Soft Tissue Reconstruction of the Posterior Trunk after Tumor Excision: A Surgical Algorithm
Abstract
Simple Summary
Abstract
1. Introduction
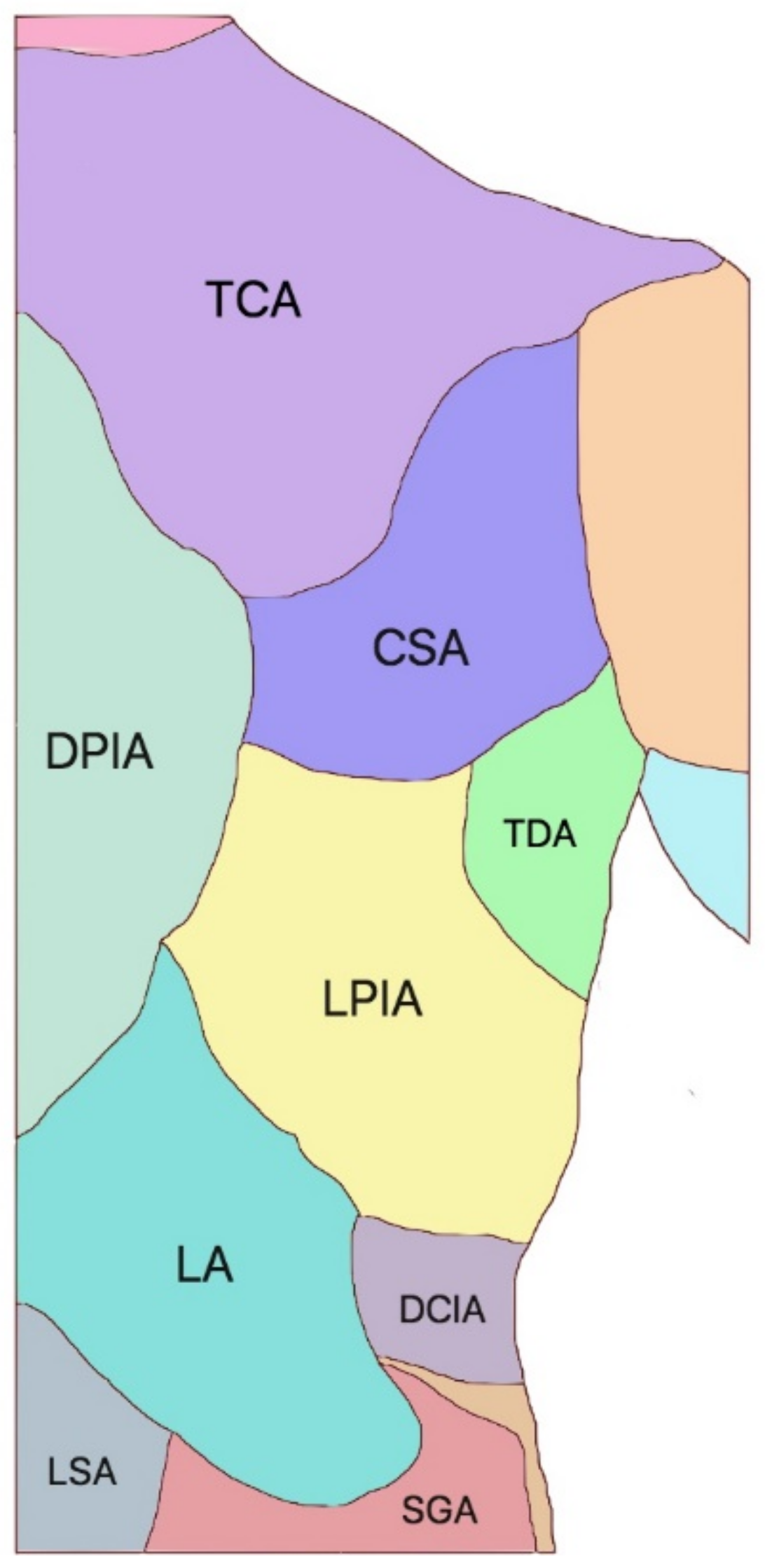
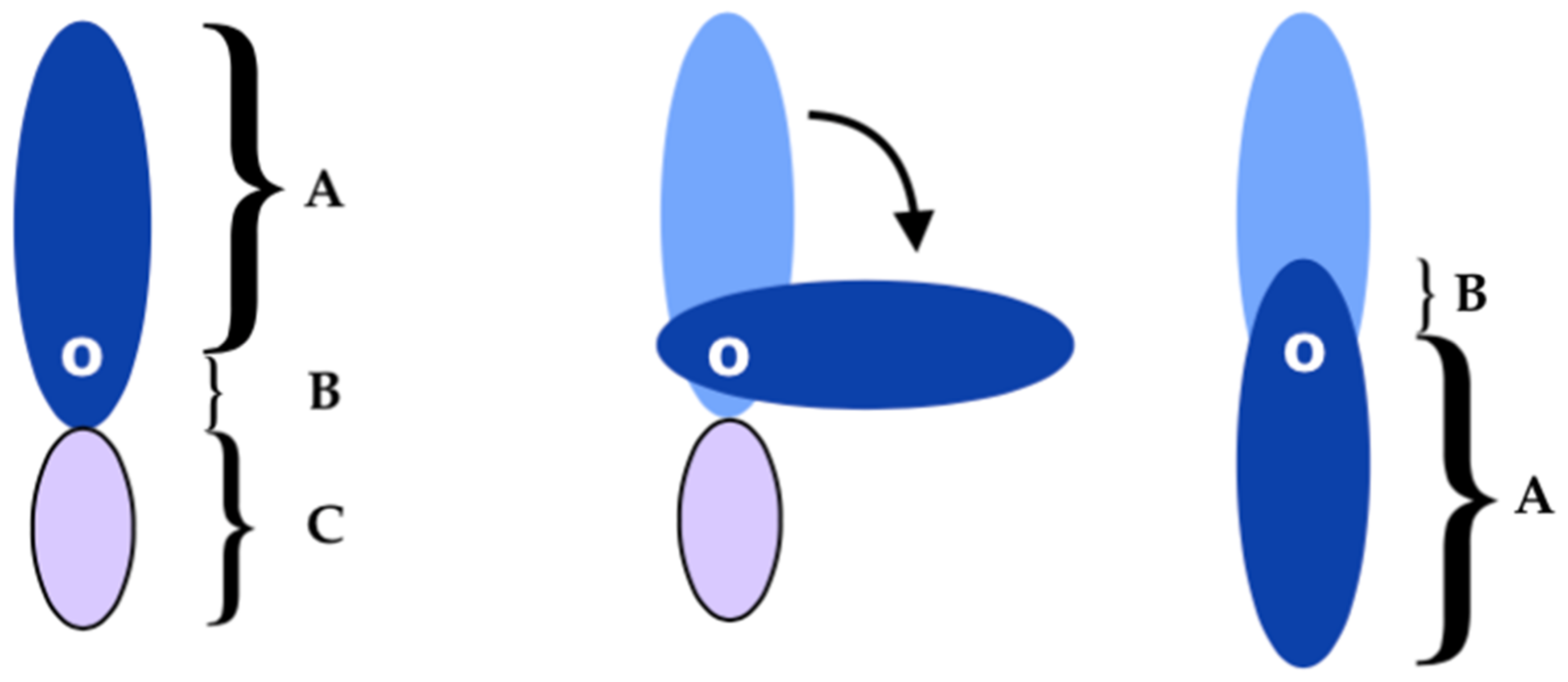
2. Pertinent Anatomy
3. Type of Flaps
3.1. Advancement Random Flaps
3.2. Myocutaneous Flaps
3.3. Locoregional Perforator Flaps
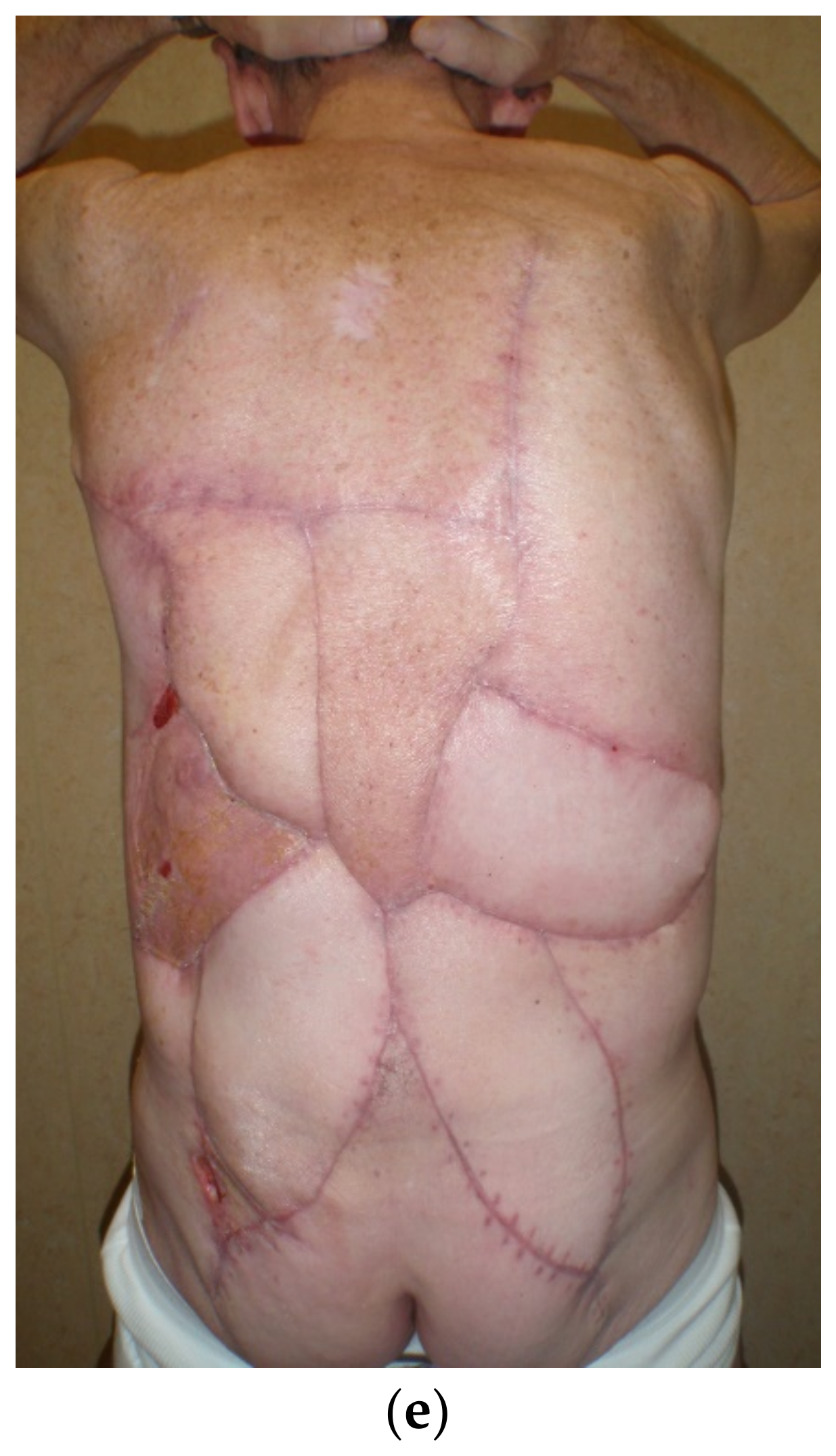
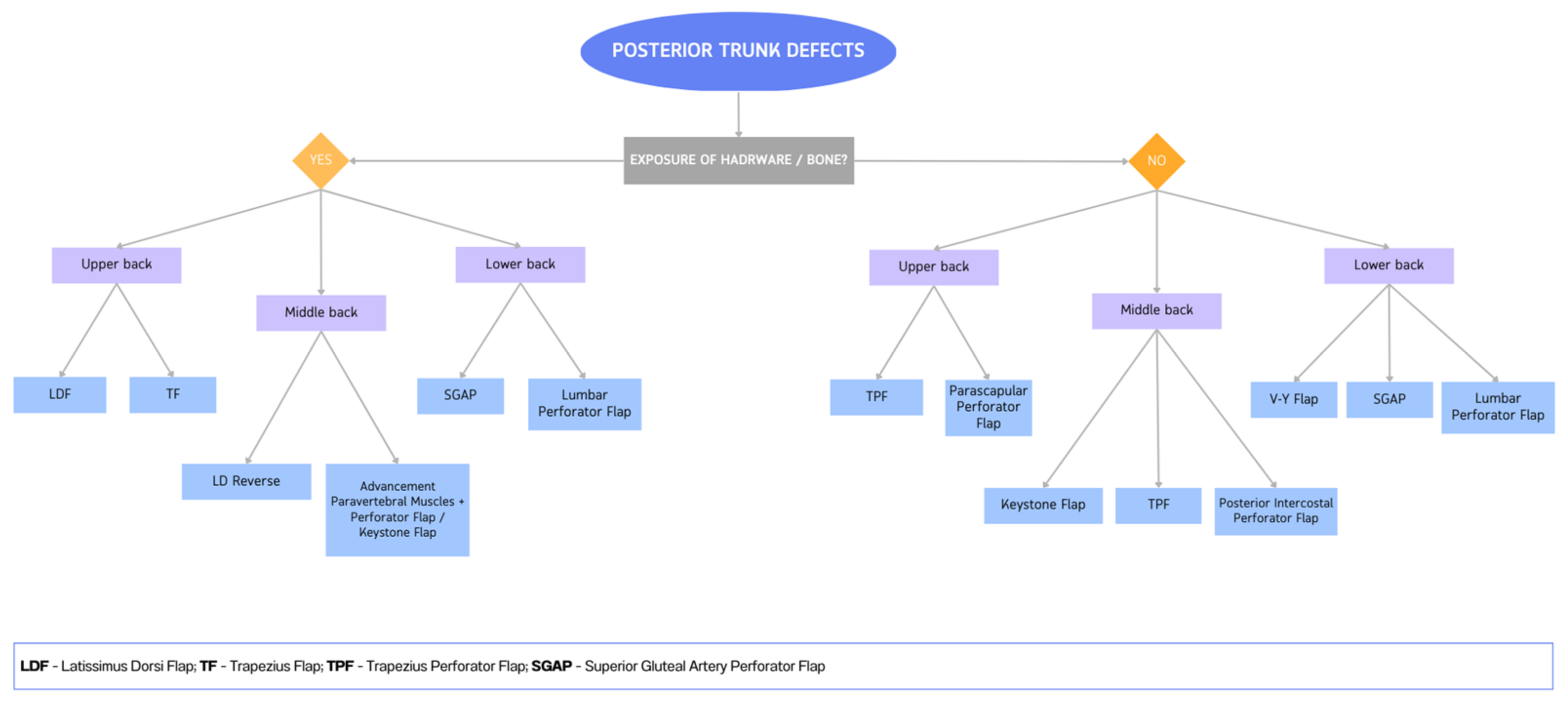
4. Reconstruction by Districts
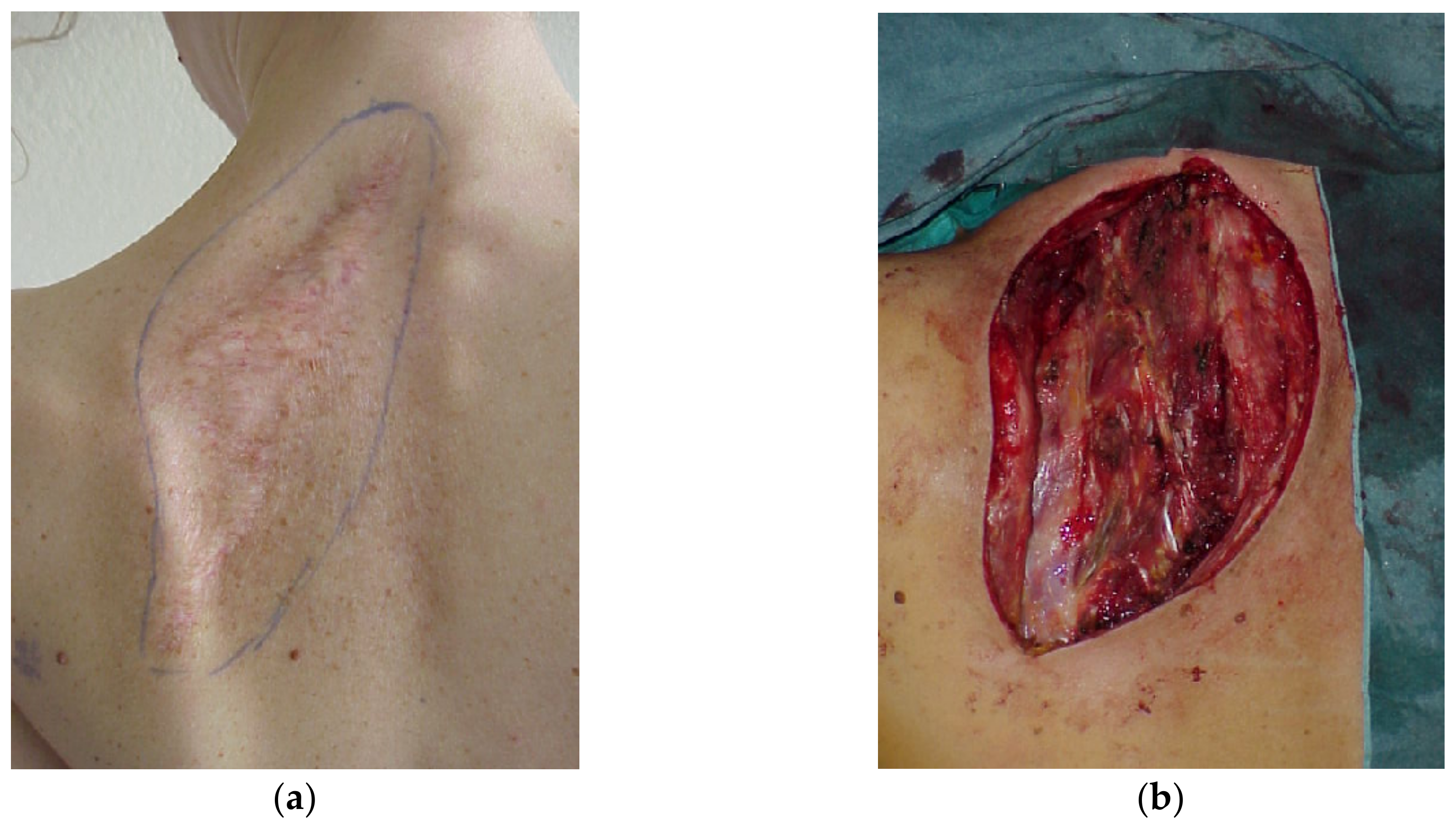
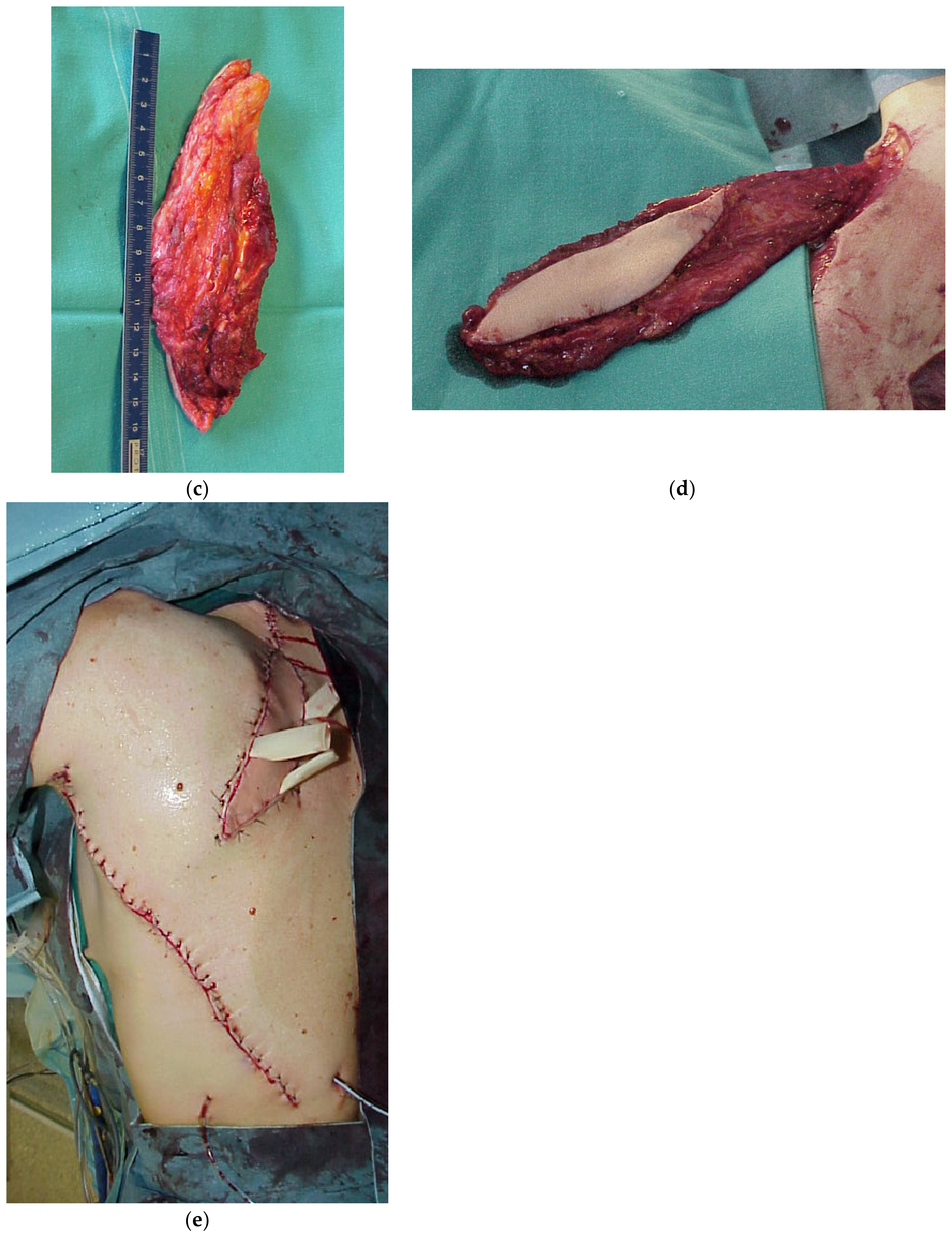
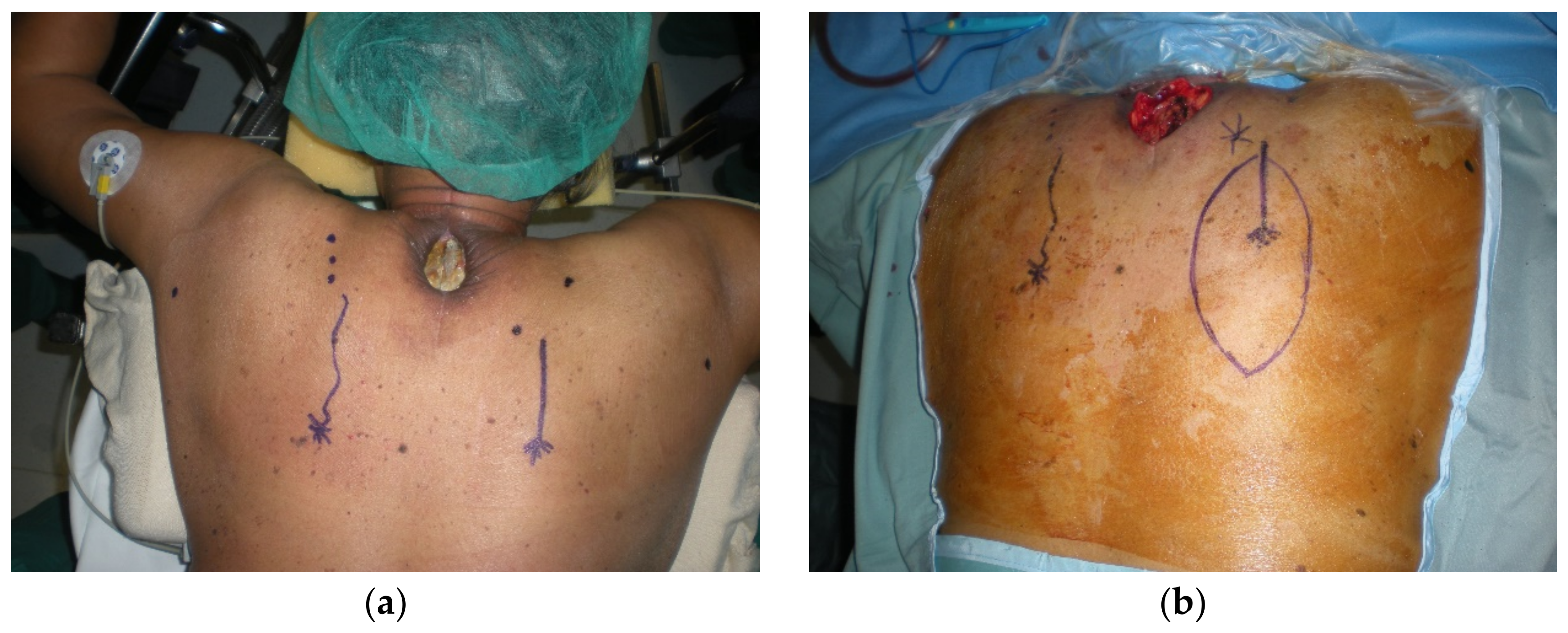
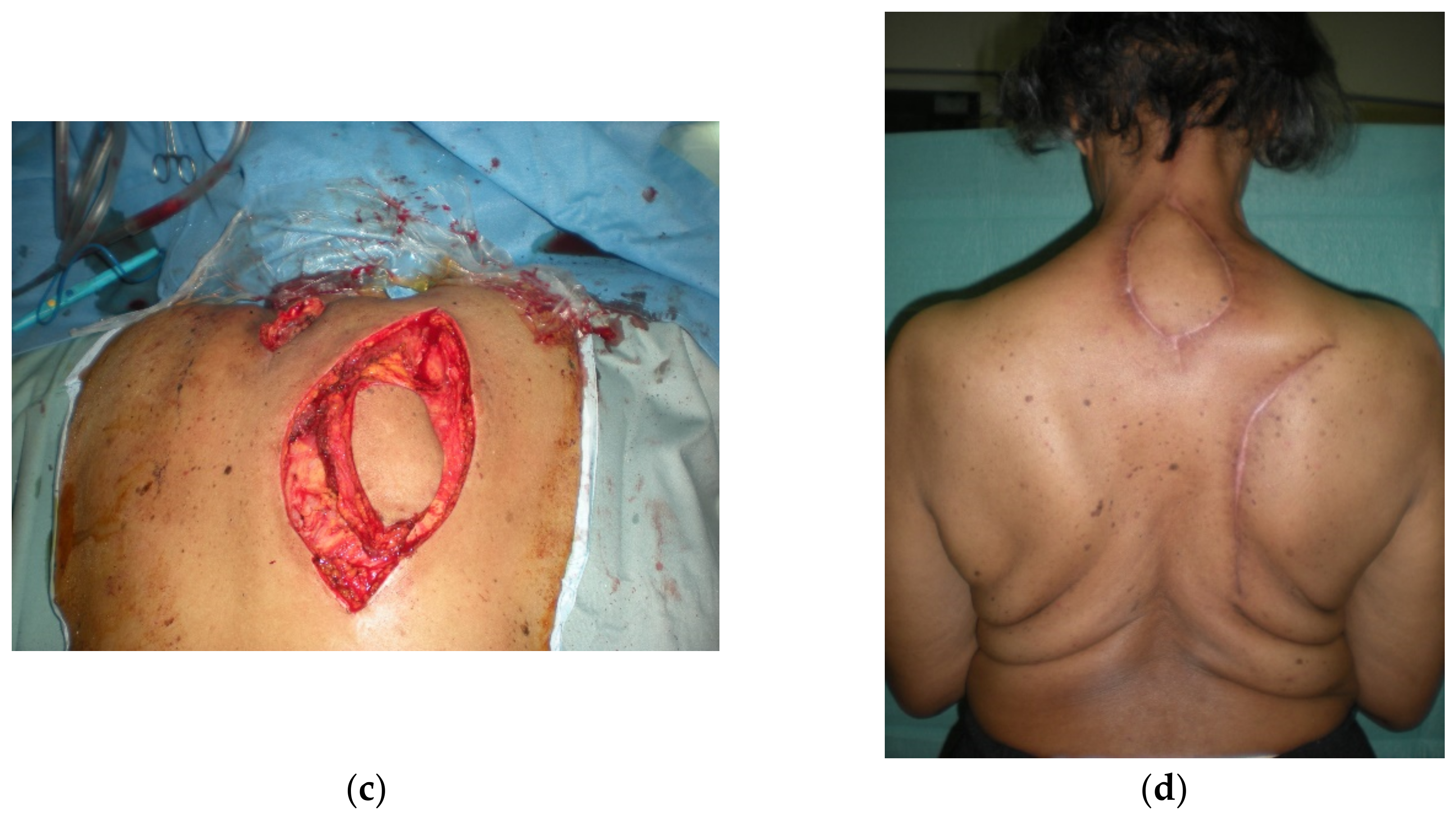
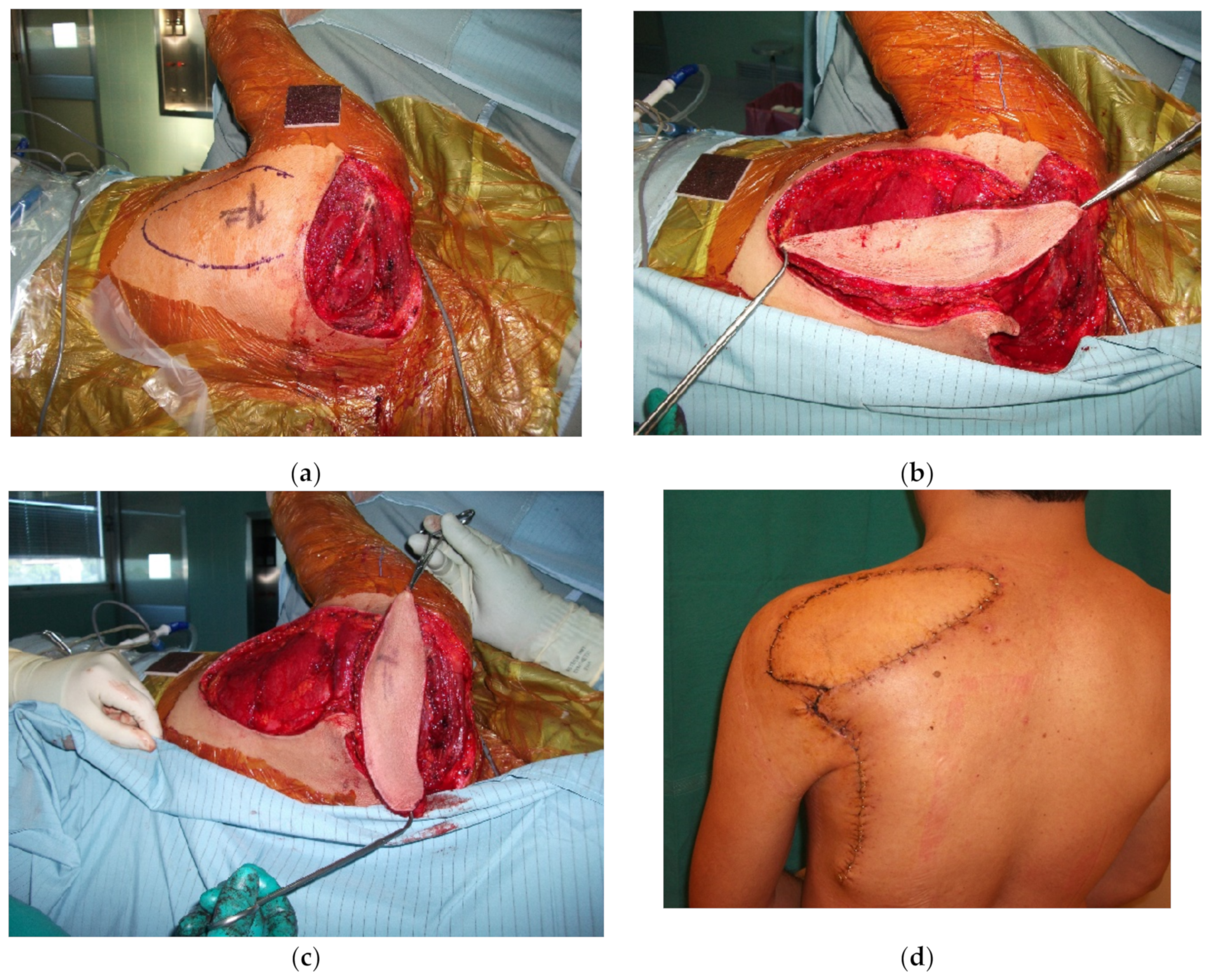
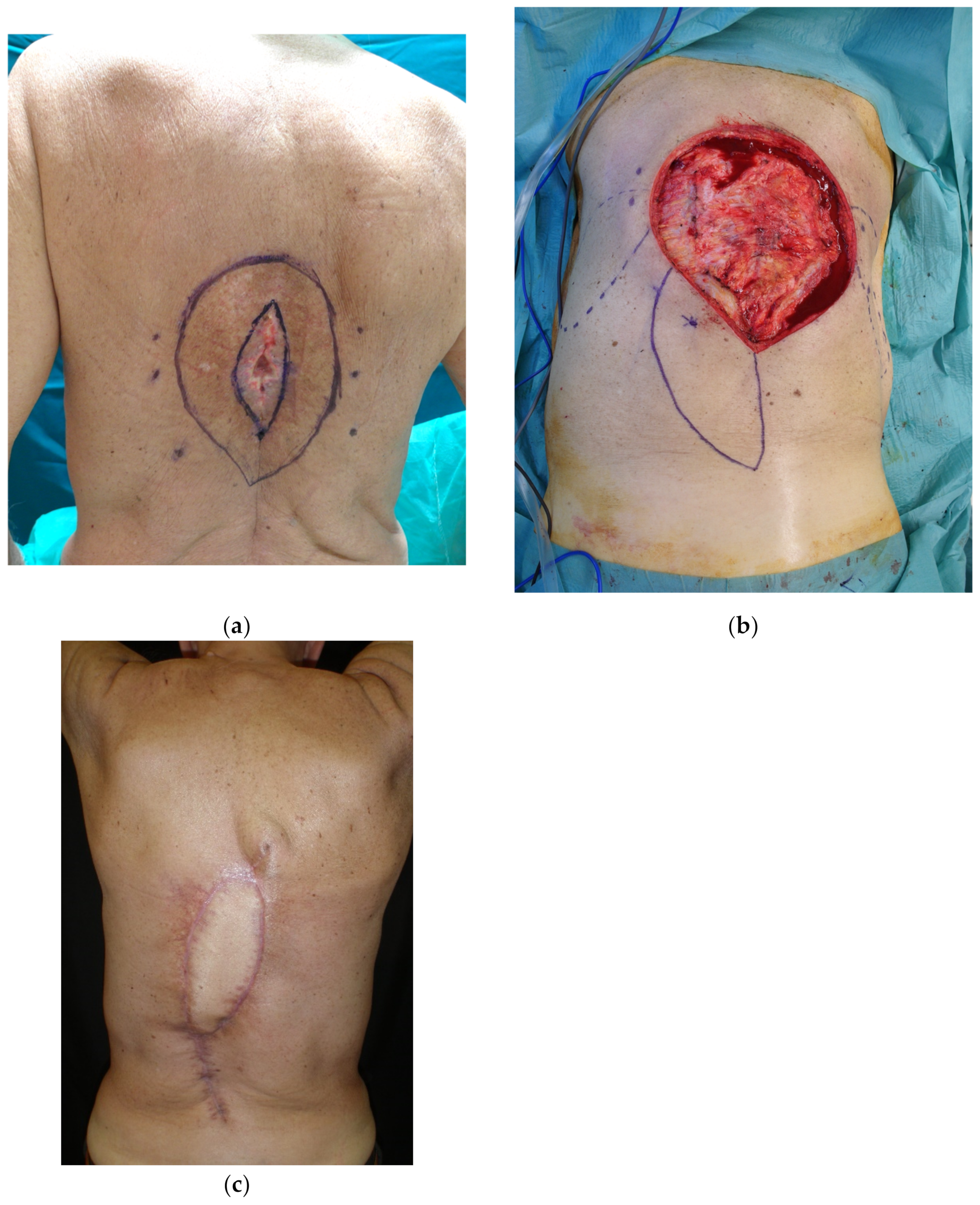
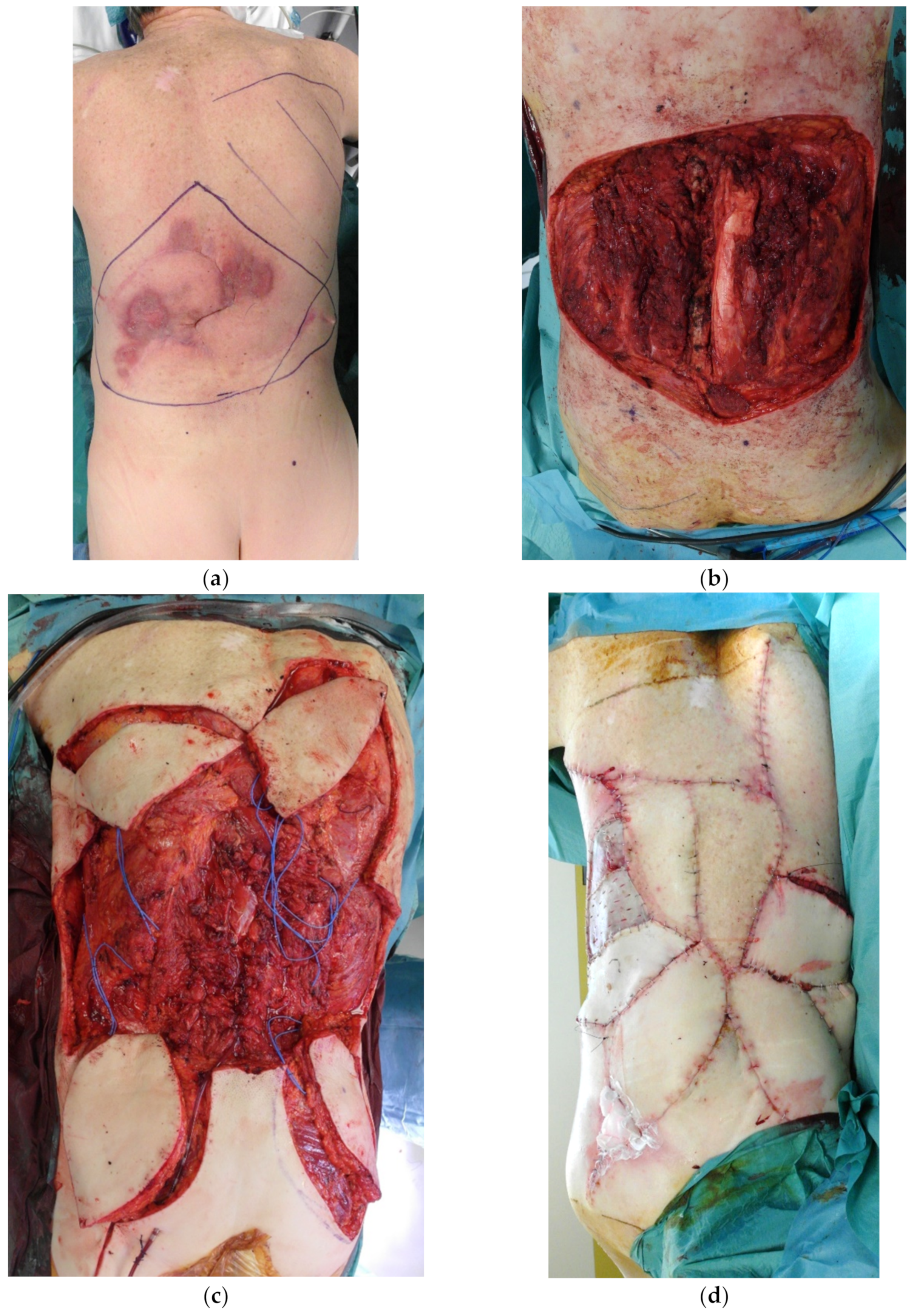
4.1. Upper Back
4.2. Midback
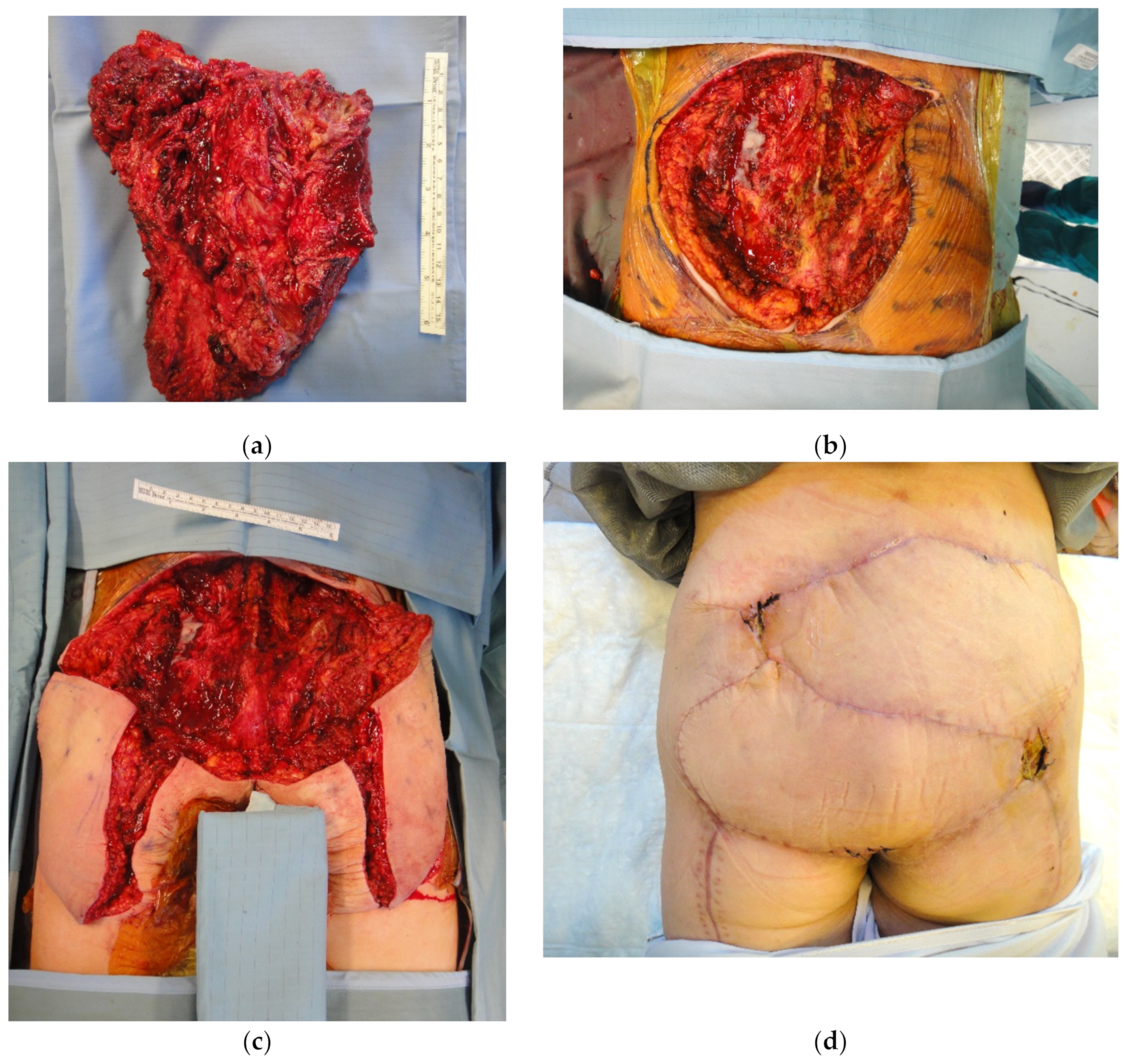
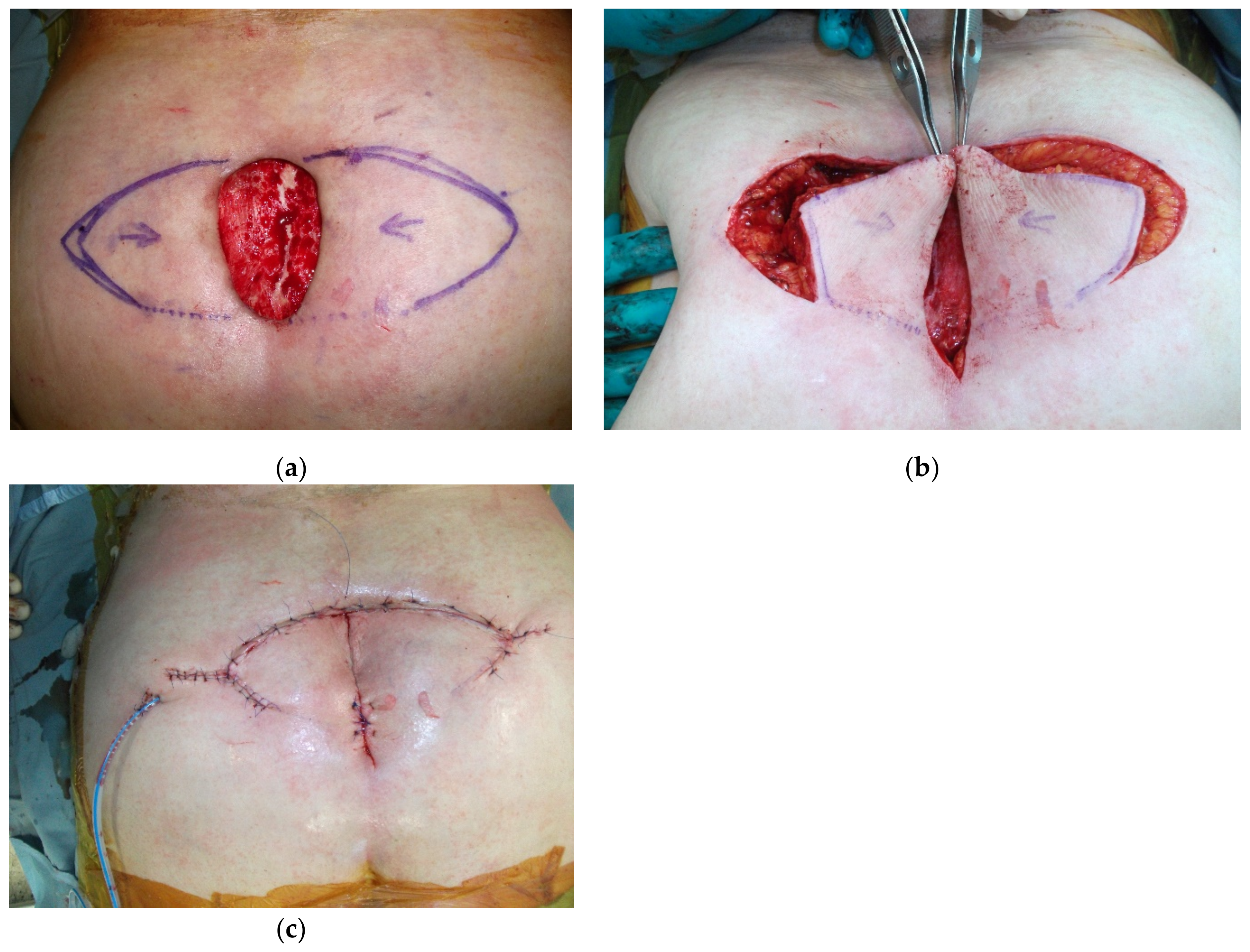
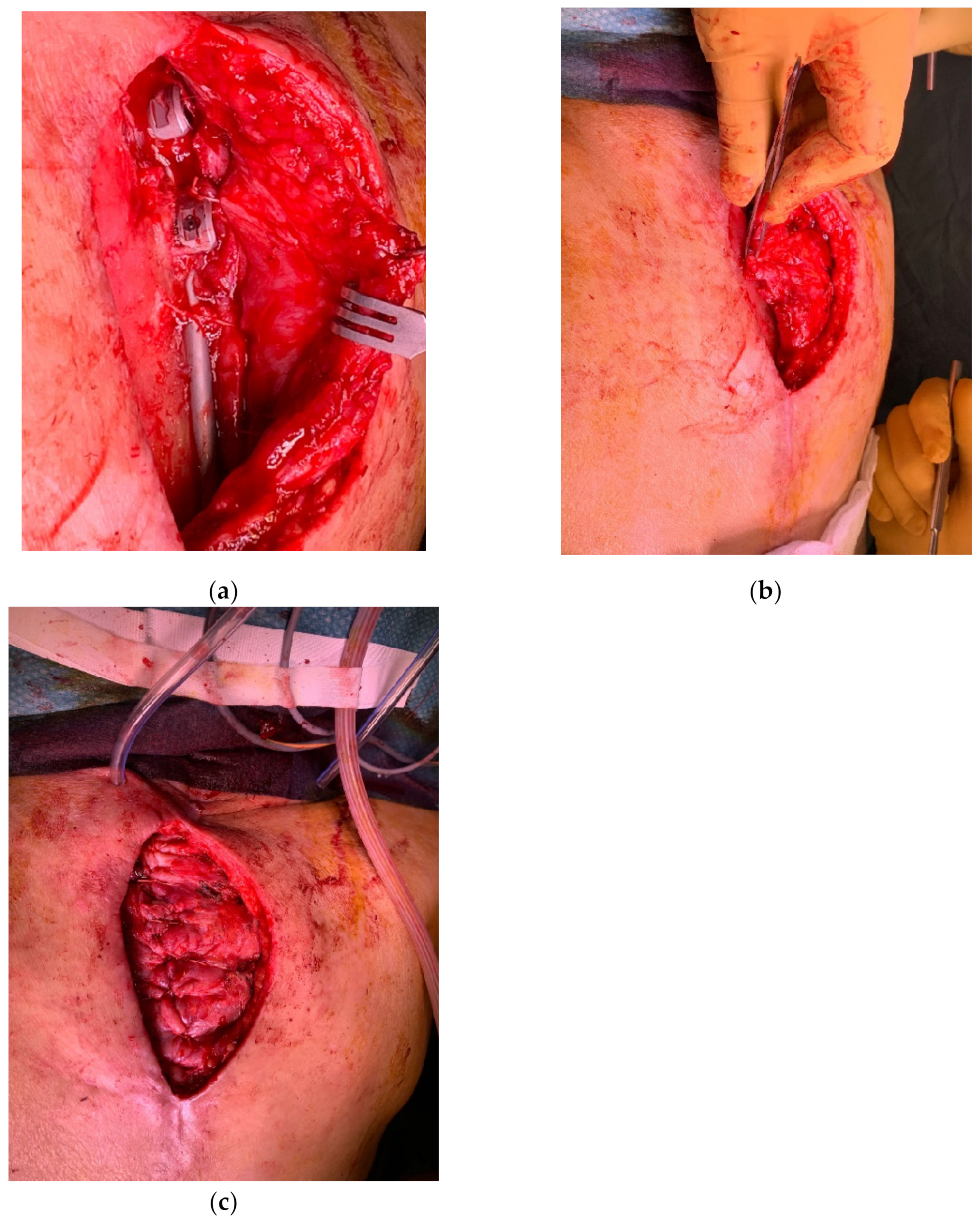
4.3. Lower Back
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft Tissue and Visceral Sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Capocaccia, R.; Botta, L.; Mallone, S.; De Angelis, R.; Ardanaz, E.; Comber, H.; Dimitrova, N.; Leinonen, M.K.; Siesling, S.; et al. Burden and Centralised Treatment in Europe of Rare Tumours: Results of RARECAREnet-a Population-Based Study. Lancet Oncol. 2017, 18, 1022–1039. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Pizzamiglio, S.; Touati, N.; Litiere, S.; Marreaud, S.; Kasper, B.; Gelderblom, H.; Stacchiotti, S.; Judson, I.; Dei Tos, A.P.; et al. The Impact of Chemotherapy on Survival of Patients with Extremity and Trunk Wall Soft Tissue Sarcoma: Revisiting the Results of the EORTC-STBSG 62931 Randomised Trial. Eur. J. Cancer 2019, 109, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Donthineni, R. Surgical Management of Metastatic Spine Tumors. Orthop. Clin. N. Am. 2006, 37, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.B.; Rhines, L.D.; Dong, W.; Chang, D.W. Immediate Soft-Tissue Reconstruction for Complex Defects of the Spine Following Surgery for Spinal Neoplasms. Plast. Reconstr. Surg. 2010, 125, 1460–1466. [Google Scholar] [CrossRef]
- Hernekamp, J.-F.; Cordts, T.; Kremer, T.; Kneser, U. Perforator-Based Flaps for Defect Reconstruction of the Posterior Trunk. Ann. Plast. Surg. 2021, 86, 72–77. [Google Scholar] [CrossRef]
- Hallock, G.G. Reconstruction of Posterior Trunk Defects. Semin. Plast. Surg. 2011, 25, 078–085. [Google Scholar] [CrossRef]
- Mericli, A.F.; Mirzabeigi, M.N.; Moore, J.H.; Fox, J.W.; Copit, S.E.; Tuma, G.A. Reconstruction of Complex Posterior Cervical Spine Wounds Using the Paraspinous Muscle Flap. Plast. Reconstr. Surg. 2011, 128, 148–153. [Google Scholar] [CrossRef]
- Chun, J.K.; Lynch, M.J.; Poultsides, G.A. Distal Trapezius Musculocutaneous Flap for Upper Thoracic Back Wounds Associated with Spinal Instrumentation and Radiation. Ann. Plast. Surg. 2003, 51, 17–22. [Google Scholar] [CrossRef]
- Mathes, D.W.; Thornton, J.F.; Rohrich, R.J. Management of Posterior Trunk Defects. Plast. Reconstr. Surg. 2006, 118, 73e. [Google Scholar] [CrossRef]
- Hallock, G.G. In an Era of Perforator Flaps, Are Muscle Flaps Passé? Plast. Reconstr. Surg. 2009, 123, 1357–1363. [Google Scholar] [CrossRef]
- Muramatsu, K.; Ihara, K.; Taguchi, T. Selection of Myocutaneous Flaps for Reconstruction Following Oncologic Resection of Sarcoma. Ann. Plast. Surg. 2010, 64, 307. [Google Scholar] [CrossRef]
- Kroll, S.S.; Rosenfield, L. Perforator-Based Flaps for Low Posterior Midline Defects. Plast. Reconstr. Surg. 1988, 81, 561–566. [Google Scholar] [CrossRef]
- Koshima, I.; Moriguchi, T.; Etoh, H.; Tsuda, K.; Tanaka, H. The Radial Artery Perforator-Based Adipofascial Flap for Dorsal Hand Coverage. Ann. Plast. Surg. 1995, 35, 474–479. [Google Scholar] [CrossRef]
- Roche, N.A.; Van Landuyt, K.; Blondeel, P.N.; Matton, G.; Monstrey, S.J. The Use of Pedicled Perforator Flaps for Reconstruction of Lumbosacral Defects. Ann. Plast. Surg. 2000, 45, 7–14. [Google Scholar] [CrossRef]
- Taylor, G.I.; Palmer, J.H. The Vascular Territories (Angiosomes) of the Body: Experimental Study and Clinical Applications. Br. J. Plast. Surg. 1987, 40, 113–141. [Google Scholar] [CrossRef]
- Taylor, G.I. The Angiosomes of the Body and Their Supply to Perforator Flaps. Clin. Plast. Surg. 2003, 30, 331–342. [Google Scholar] [CrossRef]
- Saint-Cyr, M.; Wong, C.; Schaverien, M.; Mojallal, A.; Rohrich, R.J. The Perforasome Theory: Vascular Anatomy and Clinical Implications. Plast. Reconstr. Surg. 2009, 124, 1529–1544. [Google Scholar] [CrossRef]
- Woo, S.J.; Koo, H.T.; Park, S.O.; Suami, H.; Chang, H. Evolution of Anatomical Studies on the Arterial, Venous, and Lymphatic System in Plastic Surgery. Arch. Plast. Surg. 2022, 49, 773–781. [Google Scholar] [CrossRef]
- Qian, Y.; Li, G.; Zang, H.; Cao, S.; Liu, Y.; Yang, K.; Mu, L. A Systematic Review and Meta-Analysis of Free-Style Flaps: Risk Analysis of Complications. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1651. [Google Scholar] [CrossRef]
- Innocenti, M.; Baldrighi, C.; Delcroix, L.; Adani, R. Local Perforator Flaps in Soft Tissue Reconstruction of the Upper Limb. Handchir. Mikrochir. Plast. Chir. 2009, 41, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Menichini, G.; Baldrighi, C.; Delcroix, L.; Vignini, L.; Tos, P. Are There Risk Factors for Complications of Perforator-Based Propeller Flaps for Lower-Extremity Reconstruction? Clin. Orthop. Relat. Res. 2014, 472, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Dell’Acqua, I.; Famiglietti, M.; Vignini, L.; Menichini, G.; Ghezzi, S. Free Perforator Flaps vs. Propeller Flaps in Lower Limb Reconstruction: A Cost/Effectiveness Analysis on a Series of 179 Cases. Injury 2019, 50 (Suppl. S5), S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Sadigh, P.L.; Chang, L.-R.; Hsieh, C.-H.; Feng, W.-J.; Jeng, S.-F. The Trapezius Perforator Flap: An Underused but Versatile Option in the Reconstruction of Local and Distant Soft-Tissue Defects. Plast. Reconstr. Surg. 2014, 134, 449e–456e. [Google Scholar] [CrossRef]
- Menichini, G.; Lucattelli, E.; Innocenti, A.; Brogi, M.; Cipriani, F.; Innocenti, M. Reverse-Flow Latissimus Dorsi Myocutaneous Flap in a Multi-Step Approach for Complex Back Defect Reconstruction: A Case Report. Microsurgery 2020, 40, 604–607. [Google Scholar] [CrossRef]
- Bostwick, J.; Scheflan, M.; Nahai, F.; Jurkiewicz, M.J. The “Reverse” Latissimus Dorsi Muscle and Musculocutaneous Flap: Anatomical and Clinical Considerations. Plast. Reconstr. Surg. 1980, 65, 395–399. [Google Scholar] [CrossRef]
- Stevenson, T.R.; Rohrich, R.J.; Pollock, R.A.; Dingman, R.O.; Bostwick, J. More Experience with the “Reverse” Latissimus Dorsi Musculocutaneous Flap: Precise Location of Blood Supply. Plast. Reconstr. Surg. 1984, 74, 237–243. [Google Scholar] [CrossRef]
- Hung, S.-J.; Chen, H.-C.; Wei, F.-C. Free Flaps for Reconstruction of the Lower Back and Sacral Area. Microsurgery 2000, 20, 72–76. [Google Scholar] [CrossRef]
- Behr, B.; Hirsch, T.; Goertz, O.; Ring, A.; Lehnhardt, M.; Daigeler, A. [Therapeutic options for reconstruction of the dorsal trunk wall]. Handchir. Mikrochir. Plast. Chir. 2014, 46, 90–96. [Google Scholar] [CrossRef]
- Geddes, C.R.; Tang, M.; Yang, D.; Morris, S.F. Anatomy of the Integument of the Trunk. In Perforator Flaps, Anatomy, Technology and Clinical Applications, 2nd ed.; Blondeel, P.N., Morris, S.F., Hallock, G.G., Neligan, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innocenti, M.; Mori, F.; Pedrini, F.A.; Salmaso, L.; Gennaro, A.; Sassu, P. Soft Tissue Reconstruction of the Posterior Trunk after Tumor Excision: A Surgical Algorithm. Cancers 2023, 15, 1214. https://doi.org/10.3390/cancers15041214
Innocenti M, Mori F, Pedrini FA, Salmaso L, Gennaro A, Sassu P. Soft Tissue Reconstruction of the Posterior Trunk after Tumor Excision: A Surgical Algorithm. Cancers. 2023; 15(4):1214. https://doi.org/10.3390/cancers15041214
Chicago/Turabian StyleInnocenti, Marco, Francesco Mori, Francesca Alice Pedrini, Luca Salmaso, Andrea Gennaro, and Paolo Sassu. 2023. "Soft Tissue Reconstruction of the Posterior Trunk after Tumor Excision: A Surgical Algorithm" Cancers 15, no. 4: 1214. https://doi.org/10.3390/cancers15041214
APA StyleInnocenti, M., Mori, F., Pedrini, F. A., Salmaso, L., Gennaro, A., & Sassu, P. (2023). Soft Tissue Reconstruction of the Posterior Trunk after Tumor Excision: A Surgical Algorithm. Cancers, 15(4), 1214. https://doi.org/10.3390/cancers15041214





