CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Declarations
2.2. Patient Cohorts and Study Design
2.3. Immunohistochemistry and Scoring
2.4. Statistical Analyses
2.5. Gene Expression Analyses
3. Results
3.1. CD169+ TAMs Associate with TLLSs in PTs
3.2. CD169+ TAMs and TLLSs as Prognostic Markers for Breast Cancer Patients
3.3. Treg Infiltration Impacts the Prognostic Effect of CD169+ TAMs
3.4. CD169+ TAMs and TLLSs Show Unique Independent Prognostic Effects
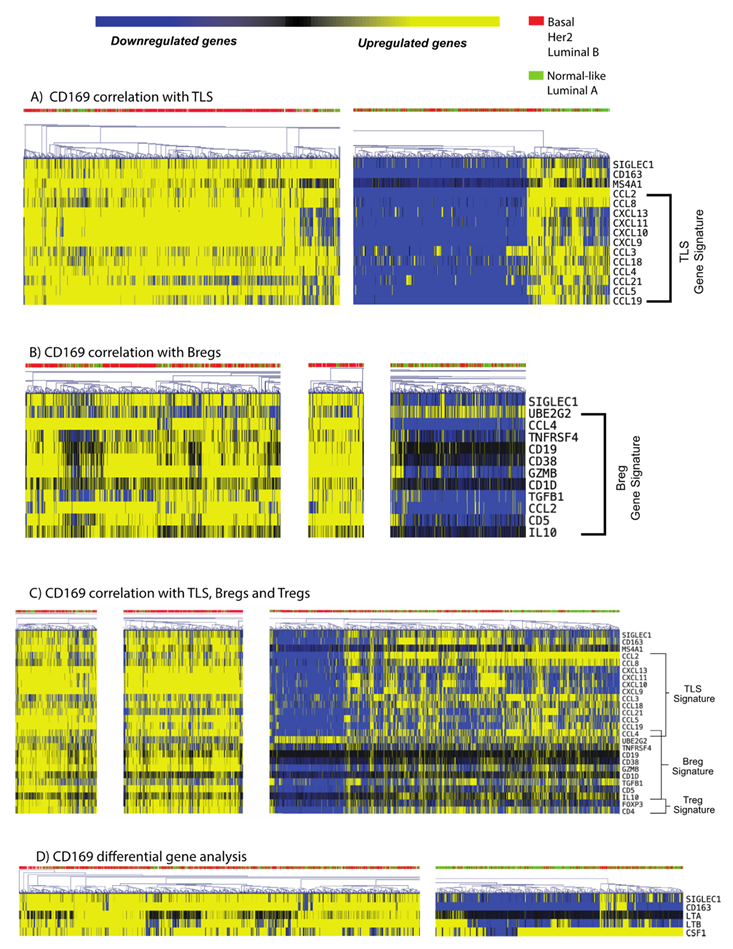
3.5. CD169+ TAMs Associate with Both Mature TLS and Breg Gene Signatures
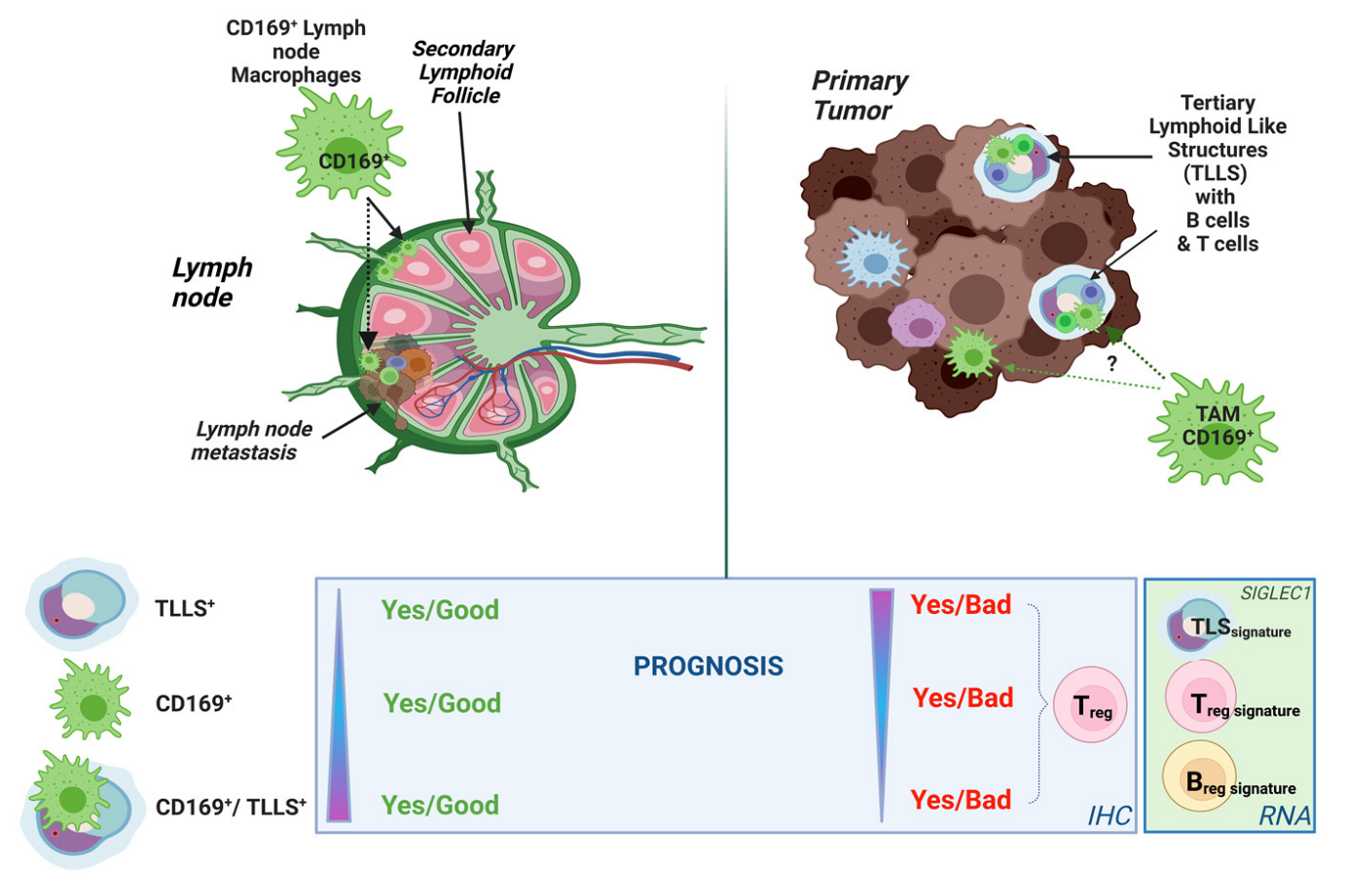
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 4 March 2022).
- Aysola, K.; Desai, A.; Welch, C.; Xu, J.; Qin, Y.; Reddy, V.; Matthews, R.; Owens, C.; Okoli, J.; Beech, D.J.; et al. Triple Negative Breast Cancer—An Overview. Hered. Genet. Curr. Res. 2013, 2013 (Suppl. S2), 1. [Google Scholar]
- Andrahennadi, S.; Sami, A.; Manna, M.; Pauls, M.; Ahmed, S. Current Landscape of Targeted Therapy in Hormone Receptor-Positive and HER2-Negative Breast Cancer. Curr. Oncol. 2021, 28, 1803–1822. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.L.; von Au, A.; Mavratzas, A.; Smetanay, K.; Schütz, F.; Schneeweiss, A. Immune Checkpoint Blockade in Patients with Triple-Negative Breast Cancer. Target. Oncol. 2020, 15, 415–428. [Google Scholar] [CrossRef]
- Tang, H.; Qiao, J.; Fu, Y.-X. Immunotherapy and tumor microenvironment. Cancer Lett. 2016, 370, 85–90. [Google Scholar] [CrossRef]
- Tang, T.; Huang, X.; Zhang, G.; Hong, Z.; Bai, X.; Liang, T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 72. [Google Scholar] [CrossRef]
- Panni, R.Z.; Linehan, D.C.; DeNardo, D.G. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy 2013, 5, 1075–1087. [Google Scholar] [CrossRef]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Qiu, C.-H. Functions of CD169 positive macrophages in human diseases (Review). Biomed. Rep. 2021, 14, 26. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Mondor, I.; Baratin, M.; Lagueyrie, M.; Saro, L.; Henri, S.; Gentek, R.; Suerinck, D.; Kastenmuller, W.; Jiang, J.X.; Bajenoff, M. Lymphatic Endothelial Cells Are Essential Components of the Subcapsular Sinus Macrophage Niche. Immunity 2019, 50, 1453–1466.e54. [Google Scholar] [CrossRef]
- Camara, A.; Cordeiro, O.G.; Alloush, F.; Sponsel, J.; Chypre, M.; Onder, L.; Asano, K.; Tanaka, M.; Yagita, H.; Ludewig, B.; et al. Lymph Node Mesenchymal and Endothelial Stromal Cells Cooperate via the RANK-RANKL Cytokine Axis to Shape the Sinusoidal Macrophage Niche. Immunity 2019, 50, 1467–1481.e66. [Google Scholar] [CrossRef]
- Martinez-Pomares, L.; Gordon, S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 2012, 33, 66–70. [Google Scholar] [CrossRef]
- Shaabani, N.; Duhan, V.; Khairnar, V.; Gassa, A.; Ferrer-Tur, R.; Häussinger, D.; Recher, M.; Zelinskyy, G.; Liu, J.; Dittmer, U.; et al. CD169+ macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infection. Cell Death Dis. 2016, 7, e2446. [Google Scholar] [CrossRef]
- Gray, E.E.; Cyster, J.G. Lymph node macrophages. J. Innate Immun. 2012, 4, 424–436. [Google Scholar] [CrossRef]
- Louie, D.A.P.; Liao, S. Lymph Node Subcapsular Sinus Macrophages as the Frontline of Lymphatic Immune Defense. Front. Immunol. 2019, 10, 347. [Google Scholar] [CrossRef]
- Affandi, A.J.; Olesek, K.; Grabowska, J.; Nijen Twilhaar, M.K.; Rodríguez, E.; Saris, A.; Zwart, E.S.; Nossent, E.J.; Kalay, H.; de Kok, M.; et al. CD169 Defines Activated CD14(+) Monocytes With Enhanced CD8(+) T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.Q.; Jiang, Z.Z.; Li, L.; Wu, Y.; Zheng, L. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J. Pathol. 2016, 239, 231–241. [Google Scholar] [CrossRef]
- Fraschilla, I.; Pillai, S. Viewing Siglecs through the lens of tumor immunology. Immunol. Rev. 2017, 276, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ohnishi, K.; Shiota, T.; Motoshima, T.; Sugiyama, Y.; Yatsuda, J.; Kamba, T.; Ishizaka, K.; Komohara, Y. CD169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018, 109, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Shiota, T.; Yagi, T.; Ohnishi, K.; Baba, Y.; Miyasato, Y.; Kiyozumi, Y.; Yoshida, N.; Takeya, M.; Baba, H.; et al. High CD169 expression in lymph node macrophages predicts a favorable clinical course in patients with esophageal cancer. Pathol. Int. 2018, 68, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Yamaguchi, M.; Erdenebaatar, C.; Saito, F.; Tashiro, H.; Katabuchi, H.; Takeya, M.; Komohara, Y. Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016, 107, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ohnishi, K.; Miyashita, A.; Nakahara, S.; Fujiwara, Y.; Horlad, H.; Motoshima, T.; Fukushima, S.; Jinnin, M.; Ihn, H.; et al. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol. Res. 2015, 3, 1356–1363. [Google Scholar] [CrossRef]
- Björk Gunnarsdottir, F.; Auoja, N.; Bendahl, P.-O.; Rydén, L.; Fernö, M.; Leandersson, K. Co-localization of CD169(+) macrophages and cancer cells in lymph node metastases of breast cancer patients is linked to improved prognosis and PDL1 expression. Oncoimmunology 2020, 9, 1848067. [Google Scholar] [CrossRef]
- Hatschek, T.; Carlsson, L.; Einbeigi, Z.; Lidbrink, E.; Linderholm, B.; Lindh, B.; Loman, N.; Malmberg, M.; Rotstein, S.; Söderberg, M.; et al. Individually tailored treatment with epirubicin and paclitaxel with or without capecitabine as first-line chemotherapy in metastatic breast cancer: A randomized multicenter trial. Breast Cancer Res. Treat. 2012, 131, 939–947. [Google Scholar] [CrossRef]
- Kimbung, S.; Kovács, A.; Bendahl, P.-O.; Malmström, P.; Fernö, M.; Hatschek, T.; Hedenfalk, I. Claudin-2 is an independent negative prognostic factor in breast cancer and specifically predicts early liver recurrences. Mol. Oncol. 2014, 8, 119–128. [Google Scholar] [CrossRef]
- Saal, L.H.; Vallon-Christersson, J.; Häkkinen, J.; Hegardt, C.; Grabau, D.; Winter, C.; Brueffer, C.; Tang, M.-H.E.; Reuterswärd, C.; Schulz, R.; et al. The Sweden Cancerome Analysis Network—Breast (SCAN-B) Initiative: A large-scale multicenter infrastructure towards implementation of breast cancer genomic analyses in the clinical routine. Genome Med. 2015, 7, 20. [Google Scholar] [CrossRef]
- Stenström, J.; Hedenfalk, I.; Hagerling, C. Regulatory T lymphocyte infiltration in metastatic breast cancer-an independent prognostic factor that changes with tumor progression. Breast Cancer Res. 2021, 23, 27. [Google Scholar] [CrossRef]
- Zhu, G.; Falahat, R.; Wang, K.; Mailloux, A.; Artzi, N.; Mulé, J.J. Tumor-Associated Tertiary Lymphoid Structures: Gene-Expression Profiling and Their Bioengineering. Front. Immunol. 2017, 8, 767. [Google Scholar] [CrossRef]
- Dubois, F.; Limou, S.; Chesneau, M.; Degauque, N.; Brouard, S.; Danger, R. Transcriptional meta-analysis of regulatory B cells. Eur. J. Immunol. 2020, 50, 1757–1769. [Google Scholar] [CrossRef]
- Bhairavabhotla, R.; Kim, Y.C.; Glass, D.D.; Escobar, T.M.; Patel, M.C.; Zahr, R.; Nguyen, C.K.; Kilaru, G.K.; Muljo, S.A.; Shevach, E.M. Transcriptome profiling of human FoxP3+ regulatory T cells. Hum. Immunol. 2016, 77, 201–213. [Google Scholar] [CrossRef]
- Brueffer, C.; Vallon-Christersson, J.; Grabau†, D.; Ehinger, A.; Häkkinen, J.; Hegardt, C.; Malina, J.; Chen, Y.; Bendahl, P.-O.; Manjer, J.; et al. Clinical Value of RNA Sequencing–Based Classifiers for Prediction of the Five Conventional Breast Cancer Biomarkers: A Report From the Population-Based Multicenter Sweden Cancerome Analysis Network—Breast Initiative. JCO Precis. Oncol. 2018, in press. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Puryear, W.B.; Akiyama, H.; Geer, S.D.; Ramirez, N.P.; Yu, X.; Reinhard, B.M.; Gummuluru, S. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013, 9, e1003291. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Zhang, R.X.; Zhang, Y.; Ou, Q.J.; Li, J.Q.; Jiang, Z.Z.; Wu, X.J.; Fang, Y.J.; Zheng, L. CD169 identifies an activated CD8(+) T cell subset in regional lymph nodes that predicts favorable prognosis in colorectal cancer patients. Oncoimmunology 2016, 5, e1177690. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Liu, S.; Yu, W.; Liu, Z. CD169(+) subcapsular sinus macrophage-derived microvesicles are associated with light zone follicular dendritic cells. Eur. J. Immunol. 2022, 52, 1581–1594. [Google Scholar] [CrossRef]
- Tumanov, A.V.; Kuprash, D.V.; Lagarkova, M.A.; Grivennikov, S.I.; Abe, K.; Shakhov, A.N.; Drutskaya, L.N.; Stewart, C.L.; Chervonsky, A.V.; Nedospasov, S.A. Distinct Role of Surface Lymphotoxin Expressed by B Cells in the Organization of Secondary Lymphoid Tissues. Immunity 2002, 17, 239–250. [Google Scholar] [CrossRef]
- Xu, H.C.; Huang, J.; Khairnar, V.; Duhan, V.; Pandyra, A.A.; Grusdat, M.; Shinde, P.; McIlwain, D.R.; Maney, S.K.; Gommerman, J.; et al. Deficiency of the B cell-activating factor receptor results in limited CD169+ macrophage function during viral infection. J. Virol. 2015, 89, 4748–4759. [Google Scholar] [CrossRef]
- Sautès-Fridman, C.; Lawand, M.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Dieu-Nosjean, M.-C. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 2016, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Qu, F.-J.; Liu, H.; Li, Z.-J.; Zhang, Y.-C.; Han, X.; Zhu, Z.-Y.; Lv, Y. Prognostic impact of tertiary lymphoid structures in breast cancer prognosis: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 536. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tsang, J.Y.S.; Hlaing, T.; Hu, J.; Ni, Y.-B.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Distinct Tertiary Lymphoid Structure Associations and Their Prognostic Relevance in HER2 Positive and Negative Breast Cancers. Oncologist 2017, 22, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, I.A.; Song, I.H.; Shin, S.-J.; Kim, J.Y.; Yu, J.H.; Gong, G. Tertiary lymphoid structures: Prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J. Clin. Pathol. 2016, 69, 422. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, S.L.; Fismen, S.; Fenton, K.A.; Fenton, C.; Mortensen, E.S. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer 2015, 15, 101. [Google Scholar] [CrossRef]
- Ishigami, E.; Sakakibara, M.; Sakakibara, J.; Masuda, T.; Fujimoto, H.; Hayama, S.; Nagashima, T.; Sangai, T.; Nakagawa, A.; Nakatani, Y.; et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer 2019, 26, 180–189. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.-j.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef]
- Joshi, N.S.; Akama-Garren, E.H.; Lu, Y.; Lee, D.Y.; Chang, G.P.; Li, A.; DuPage, M.; Tammela, T.; Kerper, N.R.; Farago, A.F.; et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity 2015, 43, 579–590. [Google Scholar] [CrossRef]
- Reticker-Flynn, N.E.; Zhang, W.; Belk, J.A.; Basto, P.A.; Escalante, N.K.; Pilarowski, G.O.W.; Bejnood, A.; Martins, M.M.; Kenkel, J.A.; Linde, I.L.; et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 2022, 185, 1942.e23. [Google Scholar] [CrossRef]
- Ravishankar, B.; Shinde, R.; Liu, H.; Chaudhary, K.; Bradley, J.; Lemos, H.P.; Chandler, P.; Tanaka, M.; Munn, D.H.; Mellor, A.L.; et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 4215–4220. [Google Scholar] [CrossRef]
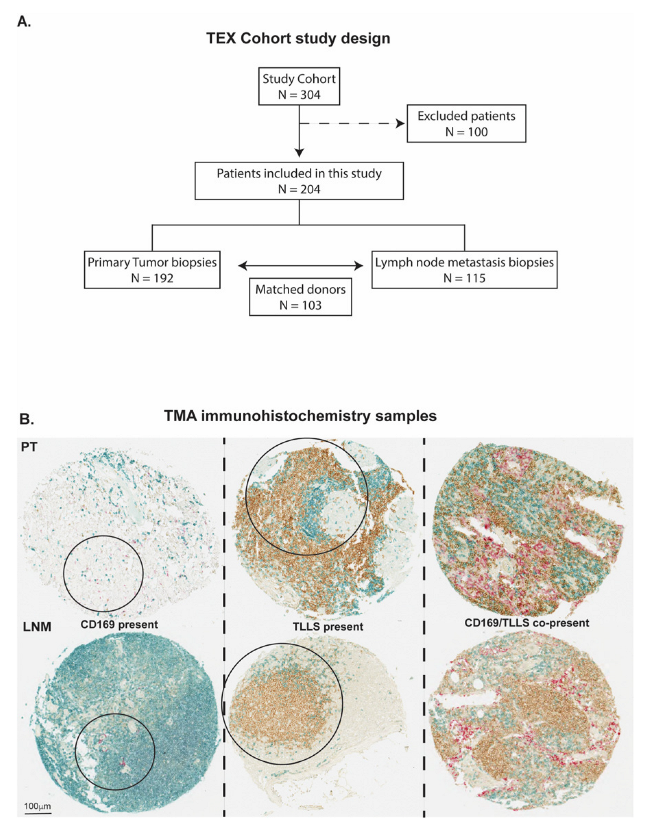


| Patient Characteristics | No. of Patients | Percent (%) | |
|---|---|---|---|
| Age | >50 | 92 | 45.1 |
| <50 | 112 | 54.9 | |
| Tumor size (T) | T1 (0–20 mm) | 83 | 40.7 |
| T2 (20–50 mm) | 95 | 46.6 | |
| T3 (>50 mm) | 16 | 7.8 | |
| T4 (Invasion) | 9 | 4.4 | |
| Missing | 1 | 0.5 | |
| Regional lymph nodes (N) | N0 | 65 | 31.9 |
| N1 | 124 | 60.8 | |
| N2 | 9 | 4.4 | |
| N3 | 2 | 1.0 | |
| Missing | 4 | 2.0 | |
| Metastasis (M) | M0 | 185 | 90.7 |
| M1 | 18 | 8.8 | |
| Missing | 1 | 0.5 | |
| PT receptor status | |||
| ER | Neg | 36 | 17.6 |
| Pos | 152 | 92.2 | |
| Missing | 16 | 7.8 | |
| PR | Neg | 80 | 39.2 |
| Pos | 107 | 52.5 | |
| Missing | 17 | 8.3 | |
| HER2 | Neg | 172 | 84.3 |
| Pos | 17 | 8.3 | |
| Missing | 15 | 7.4 | |
| LNM receptor status | |||
| ER | Neg | 28 | 13.7 |
| Pos | 74 | 36.3 | |
| Missing | 102 | 50.0 | |
| PR | Neg | 64 | 31.4 |
| Pos | 38 | 18.6 | |
| Missing | 102 | 50.0 | |
| HER2 | Neg | 77 | 37.7 |
| Pos | 13 | 6.4 | |
| Missing | 114 | 55.9 | |
| Adjuvant therapy given | |||
| Chemotherapy | No | 106 | 52.0 |
| Yes | 98 | 48.0 | |
| Endocrine | No | 92 | 45.1 |
| Yes | 112 | 54.9 | |
| Radiotherapy | No | 55 | 27.0 |
| Yes | 149 | 73.0 | |
| Clinicopathological Features | CD169+ PT | CD169+ LNM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value a | n | OR | 95% CI | p-Value a | n | ||
| Age | >50 | 85 | 1 | 48 | |||||
| <50 | 0.96 | 0.51–1.81 | 0.90 | 106 | 0.89 | 0.43–1.88 | 0.77 | 67 | |
| Overall Survival | >5 years | 1 | 67 | 1 | 42 | ||||
| <5 years | 1.21 | 0.61–2.36 | 0.59 | 124 | 2.20 | 1.01–4.79 | 0.045 | 73 | |
| Relapse free interval | >5 years | 1 | 108 | 1 | 77 | ||||
| <5 years | 0.61 | 0.31–1.22 | 0.16 | 73 | 1.37 | 0.61–3.06 | 0.44 | 35 | |
| Tumor size | T1 | 1 | 80 | 1 | 34 | ||||
| >T1 | 0.47 | 0.24–0.89 | 0.019 | 110 | 0.42 | 0.19–0.97 | 0.041 | 80 | |
| Ki67+ PT | Neg | 1 | 24 | 1 | 37 | ||||
| Pos | 2.33 | 1.183–4.600 | 0.021 | 24 | 1.26 | 0.535–2.989 | 0.67 b | 16 | |
| Ki67+ LNM | Neg | 1 | 14 | 1 | 31 | ||||
| Pos | 1.47 | 0.550–3.923 | 0.45b | 9 | 0.69 | 0.285–1.662 | 0.51b | 12 | |
| ER PT | Neg | 1 | 36 | 1 | 21 | ||||
| Pos | 0.28 | 0.13–0.60 | 0.001 | 147 | 1.43 | 0.55–3.75 | 0.63 | 85 | |
| ER LNM | Neg | 1 | 24 | 1 | 28 | ||||
| Pos | 0.76 | 0.26–2.28 | 0.77 | 69 | 0.53 | 0.22–1.29 | 0.18 | 70 | |
| PR PT | Neg | 1 | 79 | 1 | 42 | ||||
| Pos | 0.59 | 0.31–1.12 | 0.11 | 103 | 1.18 | 0.54–2.59 | 0.68 | 61 | |
| PR LNM | Neg | 1 | 57 | 1 | 60 | ||||
| Pos | 0.34 | 0.11–1.01 | 0.053 | 37 | 0.68 | 0.30–1.56 | 0.36 | 37 | |
| HER2 PT | Neg | 1 | 166 | 1 | 95 | ||||
| Pos | 1.77 | 0.61–5.17 | 0.37b | 16 | 0.65 | 0.17–2.46 | 0.74 b | 10 | |
| HER2 LNM | Neg | 1 | 69 | 1 | 77 | ||||
| Pos | 0.99 | 0.24–4.06 | 1b | 13 | 1.41 | 0.41–4.76 | 0.76 b | 12 | |
| Cell infiltration association | |||||||||
| FoxP3 PT | Neg | 1 | 68 | 1 | 40 | ||||
| Pos | 2.06 | 0.99–4.26 | 0.057 | 107 | 0.76 | 0.34–1.72 | 0.51 | 56 | |
| FoxP3 LNM | Neg | 1 | 24 | 1 | 26 | ||||
| Pos | 0.70 | 0.23–2.13 | 0.57 | 49 | 2.87 | 0.99–8.27 | 0.046 | 54 | |
| TLLS PT | Neg | 1 | 165 | 1 | 91 | ||||
| Pos | 3.77 | 1.61–8.82 | 0.004 | 26 | 2.04 | 0.58–7.27 | 0.36 b | 12 | |
| TLLS LNM | Neg | 1 | 39 | 1 | 46 | ||||
| Pos | 1.02 | 0.40–2.62 | 0.97 | 64 | 4.76 | 2.12–10.71 | 0.0001 | 69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briem, O.; Källberg, E.; Kimbung, S.; Veerla, S.; Stenström, J.; Hatschek, T.; Hagerling, C.; Hedenfalk, I.; Leandersson, K. CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer. Cancers 2023, 15, 1262. https://doi.org/10.3390/cancers15041262
Briem O, Källberg E, Kimbung S, Veerla S, Stenström J, Hatschek T, Hagerling C, Hedenfalk I, Leandersson K. CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer. Cancers. 2023; 15(4):1262. https://doi.org/10.3390/cancers15041262
Chicago/Turabian StyleBriem, Oscar, Eva Källberg, Siker Kimbung, Srinivas Veerla, Jenny Stenström, Thomas Hatschek, Catharina Hagerling, Ingrid Hedenfalk, and Karin Leandersson. 2023. "CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer" Cancers 15, no. 4: 1262. https://doi.org/10.3390/cancers15041262
APA StyleBriem, O., Källberg, E., Kimbung, S., Veerla, S., Stenström, J., Hatschek, T., Hagerling, C., Hedenfalk, I., & Leandersson, K. (2023). CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer. Cancers, 15(4), 1262. https://doi.org/10.3390/cancers15041262







