Neck Surgery for Non-Well Differentiated Thyroid Malignancies: Variations in Strategy According to Histopathology
Simple Summary
Abstract
1. Introduction
2. Lymphatic Drainage Pathways of the Thyroid Gland
3. Treatment of the Neck: Central and Lateral Neck Dissection
4. Strategy According to Histology
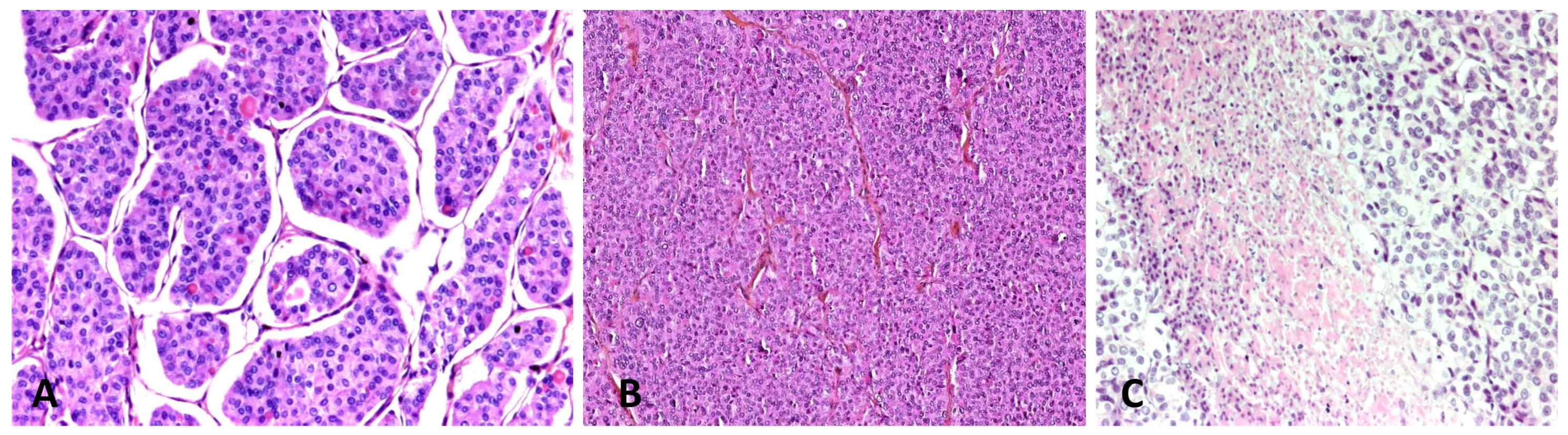
4.1. Poorly-Differentiated Thyroid Carcinoma
4.2. Anaplastic Thyroid Carcinoma
4.3. Medullary Thyroid Carcinoma
4.4. Mucoepidermoid Carcinoma
4.5. Malignant Peripheral Nerve Sheath Tumors (MPNSTs)
4.6. Angiosarcoma
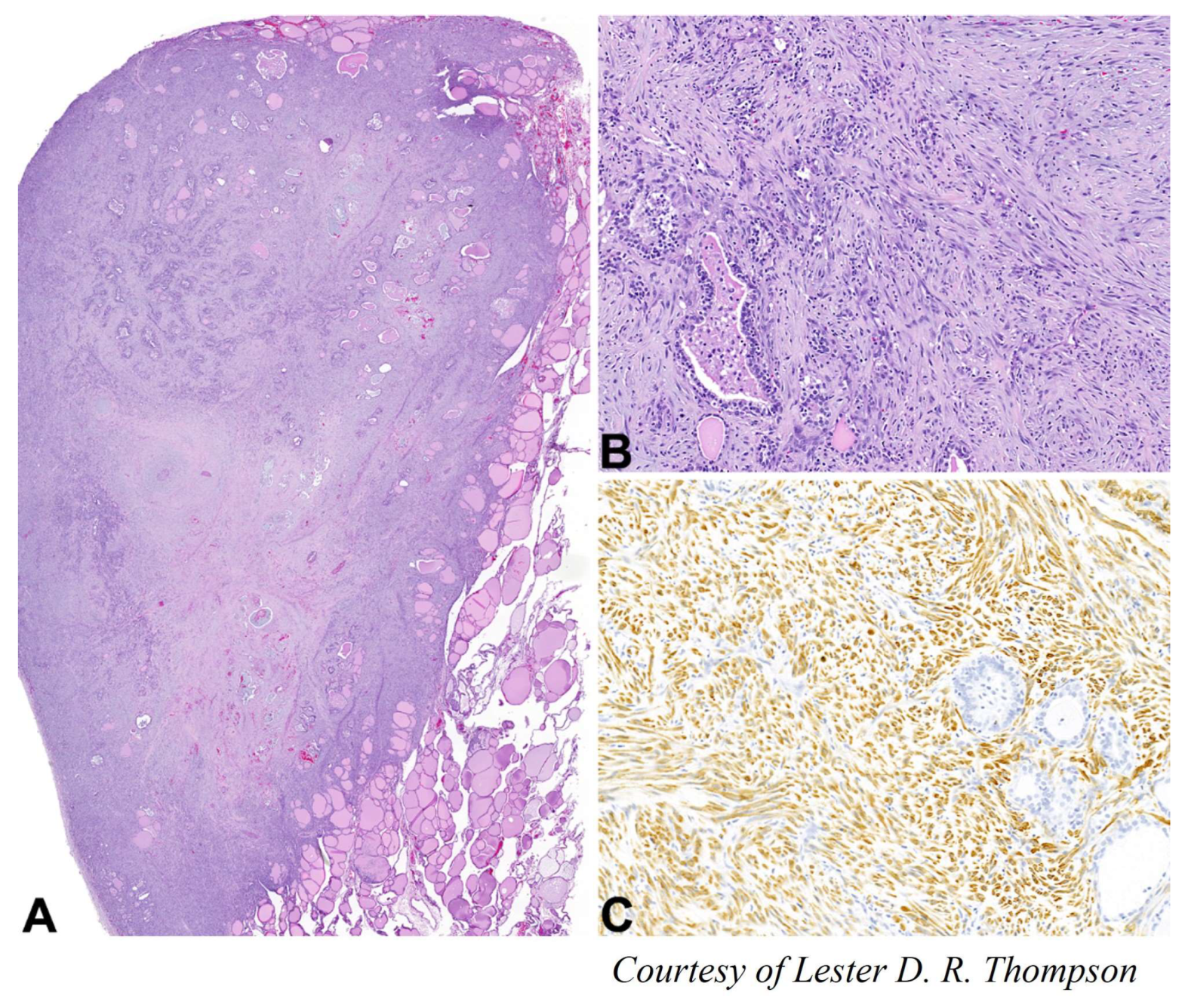
4.7. Leiomyosarcoma
4.8. Primary Malignant Thyroid Teratoma or Thyroblastoma
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asimakopoulos, P.; Shaha, A.R.; Nixon, I.J.; Shah, J.P.; Randolph, G.W.; Angelos, P.; Zafereo, M.E.; Kowalski, L.P.; Hartl, D.M.; Olsen, K.D.; et al. Management of the neck in well-differentiated thyroid cancer. Curr. Oncol. Rep. 2020, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.I. Thyroid carcinoma. Lancet 2000, 361, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimpasic, T.; Ghossein, R.; Shah, J.P.; Ganly, I. Poorly differentiated carcinoma of the thyroid gland: Current status and future prospects. Thyroid 2019, 29, 3110321. [Google Scholar] [CrossRef] [PubMed]
- American Thyroid Association Guidelines Task Force; Kloos, R.T.; Eng, C.; Evans, D.B.; Francis, G.L.; Gagel, R.F.; Gharib, H.; Moley, J.F.; Pacini, F.; Ringel, M.D.; et al. Medullary thyroid cancer: Management guidelines of the American Thyroid Association. Thyroid 2009, 19, 565–612. [Google Scholar] [CrossRef]
- Surov, A.; Gottschling, S.; Wienke, A.; Meyer, H.J.; Spielmann, R.P.; Dralle, H. Primary thyroid sarcoma: A systematic review. Anticancer Res. 2015, 35, 5185–5191. [Google Scholar]
- Kandil, E.; Abdel Khalek, M.; Abdullah, O.; Dali, D.; Faruqui, S.; Khan, A.; Friedlander, P.; Jaffe, B.M.; Crawford, B. Primary peripheral nerve sheath tumors of the thyroid gland. Thyroid 2010, 20, 583–586. [Google Scholar] [CrossRef]
- Ting, J.; Bell, D.; Ahmed, S.; Ying, A.; Waguespack, S.G.; Tu, S.M.; Weber, R.; Zafereo, M. Primary malignant thyroid teratoma: An institutional experience. Thyroid 2019, 29, 229–236. [Google Scholar] [CrossRef]
- Vander Poorten, V.; Goedseels, N.; Triantafyllou, A.; Sanabria, A.; Clement, P.M.; Cohen, O.; Golusinski, P.; Guntinas-Lichius, O.; Piazza, C.; Randolph, G.W.; et al. Effectiveness of core needle biopsy in the diagnosis of thyroid lymphoma and anaplastic thyroid carcinoma: A systematic review and meta-analysis. Front. Endocrinol. 2022, 20, 971249. [Google Scholar] [CrossRef]
- Matrone, A.; De Napoli, L.; Torregrossa, L.; Aghababyan, A.; Papini, P.; Ambrosini, C.E.; Cervelli, R.; Ugolini, C.; Basolo, F.; Molinaro, E.; et al. Core needle biopsy can early and precisely identify large thyroid masses. Front. Oncol. 2022, 5, 854755. [Google Scholar] [CrossRef]
- Wu, M.H.; Lee, Y.Y.; Lu, Y.L.; Lin, S.F. Risk factors and prognosis for metastatic follicular thyroid cancer. Front. Endocrinol. 2022, 1, 791826. [Google Scholar] [CrossRef]
- Gonçalves Filho, J.; Zafereo, M.E.; Ahmad, F.I.; Nixon, I.J.; Shaha, A.R.; Vander Poorten, V.; Sanabria, A.; Hefetz, A.K.; Robbins, K.T.; Kamani, D.; et al. Decision making for the central compartment in differentiated thyroid cancer. Eur. J. Surg. Oncol. 2018, 44, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Pilaete, K.; Delaere, P.; Decallonne, B.; Bex, M.; Hauben, E.; Nuyts, S.; Clement, P.; Hermans, R.; Vander Poorten, V. Medullary thyroid cancer: Prognostic factors for survival and recurrence, recommendations for the extent of lymph node dissection and for surgical therapy in recurrent disease. B-ENT 2012, 8, 113–121. [Google Scholar] [PubMed]
- Iyer, N.G.; Kumar, A.; Nixon, I.J.; Patel, S.G.; Ganly, I.; Tuttle, R.M.; Shah, J.P.; Shaha, A.R. Incidence and significance of Delphian node metastasis in papillary thyroid cancer. Ann. Surg. 2011, 253, 988–991. [Google Scholar] [CrossRef] [PubMed]
- De lactibus, sive, Lacteis venis, quarto vasorum mesaraicorum genere (1640). Available online: https://books.google.nl/books?id=cKdiAAAAcAAJ&printsec=frontcover&hl=nl&source=gbs_book_other_versions_r&cad=4#v=onepage&q&f=false (accessed on 1 January 2023).
- Fagher, B.; Monti, M.; Thulin, T. Selective beta 1-adrenoceptor blockade and muscle thermogenesis. Acta Med. Scand. 1988, 223, 139–145. [Google Scholar] [CrossRef]
- Mahadevan, A.; Welsh, I.C.; Sivakumar, A.; Gludish, D.W.; Shilvock, A.R.; Noden, D.M.; Huss, D.; Lansford, R.; Kurpios, N.A. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev. Cell 2014, 22, 690–706. [Google Scholar] [CrossRef]
- Pai, S.I.; Tufano, R.P. Central compartment neck dissection for thyroid cancer. Technical considerations. ORL J. Otorhinolaryngol. Relat. Spec. 2008, 70, 292–297. [Google Scholar] [CrossRef]
- Stack, B.C., Jr.; Ferris, R.L.; Goldenberg, D.; Haymart, M.; Shaha, A.; Sheth, S.; Sosa, J.A.; Tufano, R.P.; American Thyroid Association Surgical Affairs Committee. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 2012, 22, 501–508. [Google Scholar] [CrossRef]
- Nixon, I.J.; Shaha, A.R. Management of regional nodes in thyroid cancer. Oral Oncol. 2013, 49, 671–675. [Google Scholar] [CrossRef]
- Agrawal, N.; Evasovich, M.R.; Kandil, E.; Noureldine, S.I.; Felger, E.A.; Tufano, R.P.; Kraus, D.H.; Orloff, L.A.; Grogan, R.; Angelos, P.; et al. Indications and extent of central neck dissection for papillary thyroid cancer: An American Head and Neck Society Consensus Statement. Head Neck 2017, 39, 1269–1279. [Google Scholar] [CrossRef]
- Likhterov, I.; Reis, L.L.; Urken, M.L. Central compartment management in patients with papillary thyroid cancer presenting with metastatic disease to the lateral neck: Anatomic pathways of lymphatic spread. Head Neck 2017, 39, 853–859. [Google Scholar] [CrossRef]
- De Felice, M.; Di Lauro, R. Thyroid development and its disorders: Genetics and molecular mechanisms. Endocr. Rev. 2004, 25, 722–746. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, Y.S.; Kim, B.W.; Chang, H.S.; Park, C.S. Skip lateral neck node metastases in papillary thyroid carcinoma. World J. Surg. 2012, 36, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, F.; Zhou, T.; Lu, K.; Jiang, K.; Zhang, Y.; Luo, D. Risk factors of skip lateral cervical lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2022, 75, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sakamoto, H. Regional anatomy for head and neck surgery. Otolaryngol. Head Neck Surg. 1994, 66, 837–840. [Google Scholar]
- Robbins, K.T.; Woodson, G.E. Thyroid carcinoma presenting as a parapharyngeal mass. Head Neck Surg. 1985, 7, 434–436. [Google Scholar] [CrossRef]
- Harries, V.; McGill, M.; Tuttle, R.M.; Shaha, A.R.; Wong, R.J.; Shah, J.P.; Patel, S.G.; Ganly, I. Management of retropharyngeal lymph node metastases in differentiated thyroid carcinoma. Thyroid 2020, 30, 688–695. [Google Scholar] [CrossRef]
- Desuter, G.; Lonneux, M.; Plouin-Gaudon, I.; Jamar, F.; Coche, E.; Weynand, B.; Rahier, J.; Grégoire, V.; Andry, G.; Hamoir, M. Parapharyngeal metastases from thyroid cancer. Eur. J. Surg. Oncol. 2004, 30, 80–84. [Google Scholar] [CrossRef]
- Ferlito, A.; Robbins, K.T.; Silver, C.E. Neck Dissection—Management of Regional Disease in Head and Neck Cancer; Plural Publishing: San Diego, CA, USA, 2010. [Google Scholar]
- American Thyroid Association Surgery Working Group; American Association of Endocrine Surgeons; American Academy of Otolaryngology-Head and Neck Surgery; American Head and Neck Society; Carty, S.E.; Cooper, D.S.; Doherty, G.M.; Duh, Q.Y.; Kloos, R.T.; Mandel, S.J.; et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid 2009, 19, 1153–1158. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Lesnik, D.; Cunnane, M.B.; Zurakowski, D.; Acar, G.O.; Ecevit, C.; Mace, A.; Kamani, D.; Randolph, G.W. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck 2014, 36, 191–202. [Google Scholar] [CrossRef]
- Goyal, N.; Pakdaman, M.; Kamani, D.; Caragacianu, D.; Goldenberg, D.; Randolph, G.W. Mapping the distribution of nodal metastases in papillary thyroid carcinoma: Where are the nodes exactly? Laryngoscope 2017, 127, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, M.; Kyriazidis, N.; Kamani, D.; Juliano, A.F.; Kelly, H.R.; Curtin, H.D.; Barber, S.R.; Randolph, G.W. A novel thyroid cancer nodal map classification system to facilitate nodal localization and surgical management: The A to D map. Laryngoscope 2017, 127, 2429–2436. [Google Scholar] [CrossRef]
- Randolph, G.W.; Duh, Q.Y.; Heller, K.S.; Livolsi, V.A.; Mandel, S.J.; Steward, D.L.; Tufano, R.P.; Tuttle, R.M. (For The American Thyroid Association Surgical Affairs Committee’s Taskforce On Thyroid Cancer Nodal Surgery). The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012, 22, 1144–1152. [Google Scholar] [PubMed]
- Wu, G.; Fraser, S.; Pai, S.I.; Farrag, T.Y.; Ladenson, P.W.; Tufano, R.P. Determining the extent of lateral neck dissection necessary to establish regional disease control and avoid reoperation after previous total thyroidectomy and radioactive iodine for papillary thyroid cancer. Head Neck 2012, 34, 1418–1421. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Kasai, N.; Sugano, H. Poorly differentiated carcinoma of the thyroid. a clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer 1983, 52, 1849–1855. [Google Scholar] [CrossRef]
- Sanders, E.M., Jr.; LiVolsi, V.A.; Brierley, J.; Shin, J.; Randolph, G.W. An evidence-based review of poorly differentiated thyroid cancer. World J. Surg. 2007, 31, 934–945. [Google Scholar] [PubMed]
- Chao, T.C.; Lin, J.D.; Chen, M.F. Insular carcinoma: Infrequent subtype of thyroid cancer with aggressive clinical course. World J. Surg. 2004, 28, 393–396. [Google Scholar] [CrossRef]
- Bellini, M.I.; Biffoni, M.; Patrone, R.; Borcea, M.C.; Costanzo, M.L.; Garritano, T.; Melcarne, R.; Menditto, R.; Metere, A.; Scorziello, C.; et al. Poorly differentiated thyroid carcinoma: Single centre experience and review of the literature. J. Clin. Med. 2021, 10, 5258. [Google Scholar] [CrossRef]
- Kunte, S.; Sharett, J.; Wei, W.; Nasr, C.; Prendes, B.; Lamarre, E.; Ku, J.; Lorenz, R.R.; Scharpf, J.; Burkey, B.B.; et al. Poorly differentiated thyroid carcinoma: Single institution series of outcomes. Anticancer Res. 2022, 42, 2531–2539. [Google Scholar] [CrossRef]
- Brierley, J.; Tsang, R.; Panzarella, T.; Bana, N. Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin. Endocrinol. 2005, 63, 418–427. [Google Scholar] [CrossRef]
- Ibrahimpasic, T.; Ghossein, R.; Carlson, D.L.; Nixon, I.; Palmer, F.L.; Shaha, A.R.; Patel, S.G.; Tuttle, R.M.; Shah, J.P.; Ganly, I. Outcomes in patients with poorly differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Copland, J.A. Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clin. Oncol. 2010, 22, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.C.; Cote, G.; et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Coca-Pelaz, A.; Rodrigo, J.P.; Lopez, F.; Shah, J.P.; Silver, C.E.; Al Ghuzlan, A.; Menke-van der Houven van Oordt, C.W.; Smallridge, R.C.; Shaha, A.R.; Angelos, P.; et al. Evaluating new treatments for anaplastic thyroid cancer. Expert Rev. Anticancer Ther. 2022, 22, 1239–1247. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- Tan, R.K.; Finley, R.K., 3rd; Driscoll, D.; Bakamjian, V.; Hicks, W.L., Jr.; Shedd, D.P. Anaplastic carcinoma of the thyroid: A 24-year experience. Head Neck 1995, 17, 41–48. [Google Scholar] [CrossRef]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Lu, C.; Ahmed, S.; Gule-Monroe, M.K.; Williams, M.D.; Sturgis, E.M.; et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma. Thyroid 2019, 29, 1036–1043. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. American Thyroid Association guidelines task force on medullary thyroid carcinoma. revised american thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Scollo, C.; Baudin, E.; Travagli, J.P.; Caillou, B.; Bellon, N.; Leboulleux, S.; Schlumberger, M. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2003, 88, 2070–2075. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, F.; Wei, X.; Mao, Y.; Mu, J.; Zhao, L.; Wu, J.; Xin, X.; Zhang, S.; Tan, J. Ultrasound features value in the diagnosis and prognosis of medullary thyroid carcinoma. Endocrine 2021, 72, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.L.; Wajner, S.M.; Vargas, C.V. Advances and controversies in the management of medullary thyroid carcinoma. Curr. Opin. Oncol. 2017, 29, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Orloff, L.A.; Kuppersmith, R.B. American Thyroid Association’s central neck dissection terminology and classification for thyroid cancer consensus statement. Otolaryngol. Head Neck Surg. 2010, 142, 4–5. [Google Scholar] [CrossRef]
- Lindsey, S.C.; Ganly, I.; Palmer, F.; Tuttle, R.M. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid 2015, 25, 242–249. [Google Scholar] [CrossRef]
- Spanheimer, P.M.; Ganly, I.; Chou, J.F.; Capanu, M.; Nigam, A.; Ghossein, R.A.; Tuttle, R.M.; Wong, R.J.; Shaha, A.R.; Brennan, M.F.; et al. prophylactic lateral neck dissection for medullary thyroid carcinoma is not associated with improved survival. Ann. Surg. Oncol. 2021, 28, 6572–6579. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Dralle, H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J. Clin. Endocrinol. Metab. 2010, 95, 2655–2663. [Google Scholar] [CrossRef]
- Pena, I.; Clayman, G.L.; Grubbs, E.G.; Bergeron, J.M., Jr.; Waguespack, S.G.; Cabanillas, M.E.; Dadu, R.; Hu, M.I.; Fellman, B.M.; Li, Y.; et al. Management of the lateral neck compartment in patients with sporadic medullary thyroid cancer. Head Neck 2018, 40, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Hauptmann, S.; Dralle, H. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br. J. Surg. 2008, 95, 586–591. [Google Scholar] [CrossRef]
- Contrera, K.J.; Gule-Monroe, M.; Hu, M.I.; Cabanillas, M.; Busaidy, N.; Dadu, R.; Waguespack, S.G.; Wang, J.R.; Maniakas, A.; Lai, S.Y.; et al. Neoadjuvant selective RET inhibitor for medullary thyroid cancer: A case series. Thyroid 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Selpercatinib before Surgery for the Treatment of RET-Altered Thyroid Cancer, NCT04759911. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04759911 (accessed on 22 December 2022).
- Coca-Pelaz, A.; Rodrigo, J.P.; Triantafyllou, A.; Hunt, J.L.; Rinaldo, A.; Strojan, P.; Haigentz, M., Jr.; Mendenhall, W.M.; Takes, R.P.; Vander Poorten, V.; et al. Salivary mucoepidermoid carcinoma revisited. Eur. Arch. Otorhinolaryngol. 2015, 272, 799–819. [Google Scholar] [CrossRef]
- Lee, K.; Mirza, O.; Dobbs, S.; Jayaram, S. Poorly differentiated mucoepidermoid carcinoma of the thyroid. BMJ Case Rep. 2020, 13, e236539. [Google Scholar] [CrossRef] [PubMed]
- Rhatigan, R.M.; Roque, J.L.; Bucher, R.L. Mucoepidermoid carcinoma of the thyroid gland. Cancer 1977, 39, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Van Le, Q.; Ngo, D.Q.; Ngo, Q.X. primary mucoepidermoid carcinoma of the thyroid: A report of a rare case with bone metastasis and review of the literature. Case Rep. Oncol. 2019, 12, 248–259. [Google Scholar]
- Prichard, R.S.; Lee, J.C.; Gill, A.J.; Sywak, M.S.; Fingleton, L.; Robinson, B.G.; Sidhu, S.B.; Delbridge, L.W. Mucoepidermoid carcinoma of the thyroid: A report of three cases and postulated histogenesis. Thyroid 2012, 22, 205–209. [Google Scholar] [CrossRef]
- Farhat, N.A.; Faquin, W.C.; Sadow, P.M. Primary mucoepidermoid carcinoma of the thyroid gland: A report of three cases and review of the literature. Endocr. Pathol. 2013, 24, 229–233. [Google Scholar] [CrossRef]
- Wenig, B.M.; Adair, C.F.; Heffess, C.S. Primary mucoepidermoid carcinoma of the thyroid gland: A report of six cases and a review of the literature of a follicular epithelial-derived tumor. Hum. Pathol. 1995, 26, 1099–1108. [Google Scholar] [CrossRef]
- Kleihues, P.; Cavenee, W.K. Tumours of the nervous system. Pathology and genetics. In World Health Organization Classification of Tumours; IARC Press: Lyon, France, 2000. [Google Scholar]
- Al-Ghamdi, S.; Fageeh, N.; Dewan, M. Malignant schwannoma of the thyroid gland. Otolaryngol. Head Neck Surg. 2000, 122, 143–144. [Google Scholar] [CrossRef]
- Thompson, L.D.; Wenig, B.M.; Adair, C.F.; Heffess, C.S. Peripheral nerve sheath tumors of the thyroid gland: A series of four cases and a review of the literature. Endocr. Pathol. 1996, 7, 309–318. [Google Scholar] [CrossRef]
- Danish, M.H.; Wasif, M.; Din, N.U.; Awan, M.S. Malignant peripheral nerve sheath tumour of thyroid: A diagnostic dilemma. BMJ Case Rep. 2020, 13, e234374. [Google Scholar] [CrossRef]
- Kar, M.; Deo, S.V.; Shukla, N.K.; Malik, A.; DattaGupta, S.; Mohanti, B.K.; Thulkar, S. Malignant peripheral nerve sheath tumors (MPNST)- clinicopathological study and treatment outcome of twenty-four cases. World J. Surg. Oncol. 2006, 4, 55. [Google Scholar] [CrossRef]
- Pallares, J.; Perez-Ruiz, L.; Ros, S.; Panades, M.J.; Pardo-Mindan, J.; Lloreta, J.; Matias-Guiu, X. Malignant peripheral nerve sheath tumor of the thyroid: A clinicopathological and ultrastructural study of one case. Endocr. Pathol. 2004, 15, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.J.; Zagars, G.K.; Torres, K.E.; Bird, J.E.; Feig, B.W.; Guadagnolo, B.A. Malignant peripheral nerve sheath tumors: A single institution’s experience using combined surgery and radiation therapy. Am. J. Clin. Oncol. 2018, 41, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.G.; Chuah, K.L.; Goh, H.K.; Chen, Y.Y. Two cases of epithelioid angiosarcoma involving the thyroid and a brief review of non-Alpine epithelioid angiosarcoma of the thyroid. Arch. Pathol. Lab. Med. 2003, 127, 70–73. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Moscatelli, E.; Orelli, S.; Bulzonetti, N.; Musio, D.; Tombolini, V. Primary thyroid angiosarcoma: A systematic review. Oral Oncol. 2018, 82, 48–52. [Google Scholar] [CrossRef]
- Rhomberg, W.; Boehler, F.; Eiter, H.; Fritzsche, H.; Breitfellner, G. Treatment options for malignant hemangioendotheliomas of the thyroid. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Kehagias, D.; Kostopoulou, E.; Ravazoula, P.; Panagopoulos, K. Thyroid angiosarcoma (TAS)-a rare diagnosis not to be missed. Clin. Case Rep. 2020, 9, 173–176. [Google Scholar] [CrossRef]
- Astl, J.; Dusková, J.; Límanová, Z.; Povýsil, C.; Kuchynková, Z. Hemangiosarcoma of the thyroid gland. A case report. Neuroendocrinol. Lett. 2000, 21, 213–216. [Google Scholar]
- Petronella, P.; Scorzelli, M.; Luise, R.; Iannaci, G.; Sapere, P.; Ferretti, M.; Costanzo, R.M.; Freda, F.; Canonico, S.; Rossiello, R. Primary thyroid angiosarcoma: An unusual localization. World J. Surg. Oncol. 2012, 10, 73. [Google Scholar] [CrossRef]
- Bala, N.M.; Simões, P.; Aragüés, J.M.; Veiga, R.; Guerra, S.; Valadas, C. Non-alpine primary thyroid angiosarcoma. Arch. Endocrinol. Metab. 2022, 25, 2359–3997000000460. [Google Scholar] [CrossRef]
- Ryska, A.; Ludvíková, M.; Szépe, P.; Böör, A. Epithelioid haemangiosarcoma of the thyroid gland. Report of six cases from a non-Alpine region. Histopathology 2004, 44, 40–46. [Google Scholar] [CrossRef]
- Negură, I.; Bădescu, M.C.; Rezuş, C.; Dănilă, R.; Florescu, A.F.; Blaj, M.; Moroşan, E.; Apóstol, D.G.C. Morphology and one immunohistochemical marker are enough for diagnosis of primary thyroid angiosarcoma. Arch. Clin. Cases. 2021, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E.; Gaffey, M.J.; Watts, J.C.; Swanson, P.E.; Wick, M.R.; LiVolsi, V.A.; Nappi, O.; Weiss, L.M. Angiomatoid carcinoma and ‘angiosarcoma’ of the thyroid gland. A spectrum of endothelial differentiation. Am. J. Clin. Pathol. 1994, 102, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Benbella, L.; Elouarith, I.; Ouazzani, H.E.L.; Bernoussi, Z.; Lahlou, M.K.; Zouaidia, F. Thyroid angiosarcoma: A case report and review of literature. Int. J. Surg. Case Rep. 2022, 97, 107358. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; Martins, R.G.; Santos, A.P.; Matos, J.; Torres, I. Invasive thyroid angiosarcoma with a favorable outcome. Int. J. Endocrinol. Metab. 2014, 12, e15806. [Google Scholar] [CrossRef]
- Cutlan, R.T.; Greer, J.E.; Wong, F.S.; Eltorky, M. Immunohistochemical characterization of thyroid gland angiomatoid tumors. Exp. Mol. Pathol. 2000, 69, 159–164. [Google Scholar] [CrossRef]
- Tulin, A.D.; Avino, A.; Răducu, L.; Tulin, F.R.; Ştiru, O.; Balcangiu-Stroescu, A.E.; Timofte, D.; Tănăsescu, M.D.; Bălan, D.G.; Jecan, C.R.; et al. Primary thyroid angiosarcoma in a non-endemic region-a rare case. Rom. J. Morphol. Embryol. 2020, 61, 267–271. [Google Scholar] [CrossRef]
- Maiorana, A.; Collina, G.; Cesinaro, A.M.; Fano, R.A.; Eusebi, V. Epithelioid angiosarcoma of the thyroid. Clinicopathological analysis of seven cases from non-Alpine areas. Virchows Arch. 1996, 429, 131–137. [Google Scholar] [CrossRef]
- Kalitova, P.; Plzak, J.; Kodet, R.; Astl, J. Angiosarcoma of the thyroid. Eur. Arch. Otorhinolaryngol. 2009, 266, 903–905. [Google Scholar] [CrossRef]
- Bravaccini, S.; Caprara, L.; Cortecchia, S.; De Lillo, M.; Lega, S.; Poli, F.; Tasca, I.; Vacirca, A.; Cimatti, M.C.; Tumedei, M.M.; et al. Primary epithelioid angiosarcoma of the thyroid: A case report. Cytopathology 2021, 32, 519–522. [Google Scholar] [CrossRef]
- Kondapalli, A.; Redd, L.; DeBlanche, L.; Oo, Y. Primary angiosarcoma of thyroid. BMJ Case Rep. 2019, 12, e228862. [Google Scholar] [CrossRef]
- Yilmazlar, T.; Kirdak, T.; Adim, S.; Ozturk, E.; Yerci, O. A case of hemangiosarcoma in thyroid with severe anemia due to bone marrow metastasis. Endocr. J. 2005, 52, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Binesh, F.; Akhavan, A.; Navabii, H.; Dadgarnia, M.H.; Zand, V. Primary angiosarcoma of the thyroid gland in an young Iranian woman. BMJ Case Rep. 2011, 2011, 0320114042. [Google Scholar] [CrossRef] [PubMed]
- Sapalidis, K.; Kefes, N.; Romanidis, K.; Zarogoulidis, P.; Pantea, S.; Rogoveanu, O.C.; Rogoveanu, I.; Vagionas, A.; Zarampouka, K.; Tsakiridis, K.; et al. Thyroid angiosarcoma-rare case or hard to find. Curr. Health Sci. J. 2020, 46, 433–437. [Google Scholar] [PubMed]
- Wei, J.; Yang, J.; Liang, W.; Xu, C.; Wen, Y. Clinicopathological features of primary thyroid leiomyosarcoma without Epstein-Barr virus infection: A case report. Oncol. Lett. 2019, 17, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.T.; Bradish, T.; Rasul, U.; Shakeel, M. Primary thyroid leiomyosarcoma: A diagnostic and therapeutic challenge. BMJ Case Rep. 2021, 14, e236399. [Google Scholar] [CrossRef]
- Dubrava, J.; Martanovic, P.; Pavlovicova, M.; Babal, P. Primary thyroid leiomyosarcoma with transvenous extension to the right atrium: A case report. Eur. Heart J. Case Rep. 2022, 6, 193. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.; Choi, K.H.; Hwang, I.; Oh, Y.L. Metastatic leiomyosarcoma of the thyroid gland: Cytologic findings and differential diagnosis. J. Pathol. Transl. Med. 2021, 55, 360–365. [Google Scholar] [CrossRef]
- Amal, B.; El Fatemi, H.; Souaf, I.; Moumna, K.; Affaf, A. A rare primary tumor of the thyroid gland: Report a new case of leiomyosarcoma and literature review. Diagn. Pathol. 2013, 8, 36. [Google Scholar] [CrossRef]
- Thompson, L.D.; Wenig, B.M.; Adair, C.F.; Shmookler, B.M.; Heffess, C.S. Primary smooth muscle tumors of the thyroid gland. Cancer 1997, 79, 579–587. [Google Scholar] [CrossRef]
- Şahin, M.İ.; Vural, A.; Yüce, İ.; Çağlı, S.; Deniz, K.; Güney, E. Thyroid leiomyosarcoma: Presentation of two cases and review of the literature. Braz. J. Otorhinolaryngol. 2016, 82, 715–721. [Google Scholar] [CrossRef]
- Reddy, B.; Aggarwal, V.; Ajmani, A.K.; Sachan, S.; Khandelwal, D. Primary leiomyosarcoma of the thyroid gland-a rare malignancy. Eur. Endocrinol. 2019, 15, 44–46. [Google Scholar] [CrossRef]
- Kaur, M.; Chatterjee, D.; Aggarwal, P.; Saini, V. Primary leiomyosarcoma of the thyroid gland. Indian J. Pathol. Microbiol. 2022, 65, 142–144. [Google Scholar]
- Kawahara, E.; Nakanishi, I.; Terahata, S.; Ikegaki, S. Leiomyosarcoma of the thyroid gland. A case report with a comparative study of five cases of anaplastic carcinoma. Cancer 1988, 62, 2558–2563. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.Y.; Ning, N.; Li, S.Y.; Li, J.; Du, X.H.; Li, R. Primary thyroid leiomyosarcoma: A case report and literature review. Oncol. Lett. 2016, 11, 3982–3986. [Google Scholar] [CrossRef]
- Wang, T.S.; Ocal, I.T.; Oxley, K.; Sosa, J.A. Primary leiomyosarcoma of the thyroid gland. Thyroid 2008, 18, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Canu, G.L.; Bulla, J.S.; Lai, M.L.; Medas, F.; Baghino, G.; Erdas, E.; Mariotti, S.; Calò, P.G. Primary thyroid leiomyosarcoma: A case report and review of the literature. G. Chir. 2018, 39, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Conzo, G.; Candela, G.; Tartaglia, E.; Gambardella, C.; Mauriello, C.; Pettinato, G.; Bellastella, G.; Esposito, K.; Santini, L. Leiomyosarcoma of the thyroid gland: A case report and literature review. Oncol. Lett. 2014, 7, 1011–1014. [Google Scholar] [CrossRef]
- Mouaqit, O.; Belkacem, Z.; Ifrine, L.; Mohsine, R.; Belkouchi, A. A rare tumor of the thyroid gland: Report on one case of leiomyosarcoma and review of literature. Updates Surg. 2014, 66, 165–167. [Google Scholar] [CrossRef]
- Buckley, N.J.; Burch, W.M.; Leight, G.S. Malignant teratoma in the thyroid gland of an adult: A case report and a review of the literature. Surgery 1986, 100, 932–937. [Google Scholar]
- Rooper, L.M. From malignant thyroid teratoma to thyroblastoma: Evolution of a newly-recognized DICER1-associated malignancy. Adv. Anat. Pathol. 2022, 30, 136–145. [Google Scholar] [CrossRef]
- Thompson, L.D.; Rosai, J.; Heffess, C.S. Primary thyroid teratomas: A clinicopathologic study of 30 cases. Cancer 2000, 88, 1149–1158. [Google Scholar] [CrossRef]
- Beckers, K.; Faes, J.; Deprest, J.; Delaere, P.R.; Hens, G.; De Catte, L.; Naulaers, G.; Claus, F.; Hermans, R.; Vander Poorten, V.L.M. Long-term outcome of pre- and perinatal management of congenital head and neck tumors and malformations. Int. J. Pediatr. Otorhinolaryngol. 2019, 121, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Thompson, L.D.R.; Bishop, J.A.; Rooper, L.M.; Ali, S.Z. Malignant teratomas of the thyroid gland: Clinico-radiologic and cytomorphologic features of a rare entity. J. Am. Soc. Cytopathol. 2020, 9, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.W.; Brierley, J.D.; Asa, S.L.; Sturgeon, J.F. Malignant teratoma of the thyroid: Aggressive chemoradiation therapy is required after surgery. Thyroid 2003, 13, 401–404. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, F.; Al Ghuzlan, A.; Zafereo, M.; Vander Poorten, V.; Robbins, K.T.; Hamoir, M.; Nixon, I.J.; Tufano, R.P.; Randolph, G.; Pace-Asciak, P.; et al. Neck Surgery for Non-Well Differentiated Thyroid Malignancies: Variations in Strategy According to Histopathology. Cancers 2023, 15, 1255. https://doi.org/10.3390/cancers15041255
López F, Al Ghuzlan A, Zafereo M, Vander Poorten V, Robbins KT, Hamoir M, Nixon IJ, Tufano RP, Randolph G, Pace-Asciak P, et al. Neck Surgery for Non-Well Differentiated Thyroid Malignancies: Variations in Strategy According to Histopathology. Cancers. 2023; 15(4):1255. https://doi.org/10.3390/cancers15041255
Chicago/Turabian StyleLópez, Fernando, Abir Al Ghuzlan, Mark Zafereo, Vincent Vander Poorten, K. Thomas Robbins, Marc Hamoir, Iain J. Nixon, Ralph P. Tufano, Gregory Randolph, Pia Pace-Asciak, and et al. 2023. "Neck Surgery for Non-Well Differentiated Thyroid Malignancies: Variations in Strategy According to Histopathology" Cancers 15, no. 4: 1255. https://doi.org/10.3390/cancers15041255
APA StyleLópez, F., Al Ghuzlan, A., Zafereo, M., Vander Poorten, V., Robbins, K. T., Hamoir, M., Nixon, I. J., Tufano, R. P., Randolph, G., Pace-Asciak, P., Angelos, P., Coca-Pelaz, A., Khafif, A., Ronen, O., Rodrigo, J. P., Sanabria, Á., Palme, C. E., Mäkitie, A. A., Kowalski, L. P., ... Ferlito, A. (2023). Neck Surgery for Non-Well Differentiated Thyroid Malignancies: Variations in Strategy According to Histopathology. Cancers, 15(4), 1255. https://doi.org/10.3390/cancers15041255











