, 14, 153. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Sun, X.; Li, L.; Zhang, H.-Y.; Huang, Z.-L.; Wang, Y.-Q. Roles of Neuropeptides in Sleep–Wake Regulation. Int. J. Mol. Sci. 2022, 23, 4599. [Google Scholar] [CrossRef]
- Siebers, M.; Biedermann, S.V.; Bindila, L.; Lutz, B.; Fuss, J. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology 2021, 126, 105173. [Google Scholar] [CrossRef]
- Hackler, L.; Zadina, J.E.; Ge, L.J.; Kastin, A.J. Isolation of relatively large amounts of endomorphin-1 and endomorphin-2 from human brain cortex. Peptides 1997, 18, 1635–1639. [Google Scholar] [CrossRef]
- Zadina, J.E.; Hackler, L.; Ge, L.J.; Kastin, A.J. A potent and selective endogenous agonist for the mu-opiate receptor. Nature 1997, 386, 499–502. [Google Scholar] [CrossRef]
- Tabares, C.; Vicente, F.; Sánchez, S.; Aparicio, A.; Alejo, S.; Cubero, J. Quantification of hormonal changes by effects of hippotherapy in the autistic population. Neurochem. J. 2012, 6, 311–316. [Google Scholar] [CrossRef]
- Clark-Kennedy, A.E. Value judgements in medical practice. J. R. Coll. Physicians Lond. 1970, 5, 5–12. [Google Scholar]
- Epstein, R.M. Mindful practice. JAMA 1999, 282, 833–839. [Google Scholar] [CrossRef]
- Miller, F.G.; Joffe, S.; Kesselheim, A.S. Evidence, Errors, and Ethics. Perspect. Biol. Med. 2014, 57, 299–307. [Google Scholar] [CrossRef]
- Saslow, C. Understanding the perceptual world of horses. Appl. Anim. Behav. Sci. 2002, 78, 209–224. [Google Scholar] [CrossRef]
- Sacks, O. An Anthropologist on Mars: Seven Paradoxical Tales; Vintage Books: New York, NY, USA, 1996; ISBN 978-0-679-75697-2. [Google Scholar]
- de la Cruz, M.; Hui, D.; Parsons, H.A.; Bruera, E. Placebo and nocebo effects in randomized double blind clinical trials of agents for the treatment of fatigue in advanced cancer patients. Cancer 2010, 116, 766–774. [Google Scholar] [CrossRef]
| Hippotherapy Group | Control Group | p-Value | |
|---|---|---|---|
| Age (years; M ± SD) | 52.62 ± 9.93 | 52.66 ± 9.98 | ns (0.7275) |
| BMI (kg/m2; M ± SD) | 25.46 ± 5.55 | 22.22 ± 4.38 | ns (0.1137) |
| Menopausal (%) | 53 | 57 | ns (0.506) |
| Occupation (%): Retired Working life Unknown | 17 77 17 | 9 74 17 | ns (0.7207) |
| Type of breast cancer (%): Ductal carcinoma in situ Invasive ductal carcinoma Lobular carcinoma in situ Invasive lobular carcinoma Triple negative Other | 38 54 0 8 8 0 | 9 78 0 9 0 4 | * (0.0265) |
| Stage(%): T1N0 T2N0 T1N1 T2N1 T2N2 | 58 4 17 17 4 | 48 17 17 13 4 | ns (0.6839) |
| Grade (%): 1 2 3 | 42 38 21 | 22 48 30 | ns (0.3489) |
| Affected side (%): Left Right | 58 42 | 48 52 | ns (0.4441) |
| Type of treatment (%): Conservative surgery Mastectomy | 67 33 | 91 9 | (.) (0.0879) |
| Radiotherapy Chemotherapy Hormonal therapy | 83 50 63 | 91 52 70 | ns (1.0000) ns (0.8349) |
| Delay (days; M ± SD): | |||
| Q1–Q2 | 15.0 ± 5.1 | 12.9 ± 9.3 | ns (0.07) |
| Q2–Q3 | 201.0 ± 63.4 | 199.0 ± 44.8 | ns (0.22) |
| Hippotherapy Group | Control Group | p-Value | |
|---|---|---|---|
| EORTC QLQ-30 (summary score) | 70.0 ± 15.0 | 71.6 ± 11.3 | ns (0.956) |
| 57.4 ± 16.5 | 57.1 ± 14.1 | ns (0.654) |
| 80.0 ±18.6 | 85.4 ± 12.3 | ns (0.379) |
| 67.1 ± 28.0 | 72.2 ± 27.3 | ns (0.416) |
| 55.6 ± 25.0 | 55.1 ± 25.5 | ns (0.983) |
| 60.2 ± 24.1 | 64.1 ± 28.9 | ns (0.722) |
| 75.9 ± 28.0 | 69.2 ± 29.7 | ns (0.384) |
| 30.9 ± 15.6 | 28.3 ± 10.5 | ns (0.972) |
| 50.0 ± 23.7 | 51.0 ± 22.3 | ns (0.694) |
| 6.5 ± 12.8 | 2.5 ± 6.0 | ns (0.262) |
| 33.8 ± 29.4 | 29.9 ± 23.4 | ns (0.685) |
| 46.3 ± 61.9 | 23.5 ± 30.4 | * (0.025) |
| 51.9 ± 35.1 | 51.9 ± 37.4 | ns (0.994) |
| 9.26 ± 23.4 | 14.8 ± 23.3 | ns (0.110) |
| 28.7 ± 35.8 | 30.8 ± 28.2 | ns (0.478) |
| 4.63 ± 11.7 | 10.3 ± 20.6 | ns (0.307) |
| 30.6 ± 35.1 | 19.2 ± 28.6 | ns (0.244) |
| EORTC QLQ-BR23 (summary score) | 65.1 ± 12.8 | 63.6 ± 10.2 | ns (0.568) |
| 61.3 ± 32.1 | 59.8 ± 30.9 | ns (0.622) |
| 77.3 ± 24.6 | 77.4 ± 21.4 | ns (0.897) |
| 47.1 ± 26.5 | 50.0 ± 28.6 | ns (0.655) |
| 41.7 ± 30.2 | 38.1 ± 33.6 | ns (0.585) |
| 19.6 ± 13.2 | 22.8 ± 13.6 | ns (0.349) |
| 35.4 ± 17.1 | 40.7 ± 26.6 | ns (0.619) |
| 25.9 ± 20.4 | 22.2 ± 19.2 | ns (0.558) |
| FACT-Cog (summary score) | 89.3 ± 25.7 | 88.9 ± 27.4 | ns (0.872) |
| 19.2 ± 6.4 | 18.1 ± 7.7 | ns (0.556) |
| 47.1 ± 15.9 | 47.0 ± 14.9 | ns (0.962) |
| 14.4 ± 2.0 | 14.4 ± 2.7 | ns (0.560) |
| 7.7 ± 5.5 | 9.0 ± 4.6 | ns (0.329) |
| IMF (summary score) | 59.9 ± 15.1 | 60.0 ± 13.3 | ns (0.940) |
| 14.2 ± 3.6 | 14.4 ± 3.5 | ns (0.924) |
| 12.5 ± 4.4 | 12.5 ± 4.0 | ns (0.989) |
| 12.1 ± 4.4 | 11.9 ± 4.3 | ns (0.995) |
| 11.1 ± 3.7 | 10.5 ± 2.0 | ns (0.362) |
| 10.0 ± 3.5 | 10.8 ± 3.11 | ns (0.693) |
| HADS | |||
| 10.0 ± 4.1 | 10.3 ± 3.8 | ns (0.823) |
| 6.1 ± 4.3 | 7.0 ± 4.2 | ns (0.302) |
| BIS | |||
| 21.9 ± 7.9 | 20.7 ± 7.8 | ns (0.453) |
| Consulted Specialist | Frequency of Visits |
|---|---|
| Algologist | Occasionally |
| Sexologist | One group session |
| Addictologist | Occasionally |
| Occupational physician | Occasionally |
| Homeopath | Every two months |
| Acupuncturist | Monthly |
| Physical therapist | Twice a week |
| Psychologist | Every two weeks |
| Nutritionist | Two group sessions |
| Social worker | Occasionally |
| Evaluated Items | Contribution through Hippotherapy |
|---|---|
| Quality of Life—EORTC QLQ-C30, functional scales | |
|
|
|
|
|
|
|
|
|
|
|
|
| Quality of Life—EORTC QLQ-C30, symptom scales | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Quality Of Life—EORTC QLQ-BR23, functional scales | |
|
|
|
|
|
|
|
|
| Quality of Life—EORTC QLQ-BR23, symptom scales | |
|
|
|
|
|
|
| Cognitive functioning—FACT-Cog: memory, verbal fluency, concentration, mental sharpness, resistance to interference, multitasking ability | |
|
|
|
|
|
|
|
|
| Fatigue—MFI-20 | |
|
|
|
|
|
|
|
|
|
|
| Anxiety and Depression—HADS | |
|
|
|
|
| Body Image—BIS | |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viruega, H.; Galy, C.; Loriette, C.; Jacquot, S.; Houpeau, J.L.; Gaviria, M. Breast Cancer: How Hippotherapy Bridges the Gap between Healing and Recovery—A Randomized Controlled Clinical Trial. Cancers 2023, 15, 1317. https://doi.org/10.3390/cancers15041317
Viruega H, Galy C, Loriette C, Jacquot S, Houpeau JL, Gaviria M. Breast Cancer: How Hippotherapy Bridges the Gap between Healing and Recovery—A Randomized Controlled Clinical Trial. Cancers. 2023; 15(4):1317. https://doi.org/10.3390/cancers15041317
Chicago/Turabian StyleViruega, Hélène, Corinne Galy, Célia Loriette, Stéphane Jacquot, Jean Louis Houpeau, and Manuel Gaviria. 2023. "Breast Cancer: How Hippotherapy Bridges the Gap between Healing and Recovery—A Randomized Controlled Clinical Trial" Cancers 15, no. 4: 1317. https://doi.org/10.3390/cancers15041317
APA StyleViruega, H., Galy, C., Loriette, C., Jacquot, S., Houpeau, J. L., & Gaviria, M. (2023). Breast Cancer: How Hippotherapy Bridges the Gap between Healing and Recovery—A Randomized Controlled Clinical Trial. Cancers, 15(4), 1317. https://doi.org/10.3390/cancers15041317







