Uptake and Effectiveness of Risk-Reducing Surgeries in Unaffected Female BRCA1 and BRCA2 Carriers: A Single Institution Experience in the Czech Republic
Abstract
Simple Summary
Abstract
1. Introduction
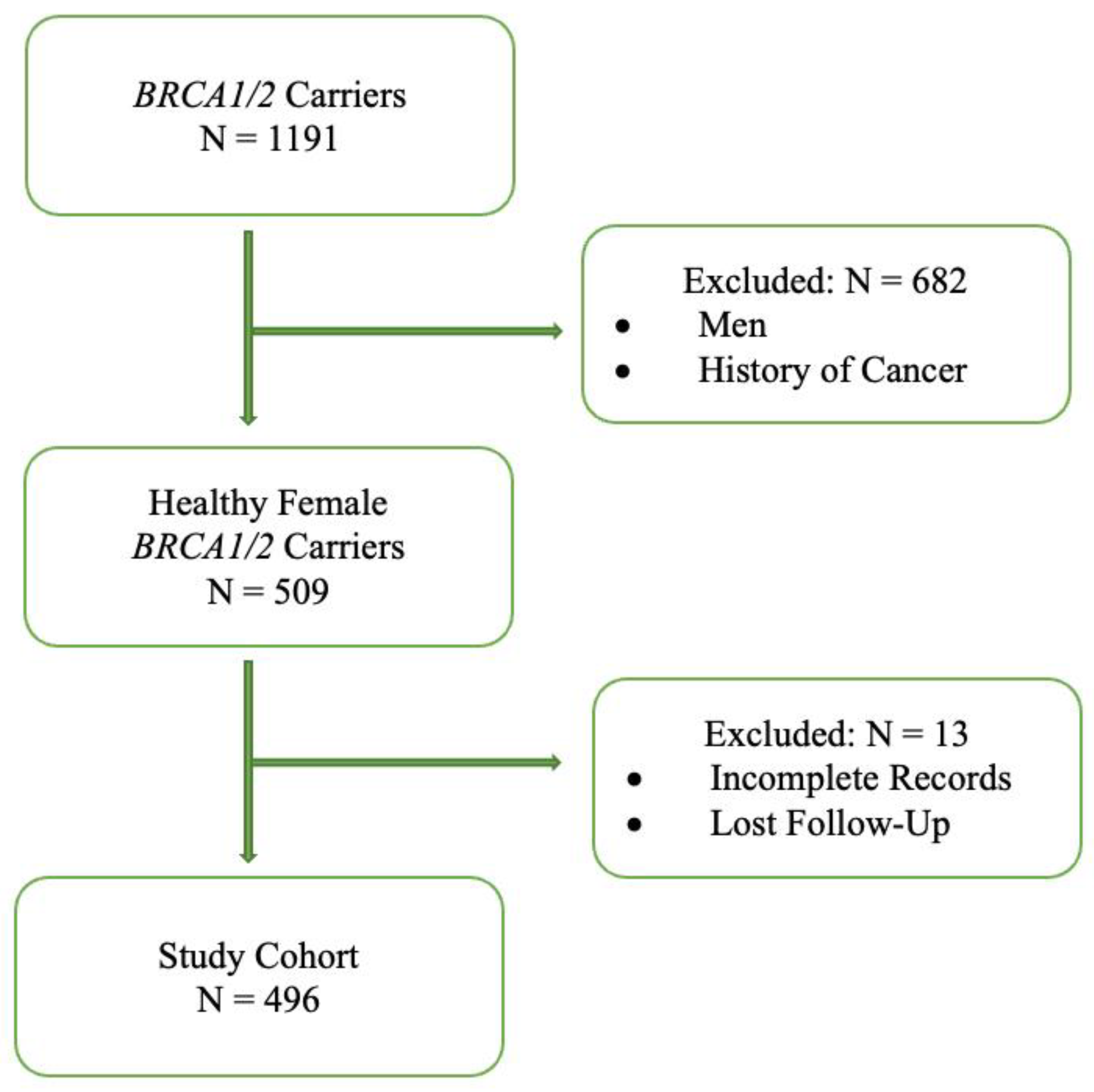
2. Materials and Methods
2.1. Study Setting
2.2. Molecular Analysis
2.3. Statistical Analysis
3. Results
3.1. BRCA1/2 Carriers Surveillance and Prophylactic Procedures
3.2. Changes in Uptake of Risk-Reducing Surgeries over the Time
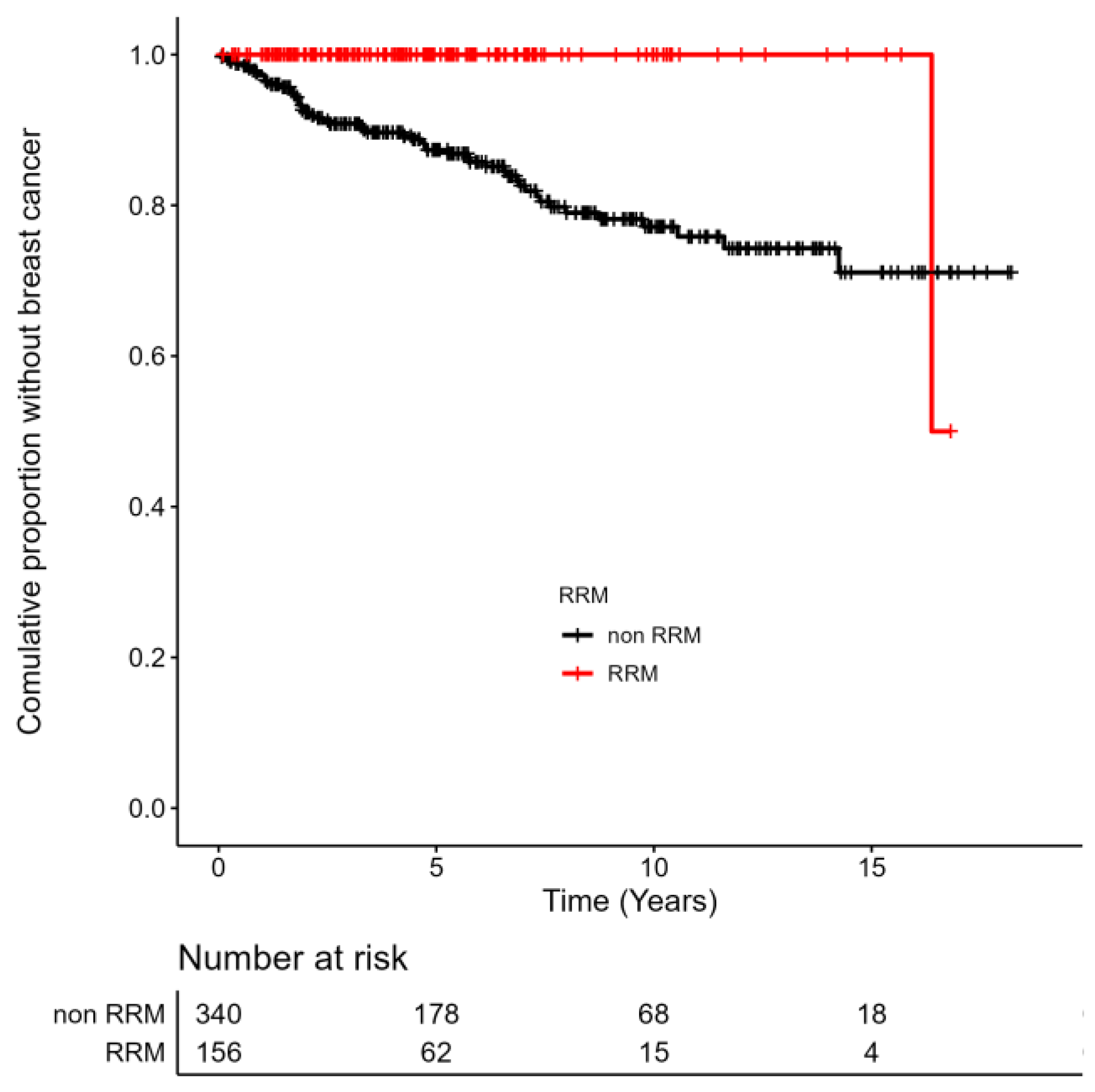
3.3. Incidence of BC in BRCA1/2 Carriers with/without RRM and BC Treatment Strategy
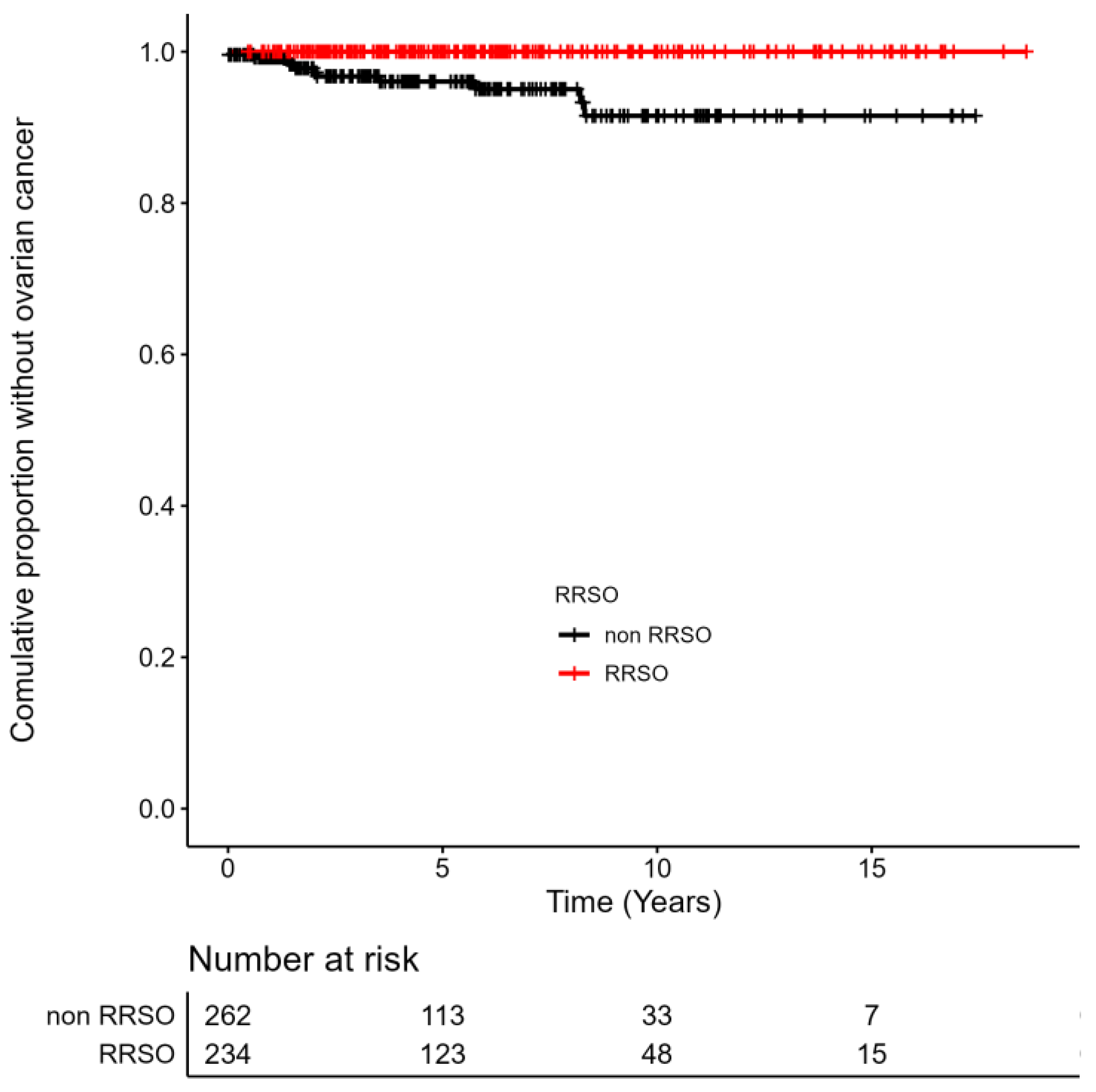
3.4. Incidence of OC in BRCA1/2 Carriers with/without RRSO and OC Treatment Strategy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, J.S.; Gutierrez-Barrera, A.M.; Young, D.; Sun, C.C.; Daniels, M.S.; Lu, K.H.; Arun, B. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J. Clin. Oncol. 2010, 28, 4214–4220. [Google Scholar] [PubMed]
- Begg, C.B.; Haile, R.W.; Borg, A.; Malone, K.E.; Concannon, P.; Thomas, D.C.; Langholz, B.; Bernstein, L.; Olsen, J.H.; Lynch, C.F.; et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA 2008, 299, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Peacock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Gareth Evans, D.; Izatt, L.; Eekes ARm Adlard, J.; Davidson, R.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.; Pharoah, P.D.P.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef]
- Risch, H.A.; McLaughlin, J.R.; Cole, D.E.; Rosen, B.; Bradley, L.; Kwan, E.; Jack, E.; Vesprini, D.J.; Kuperstein, G.; Abrahamson, J.L.; et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 2001, 68, 700–710. [Google Scholar] [CrossRef]
- Pohlreich, P.; Zikan, M.; Stribrna, J.; Kleibl, Z.; Janatova, M.; Kotlas, J.; Zidovska, J.; Novotny, J.; Petruzelka, L.; Szabo, C.; et al. High proportion of recurrent germline mutations in the BRCA1 gene in breast and ovarian cancer patients from the Prague area. Breast Cancer Res. 2005, 7, R728–R736. [Google Scholar] [CrossRef]
- Medeiros, F.; Muto, M.G.; Lee, Y.; Elvin, J.A.; Callahan, M.J.; Feltmate, C.; Garber, J.E.; Cramer, D.W.; Crum, C.P. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 2006, 30, 230–236. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Narod, S.A.; et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef]
- Sessa, C.; Balmaña, J.; Bober, S.L.; Cardoso, M.J.; Colombo, N.; Curigliano, G.; Domchek, S.M.; Evans, D.G.; Fischerova, D.; Paluch-Shimon, S.; et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 33–47. [Google Scholar] [CrossRef]
- Woodward, E.; Sleightholme, H.; Considine, A.; Williamson, S.; McHugo, J.; Cruger, D. Annual surveillance by CA125 and transvaginal ultrasound for ovarian cancer in both high-risk and population-risk women is ineffective. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 1500–1509. [Google Scholar]
- Carbine, N.E.; Lostumbo, L.; Wallace, J.; Ko, H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst. Rev. 2018, 4, CD002748. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar]
- Ludwig, K.K.; Neuner, J.; Butler, A.; Geurts, J.L.; Kong, A.L. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. Am. J. Surg. 2016, 212, 660–669. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.; Seynaeve, C.; van Asperen, C.J.; Ausems, M.G.; Collée, J.M.; van Doorn, H.C.; Gomez Garcia, E.B.; Kets, C.M.; van Leeuwen, F.E.; Meijers-Heijboer, H.E.; et al. Hereditary Breast and Ovarian Cancer Research Group Netherlands. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J. Natl. Cancer Inst. 2015, 107, djv033. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Huzarski, T.; Gronwald, J.; Singer, C.F.; Moller, P.; Lynch, H.T.; Armel, S.; Karlan, B.; Foulkes, W.D.; Neuhausen, S.L.; et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2016, 109, djw177. [Google Scholar]
- Stjepanovic, N.; Villacampa, G.; Nead, K.T.; Torres-Esquius, S.; Melis, G.G.; Nathanson, K.L.; Teule, A.; Brunet, J.; YCajal, T.R.; Llort, G.; et al. Association of premenopausal risk-reducing salpingo-oophorectomy with breast cancer risk in BRCA1/2 mutation carriers: Maximising bias-reduction. Eur. J. Cancer 2020, 132, 53–60. [Google Scholar]
- Skytte, A.B.; Gerdes, A.M.; Andersen, M.K.; Sunde, L.; Brøndum-Nielsen, K.; Waldstrøm, M.; Kølvraa, S.; Crüger, D. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA1/2 mutation carriers: Uptake and timing. Clin. Genet. 2010, 77, 342–349. [Google Scholar]
- This, P.; De La Rochefordiere, A.; Savignoni, A.; Falcon, M.C.; Tardivon, A.; Thibault, F.; Alran, S.; Fourchotte, V.; Fitoussi, A.; Couturaud, B.; et al. Breast and ovarian cancer risk management in a French cohort of 158 women carrying a BRCA1 or BRCA2 germline mutation: Patient choices and outcome. Fam. Cancer 2012, 11, 473–482. [Google Scholar]
- Collins, I.M.; Milne, R.L.; Weideman, P.C.; McLachlan, S.A.; Friedlander, M.L.; Cuningham, K.; Hopper, J.L.; Phillips, K.A. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med. J. Aust. 2013, 199, 680–683. [Google Scholar]
- Metcalfe, K.; Eisen, A.; Senter, L.; Armel, S.; Bordeleau, L.; Meschino, W.S.; Pal, T.; Lynch, H.T.; Tung, N.M.; Kwong, A.; et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br. J. Cancer 2019, 121, 15–21. [Google Scholar] [PubMed]
- Pohlreich, P.; Stribrna, J.; Kleibl, Z.; Zikan, M.; Kalbacova, R.; Petruzelka, L.; Konopasek, B. Mutations of the BRCA1 gene in hereditary breast and ovarian cancer in the Czech Republic. Med. Princ. Pract. 2003, 12, 23–29. [Google Scholar] [CrossRef]
- Pohlreich, P.; Stribrna, J.; Ticha, I.; Soukupova, J.; Kleibl, Z.; Zikan, M.; Zimovjanova, M.; Kotlas, J.; Panczak, A. Predisposing genes in hereditary breast and ovarian cancer in the Czech Republic. Eur. J. Cancer Suppl. 2010, 8, 16. [Google Scholar]
- Ticha, I.; Kleibl, Z.; Stribrna, J.; Kotlas, J.; Zimovjanova, M.; Mateju, M.; Zikan, M.; Pohlreich, P. Screening for genomic rearrangements in BRCA1 and BRCA2 genes in Czech high-risk breast/ovarian cancer patients: High proportion of population-specific alterations in BRCA1 gene. Breast Cancer Res. Treat. 2010, 124, 337–347. [Google Scholar]
- Soukupova, J.; Zemankova, P.; Lhotova, K.; Janatova, M.; Borecka, M.; Stolarova, L.; Lhota, F.; Foretova, L.; Machackova, E.; Stranecky, V.; et al. Validation of CZECANCA (CZEch CAncer paNel for Clinical Application) for targeted NGS-based analysis of hereditary cancer syndromes. PLoS ONE 2018, 13, e0195761. [Google Scholar] [CrossRef]
- Lhota, F.; Zemankova, P.; Kleiblova, P.; Soukupova, J.; Vocka, M.; Stranecky, V.; Janatova, M.; Hartmannova, H.; Hodanova, K.; Kmoch, S.; et al. Hereditary truncating mutations of DNA repair and other genes in BRCA1/BRCA2/PALB2-negatively tested breast cancer patients. Clin. Genet. 2016, 90, 324–333. [Google Scholar] [CrossRef]
- Singh, N.; Gilks, C.B.; Wilkinson, N.; McCluggage, W.G. Assessment of a new system for primary site assignment in high-grade serous carcinoma of the fallopian tube, ovary, and peritoneum. Histopathology 2015, 67, 331–337. [Google Scholar] [CrossRef]
- Rosenthal, A.N.; Fraser, L.S.M.; Philpott, S.; Manchanda, R.; Burnell, M.; Badman, P.; Hadwin, R.; Rizzuto, I.; Benjamin, E.; Jacobs, I.J.; et al. United Kingdom Familial Ovarian Cancer Screening Study collaborators. Evidence of Stage Shift in Women Diagnosed With Ovarian Cancer During Phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J. Clin. Oncol. 2017, 35, 1411–1420. [Google Scholar] [CrossRef]
- Evans, D.G.; Gandhi, A.; Wisely, J.; Clancy, T.; Woodward, E.R.; Harvey, J.; Highton, L.; Murphy, J.; Barr, L.; Howell, A.; et al. Uptake of bilateral-risk-reducing-mastectomy: Prospective analysis of 7195 women at high-risk of breast cancer. Breast 2021, 60, 45–52. [Google Scholar] [CrossRef]
- Ložar, T.; Žgajnar, J.; Perhavec, A.; Blatnik, A.; Novaković, S.; Krajc, M. Trends and timing of risk-reducing mastectomy uptake in unaffected BRCA1 and BRCA2 carriers in Slovenia. Eur. J. Surg. Oncol. 2021, 47, 1900–1906. [Google Scholar] [PubMed]
- Evans, D.G.; Wisely, J.; Clancy, T.; Lalloo, F.; Wilson, M.; Johnson, R.; Duncan, J.; Barr, L.; Gandhi, A.; Howell, A. Longer term effects of the Angelina Jolie effect: Increased risk-reducing mastectomy rates in BRCA1/2 carriers and other high-risk women. Breast Cancer Res. 2015, 17, 17–18. [Google Scholar]
- Lee, J.; Kim, S.; Kang, E.; Park, S.; Kim, Z.; Lee, M.H. Influence of the Angelina Jolie announcement and insurance reimbursement on practice patterns for hereditary breast cancer. J. Breast Cancer 2017, 20, 203–207. [Google Scholar] [CrossRef]
- Jolie, A. My Medical Choice; New York Times: New York, NY, USA, 2013; pp. 14–16. [Google Scholar]
- Hawkes, N. "Angelina effect" led to more appropriate breast cancer referrals, research shows. BMJ 2014, 349, g5755. [Google Scholar]
- Rhiem, K.; Engel, C.; Graeser, M.; Zachariae, S.; Kast, K.; Kiechle, M.; Ditsch, N.; Janni, W.; Mundhenke, C.; Golatta, M.; et al. The risk of contralateral breast cancer in patients from BRCA1/2 negative high-risk families as compared to patients from BRCA1 or BRCA2 positive families: A retrospective cohort study. Breast Cancer Res. 2012, 14, R156. [Google Scholar] [CrossRef]
- Český Statistický Úřad. Aktuální Populační vývoj V Kostce. Aktuální Populační Vývoj v Kostce|ČSÚ. Available online: https://www.czso.cz/csu/czso/aktualni-populacni-vyvoj-v-kostce (accessed on 19 January 2023).
- Manchanda, R.; Gaba, F.; Talaulikar, V.; Pundir, J.; Gessler, S.; Davies, M.; Menon, U. Risk-Reducing Salpingo-Oophorectomy and the Use of Hormone Replacement Therapy Below the Age of Natural Menopause: Scientific Impact Paper No. 66 October 2021: Scientific Impact Paper No. 66. BJOG Int. J. Obstet. Gynaecol. 2022, 129, e16–e34. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Melton, L.J., 3rd; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef]
- Jakub, J.W.; Peled, A.W.; Gray, R.J.; Greenup, R.A.; Kiluk, J.V.; Sacchini, V.; McLaughlin, S.A.; Tchou, J.C.; Vierkant, R.A.; Degnim, A.C.; et al. Oncologic safety of prophylactic nipple-40sparing mastectomy in a population with BRCA1/2 mutations: A multi-institutional study. JAMA Surg. 2018, 153, 123–129. [Google Scholar] [CrossRef]
- Stanek, K.; Zimovjanova, M.; Suk, P.; Jonas, F.; Zimovjanova, A.; Molitor, M.; Mestak, O. Bilateral Prophylactic Nipple-Sparing Mastectomy: Analysis of the Risk-Reducing Effect in BRCA1/2 Mutation Carriers. Aesthetic Plast. Surg. 2022, 46, 706–711. [Google Scholar] [CrossRef]
- Wei, C.H.; Scott, A.M.; Price, A.N.; Miller, H.C.; Klassen, A.F.; Jhanwar, S.M.; Mehrara, B.J.; Disa, J.J.; McCarthy, C.; Matros, E.; et al. Psychosocial and sexual well-being following nipple-sparing mastectomy and reconstruction. Breast J. 2016, 22, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, H.; Takei, J. Management of hereditary breast and ovarian cancer. Int. J. Clin. Oncol. 2018, 23, 45–51. [Google Scholar] [CrossRef] [PubMed]
| Total | RRM (Only) | RRSO (Only) | RRM + RRSO (Combined) | Surveillance (Only) | |
|---|---|---|---|---|---|
| BRCA1/2 Carriers, N (%) BRCA1, N (%) BRCA2, N (%) | 496 (100) 348 (100) 148 (100) | 53 (10.7) 40 (11.5) 13 (8.8) | 131 (26.4) 91 (26.1) 40 (27.0) | 103 (20.8) 71 (20.4) 32 (21.6) | 209 (42.1) 146 (41.9) 63 (42.6) |
| Mean Age, y (range) BRCA1, y, (range) BRCA2, y, (range) Median Age, y | 35.4 (18–65) 35.2 (18–65) 35.9 (18–60) 35.0 | 32.9 (22–60) 33.1 (22–56) 32.4 (26–60) 32.0 | 43.2 (28–64) 43.8 (28–64) 43.2 (34–57) 42.0 | - - - - | - - - - |
| Mean Follow-up (range) Median Follow-Up, y | 6.8 (0.5–20) 6.0 | 6.6 (0.5–20) | 7.9 (0.5–16) | - | 5.4 (0.5–17) |
| Total | RRM | RRSO | |
|---|---|---|---|
2005–2012
| 192 (100) - - - | - 23 (12.0) 2.3 39.2 | - 56 (29.2) 1.5 41.3 |
2013–2020
| 304 (100) - - - | - 96 (31.6) 1.8 40.2 | - 129 (42.4) 1.0 44.9 |
| Total BRCA1/2 N = 496 | BRCA1 N = 348 | BRCA2 N = 148 | |
|---|---|---|---|
| Breast Cancer, N (%) | 55 (11.1) | 46 (13.2) | 9 (6.1) |
Histology, N (%)
| 5 (9.1) 49 (89.1) 1 (1.8) | 4 (8.7) 41 (89.1) 1 (2.2) | 1 (11.1) 8 (88.9) 0 |
A subtype, N (%)
| 29 (52.7) 23 (41.8) 2 (3.7) 1 (1.8) | 29 (63.1) 15 (32.7) 1 (2.1) 1 (2.1) | 0 8 (88.9) 1 (11,1) 0 |
Grade, N (%)
| 3 (5.5) 15 (27.3) 37 (67.2) | 3 (6.5) 10 (21.7) 33 (71.8) | 0 5 (55.6) 4 (44.4) |
Invasive Breast Cancer-Stage, N (%)
| 27 (54.0) 23 (46.0) 0 | 22 (52.4) 20 (47.6) 0 | 5 (62.5) 3 (37.5) 0 |
Detection, N (%)
| 42 (76.3) 13 (23.7) | 34 (73.9) 12 (26.1) | 8 (88.9) 1 (11.1) |
The Sequence of Treatment, N (%)
| 5 (9.1) 5 (9.1) 25 (45.4) 20 (36.4) | 4 (8.7) 3 (6.5) 21 (45.7) 18 (39.1) | 1 (11.1) 2 (22.2) 4 (44.5) 2 (22.2) |
Type of Breast Surgery, N (%)
| 18 (32.7) 5 (9.1) 32 (58.2) | 12 (26.1) 5 (10.9) 29 (63.0) | 6 (66.7) 0 3 (33.3) |
| Patient | BRCA1/2 | Diagnosis | Ca125 | TVUS | Surgery | CT | FU (Months) | Alive/ Deceased |
|---|---|---|---|---|---|---|---|---|
| 1 | BRCA1 | STIC | Normal | Normal | RRSO | No | 30 | Alive |
| 2 | BRCA1 | STIC | Normal | Normal | RRSO | No | 34 | Alive |
| 3 | BRCA1 | STIC | Normal | Normal | RRSO | No | 79 | Alive |
| 4 | BRCA1 | STIC | Normal | Normal | RRSO | No | 97 | Alive |
| 5 | BRCA1 | FTC, st. I | Normal | Normal | SS | Yes | 32 | Alive |
| 6 | BRCA2 | FTC, st. I | Normal | Normal | SS | Yes | 49 | Alive |
| 7 | BRCA1 | OC, st. I | Elevated | Normal | SS | Yes | 17 | Alive |
| 8 | BRCA1 | OC, st. I | Normal | Normal | SS | Yes | 81 | Alive, relapse |
| 9 | BRCA1 | OC, st. III | Elevated | Abnormal | PDS | Yes | 20 | Alive |
| 10 | BRCA2 | OC, st. III | Elevated | Abnormal | PDS | Yes | 55 | Deceased |
| 11 | BRCA1 | OC, st. III | Elevated | Abnormal | PDS | Yes | 73 | Deceased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimovjanova, M.; Bielcikova, Z.; Miskovicova, M.; Vocka, M.; Zimovjanova, A.; Rybar, M.; Novotny, J.; Petruzelka, L. Uptake and Effectiveness of Risk-Reducing Surgeries in Unaffected Female BRCA1 and BRCA2 Carriers: A Single Institution Experience in the Czech Republic. Cancers 2023, 15, 1072. https://doi.org/10.3390/cancers15041072
Zimovjanova M, Bielcikova Z, Miskovicova M, Vocka M, Zimovjanova A, Rybar M, Novotny J, Petruzelka L. Uptake and Effectiveness of Risk-Reducing Surgeries in Unaffected Female BRCA1 and BRCA2 Carriers: A Single Institution Experience in the Czech Republic. Cancers. 2023; 15(4):1072. https://doi.org/10.3390/cancers15041072
Chicago/Turabian StyleZimovjanova, Martina, Zuzana Bielcikova, Michaela Miskovicova, Michal Vocka, Anna Zimovjanova, Marian Rybar, Jan Novotny, and Lubos Petruzelka. 2023. "Uptake and Effectiveness of Risk-Reducing Surgeries in Unaffected Female BRCA1 and BRCA2 Carriers: A Single Institution Experience in the Czech Republic" Cancers 15, no. 4: 1072. https://doi.org/10.3390/cancers15041072
APA StyleZimovjanova, M., Bielcikova, Z., Miskovicova, M., Vocka, M., Zimovjanova, A., Rybar, M., Novotny, J., & Petruzelka, L. (2023). Uptake and Effectiveness of Risk-Reducing Surgeries in Unaffected Female BRCA1 and BRCA2 Carriers: A Single Institution Experience in the Czech Republic. Cancers, 15(4), 1072. https://doi.org/10.3390/cancers15041072






