Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities—A Current Concept and Systematic Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
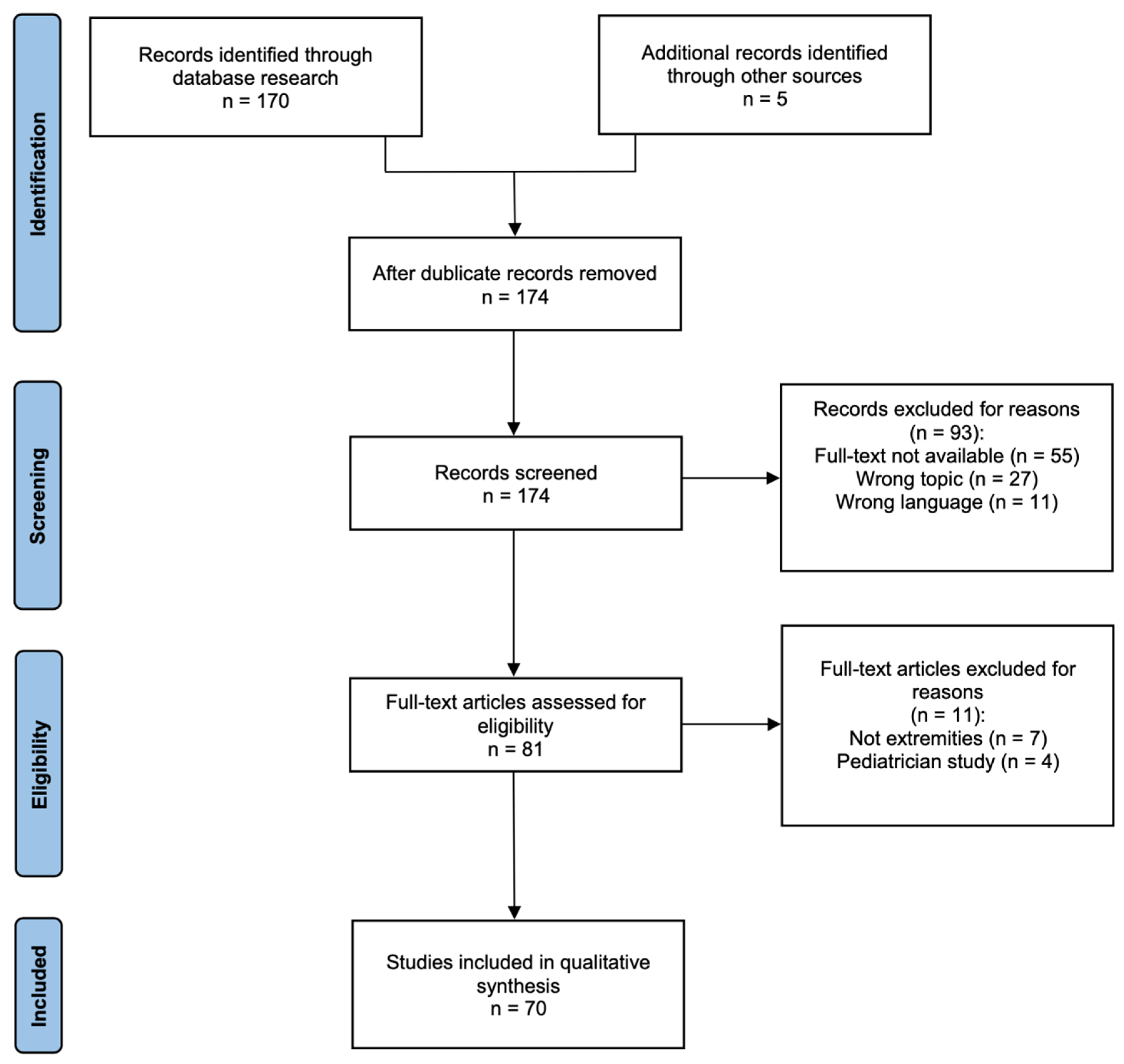
2. Material and Methods
3. Results
3.1. Local Complications
3.2. Recurrence Rate and Correlation with Margins of Resection
3.3. Brachytherapy and Tumor Grading
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Correa, R.; Gómez-Millán, J.; Lobato, M.; Fernández, A.; Ordoñez, R.; Castro, C.; Lupiañez, Y.; Medina, J.A. Radiotherapy in Soft-Tissue Sarcoma of the Extremities. Clin. Transl. Oncol. 2018, 20, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Ballo, M.T.; Lee, A.K. Current Results of Brachytherapy for Soft Tissue Sarcoma. Curr. Opin. Oncol. 2003, 15, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, A.O.; Fernandez, D.C.; Mesko, N.; Juloori, A.; Martinez, A.; Scott, J.G.; Shah, C.; Harrison, L.B. American Brachytherapy Society Consensus Statement for Soft Tissue Sarcoma Brachytherapy. Brachytherapy 2017, 16, 466–489. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, G.; Rüdiger, H.A.; Mela, M.M.; Scoccianti, G.; Livi, L.; Franchi, A.; Campanacci, D.A.; Capanna, R. Limb Salvage Surgery in Combination with Brachytherapy and External Beam Radiation for High-Grade Soft Tissue Sarcomas. Eur. J. Surg. Oncol. 2008, 34, 811–816. [Google Scholar] [CrossRef]
- Pellizzon, A.C.A. Evidence and Clinical Outcomes of Adult Soft Tissue Sarcomas of the Extremities Treated with Adjuvant High-Dose-Rate Brachytherapy—A Literature Review. J. Contemp. Brachyther. 2014, 6, 318–322. [Google Scholar] [CrossRef]
- Available online: www.cancerforum.org.au (accessed on 23 December 2022).
- Sarria, G.R.; Petrova, V.; Wenz, F.; Abo-Madyan, Y.; Sperk, E.; Giordano, F.A. Intraoperative Radiotherapy with Low Energy X-Rays for Primary and Recurrent Soft-Tissue Sarcomas. Radiat. Oncol. 2020, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.W.; Jones, K.B.; Barrott, J.J. Sarcoma–The Standard-Bearer in Cancer Discovery. Crit. Rev. Oncol. Hematol. 2018, 126, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nandra, R.; Hwang, N.; Matharu, G.; Reddy, K.; Grimer, R. One-Year Mortality in Patients with Bone and Soft Tissue Sarcomas as an Indicator of Delay in Presentation. Ann. R. Coll. Surg. Engl. 2015, 97, 425–433. [Google Scholar] [CrossRef]
- Daigeler, A.; Zmarsly, I.; Hirsch, T.; Goertz, O.; Steinau, H.-U.; Lehnhardt, M.; Harati, K. Long-Term Outcome after Local Recurrence of Soft Tissue Sarcoma: A Retrospective Analysis of Factors Predictive of Survival in 135 Patients with Locally Recurrent Soft Tissue Sarcoma. Br. J. Cancer 2014, 110, 1456–1464. [Google Scholar] [CrossRef]
- Roeder, F.; Krempien, R. Intraoperative Radiation Therapy (IORT) in Soft-Tissue Sarcoma. Radiat. Oncol. 2017, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Guzik, G.; Łyczek, J.; Kowalik, Ł. Surgical Resection with Adjuvant Brachytherapy in Soft Tissue Sarcoma of the Extremity—A Case Report. J. Contemp. Brachyther. 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-Term Results of a Prospective Randomized Trial of Adjuvant Brachytherapy in Soft Tissue Sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef]
- Ren, C.; Shi, R.; Min, L.; Zhang, W.L.; Tu, C.Q.; Duan, H.; Zhang, B.; Xiong, Y. Experience of Interstitial Permanent I125 Brachytherapy for Extremity Soft Tissue Sarcomas. Clin. Oncol. 2014, 26, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, A.; Leung, D.H.Y.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the Prognostic Significance of Microscopic Margins in 2,084 Localized Primary Adult Soft Tissue Sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef]
- Kattan, M.W.; Leung, D.H.Y.; Brennan, M.F. Postoperative Nomogram for 12-Year Sarcoma-Specific Death. JCO 2002, 20, 791–796. [Google Scholar] [CrossRef]
- Mariani, L.; Miceli, R.; Kattan, M.W.; Brennan, M.F.; Colecchia, M.; Fiore, M.; Casali, P.G.; Gronchi, A. Validation and Adaptation of a Nomogram for Predicting the Survival of Patients with Extremity Soft Tissue Sarcoma Using a Three-Grade System. Cancer 2005, 103, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Coindre, J.M.; Terrier, P.; Bui, N.B.; Bonichon, F.; Collin, F.; Le Doussal, V.; Mandard, A.M.; Vilain, M.O.; Jacquemier, J.; Duplay, H.; et al. Prognostic Factors in Adult Patients with Locally Controlled Soft Tissue Sarcoma. A Study of 546 Patients from the French Federation of Cancer Centers Sarcoma Group. JCO 1996, 14, 869–877. [Google Scholar] [CrossRef]
- Laskar, S.; Bahl, G.; Puri, A.; Agarwal, M.G.; Muckaden, M.; Patil, N.; Jambhekar, N.; Gupta, S.; Deshpande, D.D.; Shrivastava, S.K.; et al. Perioperative Interstitial Brachytherapy for Soft Tissue Sarcomas: Prognostic Factors and Long-Term Results of 155 Patients. Ann. Surg. Oncol. 2007, 14, 560–567. [Google Scholar] [CrossRef]
- Gronchi, A.; Casali, P.G.; Mariani, L.; Miceli, R.; Fiore, M.; Lo Vullo, S.; Bertulli, R.; Collini, P.; Lozza, L.; Olmi, P.; et al. Status of Surgical Margins and Prognosis in Adult Soft Tissue Sarcomas of the Extremities: A Series of Patients Treated at a Single Institution. JCO 2005, 23, 96–104. [Google Scholar] [CrossRef]
- Pasquali, S.; Palassini, E.; Stacchiotti, S.; Casali, P.G.; Gronchi, A. Neoadjuvant Treatment: A Novel Standard? Curr. Opin. Oncol. 2017, 29, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Strohmaier, S.; Zwierzchowski, G. Comparison of 60 Co and 192 Ir Sources in HDR Brachytherapy. J. Contemp. Brachytherapy 2011, 4, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Manir, K.S.; Basu, A.; Choudhury, K.B.; Basu, S.; Ghosh, K.; Gangopadhyay, S. Interstitial Brachytherapy in Soft Tissue Sarcoma: A 5 Years Institutional Experience with Cobalt 60-Based High-Dose-Rate Brachytherapy System. J. Contemp. Brachyther. 2018, 10, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Monge, R.; San Julián, M.; Amillo, S.; Cambeiro, M.; Arbea, L.; Valero, J.; González-Cao, M.; Martín-Algarra, S. Perioperative High-Dose-Rate Brachytherapy in Soft Tissue Sarcomas of the Extremity and Superficial Trunk in Adults: Initial Results of a Pilot Study. Brachytherapy 2005, 4, 264–270. [Google Scholar] [CrossRef]
- Schneider, F.; Clausen, S.; Thölking, J.; Wenz, F.; Abo-Madyan, Y. A Novel Approach for Superficial Intraoperative Radiotherapy (IORT) Using a 50 KV X-Ray Source: A Technical and Case Report. J. Appl. Clin. Med. Phys. 2014, 15, 167–176. [Google Scholar] [CrossRef]
- Arifi, S.; Belbaraka, R.; Rahhali, R.; Ismaili, N. Treatment of Adult Soft Tissue Sarcomas: An Overview. Rare Cancers Ther 2015, 3, 69–87. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Mills, E.E.; Hering, E.R. Management of Soft Tissue Tumours by Limited Surgery Combined with Tumour Bed Irradiation Using Brachytherapy and Supplementary Teletherapy. Br. J. Radiol. 1981, 54, 312–317. [Google Scholar] [CrossRef]
- Brennan, M.F.; Hilaris, B.; Shiu, M.H.; Lane, J.; Magill, G.; Friedrich, C.; Hajdu, S.I. Local Recurrence in Adult Soft-Tissue Sarcoma. A Randomized Trial of Brachytherapy. Arch. Surg. 1987, 122, 1289–1293. [Google Scholar] [CrossRef]
- Arbeit, J.M.; Hilaris, B.S.; Brennan, M.F. Wound Complications in the Multimodality Treatment of Extremity and Superficial Truncal Sarcomas. JCO 1987, 5, 480–488. [Google Scholar] [CrossRef]
- Ormsby, M.V.; Hilaris, B.S.; Nori, D.; Brennan, M.F. Wound Complications of Adjuvant Radiation Therapy in Patients with Soft-Tissue Sarcomas. Ann. Surg. 1989, 210, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Nori, D.; Shiu, M.H.; Brennan, M.F. Limb Salvage in Soft Tissue Sarcomas Involving Neurovascular Structures Using Combined Surgical Resection and Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 913–918. [Google Scholar] [CrossRef]
- Nori, D.; Schupak, K.; Shiu, M.H.; Brennan, M.F. Role of Brachytherapy in Recurrent Extremity Sarcoma in Patients Treated with Prior Surgery and Irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 1229–1233. [Google Scholar] [CrossRef]
- Brennan, M.F.; Casper, E.S.; Harrison, L.B.; Shiu, M.H.; Gaynor, J.; Hajdu, S.I. The Role of Multimodality Therapy in Soft-Tissue Sarcoma. Ann. Surg. 1991, 214, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Habrand, J.L.; Gerbaulet, A.; Pejovic, M.H.; Contesso, G.; Durand, S.; Haie, C.; Genin, J.; Schwaab, G.; Flamant, F.; Albano, M. Twenty Years Experience of Interstitial Iridium Brachytherapy in the Management of Soft Tissue Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.B.; Franzese, F.; Gaynor, J.J.; Brennan, M.F. Long-Term Results of a Prospective Randomized Trial of Adjuvant Brachytherapy in the Management of Completely Resected Soft Tissue Sarcomas of the Extremity and Superficial Trunk. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 259–265. [Google Scholar] [CrossRef]
- Pisters, P.W.; Harrison, L.B.; Woodruff, J.M.; Gaynor, J.J.; Brennan, M.F. A Prospective Randomized Trial of Adjuvant Brachytherapy in the Management of Low-Grade Soft Tissue Sarcomas of the Extremity and Superficial Trunk. J. Clin. Oncol. 1994, 12, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Janjan, N.A.; Yasko, A.W.; Reece, G.P.; Miller, M.J.; Murray, J.A.; Ross, M.I.; Romsdahl, M.M.; Oswald, M.J.; Ochran, T.G.; Pollock, R.E. Comparison of Charges Related to Radiotherapy for Soft-Tissue Sarcomas Treated by Preoperative External-Beam Irradiation versus Interstitial Implantation. Ann. Surg. Oncol. 1994, 1, 415–422. [Google Scholar] [CrossRef]
- Catton, C.; Davis, A.; Bell, R.; O’Sullivan, B.; Fornasier, V.; Wunder, J.; McLean, M. Soft Tissue Sarcoma of the Extremity. Limb Salvage after Failure of Combined Conservative Therapy. Radiother. Oncol. 1996, 41, 209–214. [Google Scholar] [CrossRef]
- Alekhteyar, K.M.; Leung, D.H.; Brennan, M.F.; Harrison, L.B. The Effect of Combined External Beam Radiotherapy and Brachytherapy on Local Control and Wound Complications in Patients with High-Grade Soft Tissue Sarcomas of the Extremity with Positive Microscopic Margin. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 321–324. [Google Scholar] [CrossRef]
- Panchal, J.I.; Agrawal, R.K.; McLean, N.R.; Dawes, P.J. Early Post-Operative Brachytherapy and Free Flap Reconstruction in the Management of Sarcomas. Eur. J. Surg. Oncol. 1996, 22, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.J.; Laskar, S.; Badhwar, R. Interstitial Brachytherapy in Soft Tissue Sarcomas. The Tata Memorial Hospital Experience. Strahlenther. Onkol. 1998, 174, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Alektiar, K.M.; Zelefsky, M.J.; Brennan, M.F. Morbidity of Adjuvant Brachytherapy in Soft Tissue Sarcoma of the Extremity and Superficial Trunk. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1273–1279. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Leung, D.; Zelefsky, M.J.; Healey, J.H.; Brennan, M.F. Adjuvant Brachytherapy for Primary High-Grade Soft Tissue Sarcoma of the Extremity. Ann. Surg. Oncol. 2002, 9, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mccarter, M.D.; Jaques, D.P.; Brennan, M.F. Randomized Clinical Trials in Soft Tissue Sarcoma. Surg. Oncol. Clin. N. Am. 2002, 11, 11–22. [Google Scholar] [CrossRef]
- Rachbauer, F.; Sztankay, A.; Kreczy, A.; Sununu, T.; Bach, C.; Nogler, M.; Krismer, M.; Eichberger, P.; Schiestl, B.; Lukas, P. High-Dose-Rate Intraoperative Brachytherapy (IOHDR) Using Flab Technique in the Treatment of Soft Tissue Sarcomas. Strahlenther. Onkol. 2003, 179, 480–485. [Google Scholar] [CrossRef]
- Strander, H.; Turesson, I.; Cavallin-Ståhl, E. A Systematic Overview of Radiation Therapy Effects in Soft Tissue Sarcomas. Acta Oncol. 2003, 42, 516–531. [Google Scholar] [CrossRef]
- Murray, P.M. Soft Tissue Sarcoma of the Upper Extremity. Hand Clin. 2004, 20, 325–333. [Google Scholar] [CrossRef]
- Maples, W.J.; Buskirk, S.J. Multimodality Treatment of Upper Extremity Bone and Soft Tissue Sarcomas. Hand Clin. 2004, 20, 221–225. [Google Scholar] [CrossRef]
- Kretzler, A.; Molls, M.; Gradinger, R.; Lukas, P.; Steinau, H.-U.; Würschmidt, F. Intraoperative Radiotherapy of Soft Tissue Sarcoma of the Extremity. Strahlenther. Onkol. 2004, 180, 365–370. [Google Scholar] [CrossRef]
- Fontanesi, J.; Mott, M.P.; Lucas, D.R.; Miller, P.R.; Kraut, M.J. The Role of Irradiation in the Management of Locally Recurrent Non-Metastatic Soft Tissue Sarcoma of Extremity/Trunkal Locations. Sarcoma 2004, 8, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.G.; Infanger, M.; Reiner, B.; Davis, J.B. A Novel Technique Using Customised Templates for the Application of Fractionated Interstitial HDR Brachytherapy to the Tumour Bed in Soft-Tissue Sarcomas Located in the Extremities. Clin. Oncol. 2004, 16, 457–460. [Google Scholar] [CrossRef]
- Moureau-Zabotto, L.; Thomas, L.; Bui, B.N.; Chevreau, C.; Stockle, E.; Martel, P.; Bonneviale, P.; Marques, B.; Coindre, J.-M.; Kantor, G.; et al. Management of Soft Tissue Sarcomas (STS) in First Isolated Local Recurrence: A Retrospective Study of 83 Cases. Radiother. Oncol. 2004, 73, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, J.; Mott, M.P.; Kraut, M.J.; Lucas, D.P.; Miller, P.R. The Role of Postoperative Irradiation in the Treatment of Locally Recurrent Incompletely Resected Extra-Abdominal Desmoid Tumors. Sarcoma 2004, 8, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, M.S.; Ray, M.E. Adjuvant Therapy for Soft Tissue Sarcoma. J. Natl. Compr. Cancer Netw. 2005, 3, 207–213. [Google Scholar] [CrossRef] [PubMed]
- DeLaney, T.F.; Trofimov, A.V.; Engelsman, M.; Suit, H.D. Advanced-Technology Radiation Therapy in the Management of Bone and Soft Tissue Sarcomas. Cancer Control 2005, 12, 27–35. [Google Scholar] [CrossRef]
- Lazzaro, G.; Lazzari, R.; Pelosi, G.; De Pas, T.; Mariani, L.; Mazzarol, G.; Sances, D.; Tosti, G.; Baldini, F.; Mosconi, M.; et al. Pulsed Dose-Rate Perioperative Interstitial Brachytherapy for Soft Tissue Sarcomas of the Extremities and Skeletal Muscles of the Trunk. Ann. Surg. Oncol. 2005, 12, 935–942. [Google Scholar] [CrossRef]
- Aronowitz, J.N.; Pohar, S.S.; Liu, L.; Haq, R.; Damron, T.A. Adjuvant High Dose Rate Brachytherapy in the Management of Soft Tissue Sarcoma: A Dose-Toxicity Analysis. Am. J. Clin. Oncol. 2006, 29, 508–513. [Google Scholar] [CrossRef]
- Mierzwa, L.M.; McCluskey, C.M.; Barrett, W.L.; Lowy, A.; Sussman, J.; Sorger, J. Interstitial Brachytherapy for Soft Tissue Sarcoma: A Single Institution Experience. Brachytherapy 2007, 6, 298–303. [Google Scholar] [CrossRef]
- Torres, M.A.; Ballo, M.T.; Butler, C.E.; Feig, B.W.; Cormier, J.N.; Lewis, V.O.; Pollock, R.E.; Pisters, P.W.; Zagars, G.K. Management of Locally Recurrent Soft-Tissue Sarcoma after Prior Surgery and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1124–1129. [Google Scholar] [CrossRef]
- Pohar, S.; Haq, R.; Liu, L.; Koniarczyk, M.; Hahn, S.; Damron, T.; Aronowitz, J.N. Adjuvant High-Dose-Rate and Low-Dose-Rate Brachytherapy with External Beam Radiation in Soft Tissue Sarcoma: A Comparison of Outcomes. Brachytherapy 2007, 6, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Muhic, A.; Hovgaard, D.; Mørk Petersen, M.; Daugaard, S.; Højlund Bech, B.; Roed, H.; Kjaer-Kristoffersen, F.; Aage Engelholm, S. Local Control and Survival in Patients with Soft Tissue Sarcomas Treated with Limb Sparing Surgery in Combination with Interstitial Brachytherapy and External Radiation. Radiother. Oncol. 2008, 88, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, A.; Citrin, D. The Role of Radiation Therapy in the Management of Sarcomas. Surg. Clin. N. Am. 2008, 88, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Rimner, A.; Brennan, M.F.; Zhang, Z.; Singer, S.; Alektiar, K.M. Influence of Compartmental Involvement on the Patterns of Morbidity in Soft Tissue Sarcoma of the Thigh. Cancer 2009, 115, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Rudert, M.; Burgkart, R.; Gradinger, R.; Rechl, H. [Surgical treatment of musculoskeletal soft tissue sarcomas]. Chirurg 2009, 80, 194–201. [Google Scholar] [CrossRef]
- Petera, J.; Soumarová, R.; Růzicková, J.; Neumanová, R.; Dusek, L.; Sirák, I.; Macingová, Z.; Paluska, P.; Kasaová, L.; Hodek, M.; et al. Perioperative Hyperfractionated High-Dose Rate Brachytherapy for the Treatment of Soft Tissue Sarcomas: Multicentric Experience. Ann. Surg. Oncol. 2010, 17, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.K.; Deo, S.V.S. Soft Tissue Sarcoma—Review of Experience at a Tertiary Care Cancer Centre. Indian J. Surg. Oncol. 2011, 2, 309–312. [Google Scholar] [CrossRef]
- Bradley, J.A.; Kleinman, S.H.; Rownd, J.; King, D.; Hackbarth, D.; Whitfield, R.; Wang, D. Adjuvant High Dose Rate Brachytherapy for Soft Tissue Sarcomas: Initial Experience Report. J. Contemp. Brachyther. 2011, 3, 3–10. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Brennan, M.F.; Singer, S. Local Control Comparison of Adjuvant Brachytherapy to Intensity-Modulated Radiotherapy in Primary High-Grade Sarcoma of the Extremity. Cancer 2011, 117, 3229–3234. [Google Scholar] [CrossRef]
- Atean, I.; Pointreau, Y.; Rosset, P.; Garaud, P.; De-Pinieux, G.; Calais, G. Prognostic Factors of Extremity Soft Tissue Sarcoma in Adults. A Single Institutional Analysis. Cancer Radiother. 2012, 16, 661–666. [Google Scholar] [CrossRef]
- Emory, C.L.; Montgomery, C.O.; Potter, B.K.; Keisch, M.E.; Conway, S.A. Early Complications of High-Dose-Rate Brachytherapy in Soft Tissue Sarcoma: A Comparison with Traditional External-Beam Radiotherapy. Clin. Orthop. Relat. Res. 2012, 470, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Delaney, T.F. Radiation Therapy: Neoadjuvant, Adjuvant, or Not at All. Surg. Oncol. Clin. N. Am. 2012, 21, 215–241. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, M.P.H.; Rehders, A.; Knoefel, W.T. [Multimodal management in soft tissue sarcoma of the trunk and extremities]. Chirurg 2014, 85, 378–382. [Google Scholar] [CrossRef]
- Miller, E.D.; Xu-Welliver, M.; Haglund, K.E. The Role of Modern Radiation Therapy in the Management of Extremity Sarcomas. J. Surg. Oncol. 2015, 111, 599–603. [Google Scholar] [CrossRef]
- Röper, B.; Heinrich, C.; Kehl, V.; Rechl, H.; Specht, K.; Wörtler, K.; Töpfer, A.; Molls, M.; Kampfer, S.; von Eisenharth-Rothe, R.; et al. Study of Preoperative Radiotherapy for Sarcomas of the Extremities with Intensity-Modulation, Image-Guidance and Small Safety-Margins (PREMISS). BMC Cancer 2015, 15, 904. [Google Scholar] [CrossRef]
- Larrier, N.A.; Czito, B.G.; Kirsch, D.G. Radiation Therapy for Soft Tissue Sarcoma: Indications and Controversies for Neoadjuvant Therapy, Adjuvant Therapy, Intraoperative Radiation Therapy, and Brachytherapy. Surg. Oncol. Clin. N. Am. 2016, 25, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, A.O.; Gonzalez, R.J.; Scott, J.G.; Mullinax, J.E.; Abuodeh, Y.A.; Kim, Y.; Binitie, O.; Ahmed, A.K.; Bui, M.M.; Saini, A.S.; et al. Implications of Staged Reconstruction and Adjuvant Brachytherapy in the Treatment of Recurrent Soft Tissue Sarcoma. Brachytherapy 2016, 15, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Sinnatamby, M. Malignant Soft Tissue Sarcoma of the Shoulder Treated by Surface Mould Brachytherapy Boost in an Adjuvant Setting. J. Contemp. Brachyther. 2017, 9, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, A.; Galuppi, A.; Frakulli, R.; Arcelli, A.; Romani, F.; Mattiucci, G.C.; Bianchi, G.; Ferrari, S.; Ferraro, A.; Farioli, A.; et al. Adjuvant Radiotherapy with Brachytherapy Boost in Soft Tissue Sarcomas. J. Contemp. Brachyther. 2017, 9, 256–262. [Google Scholar] [CrossRef]
- Klein, J.; Ghasem, A.; Huntley, S.; Donaldson, N.; Keisch, M.; Conway, S. Does an Algorithmic Approach to Using Brachytherapy and External Beam Radiation Result in Good Function, Local Control Rates, and Low Morbidity in Patients with Extremity Soft Tissue Sarcoma? Clin. Orthop. Relat. Res. 2018, 476, 634–644. [Google Scholar] [CrossRef]
- Healey, J.H. CORR Insights®: Does an Algorithmic Approach to Using Brachytherapy and External Beam Radiation Result in Good Function, Local Control Rates, and Low Morbidity in Patients with Extremity Soft Tissue Sarcoma? Clin. Orthop. Relat. Res. 2018, 476, 645–647. [Google Scholar] [CrossRef]
- Gimeno, M.; San Julián, M.; Cambeiro, M.; Arbea, L.; Jablonska, P.; Moreno-Jiménez, M.; Amillo, S.; Aristu, J.; Lecanda, F.; Martinez-Monge, R. Long-Term Results of Perioperative High Dose Rate Brachytherapy (PHDRB) and External Beam Radiation in Adult Patients with Soft Tissue Sarcomas of the Extremities and the Superficial Trunk: Final Results of a Prospective Controlled Study. Radiother. Oncol. 2019, 135, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Spoto, R.; Vavassori, A.; Dicuonzo, S.; Pepa, M.; Volpe, S.; Alessandro, O.; Gandini, S.; Di Venosa, B.; Miglietta, E.; Fodor, C.; et al. Adjuvant Radiotherapy Treatment for Soft Tissue Sarcoma of Extremities and Trunk. A Retrospective Mono-Institutional Analysis. Neoplasma 2020, 67, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F. Radiation Therapy in Adult Soft Tissue Sarcoma—Current Knowledge and Future Directions: A Review and Expert Opinion. Cancers 2020, 12, 3242. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, A.; Pennacchioli, E.; Augugliaro, M.; Durante, S.; Dicuonzo, S.; Orsolini, G.M.; Prestianni, P.; Cambria, R.; Comi, S.; Mazzarol, G.; et al. Adjuvant High-Dose-Rate Interstitial Brachytherapy for Malignant Peripheral Nerve Sheath Tumor of the Foot: A Case Report. J. Contemp. Brachyther. 2021, 13, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Lawrenz, J.M.; Johnson, S.R.; Zhu, K.; McKeon, M.; Moran, C.P.; Vega, J.; Hajdu, K.S.; Norris, J.P.; Luo, L.Y.; Shinohara, E.T.; et al. Adjuvant Radiation after Primary Resection of Atypical Lipomatous Tumors of the Extremity Reduces Local Recurrence but Increases Complications: A Multicenter Evaluation. Sarcoma 2022, 2022, 2091677. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F.; Lehner, B.; Saleh-Ebrahimi, L.; Hensley, F.W.; Ulrich, A.; Alldinger, I.; Mechtersheimer, G.; Huber, P.E.; Krempien, R.; Bischof, M.; et al. Intraoperative Electron Radiation Therapy Combined with External Beam Radiation Therapy and Limb Sparing Surgery in Extremity Soft Tissue Sarcoma: A Retrospective Single Center Analysis of 183 Cases. Radiother. Oncol. 2016, 119, 22–29. [Google Scholar] [CrossRef]
- Alektiar, K.M.; Hong, L.; Brennan, M.F.; Della-Biancia, C.; Singer, S. Intensity Modulated Radiation Therapy for Primary Soft Tissue Sarcoma of the Extremity: Preliminary Results. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 458–464. [Google Scholar] [CrossRef]
- Casali, P.G.; Jost, L.; Sleijfer, S.; Verweij, J.; Blay, J.-Y. Soft Tissue Sarcomas: ESMO Clinical Recommendations for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2008, 19, ii89–ii93. [Google Scholar] [CrossRef]
- Adjuvant Chemotherapy for Localised Resectable Soft-Tissue Sarcoma of Adults: Meta-Analysis of Individual Data. Lancet 1997, 350, 1647–1654. [CrossRef]
- Abarca, T.; Gao, Y.; Monga, V.; Tanas, M.R.; Milhem, M.M.; Miller, B.J. Improved Survival for Extremity Soft Tissue Sarcoma Treated in High-Volume Facilities. J. Surg. Oncol. 2018, 117, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
| ID | Study | Year | Region | Country | Sample Size | Follow-Up | Treatment | Study Type | LoE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mills et al. [29] | 1981 | Africa | South Africa | 17 | 28 months | HD-BRT | Retrospective study | 3 |
| 2 | Brennan et al. [30] | 1987 | North America | USA | 117 | 16 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 3 | Arbeit et al. [31] | 1987 | North America | USA | 105 | 11.9 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 4 | Ormsby et al. [32] | 1989 | North America | USA | 52 | 3 months | BRT vs. No BRT | Retrospective study | 3 |
| 5 | Zelefsky et al. [33] | 1990 | North America | USA | 45 | 4 years | BRT | Retrospective study | 3 |
| 6 | Nori et al. [34] | 1991 | North America | USA | 40 | 36 months | BRT | Retrospective study | 3 |
| 7 | Brennan et al. [35] | 1991 | North America | USA | 126 | 40.8 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 8 | Habrand et al. [36] | 1991 | Europe | France | 48 | 82 months | BRT | Retrospective study | 3 |
| 9 | Harrison et al. [37] | 1993 | North America | USA | 126 | 66.5 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 10 | Pisters et al. [38] | 1994 | North America | USA | 45 | 67 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 11 | Janjan et al. [39] | 1994 | North America | USA | 35 | n.a. | BRT vs. EBRT | Comparative study | 3 |
| 12 | Catton et al. [40] | 1996 | North America | Canada | 25 | 24 months | BRT or EBRT or BRT + EBRT vs. Surgery alone | Retrospective study | 3 |
| 13 | Alekhteyar et al. [41] | 1996 | North America | USA | 105 | 22 months | BRT vs. BRT + EBRT | Retrospective study | 3 |
| 14 | Pisters et al. [14] | 1996 | North America | USA | 164 | 76 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 15 | Panchal et al. [42] | 1996 | Europe | United Kingdom | 4 | 27.5 months | Surgery + BRT | Retrospective study | 3 |
| 16 | Chaudhary et al. [43] | 1998 | Asia | India | 151 | 24 months | BRT vs. BRT + EBRT | Comparative study | 3 |
| 17 | Alektiar et al. [44] | 2000 | North America | USA | 164 | 100 months | BRT vs. No BRT | Prospective randomized trial | 2 |
| 18 | Alektiar et al. [45] | 2002 | North America | USA | 202 | 61 months | BRT | Retrospective study | 3 |
| 19 | Mccarter et al. [46] | 2002 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 20 | Ballo et al. [3] | 2003 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 21 | Rachbauer et al. [47] | 2003 | Europe | Austria | 39 | 26 months | HD-BRT + EBRT | Prospective study | 2 |
| 22 | Strander et al. [48] | 2003 | Europe | Sweden | n.a. | n.a. | n.a. | Review | 5 |
| 23 | Murray et al. [49] | 2004 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 24 | Maples et al. [50] | 2004 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 25 | Kretzler et al. [51] | 2004 | Europe | Germany | 28 | 4.3 years | BRT ± EBRT | Retrospective study | 3 |
| 26 | Fontanesi et al. [52] | 2004 | North America | USA | 31 | 60.5 months | Surgery ± BRT ± EBRT | Retrospective study | 3 |
| 27 | Baumert et al. [53] | 2004 | Europe | Switzerland | 1 | n.a. | BRT | Case report | 4 |
| 28 | Moureau-Zabotto et al. [54] | 2004 | Europe | France | 83 | 13 years | Surgery ± BRT ± EBRT | Retrospective study | 3 |
| 29 | Fontanesi et al. [55] | 2004 | North America | USA | 13 | 76 months | Surgery ± BRT ± EBRT | Retrospective study | 3 |
| 30 | Schuetze et al. [56] | 2005 | North America | USA | n.a | n.a. | n.a. | Review | 5 |
| 31 | DeLaney et al. [57] | 2005 | North America | USA | n.a | n.a. | n.a. | Review | 5 |
| 32 | Martínez-Monge et al. [25] | 2005 | Europe | Spain | 25 | 23.2 months | HD-BRT + EBRT | Retrospective study | 3 |
| 33 | Lazzaro et al. [58] | 2005 | Europe | Italy | 42 | 34 months | BRT ± EBRT | Retrospective study | 3 |
| 34 | Aronowitz et al. [59] | 2006 | North America | USA | 12 | 34 months | HD-BRT | Retrospective study | 3 |
| 35 | Mierzwa et al. [60] | 2007 | North America | USA | 43 | 39 months | BRT ± EBRT | Retrospective study | 3 |
| 36 | Torres et al. [61] | 2007 | North America | USA | 62 | 6 years | BRT vs. No BRT | Retrospective study | 3 |
| 37 | Laskar et al. [20] | 2007 | Asia | India | 155 | 45 months | BRT ± EBRT | Retrospective study | 3 |
| 38 | Pohar et al. [62] | 2007 | North America | USA | 37 | 47 vs. 17 months | LD-BRT + EBRT vs. HD-BRT + EBRT | Retrospective study | 3 |
| 39 | Beltrami et al. [5] | 2008 | Europe | Italy | 112 | 75 months | BRT + EBRT | Retrospective study | 3 |
| 40 | Muhic et al. [63] | 2008 | Europe | Denmark | 39 | 3.4 years | PDR-BRT + EBRT | Retrospective study | 3 |
| 41 | Kaushal et al. [64] | 2008 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 42 | Rimner et al. [65] | 2009 | North America | USA | 255 | 71 months | BRT or EBRT or BRT + EBRT | Retrospective study | 3 |
| 43 | Rudert et al. [66] | 2009 | Europe | Germany | n.a. | n.a. | n.a. | Review | 5 |
| 44 | Petera et al. [67] | 2010 | Europe | Czech Republic | 45 | 3.2 years | BRT ± EBRT | Retrospective study | 3 |
| 45 | Shukla et al. [68] | 2011 | Asia | India | 300 | n.a. | BRT ± EBRT | Retrospective study | 3 |
| 46 | Bradley et al. [69] | 2011 | North America | USA | 11 | 20.8 months | HD-BRT | Retrospective study | 3 |
| 47 | Alektiar et al. [70] | 2011 | North America | USA | 134 | 46 months | LD-BRT or IMRT | Retrospective study | 3 |
| 48 | Atean et al. [71] | 2012 | Europe | France | 87 | 69 months | EBRT vs. EBRT + BRT | Retrospective study | 3 |
| 49 | Guzik et al. [13] | 2012 | Europe | Poland | 1 | n.a. | BRT | Case report | 4 |
| 50 | Emory et al. [72] | 2012 | North America | USA | 190 | 40 months | EBRT or BRT or BRT + EBRT | Retrospective study | 3 |
| 51 | Delaney et al. [73] | 2012 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 52 | Ghadimi et al. [74] | 2014 | Europe | Germany | n.a. | n.a. | n.a. | Review | 5 |
| 53 | Pellizzon et al. [6] | 2014 | South America | Brazil | n.a. | n.a. | n.a. | Review | 5 |
| 54 | Ren et al. [15] | 2014 | Asia | China | 110 | 43.7 months | BRT | Retrospective study | 3 |
| 55 | Miller et al. [75] | 2015 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 56 | Röper et al. [76] | 2015 | Europe | Germany | n.a | n.a | n.a | Prospective study | 3 |
| 57 | Larrier et al. [77] | 2016 | North America | USA | n.a. | n.a | n.a | Review | 5 |
| 58 | Naghavi et al. [78] | 2016 | North America | USA | 40 | 27 months | BRT | Retrospective study | 3 |
| 59 | Mukherji et al. [79] | 2017 | Asia | India | 3 | 34 months | BRT | Case report | 4 |
| 60 | Naghavi et al. [4] | 2017 | North America | USA | n.a. | n.a. | n.a. | Review | 5 |
| 61 | Cortesi et al. [80] | 2017 | Europe | Italy | 107 | 100 months | BRT + EBRT | Retrospective study | 3 |
| 62 | Correa et al. [1] | 2018 | Europe | Spain | n.a. | n.a. | n.a. | Review | 5 |
| 63 | Klein et al. [81] | 2018 | North America | USA | 171 | 71.8 months | HD-BRT or EBRT or HD-BRT + EBRT | Retrospective study | 3 |
| 64 | Healey et al. [82] | 2018 | North America | USA | n.a. | n.a. | n.a. | Expert opinion | 7 |
| 65 | Manir et al. [24] | 2018 | Asia | India | 27 | 20 months | BRT ± EBRT | Retrospective study | 3 |
| 66 | Gimeno et al. [83] | 2019 | Europe | Spain | 106 | 7.1 years | HD-BRT + EBRT | Prospective controlled study | 2 |
| 67 | Spoto et al. [84] | 2020 | Europe | Italy | 90 | 4.2 years | BRT vs. EBRT vs. BRT + EBRT | Retrospective study | 3 |
| 68 | Roeder et al. [85] | 2020 | Europe | Austria | n.a. | n.a. | n.a. | Review | 5 |
| 69 | Sarria et al. [8] | 2020 | Europe | Germany | 31 | 4.9 years | BRT | Retrospective study | 3 |
| 70 | Vavassori et al. [86] | 2021 | Europe | Italy | 1 | 40 months | HD-BRT | Case report | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neugebauer, J.; Blum, P.; Keiler, A.; Süß, M.; Neubauer, M.; Moser, L.; Dammerer, D. Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities—A Current Concept and Systematic Review of the Literature. Cancers 2023, 15, 1133. https://doi.org/10.3390/cancers15041133
Neugebauer J, Blum P, Keiler A, Süß M, Neubauer M, Moser L, Dammerer D. Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities—A Current Concept and Systematic Review of the Literature. Cancers. 2023; 15(4):1133. https://doi.org/10.3390/cancers15041133
Chicago/Turabian StyleNeugebauer, Johannes, Philipp Blum, Alexander Keiler, Markus Süß, Markus Neubauer, Lukas Moser, and Dietmar Dammerer. 2023. "Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities—A Current Concept and Systematic Review of the Literature" Cancers 15, no. 4: 1133. https://doi.org/10.3390/cancers15041133
APA StyleNeugebauer, J., Blum, P., Keiler, A., Süß, M., Neubauer, M., Moser, L., & Dammerer, D. (2023). Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities—A Current Concept and Systematic Review of the Literature. Cancers, 15(4), 1133. https://doi.org/10.3390/cancers15041133





