Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell lines
2.3. Analysis of CEA Expression on the Surface
2.4. Western Blot Analysis
2.5. Enzyme Linked Immunosorbent Assay (ELISA)
2.6. Flow Cytometry
2.7. Vector Construction and Preparation of Virus Solutions
2.8. CAR-T Cell Production
2.9. CAR-T Cell Sorting
2.10. Cytotoxicity Assay
2.11. Orthotopic Pancreatic Ductal Adenocarcinoma Xenograft Models
2.12. In Vivo CAR-T Cell Treatment
2.13. Patients
2.14. Immunohistochemical Staining
2.15. Statistical Analysis
3. Results
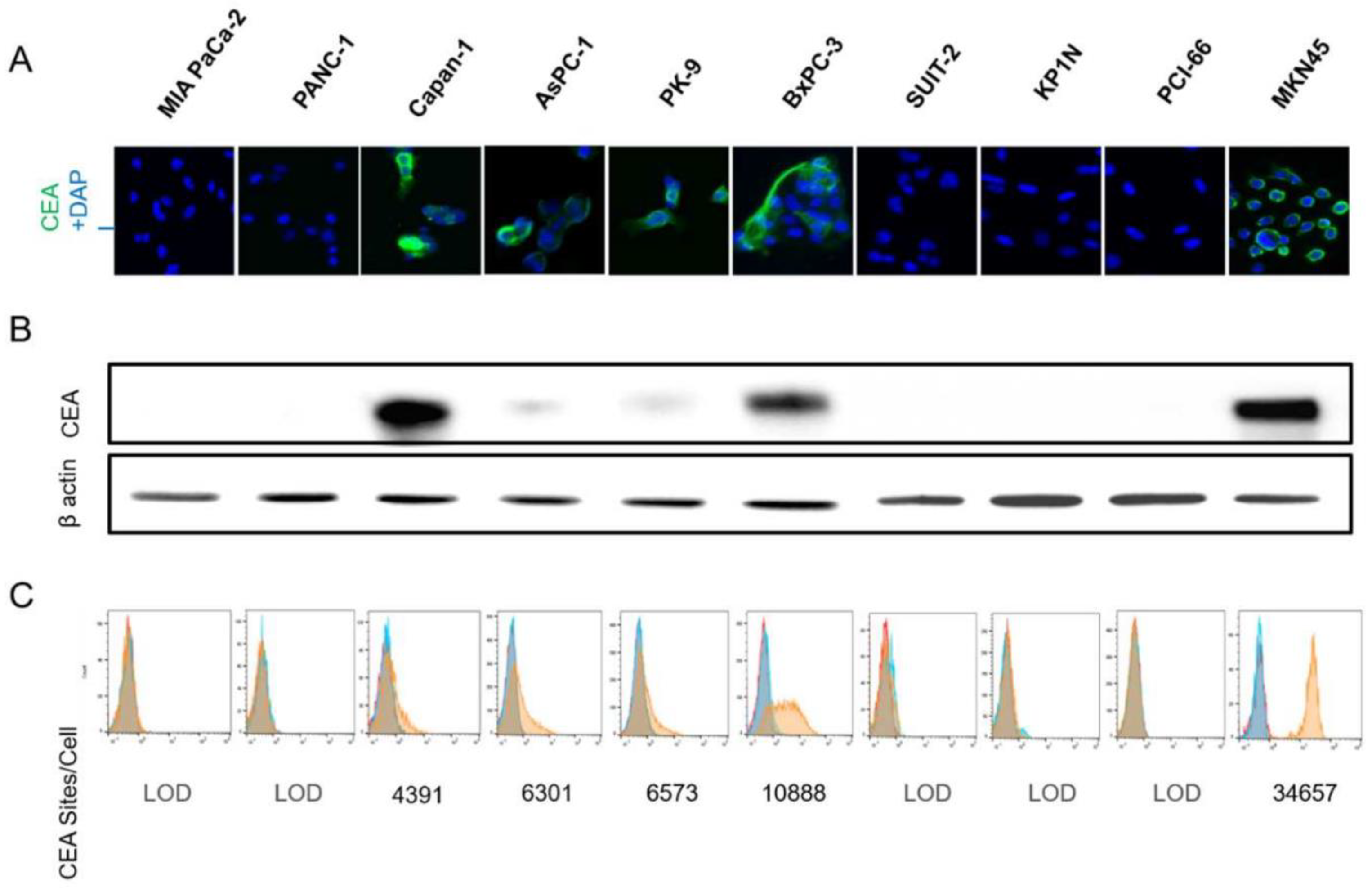
3.1. CEA Expression on Human Pancreatic Cells
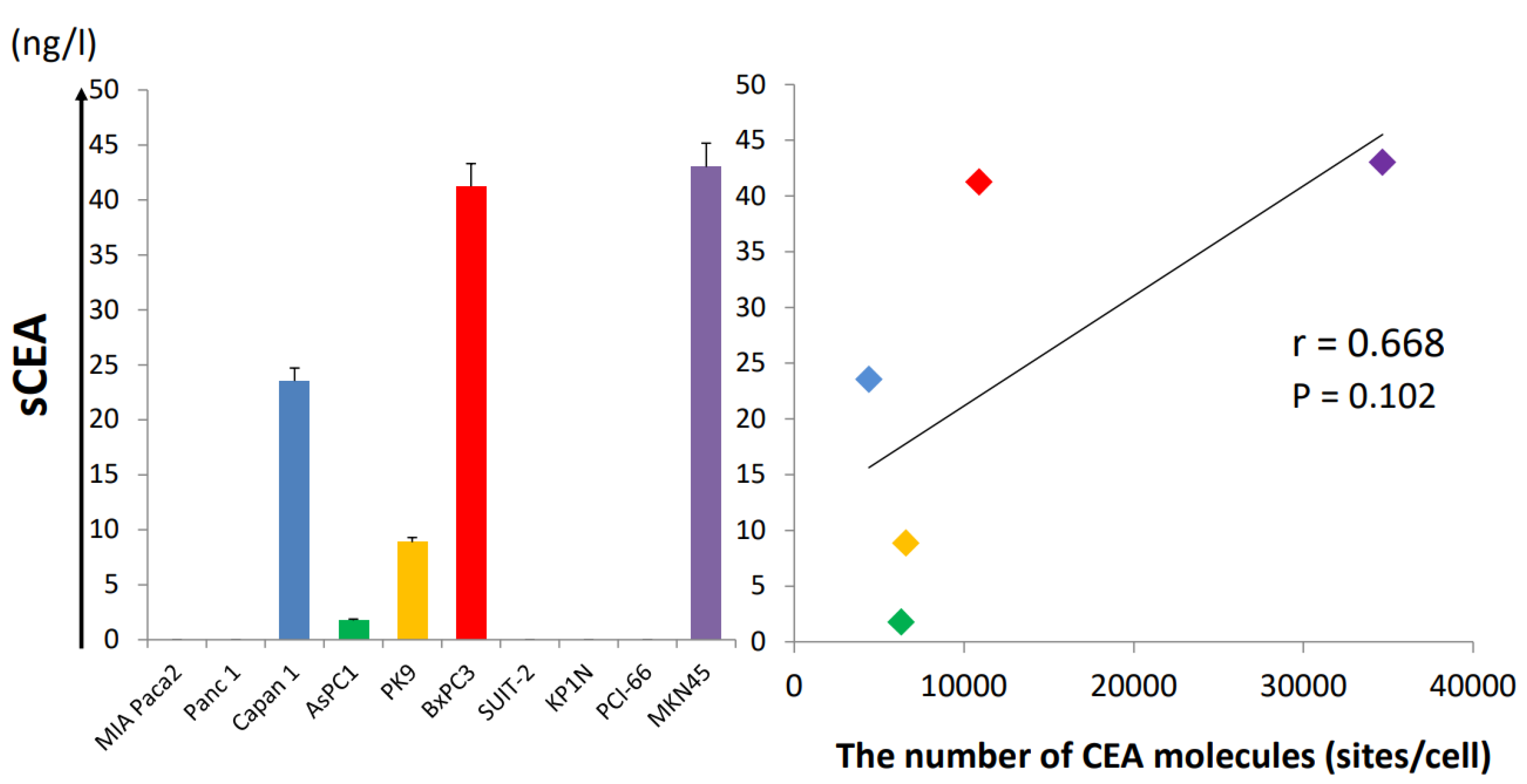
3.2. Soluble CEA Secretion by Pancreatic Cell Lines In Vitro
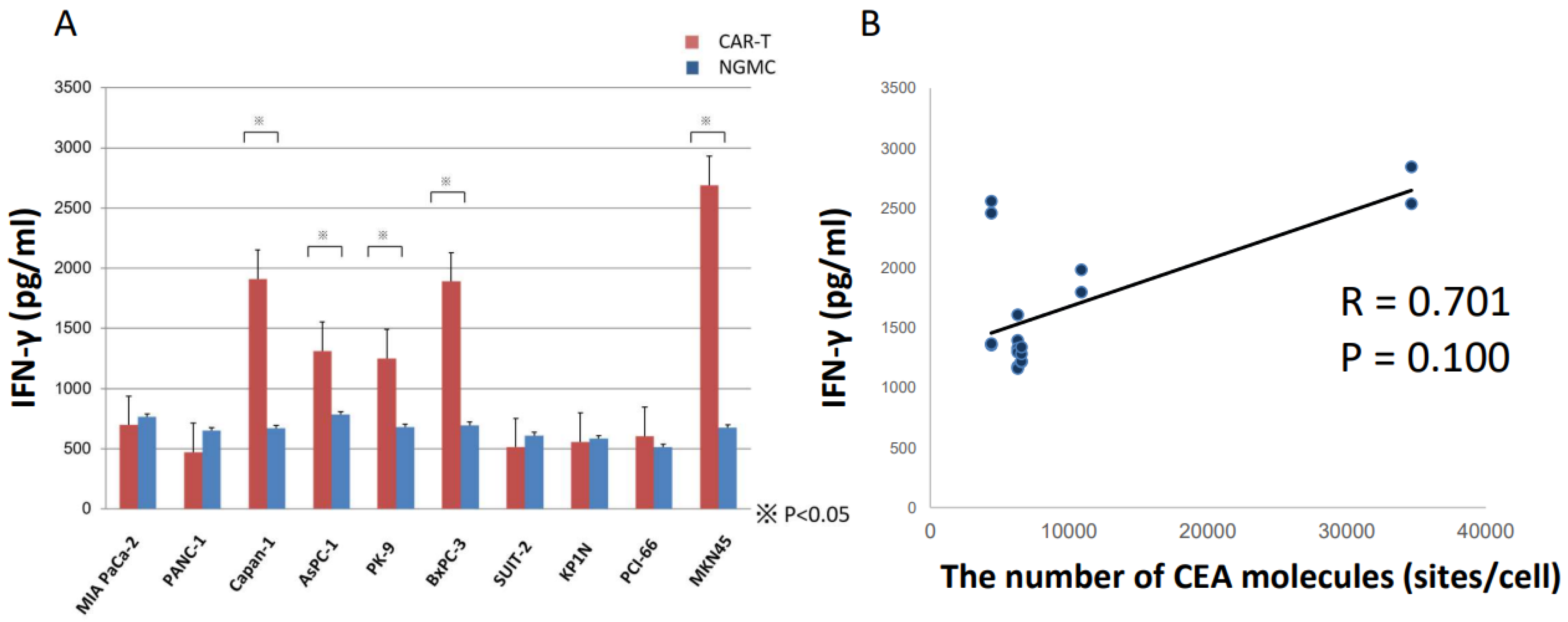
3.3. ELISA Revealed Anti-CEA-CAR-T Activation against CEA Positive Cells
3.4. Cytotoxicity Assay Showed Anti-CEA-CAR-T Activation against CEA Positive Cells
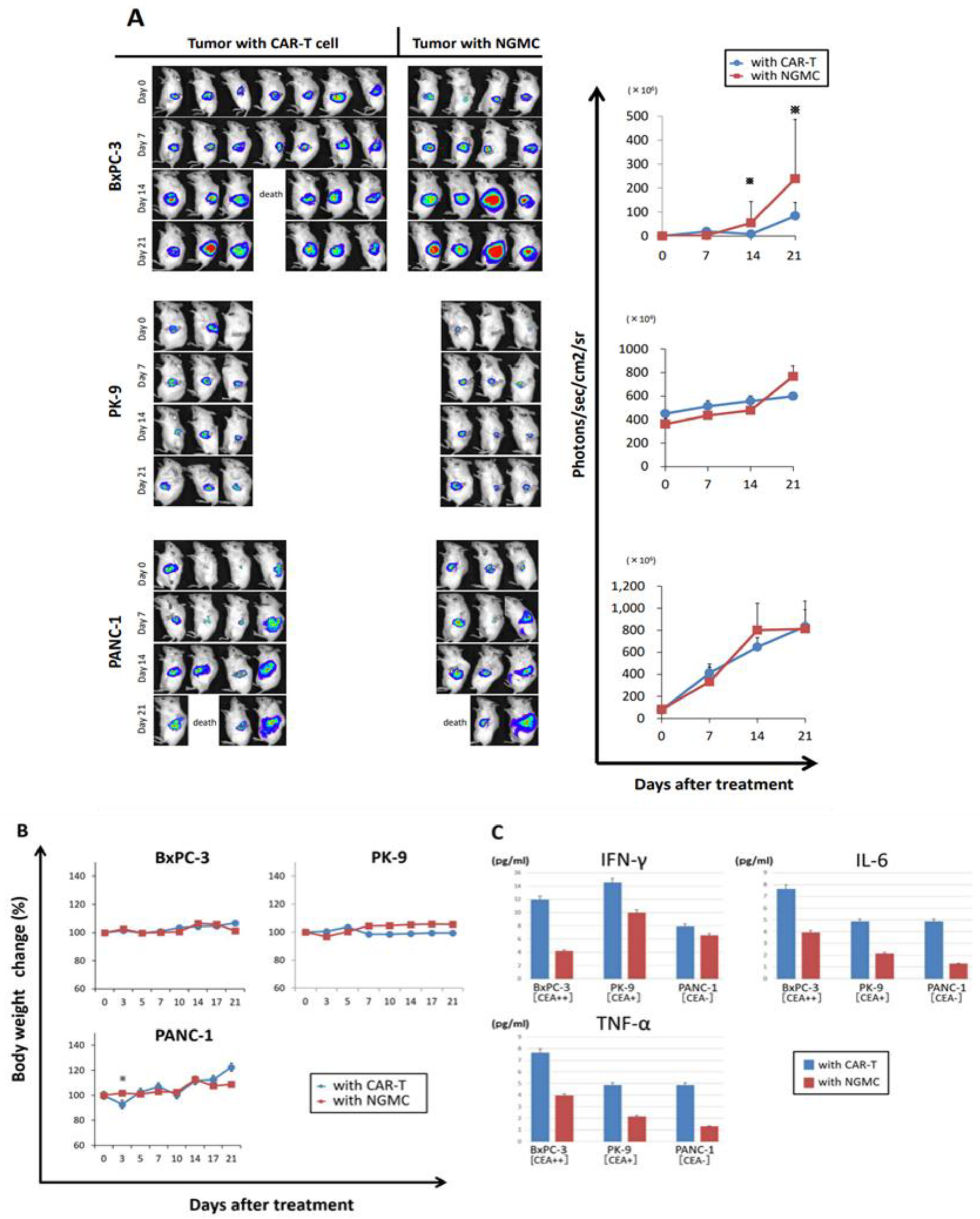
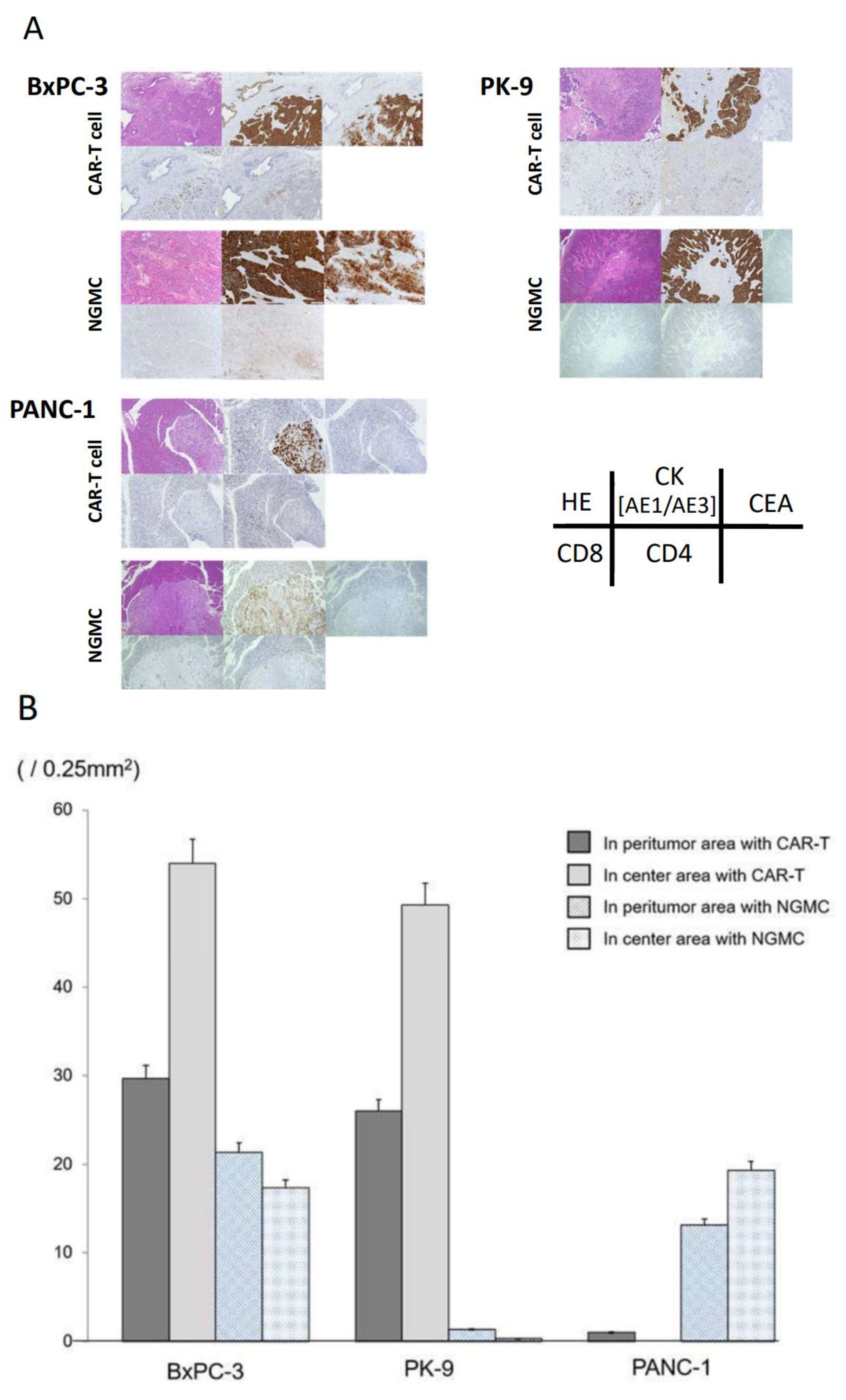
3.5. Adaptive Therapy with Anti-CEA-CAR-T Caused Differential Antigen Expression on Orthotopic Xenograft Models of CEA Positive PDAC
3.6. Immunohistochemical Evaluation of Transplanted Tumor following Anti-CEA-CAR-T Treatment
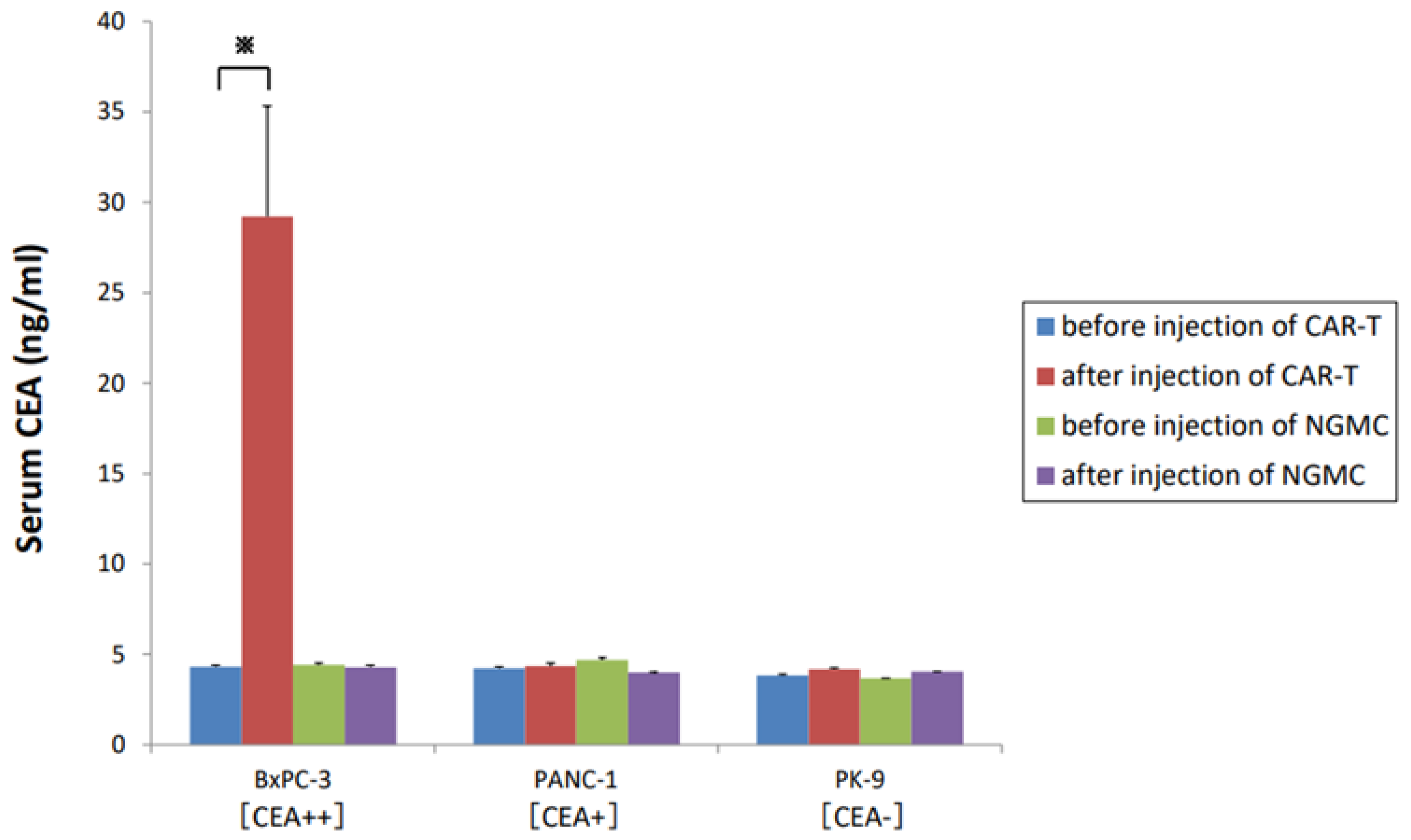
3.7. Soluble CEA Did Not Markedly Block Anti-CEA-CAR-T Activation
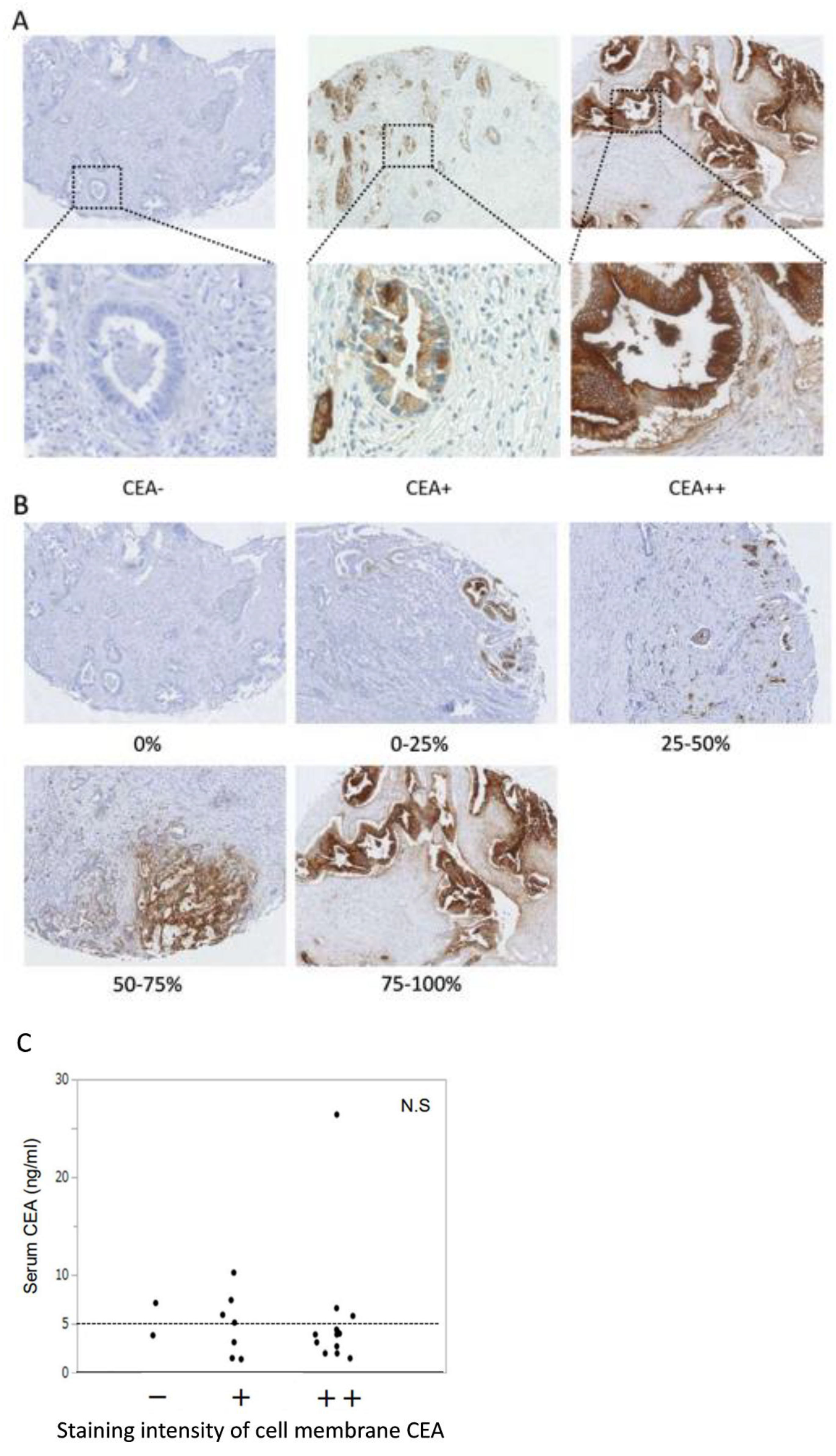
3.8. Identification of Surrogate Markers by Retrospective Analysis of PDAC Cases to Select PDAC Patients for Anti-CEA-CAR-T Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gerda, E.; Jacques, F.; Niels, C.; Freddie, B.; Marianne, L.G.; Asa, K.; Jóanis, E.K.; Elínborg, O.; Eero, P.; Hans, H.S. NORDCAN—A Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef]
- Cielito, C.R.; Wenyaw, C.; James, L.A.; Henry, Q.X.; Linus, H.; Douglas, B.E.; Gauri, V.; Samrat, B.; Robert, A.W.; Christopher, C. Patterns of self-reported symptoms in pancreatic cancer patients receiving chemoradiation. J. Pain Symptom. Manag. 2007, 34, 244–252. [Google Scholar] [CrossRef]
- Rebecca, L.; Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2016, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kawalekar, O.U.; O’ Connor, R.S.; Fraietta, J.A.; Guo, L.; McGettigan, S.E.; Posey Jr, A.D.; Patel, P.R.; Guedan, S.; Scholler, J.; Keith, B.; et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity 2016, 44, 712. [Google Scholar] [CrossRef]
- Erhao, Z.; Peiwei, Y.; Jieyi, G.; Heming, W.; Xiaowei, C.; Chen, L.; Ying, W.; Jianpeng, X.; Weiyan, Q.; Qingbo, S.; et al. Recombination of a dual-CAR-modified T lymphocyte to accurately eliminate pancreatic malignancy. J. Hematol. Oncol. 2018, 11, 102. [Google Scholar] [CrossRef]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. NIH Public Access—Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2012, 365, 725–733. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.E.; Somerville, R.P.T.; Carpenter, R.O.; Stetler-Stevenson, M.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Davila, M.L.; Riviere, I.; Wang, X.; Bartido, S.; Park, J.; Curran, K.; Chung, S.S.; Stefanski, J.; Borquez-Ojeda, O.; Olszewska, M.; et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy. Sci. Transl. Med. 2014, 6, 224–225. [Google Scholar] [CrossRef] [PubMed]
- Gansauge, S.; Gansauge, F.; Beger, H.G. Molecular oncology in pancreatic cancer. J. Mol. Med. 1996, 74, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Hahn, O.; Rappl, G.; Michael, N.; Ingo, H.S.; Andreas, A.H.; Hinrich, A. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology 2012, 43, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, N.; Okamoto, S.; Amaishi, Y.; Sato, E.; Seo, N.; Mineno, J.; Takesako, K.; Kato, T.; Shiku, H. Efficient tumor regression by adoptively transferred CEA-specific CAR-T cells associated with symptoms of mild cytokine release syndrome. Oncoimmunology 2016, 5, e1211218. [Google Scholar] [CrossRef] [PubMed]
- Mehmet, A.; Mohammad, Y.Z.; Edmund, K.W.; Bassel, F.E.; Gregory, B.L. The Potential of CAR T Cell Therapy in Pancreatic Cancer. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Kuroki, M.; Hachimine, K.; Huang, J.; Shibaguchi, H. Re-targeting of Cytotoxic T Lymphocytes and / or Natural Killer Cells to CEA-expressing Tumor Cells with Anti-CEA Antibody Activity. Anticancer Res. 2005, 6A, 3725–3732. [Google Scholar]
- Okamoto, S.; Amaishi, Y.; Goto, Y.; Ikeda, H.; Fujiwara, H.; Kuzushima, K.; Yasukawa, M.; Shiku, H.; Mineno, J. A promising vector for TCR gene therapy: Differential effect of siRNA, 2A peptide, and disulfide bond on the introduced TCR expression. Mol. Ther. Nucleic Acids 2012, 1, e63. [Google Scholar] [CrossRef]
- Simona, R.; Ornella, Z.; Stefano, B.; Massimiliano, A.; Rodolfo, B.; Giuseppe, N.; Emira, A.; Carlo, R. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol. 2004, 34, 613–622. [Google Scholar] [CrossRef]
- Gouin, E.; Ouary, M.; Pogu, S.; Sai, P. Release of Carcinoembryonic Antigen from Human Tumor Cells by Phosphatidylinositol-Specific Phospholipase C: Highly Effective Extraction and Upregulation from LS-174T Colonic Adenocarcinoma Cells. Arch. Biochem. Biophys. 1993, 306, 125–132. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Shao, Y.; Zhang, Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J Hematol Oncol. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Liu, C.G.; Dakhova, O.; Ashoori, A.; Corder, A.; Gray, T.; et al. Human Epidermal Growth Factor Receptor 2 (HER2)—Specific Chimeric Antigen Receptor–Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Haas, A.R.; Beatty, G.L.; Morgan, M.A.; Stashwick, C.J.; O’Hara, M.H.; Porter, D.L.; Maus, M.V.; Levine, B.L.; Lacey, S.F.; et al. Abstract CT105: Safety and feasibility of chimeric antigen receptor modified T cells directed against mesothelin (CART-meso) in patients with mesothelin expressing cancers. Ancer Res. 2015, 75, CT105. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Torigian, D.A.; Nazimuddin, F.; Chen, F.; Kulikovskaya, I.M.; Soulen, M.C.; McGarvey, M.; Nelson, A.M.; et al. Activity of Mesothelin-specific Chimeric Antigen RAeceptor T cells against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology 2019, 155, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B.; Yang, Y. Chimeric antigen receptor T cell therapy for solid tumors: Current status, obstacles and future strategies. Cancers 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Jindal, V.; Arora, E.; Gupta, S. Challenges and prospects of chimeric antigen receptor T cell therapy in solid tumors. Med. Oncol. 2018, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jindal, V.; Arora, E.; Masab, M.; Gupta, S. Chimeric antigen receptor T cell therapy in pancreatic cancer: From research to practice. Med. Oncol. 2018, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Majzner, R.G.; Mackall, C.L. Tumor antigen escape from car t-cell therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Moon, E.K. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019, 10, 1–21. [Google Scholar] [CrossRef]
- De, V.A.; Orecchia, P.; Balza, E.; Reverberi, D.; Scaldaferri, D.; Taramelli, R.; Noonan, D.M.; Aqcuati, F.; Mortara, L. Overexpression of murine rnaset2 in a colon syngeneic mouse carcinoma model leads to rebalance of intra-tumor m1/m2 macrophage ratio, activation of t cells, delayed tumor growth, and rejection. Cancers 2020, 12, 717. [Google Scholar] [CrossRef]
- Feng, K.; Liu, Y.; Guo, Y.; Qiu, J.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Han, W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018, 9, 838–847. [Google Scholar] [CrossRef]
- Girgis, M.D.; Olafsen, T.; Kenanova, V.; Tomlinson, J.S.; McCabe, K.E.; Wu, A.M. Targeting CEA in Pancreas Cancer Xenografts with a Mutated scFv-Fc Antibody Fragment. EJNMMI Res. 2011, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef]
- Gross, G. Chimeric T cell receptors specific to a B-lymphoma idiotype: A model Search results. Biochem. Soc. Trans. 1995, 23, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Eshhar, Z.; Bach, N.; Fitzer-Attas, C.J.; Gross, G.; Lustgarten, J.; Waks, T.; Schindler, D.G. The T-body approach: Potential for cancer immunotherapy. Semin Immunopathol. 1996, 18, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Terai, K.; Jiang, M.; Tokuyama, W.; Murano, T.; Takada, N.; Fujimura, K.; Ebinuma, h.; Kishimoto, T.; Hiruta, N.; Schneider, W.J.; et al. Levels of soluble LR11/SorLA are highly increased in the bile of patients with biliary tract and pancreatic cancers. Clin. Chim. Acta 2016, 457, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Cros, J.; Raffenne, J.; Couvelard, A.; Poté, N. Tumor Heterogeneity in Pancreatic Adenocarcinoma. Pathobiology 2018, 85, 64–71. [Google Scholar] [CrossRef]
- Yao, W.; Maitra, A.; Ying, H. Recent insights into the biology of pancreatic cancer. EBioMedicine 2020, 53, 102655. [Google Scholar] [CrossRef]
- Behr, T.M.; Sharkey, R.M.; Juweid, M.I.; Robert, M.D.; Zhiliang, Y.; Cun-H., Z.; Jeffry, A.S.; David, V.G.; David, M.G. Factors influencing the pharmacokinetics, dosimetry, and diagnostic accuracy of radioimmunodetection and radioimmunotherapy of carcinoembryonic antigen-expressing tumors. Cancer Res. 1996, 56, 1805–1816. [Google Scholar]
- Zhang, J.; Fujimoto, J.; Zhang, J.; Wedge, D.C.; Song, X.; Zhang, J.; Seth, S.; Chow, C.; Cao, Y.; Gumbs, C.; et al. Intra-tumor heterogeneity in localized lung adenocarcinomas delineated by multi-region sequencing. Science 2014, 346, 256–259. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Thress, K.S.; Alden, R.S.; Lawrance, R.; Paweletz, C.P.; Cantarini, M.; Yang, J.C.; Barrett, J.C.; Janne, P.A. Association Between Plasma Genotyping and Outcomes of Treatment with Osimertinib (AZD 9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2016, 34, 3375–3382. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of All Postoperative Patients | |
| n = 151 | |
| Gender | |
| Male | 89 (58.9) |
| Female | 62 (41.1) |
| Age (years old) | 68 (42–83) |
| Serum CEA (ng/mL) | 4.2 (0.6–212) |
| <5 | 96 (63.6) |
| ≥5 | 55 (36.4) |
| Characteristics of randomly selected patients for Tissue Microarray | |
| n = 22 | |
| Gender | |
| Male | 12 (54.5) |
| Female | 10 (45.5) |
| Age (years old) | 68.5 (55–82) |
| Serum CEA (ng/mL) | 3.95 (1.5–26.4) |
| <5 | 14 (63.6) |
| ≥5 | 8 (36.4) |
| Heterogeneity (%) | p Value | |||||
|---|---|---|---|---|---|---|
| 0 | 0–25 | 25–50 | 50–75 | 75–100 | ||
| Serum CEA (ng/mL) | 0.731 | |||||
| <5 | 1 | 1 | 1 | 2 | 10 | |
| ≥5 | 1 | 0 | 1 | 0 | 5 | |
| Intensity | <0.001 * | |||||
| CEA− | 2 | 0 | 0 | 0 | 0 | |
| CEA+ | 0 | 1 | 2 | 2 | 2 | |
| CEA++ | 0 | 0 | 0 | 0 | 13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, O.; Tsuchikawa, T.; Kato, T.; Amaishi, Y.; Okamoto, S.; Mineno, J.; Takeuchi, Y.; Sasaki, K.; Nakamura, T.; Umemoto, K.; et al. Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells. Cancers 2023, 15, 601. https://doi.org/10.3390/cancers15030601
Sato O, Tsuchikawa T, Kato T, Amaishi Y, Okamoto S, Mineno J, Takeuchi Y, Sasaki K, Nakamura T, Umemoto K, et al. Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells. Cancers. 2023; 15(3):601. https://doi.org/10.3390/cancers15030601
Chicago/Turabian StyleSato, Osamu, Takahiro Tsuchikawa, Takuma Kato, Yasunori Amaishi, Sachiko Okamoto, Junichi Mineno, Yuta Takeuchi, Katsunori Sasaki, Toru Nakamura, Kazufumi Umemoto, and et al. 2023. "Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells" Cancers 15, no. 3: 601. https://doi.org/10.3390/cancers15030601
APA StyleSato, O., Tsuchikawa, T., Kato, T., Amaishi, Y., Okamoto, S., Mineno, J., Takeuchi, Y., Sasaki, K., Nakamura, T., Umemoto, K., Suzuki, T., Wang, L., Wang, Y., Hatanaka, K. C., Mitsuhashi, T., Hatanaka, Y., Shiku, H., & Hirano, S. (2023). Tumor Growth Suppression of Pancreatic Cancer Orthotopic Xenograft Model by CEA-Targeting CAR-T Cells. Cancers, 15(3), 601. https://doi.org/10.3390/cancers15030601





