Chimp Optimization Algorithm Influenced Type-2 Intuitionistic Fuzzy C-Means Clustering-Based Breast Cancer Detection System
Abstract
:Simple Summary
Abstract
1. Introduction
2. Related Works
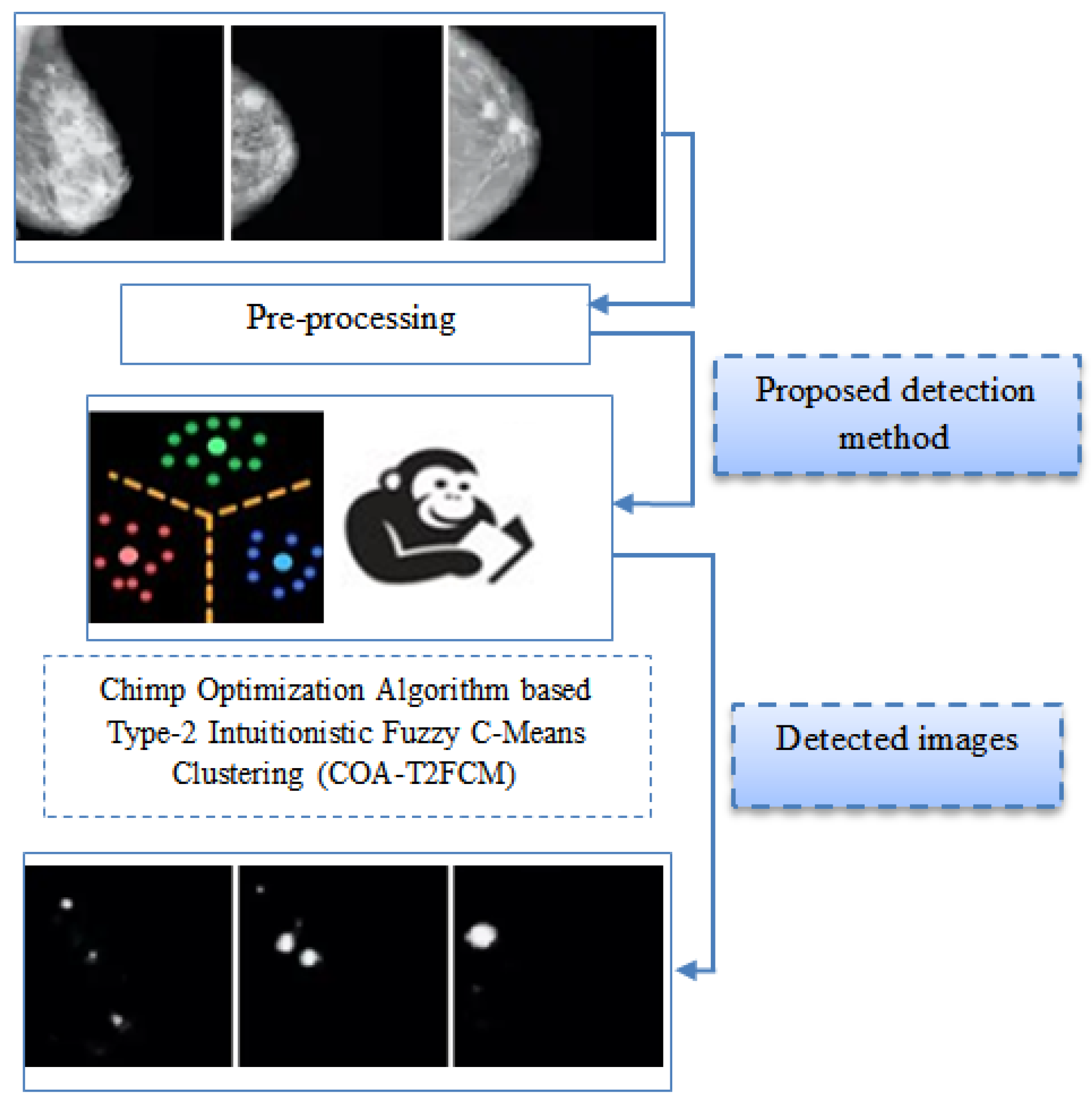
- The proposed detection method incorporates type-2 intuitionistic fuzzy c-means clustering for clustering suspicious region from the mammogram. Initially, the type-2 intuitionistic fuzzy c-means clustering objective function is considered with the consideration of intuitionistic fuzzy data extracted from the MRI images.
- The chimp optimization approach can be used to identify the most efficient cluster center in type-2 intuitionistic fuzzy c-means clustering. Chimp optimization algorithm is utilized to optimize the cluster center and fuzzifier from the clustering method.
- The projected technique is executed in Python to evaluate the performance and similarity measure of the proposed system. The projected technique is compared with the conventional techniques such as fuzzy c means clustering and k mean clustering methods.
3. Proposed System Model
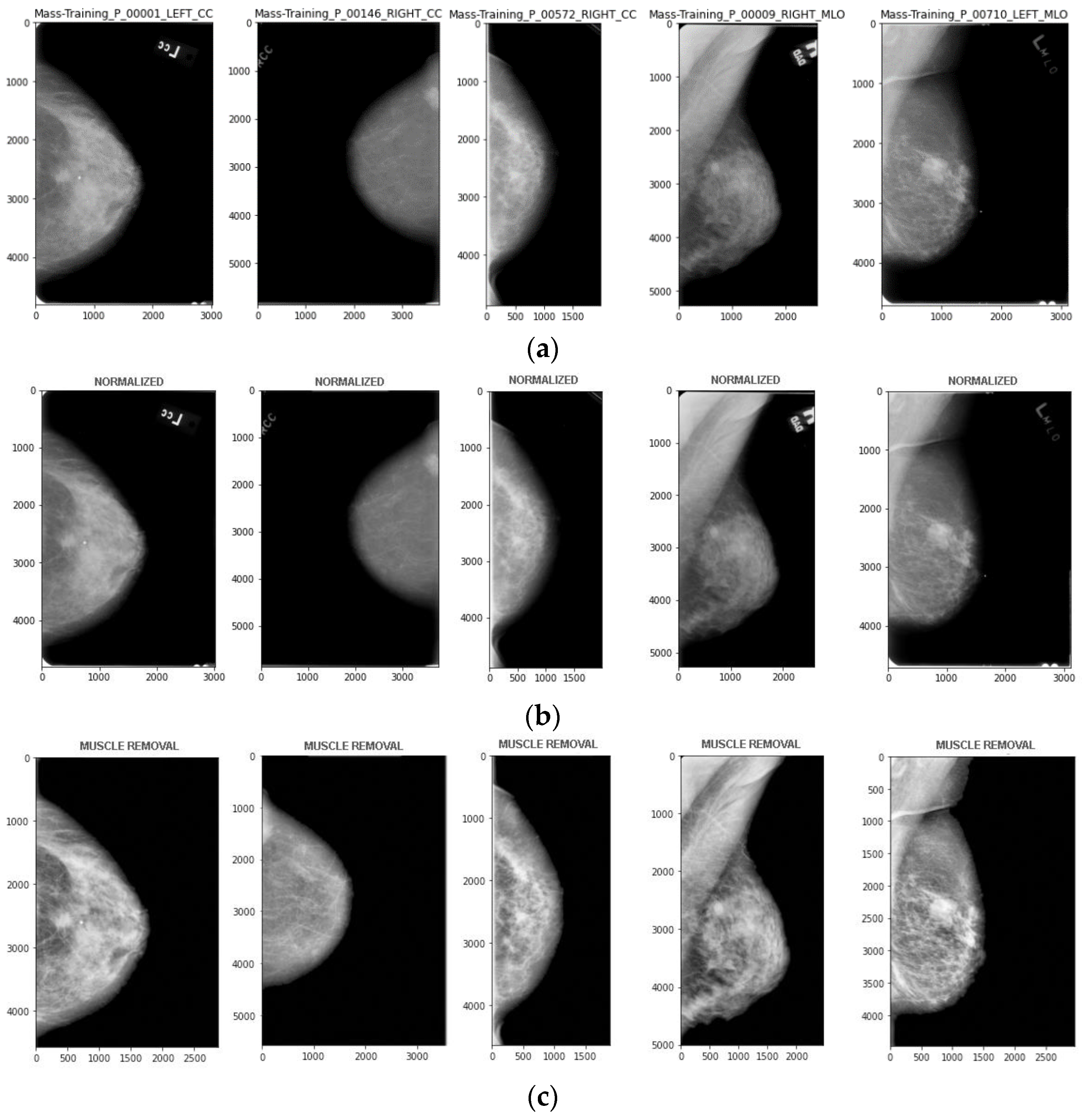
3.1. Stages of Pre-Processing
- (a)
- Intensity normalization
- (b)
- Pectoral Muscle removal
3.2. Intuitionistic Fuzzy C-Means Clustering of Type2
3.3. Chimp Optimization Algorithm
- Inspiration
- Track and dynamic the prey
- Investigation phase
- Development phase
- A phase of exploitation with the help of a social incentive
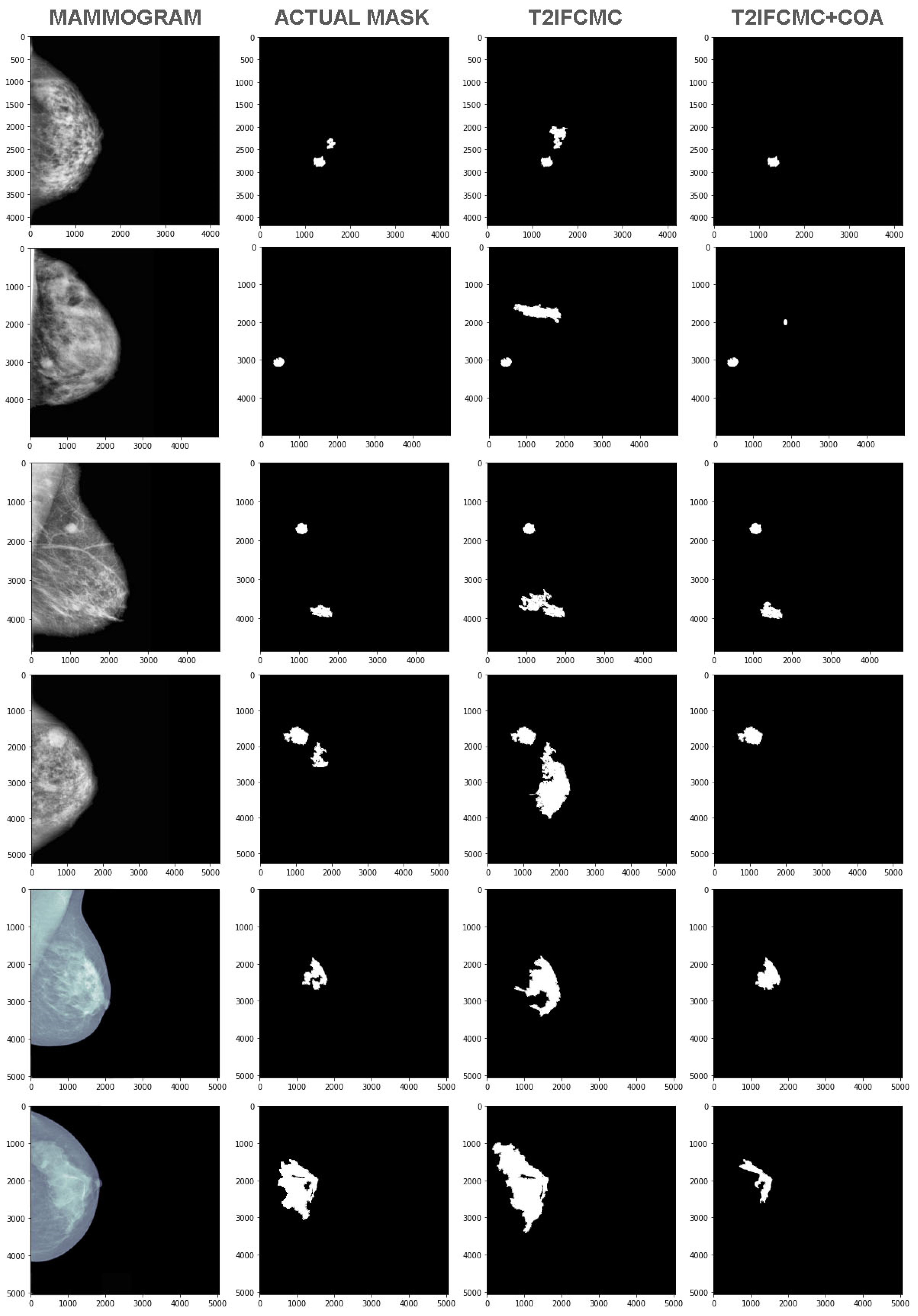
4. Results and Discussion
- The malignant regions are introduced and shown as detected which is termed as False Negative (FN).
- An actual malignant region is not detected, which is called a False Positive (FP).
- An actual malignant region is not detected, which is called an undiagnosed true negative (TN).
- An actual malignant region is detected as occurred and called as True Positive (TP).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elawady, I.; Bensaada, A.K.; Litim, E.M. Breast Cancer Detection Using Deep Convolutional Neural Networks and Raspberry Pi 3 Board. In Advances in Deep Learning, Artificial Intelligence and Robotics; Springer: Cham, Switzerland, 2022; pp. 99–110. [Google Scholar]
- Pantanowitz, L.; Hartman, D.; Qi, Y.; Cho, E.Y.; Suh, B.; Paeng, K.; Dhir, R.; Michelow, P.; Hazelhurst, S.; Song, S.Y.; et al. Accuracy and efficiency of an artificial intelligence tool when counting breast mitoses. Diagn. Pathol. 2020, 15, 80. [Google Scholar] [PubMed]
- Kumari, L.K.; Jagadesh, B.N. A Robust Feature Extraction Technique for Breast Cancer Detection using Digital Mammograms based on Advanced GLCM Approach. Pervasive Health Technol. 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Shoshan, Y.; Bakalo, R.; Gilboa-Solomon, F.; Ratner, V.; Barkan, E.; Ozery-Flato, M.; Amit, M.; Khapun, D.; Ambinder, E.B.; Oluyemi, E.T.; et al. Artificial Intelligence for Reducing Workload in Breast Cancer Screening with Digital Breast Tomosynthesis. Radiology 2022, 303, 69–77. [Google Scholar] [PubMed]
- Onega, T.; Zhu, W.; Kerlikowske, K.; Miglioretti, D.L.; Lee, C.I.; Henderson, L.M.; Tosteson, A.N.A.; Wernli, K.J.; diFlorio, R.; Weaver, D.L.; et al. Preoperative MRI in breast cancer: Effect of breast density on biopsy rate and yield. Breast Cancer Res. Treat. 2022, 191, 177–190. [Google Scholar]
- Hadadi, I.; Rae, W.; Clarke, J.; McEntee, M.; Ekpo, E. Breast cancer detection across dense and non-dense breasts: Markers of diagnostic confidence and efficacy. Acta Radiol. Open 2022, 11, 20584601211072279. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Loke, S.Y.; Tang, Y.C.; Too, H.P.; Zhou, L.; Lee, A.S.; Hartman, M. Development and validation of a circulating microRNA panel for the early detection of breast cancer. Br. J. Cancer 2022, 126, 472–481. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, M. Breast Cancer Detection Based on Feature Selection Using Enhanced Grey Wolf Optimizer and Support Vector Machine Algorithms. Vietnam. J. Comput. Sci. 2021, 8, 177–197. [Google Scholar]
- Rana, P.; Gupta, P.K.; Sharma, V. A Novel Deep Learning-based Whale Optimization Algorithm for Prediction of Breast Cancer. Braz. Arch. Biol. Technol. 2021, 64, 1–16. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Wylie, E.; Lotter, W.; Pearce, A.; Carter, S.M.; Lund, H.; Waddell, A.; Kim, J.G.; Pereira, G.F.; Lee, C.I. Artificial intelligence (AI) to enhance breast cancer screening: Protocol for population-based cohort study of cancer detection. BMJ Open 2022, 12, e054005. [Google Scholar] [CrossRef]
- Alruwaili, M.; Gouda, W. Automated Breast Cancer Detection Models Based on Transfer Learning. Sensors 2022, 22, 876. [Google Scholar] [CrossRef]
- Adachi, M.; Nakagawa, T.; Fujioka, T.; Mori, M.; Kubota, K.; Oda, G.; Kikkawa, T. Feasibility of Portable Microwave Imaging Device for Breast Cancer Detection. Diagnostics 2022, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Rashed, E.A.; Gaber, T.; Karam, O. Deep learning model for fully automated breast cancer detection system from thermograms. PLoS ONE 2022, 17, e0262349. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, L.; Ali Asgharzadeholiaee, N. A Homogeneous Ensemble Classifier for Breast Cancer Detection Using Parameters Tuning of MLP Neural Network. Appl. Artif. Intell. 2022, 36, 2031820. [Google Scholar] [CrossRef]
- Escorcia-Gutierrez, J.; Mansour, R.F.; Beleño, K.; Jiménez-Cabas, J.; Pérez, M.; Madera, N.; Velasquez, K. Automated Deep Learning Empowered Breast Cancer Diagnosis Using Biomedical Mammogram Images. Comput. Mater. Contin. 2022, 71, 3–4221. [Google Scholar] [CrossRef]
- Gamarra, M.; Manjarres, Y.; Torres, M.T.; Escorcia-Gutierrez, J.; Zurek, E. C-Kmeans: An Approach to Cell Image Segmentation Using Clustering Algorithms. Int. J. Artif. Intell. 2021, 19, 2021. [Google Scholar]
- Rouhi, R.; Jafari, M.; Kasaei, S.; Keshavarzian, P. Benign and malignant breast tumors classification based on region growing and CNN segmentation. Expert Syst. Appl. 2015, 42, 990–1002. [Google Scholar] [CrossRef]
- Setiawan, A.S.; Wesley, E.J.; Purnama, Y. Mammogram Classification using Law’s Texture Energy Measure and Neural Networks. Procedia Comput. Sci. 2015, 59, 92–97. [Google Scholar] [CrossRef]
- Kashyap, K.L.; Bajpai, M.K.; Khanna, P. Breast cancer detection in digital mammograms. In Proceedings of the 2015 IEEE International Conference on Imaging Systems and Techniques (IST), Macau, China, 16–18 September 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Patel, B.C.; Sinha, G.R. Mammography Feature Analysis and Mass Detection in Breast Cancer Images. In Proceedings of the 2014 International Conference on Electronic Systems, Signal Processing and Computing Technologies, Nagpur, India, 9–11 January 2014; pp. 474–478. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Ma, Y. Breast mass classification in digital mammography based on extreme learning machine. Neurocomputing 2016, 173, 930–941. [Google Scholar] [CrossRef]
- Mini-MIAS Database. Available online: https://www.kaggle.com/datasets/kmader/mias-mammography (accessed on 15 August 2022).
- DDIS Database. Available online: http://www.eng.usf.edu/cvprg/mammography/database.html (accessed on 15 August 2022).
- Moreira, I.C.; Amaral, I.; Domingues, I.; Cardoso, A.; Cardoso, M.J.; Cardoso, J.S. INbreast: Toward a full-field digital mammographic database. Acad Radiol. 2012, 19, 236–248. [Google Scholar] [CrossRef]
- Dey, S.; Roychoudhury, R.; Malakar, S.; Sarkar, R. Screening of breast cancer from thermogram images by edge detection aided deep transfer learning model. Multimed. Tools Appl. 2022, 81, 9331–9349. [Google Scholar]
- Nguyen, D.D.; Ngo, L.T.; Pham, L.T. Interval type-2 fuzzy c-means clustering using intuitionistic fuzzy sets. In Proceedings of the 2013 Third World Congress on Information and Communication Technologies (WICT 2013), Hanoi, Vietnam, 15–18 December 2013; pp. 299–304. [Google Scholar] [CrossRef]
- Al Husaini, M.A.S.; Habaebi, M.H.; Gunawan, T.S.; Islam, M.R.; Elsheikh, E.A.; Suliman, F.M. Thermal-based early breast cancer detection using inception V3, inception V4 and modified inception MV4. Neural Comput. Appl. 2022, 34, 333–348. [Google Scholar] [PubMed]
- Parvathavarthini, S.; Shanthi, S. Breast Cancer Detection using Crow Search Optimization based Intuitionistic Fuzzy Clustering with Neighborhood Attraction. Asian Pac. J. Cancer Prev. 2019, 25, 157–165. [Google Scholar] [CrossRef]
- Mathew, T.; Ajith, B.; Kini, J.R.; Rajan, J. Deep learning-based automated mitosis detection in histopathology images for breast cancer grading. Int. J. Imaging Syst. Technol. 2022, 32, 1192–1208. [Google Scholar] [CrossRef]

| Reference | ML/DL Methods | Database | Accuracy |
|---|---|---|---|
| [17] | Random forest, NB, SVM, and KNN | MIAS and DDSM | 96.47% |
| [18] | Fast Forward Neural Network | MIAS | 93.90% |
| [19] | SVM using (RBFK) | mini-MIAS | 96.92% |
| [20] | Multilayer perceptron neural network | DDSM | 95.60% |
| [21] | ELM and SVM | mini-MIAS + DDSM | 96.02% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaji, P.; Muniasamy, V.; Bilfaqih, S.M.; Muniasamy, A.; Tharanidharan, S.; Mani, D.; Alsid, L.E.G. Chimp Optimization Algorithm Influenced Type-2 Intuitionistic Fuzzy C-Means Clustering-Based Breast Cancer Detection System. Cancers 2023, 15, 1131. https://doi.org/10.3390/cancers15041131
Balaji P, Muniasamy V, Bilfaqih SM, Muniasamy A, Tharanidharan S, Mani D, Alsid LEG. Chimp Optimization Algorithm Influenced Type-2 Intuitionistic Fuzzy C-Means Clustering-Based Breast Cancer Detection System. Cancers. 2023; 15(4):1131. https://doi.org/10.3390/cancers15041131
Chicago/Turabian StyleBalaji, Prasanalakshmi, Vasanthi Muniasamy, Syeda Meraj Bilfaqih, Anandhavalli Muniasamy, Sridevi Tharanidharan, Devi Mani, and Linda Elzubir Gasm Alsid. 2023. "Chimp Optimization Algorithm Influenced Type-2 Intuitionistic Fuzzy C-Means Clustering-Based Breast Cancer Detection System" Cancers 15, no. 4: 1131. https://doi.org/10.3390/cancers15041131
APA StyleBalaji, P., Muniasamy, V., Bilfaqih, S. M., Muniasamy, A., Tharanidharan, S., Mani, D., & Alsid, L. E. G. (2023). Chimp Optimization Algorithm Influenced Type-2 Intuitionistic Fuzzy C-Means Clustering-Based Breast Cancer Detection System. Cancers, 15(4), 1131. https://doi.org/10.3390/cancers15041131







