New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Liver Transplantation in Cholangiocarcinoma
2.1. Liver Transplantation in iCCA
2.2. Locally Advanced, Unresectable iCCA and Liver Transplantation: Role of Neoadjuvant Therapy
2.3. Perihiliar CCA (pCCA) and Liver Transplantation
3. Locoregional Therapies
3.1. Thermal Ablation
3.2. Transarterial Chemoembolization, Radioembolization, and Chemotherapy Hepatic Arterial Infusion
3.3. External Beam Radiotherapy
4. Systemic Therapy
Targeted Therapy in CCA
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.; Bachini, M.; Ilyas, S.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical Presentation, Diagnosis and Staging of Cholangiocarcinoma. Liver Int. 2019, 39, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Sanchez, L.; Lamarca, A.; la Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.-J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma Landscape in Europe: Diagnostic, Prognostic and Therapeutic Insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Gorgen, A.; Roayaie, S.; Droz dit Busset, M.; Sapisochin, G. Liver Resection and Transplantation for Intrahepatic Cholangiocarcinoma. J. Hepatol. 2020, 72, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Ferrer-Fábrega, J.; Sampson-Dávila, J.; Díaz, A.; Ayuso, C.; Forner, A.; Fondevila, C.; García-Valdecasas, J.C.; Bruix, J.; Fuster, J. Intention-to-Treat Curative Liver Resection in Patients with “Very Early” Intrahepatic Cholangiocarcinoma. Langenbecks Arch. Surg. 2020, 405, 967–975. [Google Scholar] [CrossRef]
- Amini, N.; Ejaz, A.; Spolverato, G.; Kim, Y.; Herman, J.M.; Pawlik, T.M. Temporal Trends in Liver-Directed Therapy of Patients with Intrahepatic Cholangiocarcinoma in the United States: A Population-Based Analysis. J. Surg. Oncol. 2014, 110, 163–170. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine Compared with Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Edeline, J.; Hirano, S.; Bertaut, A.; Konishi, M.; Benabdelghani, M.; Uesaka, K.; Watelet, J.; Ohtsuka, M.; Hammel, P.; Kaneoka, Y.; et al. Individual Patient Data Meta-Analysis of Adjuvant Gemcitabine-Based Chemotherapy for Biliary Tract Cancer: Combined Analysis of the BCAT and PRODIGE-12 Studies. Eur. J. Cancer 2022, 164, 80–87. [Google Scholar] [CrossRef]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional Therapies in Patients with Intrahepatic Cholangiocarcinoma: A Systematic Review and Pooled Analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Volk, M.L.; Vijan, S.; Marrero, J.A. A Novel Model Measuring the Harm of Transplanting Hepatocellular Carcinoma Exceeding Milan Criteria. Am. J. Transpl. 2008, 8, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Lesurtel, M.; Bossuyt, P.M.M.; Gores, G.J.; Langer, B.; Perrier, A. Recommendations for Liver Transplantation for Hepatocellular Carcinoma: An International Consensus Conference Report. Lancet Oncol. 2012, 13, e11. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.S.; Rodriguez, J.A.; Barshes, N.R.; O’Mahony, C.A.; Goss, J.A.; Aloia, T.A. Outcomes Analysis for 280 Patients with Cholangiocarcinoma Treated with Liver Transplantation over an 18-Year Period. J. Gastrointest. Surg. 2008, 12, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.M.; Stone, M.; Tillery, G.W.; Senzer, N.; Levy, M.; Husberg, B.S.; Gonwa, T.; Klintmalm, G. Is Liver Transplantation Indicated for Cholangiocarcinoma? Am. J. Surg. 1993, 166, 768–772. [Google Scholar] [CrossRef]
- Meyer, C.G.; Penn, I.; James, L. Liver Transplantation for Cholangiocarcinoma: Results in 207 Patients. Transplantation 2000, 69, 1633–1637. [Google Scholar] [CrossRef]
- Sapisochin, G.; Facciuto, M.; Rubbia-Brandt, L.; Marti, J.; Mehta, N.; Yao, F.Y.; Vibert, E.; Cherqui, D.; Grant, D.R.; Hernandez-Alejandro, R.; et al. Liver Transplantation for “Very Early” Intrahepatic Cholangiocarcinoma: International Retrospective Study Supporting a Prospective Assessment. Hepatology 2016, 64, 1178–1188. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Giannis, D.; Economopoulos, K.P.; Hayat, M.H.; Montenovo, M.I.; Matsuoka, L.K.; Alexopoulos, S.P. Liver Transplantation for Intrahepatic Cholangiocarcinoma: A Meta-Analysis and Meta-Regression of Survival Rates. Transplantation 2021, 105, 2263–2271. [Google Scholar] [CrossRef]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor, D.W.; Shetty, A.; et al. Survival Following Liver Transplantation for Locally Advanced, Unresectable Intrahepatic Cholangiocarcinoma. Am. J. Transpl. 2022, 22, 823–832. [Google Scholar] [CrossRef]
- Ito, T.; Butler, J.R.; Noguchi, D.; Ha, M.; Aziz, A.; Agopian, V.G.; DiNorcia, J.; Yersiz, H.; Farmer, D.G.; Busuttil, R.W.; et al. A 3-Decade, Single-Center Experience of Liver Transplantation for Cholangiocarcinoma: Impact of Era, Tumor Size, Location, and Neoadjuvant Therapy. Liver Transpl. 2022, 28, 386–396. [Google Scholar] [CrossRef]
- Tan, E.K.; Taner, T.; Heimbach, J.K.; Gores, G.J.; Rosen, C.B. Liver Transplantation for Peri-Hilar Cholangiocarcinoma. J. Gastrointest. Surg. 2020, 24, 2679–2685. [Google Scholar] [CrossRef]
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD Practice Guidance on Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology 2022, 77, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, T.; Hibi, T.; Moonka, D.; Sapisochin, G.; Abouljoud, M.S.; Nagai, S. Center Experience Affects Liver Transplant Outcomes in Patients with Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 5209–5221. [Google Scholar] [CrossRef] [PubMed]
- Mantel, H.T.J.; Westerkamp, A.C.; Adam, R.; Bennet, W.F.; Seehofer, D.; Settmacher, U.; Sánchez-Bueno, F.; Prous, J.F.; Boleslawski, E.; Friman, S.; et al. Strict Selection Alone of Patients Undergoing Liver Transplantation for Hilar Cholangiocarcinoma Is Associated with Improved Survival. PLoS ONE 2016, 11, e0156127. [Google Scholar] [CrossRef] [PubMed]
- Loveday, B.P.T.; Knox, J.J.; Dawson, L.A.; Metser, U.; Brade, A.; Horgan, A.M.; Gallinger, S.; Greig, P.D.; Moulton, C.A. Neoadjuvant Hyperfractionated Chemoradiation and Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma in Canada. J. Surg. Oncol. 2018, 117, 213–219. [Google Scholar] [CrossRef]
- Duignan, S.; Maguire, D.; Ravichand, C.S.; Geoghegan, J.; Hoti, E.; Fennelly, D.; Armstrong, J.; Rock, K.; Mohan, H.; Traynor, O. Neoadjuvant Chemoradiotherapy Followed by Liver Transplantation for Unresectable Cholangiocarcinoma: A Single-Centre National Experience. HPB 2014, 16, 91–98. [Google Scholar] [CrossRef]
- Cambridge, W.A.; Fairfield, C.; Powell, J.J.; Harrison, E.M.; Søreide, K.; Wigmore, S.J.; Guest, R.V. Meta-Analysis and Meta-Regression of Survival After Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Ann. Surg. 2021, 273, 240–250. [Google Scholar] [CrossRef]
- van Keulen, A.M.; Franssen, S.; van der Geest, L.G.; de Boer, M.T.; Coenraad, M.; van Driel, L.M.J.W.; Erdmann, J.I.; Haj Mohammad, N.; Heij, L.; Klümpen, H.J.; et al. Nationwide Treatment and Outcomes of Perihilar Cholangiocarcinoma. Liver Int. 2021, 41, 1945–1953. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-Peritoneal Fine Needle Aspiration Biopsy of Hilar Cholangiocarcinoma Is Associated with Disease Dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef]
- Bhat, M.; Hathcock, M.; Kremers, W.K.; Darwish Murad, S.; Schmit, G.; Martenson, J.; Alberts, S.; Rosen, C.B.; Gores, G.J.; Heimbach, J. Portal Vein Encasement Predicts Neoadjuvant Therapy Response in Liver Transplantation for Perihilar Cholangiocarcinoma Protocol. Transpl. Int. 2015, 28, 1383–1391. [Google Scholar] [CrossRef]
- Sio, T.T.; Martenson, J.A.; Haddock, M.G.; Novotny, P.J.; Gores, G.J.; Alberts, S.R.; Miller, R.C.; Heimbach, J.K.; Rosen, C.B. Outcome of Transplant-Fallout Patients With Unresectable Cholangiocarcinoma. Am. J. Clin. Oncol. 2016, 39, 271–275. [Google Scholar] [CrossRef]
- Shin, S.-P.; Koh, D.-H. Clinical Impact of Sarcopenia on Cholangiocarcinoma. Life 2022, 12, 815. [Google Scholar] [CrossRef]
- Mantel, H.T.J.; Rosen, C.B.; Helmbach, J.K.; Nyberg, S.L.; Ishitani, M.B.; Andrews, J.C.; McKusick, M.A.; Haddock, M.G.; Alberts, S.R.; Gores, G.J. Vascular Complications after Orthotopic Liver Transplantation after Neoadjuvant Therapy for Hilar Cholangiocarcinoma. Liver Transpl. 2007, 13, 1372–1381. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Gores, G.J.; Haddock, M.G.; Alberts, S.R.; Pedersen, R.; Kremers, W.; Nyberg, S.L.; Ishitani, M.B.; Rosen, C.B. Predictors of Disease Recurrence Following Neoadjuvant Chemoradiotherapy and Liver Transplantation for Unresectable Perihilar Cholangiocarcinoma. Transplantation 2006, 82, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Ethun, C.G.; Lopez-Aguiar, A.G.; Anderson, D.J.; Adams, A.B.; Fields, R.C.; Doyle, M.B.; Chapman, W.C.; Krasnick, B.A.; Weber, S.M.; Mezrich, J.D.; et al. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann. Surg. 2018, 267, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Breuer, E.; Mueller, M.; Doyle, M.B.; Yang, L.; Darwish Murad, S.; Anwar, I.J.; Merani, S.; Limkemann, A.; Jeddou, H.; Kim, S.C.; et al. Liver Transplantation as a New Standard of Care in Patients With Perihilar Cholangiocarcinoma? Results From an International Benchmark Study. Ann. Surg. 2022, 276, 846–853. [Google Scholar] [CrossRef]
- Rosen, C.B. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Paradigms for Resectable Disease in Annals of Surgery 2018. Ann. Surg. 2018, 267, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Adeva, J.; Sangro, B.; Salati, M.; Edeline, J.; la Casta, A.; Bittoni, A.; Berardi, R.; Bruix, J.; Valle, J.W. Medical Treatment for Cholangiocarcinoma. Liver Int. 2019, 39, 123–142. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. On behalf of the ESMO Guidelines Committee Biliary Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Kim, J.H.; Won, H.J.; Shin, Y.M.; Kim, K.A.; Kim, P.N. Radiofrequency Ablation for the Treatment of Primary Intrahepatic Cholangiocarcinoma. AJR Am. J. Roentgenol. 2011, 196, W205–W209. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, W.; Wu, W.; Yan, K.; Xing, B.C.; Chen, M.H. Radiofrequency Ablation in the Management of Unresectable Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2012, 23, 642–649. [Google Scholar] [CrossRef]
- Díaz-González, Á.; Vilana, R.; Bianchi, L.; García-Criado, Á.; Rimola, J.; Rodríguez de Lope, C.; Ferrer, J.; Ayuso, C.; da Fonseca, L.G.; Reig, M.; et al. Thermal Ablation for Intrahepatic Cholangiocarcinoma in Cirrhosis: Safety and Efficacy in Non-Surgical Patients. J. Vasc. Interv. Radiol. 2020, 31, 710–719. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Simo, K.A.; Hansen, P.; Rocha, F.; Philips, P.; McMasters, K.M.; Tatum, C.M.; Kelly, L.R.; Driscoll, M.; Sharma, V.R.; et al. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann. Surg. Oncol. 2022, 29, 5462–5473. [Google Scholar] [CrossRef] [PubMed]
- Paprottka, K.J.; Galiè, F.; Ingrisch, M.; Geith, T.; Ilhan, H.; Todica, A.; Michl, M.; Nadjiri, J.; Paprottka, P.M. Outcome and Safety after 103 Radioembolizations with Yttrium-90 Resin Microspheres in 73 Patients with Unresectable Intrahepatic Cholangiocarcinoma-An Evaluation of Predictors. Cancers 2021, 13, 5399. [Google Scholar] [CrossRef] [PubMed]
- Gangi, A.; Shah, J.; Hatfield, N.; Smith, J.; Sweeney, J.; Choi, J.; El-Haddad, G.; Biebel, B.; Parikh, N.; Arslan, B.; et al. Intrahepatic Cholangiocarcinoma Treated with Transarterial Yttrium-90 Glass Microsphere Radioembolization: Results of a Single Institution Retrospective Study. J. Vasc. Interv. Radiol. 2018, 29, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; Braat, A.J.A.T.; Margonis, G.A.; Brown, D.B.; Taylor, K.B.; Borgmann, A.J.; Kappadath, S.C.; Mahvash, A.; IJzermans, J.N.M.; Weiss, M.J.; et al. Yttrium-90 Radioembolization in Intrahepatic Cholangiocarcinoma: A Multicenter Retrospective Analysis. J. Vasc. Interv. Radiol. 2020, 31, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Mouli, S.; Memon, K.; Baker, T.; Benson, A.B.; Mulcahy, M.F.; Gupta, R.; Ryu, R.K.; Salem, R.; Lewandowski, R.J. Yttrium-90 Radioembolization for Intrahepatic Cholangiocarcinoma: Safety, Response, and Survival Analysis. J. Vasc. Interv. Radiol. 2013, 24, 1227–1234. [Google Scholar] [CrossRef]
- Levillain, H.; Duran Derijckere, I.; Ameye, L.; Guiot, T.; Braat, A.; Meyer, C.; Vanderlinden, B.; Reynaert, N.; Hendlisz, A.; Lam, M.; et al. Personalised Radioembolization Improves Outcomes in Refractory Intra-Hepatic Cholangiocarcinoma: A Multicenter Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2270–2279. [Google Scholar] [CrossRef]
- Bargellini, I.; Mosconi, C.; Pizzi, G.; Lorenzoni, G.; Vivaldi, C.; Cappelli, A.; Vallati, G.E.; Boni, G.; Cappelli, F.; Paladini, A.; et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc. Intervent. Radiol. 2020, 43, 1305–1314. [Google Scholar] [CrossRef]
- White, J.; Carolan-Rees, G.; Dale, M.; Patrick, H.E.; See, T.C.; Bell, J.K.; Manas, D.M.; Crellin, A.; Slevin, N.J.; Sharma, R.A. Yttrium-90 Transarterial Radioembolization for Chemotherapy-Refractory Intrahepatic Cholangiocarcinoma: A Prospective, Observational Study. J. Vasc. Interv. Radiol. 2019, 30, 1185–1192. [Google Scholar] [CrossRef]
- Köhler, M.; Harders, F.; Lohöfer, F.; Paprottka, P.M.; Schaarschmidt, B.M.; Theysohn, J.; Herrmann, K.; Heindel, W.; Schmidt, H.H.; Pascher, A.; et al. Prognostic Factors for Overall Survival in Advanced Intrahepatic Cholangiocarcinoma Treated with Yttrium-90 Radioembolization. J. Clin. Med. 2019, 9, 56. [Google Scholar] [CrossRef]
- Schartz, D.A.; Porter, M.; Schartz, E.; Kallas, J.; Gupta, A.; Butani, D.; Cantos, A. Transarterial Yttrium-90 Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2022, 33, 679–686. [Google Scholar] [CrossRef]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-Line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, M.S.; Cho, C.K.; Yoo, H.J.; Jang, W.I.; Seo, Y.S.; Paik, E.K.; Kim, K.B.; Han, C.J.; Kim, S.B. Outcomes of Stereotactic Body Radiotherapy for Unresectable Primary or Recurrent Cholangiocarcinoma. Radiat. Oncol. J. 2014, 32, 163–169. [Google Scholar] [CrossRef]
- De, B.; Tran Cao, H.S.; Vauthey, J.N.; Manzar, G.S.; Corrigan, K.L.; Raghav, K.P.S.; Lee, S.S.; Tzeng, C.W.D.; Minsky, B.D.; Smith, G.L.; et al. Ablative Liver Radiotherapy for Unresected Intrahepatic Cholangiocarcinoma: Patterns of Care and Survival in the United States. Cancer 2022, 128, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. New Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Lamarca, A.; Ross, P.; Wasan, H.S.; Hubner, R.A.; McNamara, M.G.; Lopes, A.; Manoharan, P.; Palmer, D.; Bridgewater, J.; Valle, J.W. Advanced Intrahepatic Cholangiocarcinoma: Post Hoc Analysis of the ABC-01, -02, and -03 Clinical Trials. J. Natl. Cancer Inst. 2020, 112, 200–210. [Google Scholar] [CrossRef]
- Phelip, J.-M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Morizane, C.; Okusaka, T.; Mizusawa, J.; Katayama, H.; Ueno, M.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Fukutomi, A.; et al. Combination Gemcitabine plus S-1 versus Gemcitabine plus Cisplatin for Advanced/Recurrent Biliary Tract Cancer: The FUGA-BT (JCOG1113) Randomized Phase III Clinical Trial. Ann. Oncol. 2019, 30, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kang, J.H.; Lee, J.; Lee, H.W.; Oh, S.Y.; Jang, J.S.; Lee, M.A.; Sohn, B.S.; Yoon, S.Y.; Choi, H.J.; et al. Capecitabine plus Oxaliplatin versus Gemcitabine plus Oxaliplatin as First-Line Therapy for Advanced Biliary Tract Cancers: A Multicenter, Open-Label, Randomized, Phase III, Noninferiority Trial. Ann. Oncol. 2019, 30, 788–795. [Google Scholar] [CrossRef]
- McNamara, M.G.; Bridgewater, J.; Palmer, D.H.; Faluyi, O.; Wasan, H.; Patel, A.; Ryder, W.D.; Barber, S.; Gnanaranjan, C.; Ghazaly, E.; et al. A Phase Ib Study of NUC-1031 in Combination with Cisplatin for the First-Line Treatment of Patients with Advanced Biliary Tract Cancer (ABC-08). Oncologist 2021, 26, e669–e678. [Google Scholar] [CrossRef]
- Kang, S.; El-rayes, B.F.; Akce, M. Evolving Role of Immunotherapy in Advanced Biliary Tract Cancers. Cancers (Basel) 2022, 14, 1748. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Ruth, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Oh, D.-Y. Updated Overall Survival from the Phase 3 TOPAZ-1 Study of Durvalumab or Placebo plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer. (Abstract 56P). Ann. Oncol. 2022, 33, S19–S26. [Google Scholar] [CrossRef]
- Vogel, A.; Boeck, S.; Waidmann, O.; Bitzer, M.; Wenzel, P.; Belle, S.; Springfeld, C.; Schulze, K.; Weinmann, A.; Lindig, U.; et al. A Randomized Phase II Trial of Durvalumab and TremelIMUmab with Gemcitabine or Gemcitabine and Cisplatin Compared to Gemcitabine and Cisplatin in Treatment-Naïve Patients with CHolangio- and GallbladdEr Carcinoma (IMMUCHEC). Ann. Oncol. 2022, 33, S563. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal Irinotecan plus Fluorouracil and Leucovorin versus Fluorouracil and Leucovorin for Metastatic Biliary Tract Cancer after Progression on Gemcitabine plus Cisplatin (NIFTY): A Multicentre, Open-Label, Randomised, Phase 2b Study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, K.-P.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Jeong, J.; Lee, J.S.; Kim, K.W.; et al. Final Results from the NIFTY Trial, a Phase IIb, Randomized, Open-Label Study of Liposomal Irinotecan (Nal-IRI) plus Fluorouracil (5-FU)/Leucovorin (LV) in Patients (Pts) with Previously Treated Metastatic Biliary Tract Cancer (BTC). Ann. Oncol. 2022, 33, S565. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-Line FOLFOX Chemotherapy versus Active Symptom Control for Advanced Biliary Tract Cancer (ABC-06): A Phase 3, Open-Label, Randomised, Controlled Trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, H.S.; Lee, S.H.; Woo, S.M.; Kim, D.U.; Bang, S. Efficacy and Safety of Pembrolizumab for Gemcitabine/Cisplatin-Refractory Biliary Tract Cancer: A Multicenter Retrospective Study. J. Clin. Med. 2020, 9, 1769. [Google Scholar] [CrossRef]
- Naganuma, A.; Sakuda, T.; Murakami, T.; Aihara, K.; Watanuki, Y.; Suzuki, Y.; Shibasaki, E.; Masuda, T.; Uehara, S.; Yasuoka, H.; et al. Microsatellite Instability-High Intrahepatic Cholangiocarcinoma with Portal Vein Tumor Thrombosis Successfully Treated with Pembrolizumab. Intern. Med. 2020, 59, 2261–2267. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Maio, M.; Ascierto, P.A.; Manzyuk, L.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.M.; de Jesus-Acosta, A.; Doi, T.; Longo, F.; et al. Pembrolizumab in Microsatellite Instability High or Mismatch Repair Deficient Cancers: Updated Analysis from the Phase II KEYNOTE-158 Study. Ann. Oncol. 2022, 33, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Montal, R.; Sia, D.; Montironi, C.; Leow, W.Q.; Esteban-Fabró, R.; Pinyol, R.; Torres-Martin, M.; Bassaganyas, L.; Moeini, A.; Peix, J.; et al. Molecular Classification and Therapeutic Targets in Extrahepatic Cholangiocarcinoma. J. Hepatol. 2020, 73, 315–327. [Google Scholar] [CrossRef]
- Sia, D.; Hoshida, Y.; Villanueva, A.; Roayaie, S.; Ferrer, J.; Tabak, B.; Peix, J.; Sole, M.; Tovar, V.; Alsinet, C.; et al. Integrative Molecular Analysis of Intrahepatic Cholangiocarcinoma Reveals 2 Classes That Have Different Outcomes. Gastroenterology 2013, 144, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic Spectra of Biliary Tract Cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, H.; Saurí, T.; Acosta, D.A.; Guardiola, M.; Sierra, A.; Hernando, J.; Nuciforo, P.; Miquel, J.M.; Molero, C.; Peiró, S.; et al. ESMO Scale for Clinical Actionability of Molecular Targets Driving Targeted Treatment in Patients with Cholangiocarcinoma. Clin. Cancer Res. 2022, 28, 1662–1671. [Google Scholar] [CrossRef]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast Growth Factor Receptor 2 Tyrosine Kinase Fusions Define a Unique Molecular Subtype of Cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef]
- Sia, D.; Losic, B.; Moeini, A.; Cabellos, L.; Hao, K.; Revill, K.; Bonal, D.; Miltiadous, O.; Zhang, Z.; Hoshida, Y.; et al. Massive Parallel Sequencing Uncovers Actionable FGFR2-PPHLN1 Fusion and ARAF Mutations in Intrahepatic Cholangiocarcinoma. Nat. Commun. 2015, 6, 6087. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in Previously Treated Patients with Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusions or Rearrangements: Mature Results from a Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. New Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Jeong, J.H.; Kim, K.P.; Lee, S.S.; Oh, D.W.; Park, D.H.; Song, T.J.; Park, Y.; Hong, S.M.; Ryoo, B.Y.; et al. Feasibility of HER2-Targeted Therapy in Advanced Biliary Tract Cancer: A Prospective Pilot Study of Trastuzumab Biosimilar in Combination with Gemcitabine Plus Cisplatin. Cancers 2021, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chon, H.J.; Cheon, J.; Lee, M.A.; Im, H.-S.; Jang, J.-S.; Kim, M.H.; Park, S.; Kang, B.; Hong, M.; et al. Trastuzumab plus FOLFOX for HER2-Positive Biliary Tract Cancer Refractory to Gemcitabine and Cisplatin: A Multi-Institutional Phase 2 Trial of the Korean Cancer Study Group (KCSG-HB19–14). Lancet Gastroenterol. Hepatol. 2023, 8, 56–65. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600E-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary Tract Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 34, 127–140. [Google Scholar] [CrossRef]
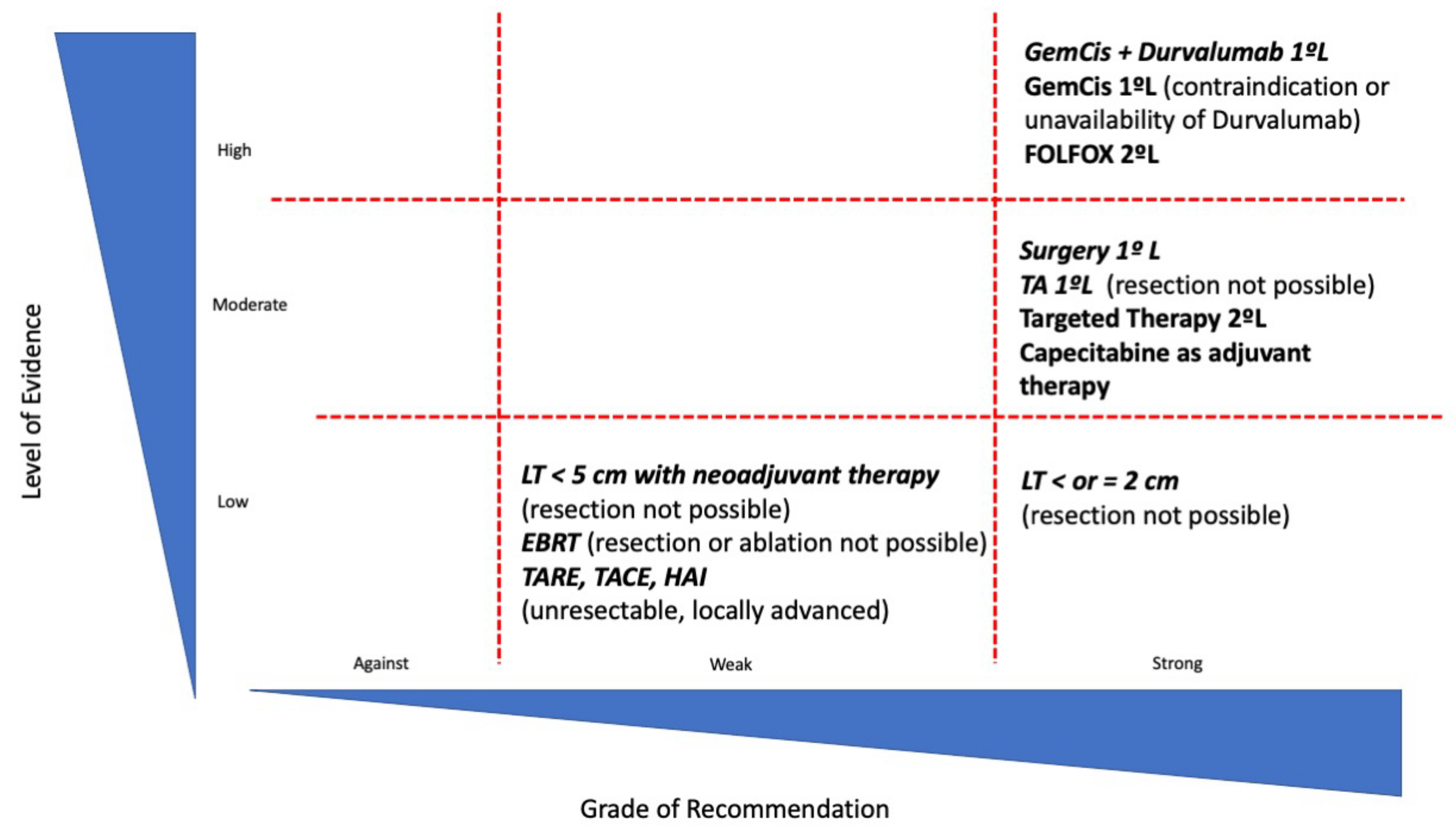
| Study | Design | Number of Patients | Neoadjuvant Therapy | Overall Survival |
|---|---|---|---|---|
| Sapisochin et al. (2016) [16] | Retrospective cohort multicenter. Incidental iCCA by pathological study. | 48 | 1 year: 93% 3 years: 84% 5 years: 65% | |
| McMillan et al. (2022) [18] | Prospective single-center case series. | 18 | Neoadjuvant chemotherapy (GemCis) and disease stability were required by radiological evaluation for at least six months. Treatments in addition to GemCis were heterogeneous: locoregional therapies, liver resection, and TT (IDH-1, FGFR, and PARP). | 1 year: 100% 3 years: 71% 5 years: 57% |
| Ito et al. (2022) [19] | Retrospective, single-center, case series. | 30 | Neoadjuvant chemotherapy and or locoregional therapies. | 1 year: 80% 3 years: 63% 5 years: 49% |
| Agent | Trial id and/or Name | Mechanism or Pathway | Phase | Study Population | Arms | Outcomes |
|---|---|---|---|---|---|---|
| IDH mutations | ||||||
| Ivosidenib | NCT02989857ClarIDHy trial | IDH-1 inhibitor (decreases oncometabolite 2-HG) | 3 | Previously treated, advanced, IDH1-mutant CCA. | Ivosidenib vs. Placebo | mPFS (months): 2.7 (95% CI, 1.6–4.2) vs. 1.4 (1.4–1.6); HR: 0.37; (95% CI, 0.25–0.54) p < 0.0001 mOS (months): 10.3 (95% CI, 7.8–12.4) vs. 7.5 (95% CI, 4.8–11.1) |
| FGFR alterations | ||||||
| Pemigatinib | NCT02924376 FIGHT-202 | FGFR 1, 2, and 3 reversible inhibitors; FGFR fusions or rearrangements | 2 | Advanced, previously treated CCA with and without FGFR2 fusions/rearrangements/alterations. | FGFR2 rearrangements or fusion CCA Other FGF/FGFR alterations No FGF/FGFR alterations | ORR (%): 37 (95% CI, 27.9 –46.9) mOS (months): 17.5 (95% CI, 14.4–22.9) vs. 6.7 (95% CI, 2.1–10.5) vs. 4.0 (95% CI, 2.0–4.6) |
| Infigratinib (BGJ398) | NCT02150967PROOF-201 | ATP-competitive FGFR 1, 2, and 3 tyrosine kinase reversible inhibitor | 2 | Locally advanced or metastatic CCA with FGFR2 fusions or rearrangements, previously treated with at least one gemcitabine-containing regimen. | Single arm | ORR (%): 23.1 (95% CI, 15.6–32.2) |
| Futibatinib (TAS-120) | NCT02052778FOENIX-CCA2 | Highly selective, irreversible pan-FGFR antagonist | 2 | Advanced, previously treated iCCA with FGFR2 fusions/other rearrangements. | Single arm | ORR (%): 41.7 mPFS (months): 9 mOS (months): 21.7 |
| Erdafitinib | NCT02699606LUC2001 | Pan-FGFR kinase inhibitor | 2 | Patients previously treated, aCCA with FGFR alterations. | Single arm | ORR (%): 50.0 |
| HER2 alterations | ||||||
| Pertuzumab and trastuzumab | NCT02091141MyPathway | Monoclonal ab targeting HER2 domain II; monoclonal ab binds to domain IV of HER2 | 2 | Previously treated, advanced BTC withHER2 amplification, overexpression, or both. | Single arm | ORR (%): 23 (95% CI, 11–39) |
| Neratinib | NCT01953926(SUMMIT trial) | Pan-HER irreversible TKI, with clinical activity against HER2 | 2 | Previously treated, advanced BTC harboring HER2 somatic mutations. | Single arm | ORR (%): 12 (95% CI, 3–31) mPFS (months): 1.8 (95% CI, 1.1–3.7) |
| BRAF V600E mutation | ||||||
| Dabrafenib and trametinib | NCT02034110ROAR trial | B-type Raf proto-oncogene, tyrosine kinase in the MAPK pathway | 2 | Previously treated, advanced BTC withBRAF V600E mutation. | Single arm | ORR (%): 47 (95% CI, 31 to 62) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, E.; Ferrer-Fàbrega, J.; Sauri, T.; Soler, A.; Cobo, A.; Burrel, M.; Iserte, G.; Forner, A. New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy. Cancers 2023, 15, 1244. https://doi.org/10.3390/cancers15041244
Mauro E, Ferrer-Fàbrega J, Sauri T, Soler A, Cobo A, Burrel M, Iserte G, Forner A. New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy. Cancers. 2023; 15(4):1244. https://doi.org/10.3390/cancers15041244
Chicago/Turabian StyleMauro, Ezequiel, Joana Ferrer-Fàbrega, Tamara Sauri, Alexandre Soler, Amparo Cobo, Marta Burrel, Gemma Iserte, and Alejandro Forner. 2023. "New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy" Cancers 15, no. 4: 1244. https://doi.org/10.3390/cancers15041244
APA StyleMauro, E., Ferrer-Fàbrega, J., Sauri, T., Soler, A., Cobo, A., Burrel, M., Iserte, G., & Forner, A. (2023). New Challenges in the Management of Cholangiocarcinoma: The Role of Liver Transplantation, Locoregional Therapies, and Systemic Therapy. Cancers, 15(4), 1244. https://doi.org/10.3390/cancers15041244





