Association between Tumor Mutational Burden, Stromal CD8+ Tumor-Infiltrating Lymphocytes, and Clinical Factors in Cervical Cancers Treated with Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Radiotherapy
2.3. Assessment of TMB
2.4. Human Papillomavirus Genotyping
2.5. Immunohistochemical Analysis of CD8+TILs
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Giannini, A.; Bogani, G.; Vizza, E.; Chiantera, V.; Laganà, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; D’Oria, O. Advances on prevention and screening of gynecologic tumors: Are we stepping forward? Healthcare 2022, 10, 1605. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; ESMO Guidelines Committee. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahant-shetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- D’Oria, O.; Corrado, G.; Laganà, A.S.; Chiantera, V.; Vizza, E.; Giannini, A. New advances in cervical cancer: From bench to bedside. Int. J. Environ. Res. Public Health 2022, 19, 7094. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Demaria, S.; Ohno, T. The role of radiotherapy in the age of immunotherapy. Jpn. J. Clin. Oncol. 2021, 51, 513–522. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Leaman, O.; López-Campos, F.; Montero, A.; Conde, A.J.; Aristu, J.J.; Lara, P.; Calvo, F.M.; Melero, I. Immune mechanisms mediating abscopal effects in radioimmunotherapy. Pharmacol. Ther. 2019, 196, 195–203. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Ahmadzadeh, M.; Lu, Y.C.; Gros, A.; Turcotte, S.; Robbins, P.F.; Gartner, J.J.; Zheng, Z.; Li, Y.F.; Ray, S.; et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015, 350, 1387–1390. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Ota, N.; Yoshimoto, Y.; Darwis, N.D.M.; Sato, H.; Ando, K.; Oike, T.; Ohno, T. High tumor mutational burden predicts worse prognosis for cervical cancer treated with radiotherapy. Jpn. J. Radiol. 2022, 40, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xiang, L.; Cao, K.; Zhang, J.; Luo, Y.; Sun, W.; Zhang, N.; Ren, J.; Zhang, J.; Gong, Y.; et al. The prognostic value of tumor mutational burden and immune cell infiltration in esophageal cancer patients with or without radiotherapy. Aging 2020, 12, 4603–4616. [Google Scholar] [CrossRef]
- Mori, Y.; Sato, H.; Kumazawa, T.; Permata, T.B.M.; Yoshimoto, Y.; Murata, K.; Noda, S.E.; Kaminuma, T.; Ando, K.; Oike, T.; et al. Analysis of radiotherapy-induced alteration of CD8+ T cells and PD-L1 expression in patients with uterine cervical squamous cell carcinoma. Oncol. Lett. 2021, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Yoshimoto, Y.; Murata, K.; Noda, S.E.; Ando, K.; Ebara, T.; Okonogi, N.; Kaminuma, T.; Yamada, S.; Ikota, H.; et al. Treatment outcomes of patients with adenocarcinoma of the uterine cervix after definitive radiotherapy and the prognostic impact of tumor-infiltrating CD8+ lymphocytes in pre-treatment biopsy specimens: A multi-institutional retrospective study. J. Radiat. Res. 2020, 61, 275–284. [Google Scholar] [CrossRef]
- Chen, H.; Xia, B.; Zheng, T.; Lou, G. Immunoscore system combining CD8 and PD-1/PD-L1: A novel approach that predicts the clinical outcomes for cervical cancer. Int. J. Biol. Mark. 2020, 35, 65–73. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Someya, M.; Takada, Y.; Hasegawa, T.; Kitagawa, M.; Fukushima, Y.; Gocho, T.; Hori, M.; Nakata, K.; Hirohashi, Y.; et al. Association between radiotherapy-induced alteration of programmed death ligand 1 and survival in patients with uterine cervical cancer undergoing preoperative radiotherapy. Strahlenther. Onkol. 2020, 196, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Noda, S.E.; Okonogi, N.; Murata, K.; Shibuya, K.; Kiyohara, H.; Tamaki, T.; Ando, K.; Oike, T.; Ohkubo, Y.; et al. In-room computed tomography-based brachytherapy for uterine cervical cancer: Results of a 5-year retrospective study. J. Radiat. Res. 2017, 58, 543–551. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Sasaki, Y.; Murata, K.; Noda, S.E.; Miyasaka, Y.; Hamamoto, J.; Furuya, M.; Hirato, J.; Suzuki, Y.; Ohno, T.; et al. Mutation profiling of uterine cervical cancer patients treated with definitive radiotherapy. Gynecol. Oncol. 2020, 159, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Donnem, T.; Hald, S.M.; Paulsen, E.E.; Richardsen, E.; Al-Saad, S.; Kilvaer, T.K.; Brustugun, O.T.; Helland, A.; Lund-Iversen, M.; Poehl, M.; et al. Stromal CD8+ T-cell Density–A promising supplement to TNM staging in non-small cell lung cancer. Clin. Cancer Res. 2015, 21, 2635–2643. [Google Scholar] [CrossRef]
- Okazaki, S.; Murata, K.; Noda, S.E.; Kumazaki, Y.; Hirai, R.; Igari, M.; Abe, T.; Komatsu, S.; Nakano, T.; Kato, S. Dose-volume parameters and local tumor control in cervical cancer treated with central-shielding external-beam radiotherapy and CT-based image-guided brachytherapy. J. Radiat. Res. 2019, 60, 490–500. [Google Scholar] [CrossRef]
- Zolciak-Siwinska, A.; Gruszczynska, E.; Bijok, M.; Jonska-Gmyrek, J.; Dabkowski, M.; Staniaszek, J.; Michalski, W.; Kowalczyk, A.; Milanowska, K. Computed tomography-planned high-dose-rate brachytherapy for treating uterine cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 87–92. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Schilling, B.; Liu, D.; Sucker, A.; Livingstone, E.; Jerby-Arnon, L.; Zimmer, L.; Gutzmer, R.; Satzger, I.; Loquai, C.; et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat. Med. 2019, 25, 1916–1927. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 2018, 33, 843–852. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Federico, L.; Li, Y.; Dai, H.; Lu, Y.; Mills, G.B.; Yi, S.; Lin, S.Y.; Sahni, N. Multi-omics analysis reveals neoantigen-independent immune cell infiltration in copy-number driven cancers. Nat. Commun. 2018, 9, 1317. [Google Scholar] [CrossRef]
- Tokunaga, R.; Xiu, J.; Goldberg, R.M.; Philip, P.A.; Seeber, A.; Battaglin, F.; Arai, H.; Lo, J.H.; Naseem, M.; Puccini, A.; et al. The impact of ARID1A mutation on molecular characteristics in colorectal cancer. Eur. J. Cancer 2020, 140, 119–129. [Google Scholar] [CrossRef]
- Okamura, R.; Kato, S.; Lee, S.; Jimenez, R.E.; Sicklick, J.K.; Kurzrock, R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J. Immunother. Cancer 2020, 8, e000438. [Google Scholar] [CrossRef]
- Kamori, T.; Oki, E.; Shimada, Y.; Hu, Q.; Hisamatsu, Y.; Ando, K.; Shimokawa, M.; Wakai, T.; Oda, Y.; Mori, M. The effects of ARID1A mutations on colorectal cancer and associations with PD-L1 expression by stromal cells. Cancer Rep. 2022, 5, e1420. [Google Scholar] [CrossRef]
- Kuroda, Y.; Chiyoda, T.; Kawaida, M.; Nakamura, K.; Aimono, E.; Yoshimura, T.; Takahashi, M.; Saotome, K.; Yoshihama, T.; Iwasa, N.; et al. ARID1A mutation/ARID1A loss is associated with a high immunogenic profile in clear cell ovarian cancer. Gynecol. Oncol. 2021, 162, 679–685. [Google Scholar] [CrossRef]
- Jones, C.A.; Tansey, W.P.; Weissmiller, A.M. Emerging themes in mechanisms of tumorigenesis by SWI/SNF subunit mutation. Epigenet. Insights 2022, 15, 25168657221115656. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Kato, S.; Lippman, S.; Kurzrock, R. Chromatin remodeling (SWI/SNF) complexes, cancer, and response to immunotherapy. J. Immunother. Cancer 2022, 10, e004669. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, P.; Wang, J.; Lin, C.; Liu, H.; Li, H.; He, H.; Li, R.; Zhang, H.; Zhang, W. Somatic ARID1A mutation stratifies patients with gastric cancer to PD-1 blockade and adjuvant chemotherapy. Cancer Immunol. Immunother. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Luvero, D.; Lopez, S.; Bogani, G.; Raspagliesi, F.; Angioli, R. From the infection to the immunotherapy in cervical cancer: Can we stop the natural course of the disease? Vaccines 2020, 8, 597. [Google Scholar] [CrossRef]
- Tomao, F.; Santangelo, G.; Musacchio, L.; Di Donato, V.; Fischetti, M.; Giancotti, A.; Perniola, G.; Petrella, M.C.; Monti, M.; Palaia, I.; et al. Targeting cervical cancer: Is there a role for poly (ADP-ribose) polymerase inhibition? J. Cell. Physiol. 2020, 235, 5050–5058. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, A.X.J.; Chen, G.; Wu, Y.; Gu, W. Prognostic and therapeutic TILs of cervical cancer-current advances and future perspectives. Mol. Ther. Oncolytics 2021, 22, 410–430. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, X.; Feng, Y.; Cai, H.; Dong, D.; Peng, Y.; Yao, X.; Guo, Y.; Ma, M.; Dong, T.; et al. PD-1 expression status on CD8+ tumour infiltrating lymphocytes associates with survival in cervical cancer. Front. Oncol. 2021, 11, 678758. [Google Scholar] [CrossRef] [PubMed]
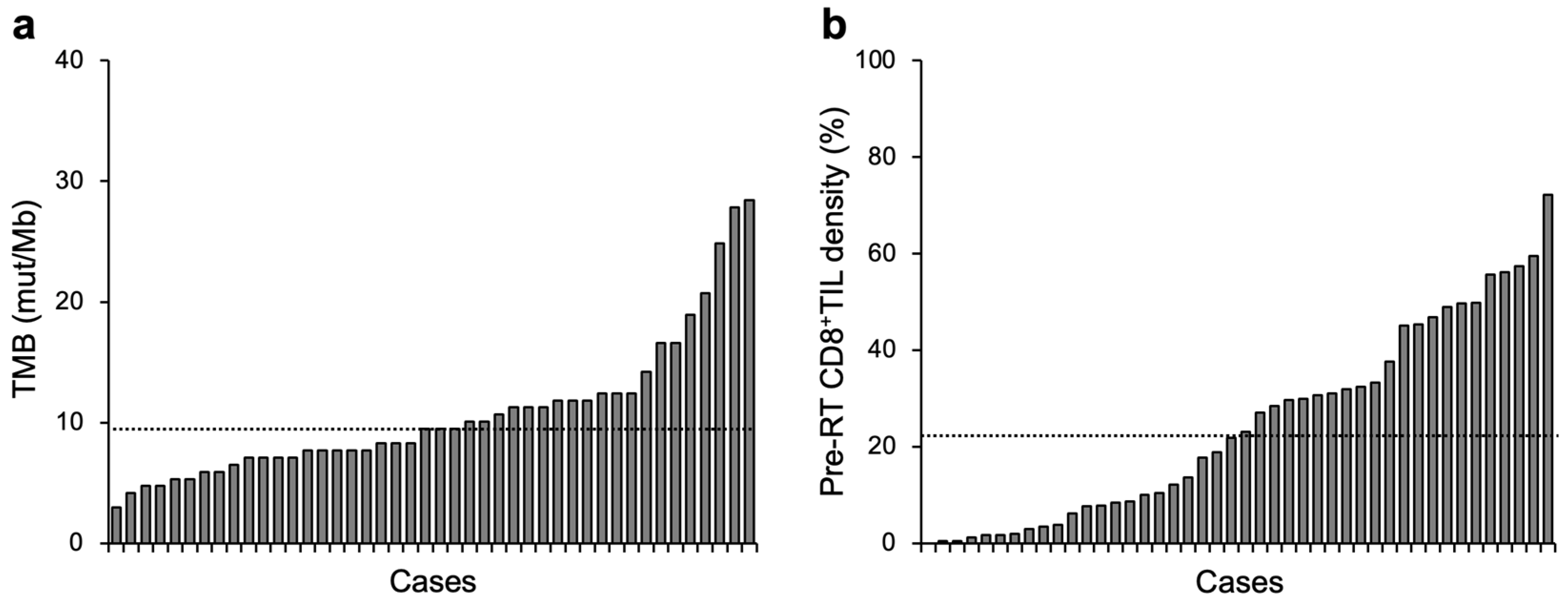


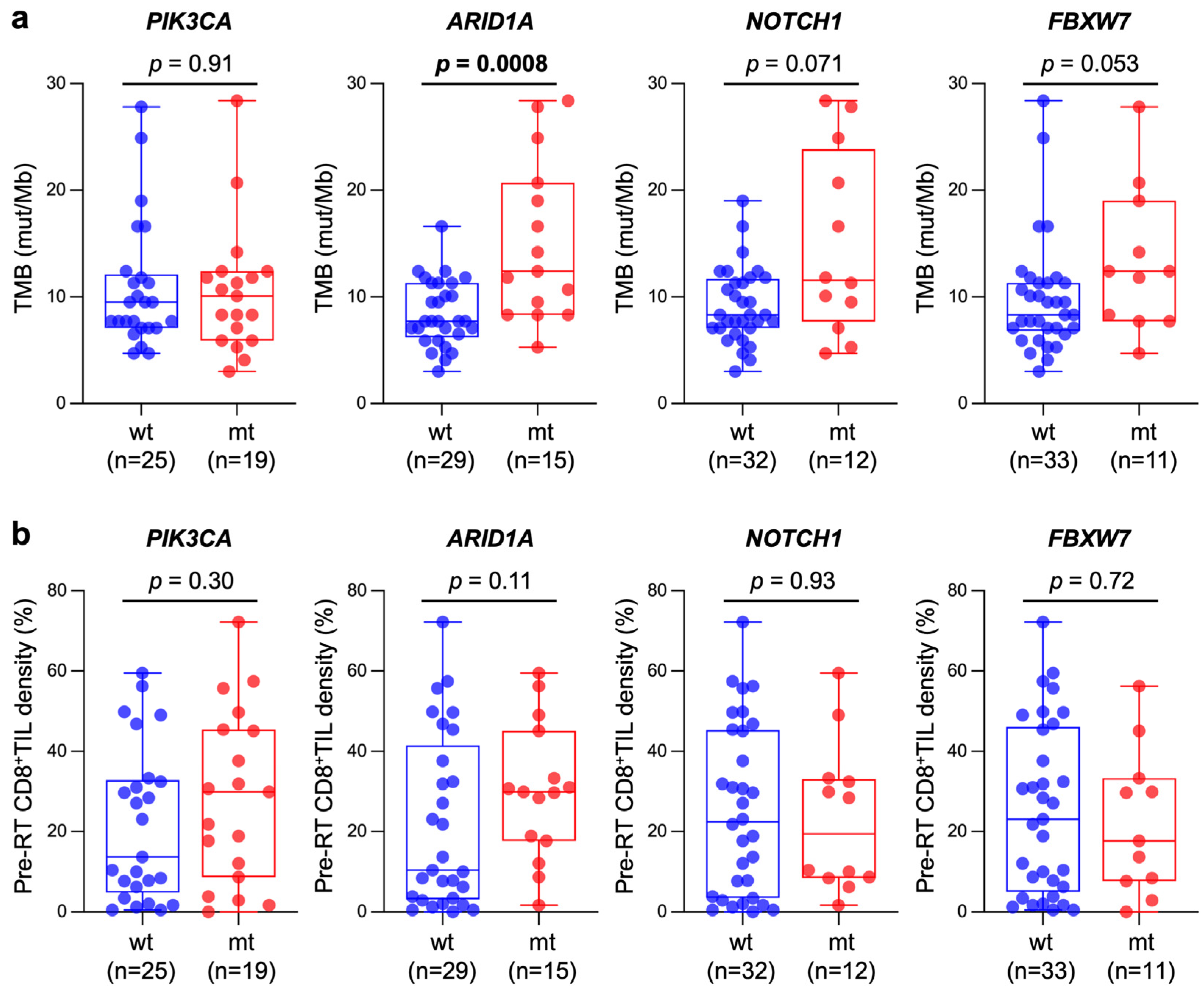
| Characteristics | Number (%) | |
|---|---|---|
| Follow-up period (M) | 61 (8–108) | |
| Age | 62 (33–87) | |
| FIGO stage | ||
| IB | 4 (9.1%) | |
| II | 17 (38.6%) | |
| III | 21 (47.7%) | |
| IVA | 2 (4.5%) | |
| Tumor diameter | ||
| ≤40 mm | 8 (18.2%) | |
| 41–60 mm | 24 (54.5%) | |
| >60 mm | 12 (27.3%) | |
| Pelvic LN status | ||
| Positive | 24 (54.5%) | |
| Negative | 20 (45.5%) | |
| PALN status | ||
| Positive | 6 (13.6%) | |
| Negative | 38 (86.4%) | |
| HPV status | ||
| Positive | 34 (77.3%) | |
| Negative | 10 (22.7%) | |
| Concurrent CT | ||
| Yes | 30 (68.2%) | |
| No | 14 (31.8%) | |
| Characteristics | TMB | Pre-RT CD8+TIL Density | |||||
|---|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | ||
| (n = 24) | (n = 20) | (n = 22) | (n = 22) | ||||
| Follow-up period (M) | 61 (9–108) | 62 (8–105) | 0.78 | 61 (8–108) | 62 (18–104) | 0.57 | |
| Age | 58 (33–87) | 63 (37–80) | 0.31 | 65 (35–87) | 59 (33–77) | 0.37 | |
| FIGO stage | |||||||
| IB | 1 (4.2%) | 3 (15%) | 0.45 | 3 (13.6%) | 1 (6.0%) | 0.37 | |
| II | 10 (41.7%) | 7 (35%) | 8 (36.4%) | 9 (40.9%) | |||
| III | 11 (45.8%) | 10 (50%) | 9 (40.9%) | 12 (54.5%) | |||
| IVA | 2 (8.3%) | 0 (0%) | 2 (9.1%) | 0 (0.0%) | |||
| Tumor diameter | |||||||
| ≤40 mm | 5 (20.8%) | 3 (15.0%) | 0.47 | 5 (22.7%) | 3 (13.6%) | 0.18 | |
| 41–60 mm | 11 (45.9%) | 13 (65.0%) | 9 (40.9%) | 15 (68.2%) | |||
| >60 mm | 8 (33.3%) | 4 (20.0%) | 8 (36.4%) | 4 (18.2%) | |||
| Pelvic LN status | |||||||
| Positive | 11 (45.8%) | 13 (65.0%) | 11 (50.0%) | 13 (59.1%) | 0.56 | ||
| Negative | 13 (54.2%) | 7 (35.0%) | 0.24 | 11 (50.0%) | 9 (40.9%) | ||
| PALN status | |||||||
| Positive | 4 (16.7%) | 2 (10.0%) | 4 (18.2%) | 2 (9.1%) | 0.66 | ||
| Negative | 20 (83.3%) | 18 (90.0%) | 0.67 | 18 (81.8%) | 20 (90.9%) | ||
| HPV status | |||||||
| Positive | 18 (75.0%) | 16 (80.0%) | 0.73 | 19 (86.4%) | 15 (68.2%) | 0.17 | |
| Negative | 6 (25.0%) | 4 (20.0%) | 3 (13.6%) | 7 (31.8%) | |||
| Concurrent CT | |||||||
| Yes | 16 (66.7%) | 14 (70.0%) | >0.99 | 13 (59.1%) | 17 (77.3%) | 0.33 | |
| No | 8 (33.3%) | 6 (30.0%) | 9 (40.9%) | 5 (22.7%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, H.; Oike, T.; Sato, H.; Ando, K.; Ohno, T. Association between Tumor Mutational Burden, Stromal CD8+ Tumor-Infiltrating Lymphocytes, and Clinical Factors in Cervical Cancers Treated with Radiotherapy. Cancers 2023, 15, 1210. https://doi.org/10.3390/cancers15041210
Ruan H, Oike T, Sato H, Ando K, Ohno T. Association between Tumor Mutational Burden, Stromal CD8+ Tumor-Infiltrating Lymphocytes, and Clinical Factors in Cervical Cancers Treated with Radiotherapy. Cancers. 2023; 15(4):1210. https://doi.org/10.3390/cancers15041210
Chicago/Turabian StyleRuan, Hanguang, Takahiro Oike, Hiro Sato, Ken Ando, and Tatsuya Ohno. 2023. "Association between Tumor Mutational Burden, Stromal CD8+ Tumor-Infiltrating Lymphocytes, and Clinical Factors in Cervical Cancers Treated with Radiotherapy" Cancers 15, no. 4: 1210. https://doi.org/10.3390/cancers15041210
APA StyleRuan, H., Oike, T., Sato, H., Ando, K., & Ohno, T. (2023). Association between Tumor Mutational Burden, Stromal CD8+ Tumor-Infiltrating Lymphocytes, and Clinical Factors in Cervical Cancers Treated with Radiotherapy. Cancers, 15(4), 1210. https://doi.org/10.3390/cancers15041210






