Metronomic Chemotherapy Based on Topotecan or Topotecan and Cyclophosphamide Combination (CyTo) in Advanced, Pretreated Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Analysis of Treatment Efficacy
2.4. Safety Analysis
2.5. Statistical Considerations
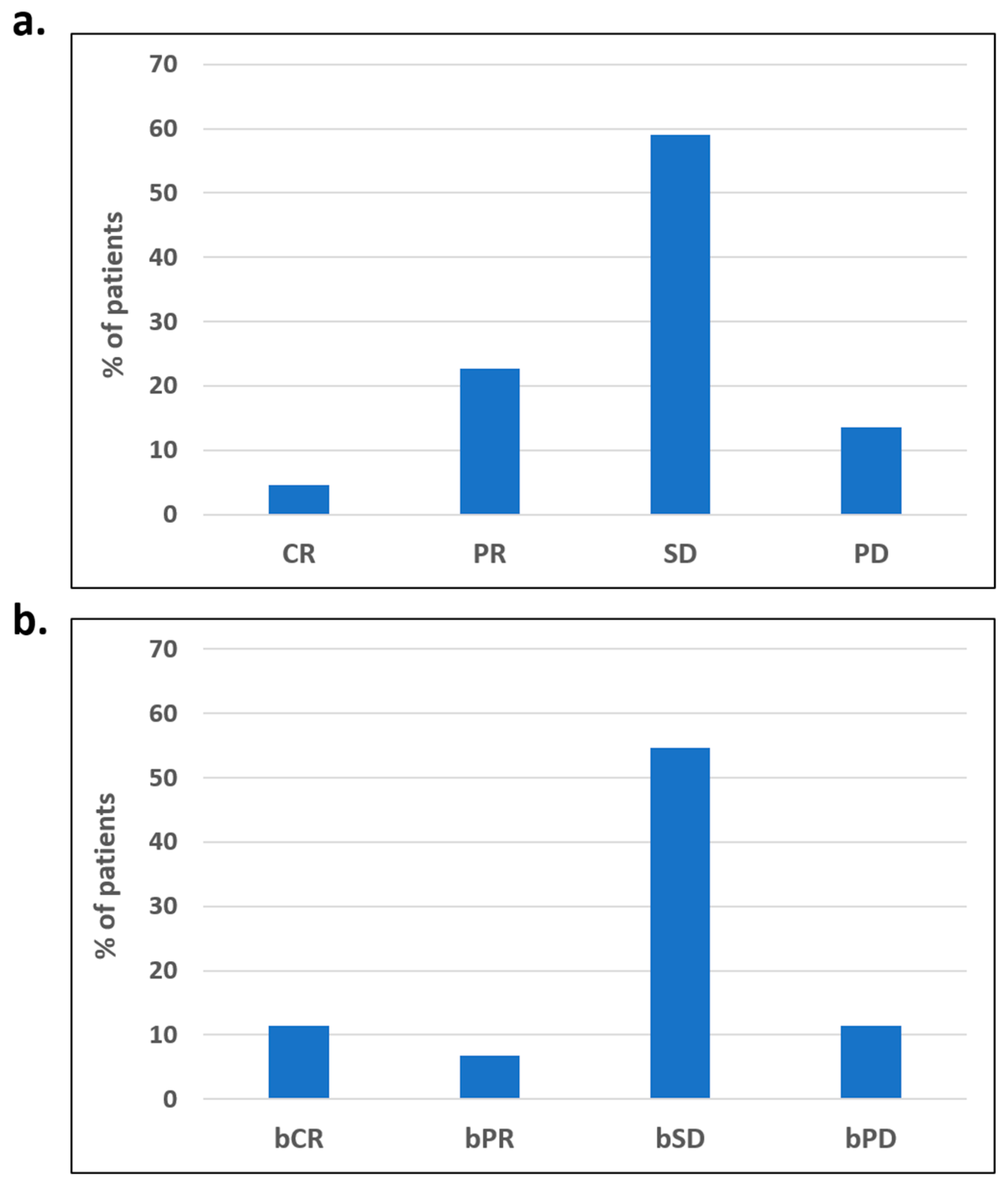
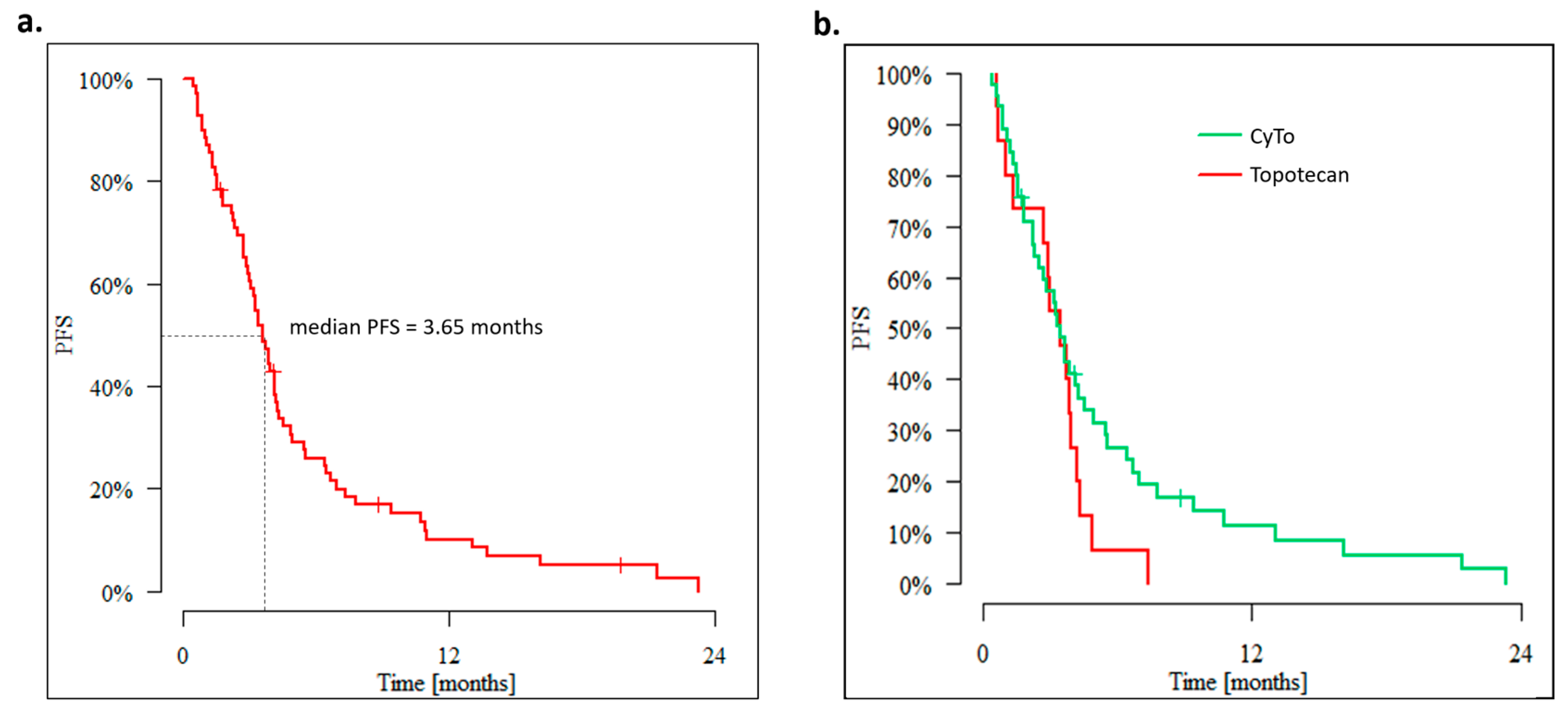
3. Results
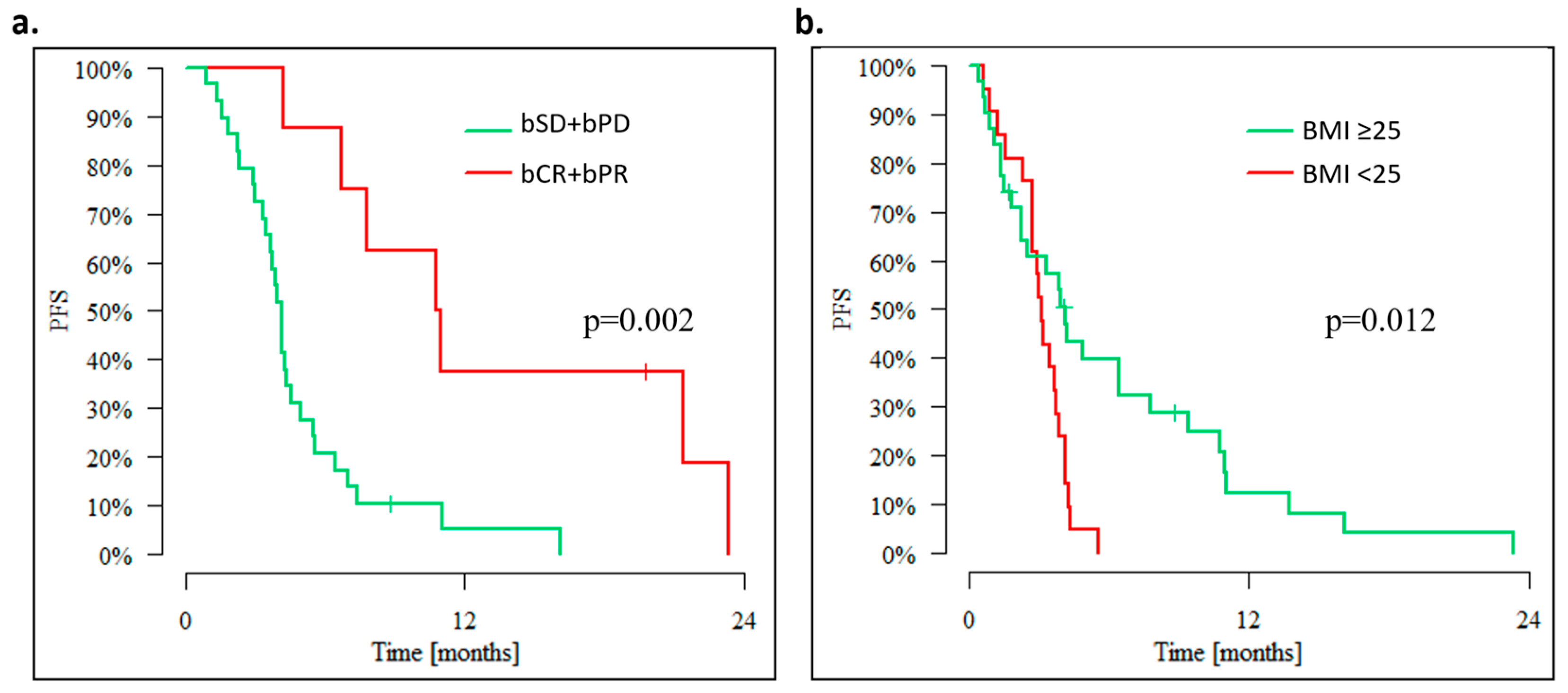
3.1. Outcomes of Overweight Advanced OC Patients
3.2. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 284. [Google Scholar] [CrossRef]
- Cancer Registration Statistics, England Statistical Bulletins—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/previousReleases (accessed on 28 December 2022).
- Dion, L.; Mimoun, C.; Timoh, K.N.; Bendifallah, S.; Bricou, A.; Collinet, P.; Touboul, C.; Ouldamer, L.; Azaïs, H.; Dabi, Y.; et al. Ovarian Cancer in the Elderly: Time to Move towards a More Logical Approach to Improve Prognosis—A Study from the FRANCOGYN Group. J. Clin. Med. 2020, 9, 1339. [Google Scholar] [CrossRef]
- Tew, W.P. Ovarian Cancer in the Older Woman. J. Geriatr. Oncol. 2016, 7, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Ballatori, E.; Favalli, G.; Scambia, G. Quality of Life: Gynaecological Cancers. Ann. Oncol. 2001, 12, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.; Kuang, R.; Landen, C.; Shridhar, V. Platinum-Sensitive Recurrence in Ovarian Cancer: The Role of Tumor Microenvironment. Front. Oncol. 2013, 3, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum Resistant” Ovarian Cancer: What Is It, Who to Treat and How to Measure Benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef]
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; Du Bois, A. The Impact of Second to Sixth Line Therapy on Survival of Relapsed Ovarian Cancer after Primary Taxane/Platinum-Based Therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Tillmanns, T.D.; Buller, R.; Stewart, C.F.; MacEachern, J.; Schaiquevich, P.; Walker, M.S.; Stepanski, E.J. Daily Oral Topotecan: Utilization of a Metronomic Dosing Schedule to Treat Recurrent or Persistent Solid Tumors. J. Clin. Oncol. 2008, 26, 2571. [Google Scholar] [CrossRef]
- Turner, D.C.; Tillmanns, T.D.; Harstead, K.E.; Throm, S.L.; Stewart, C.F. Combination Metronomic Oral Topotecan and Pazopanib: A Pharmacokinetic Study in Cancer Patients with Gynecological Cancer. Anticancer Res. 2013, 33, 3823. [Google Scholar] [PubMed]
- Minturn, J.E.; Janss, A.J.; Fisher, P.G.; Allen, J.C.; Patti, R.; Phillips, P.C.; Belasco, J.B. A Phase II Study of Metronomic Oral Topotecan for Recurrent Childhood Brain Tumors. Pediatr. Blood Cancer 2011, 56, 39–44. [Google Scholar] [CrossRef]
- Morgan, R.D.; McNeish, I.A.; Cook, A.D.; James, E.C.; Lord, R.; Dark, G.; Glasspool, R.M.; Krell, J.; Parkinson, C.; Poole, C.J.; et al. Objective Responses to First-Line Neoadjuvant Carboplatin–Paclitaxel Regimens for Ovarian, Fallopian Tube, or Primary Peritoneal Carcinoma (ICON8): Post-Hoc Exploratory Analysis of a Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 277–288. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; Van Der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Clamp, A.R.; James, E.C.; McNeish, I.A.; Dean, A.; Kim, J.W.; O’Donnell, D.M.; Hook, J.; Coyle, C.; Blagden, S.; Brenton, J.D.; et al. Weekly Dose-Dense Chemotherapy in First-Line Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Carcinoma Treatment (ICON8): Primary Progression Free Survival Analysis Results from a GCIG Phase 3 Randomised Controlled Trial. Lancet 2019, 394, 2084–2095. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard Chemotherapy with or without Bevacizumab for Women with Newly Diagnosed Ovarian Cancer (ICON7): Overall Survival Results of a Phase 3 Randomised Trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib Tablets as Maintenance Therapy in Patients with Platinum-Sensitive Relapsed Ovarian Cancer and a BRCA1/2 Mutation (SOLO2/ENGOT-Ov21): A Final Analysis of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pignata, S.; Lorusso, D.; Perrone, F.; Zara, D.; Puglisi, F. Number Needed to Treat in Trials of Targeted Therapies for Advanced Ovarian Cancer. JAMA Netw. Open 2022, 5, e2245077. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Cordani, N.; Capici, S.; Cogliati, V.; Riva, F.; Cerrito, M. Metronomic Chemotherapy. Cancers 2021, 13, 2236. [Google Scholar] [CrossRef]
- Wysocki, P.J.; Lubas, M.T.; Wysocka, M.L. Metronomic Chemotherapy in Prostate Cancer. J. Clin. Med. 2022, 11, 2853. [Google Scholar] [CrossRef]
- Mpekris, F.; Baish, J.W.; Stylianopoulos, T.; Jain, R.K. Role of Vascular Normalization in Benefit from Metronomic Chemotherapy. Proc. Natl. Acad. Sci. USA 2017, 114, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Hsu, C.C.; Pai, V.C.; Liao, W.Y.; Huang, S.S.; Tan, K.T.; Yen, C.J.; Hsu, S.C.; Chen, W.Y.; Shan, Y.S.; et al. Metronomic Chemotherapy Prevents Therapy-Induced Stromal Activation and Induction of Tumor-Initiating Cells. J. Exp. Med. 2016, 213, 2967. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Improving Conventional or Low Dose Metronomic Chemotherapy with Targeted Antiangiogenic Drugs. Cancer Res. Treat. 2007, 39, 150. [Google Scholar] [CrossRef]
- Merritt, W.M.; Danes, C.G.; Shahzad, M.M.K.; Lin, Y.G.; Kamat, A.A.; Han, L.Y.; Spannuth, W.A.; Nick, A.M.; Mangala, L.S.; Stone, R.L.; et al. Anti-Angiogenic Properties of Metronomic Topotecan in Ovarian Carcinoma. Cancer Biol. Ther. 2009, 8, 1596–1603. [Google Scholar] [CrossRef]
- Banissi, C.; Ghiringhelli, F.; Chen, L.; Carpentier, A.F. Treg Depletion with a Low-Dose Metronomic Temozolomide Regimen in a Rat Glioma Model. Cancer Immunol. Immunother. 2009, 58, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.E.; Munzone, E.; Bocci, G.; Afonso, N.; Gomez, P.; Langkjer, S.; Petru, E.; Pivot, X.; Sánchez Rovira, P.; Wysocki, P.; et al. Pan-European Expert Meeting on the Use of Metronomic Chemotherapy in Advanced Breast Cancer Patients: The PENELOPE Project. Adv. Ther. 2019, 36, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou, P.; Hinsley, S.; McNeish, I.A.; Roxburgh, P.; Glasspool, R. Metronomic Oral Cyclophosphamide in Relapsed Ovarian Cancer. Int. J. Gynecol. Cancer 2021, 31, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Gebbia, V.; Boussen, H.; Valerio, M.R. Oral Metronomic Cyclophosphamide with and without Methotrexate as Palliative Treatment for Patients with Metastatic Breast Carcinoma. Anticancer Res. 2012, 32, 529–536. [Google Scholar] [PubMed]
- Montagna, E.; Bagnardi, V.; Cancello, G.; Sangalli, C.; Pagan, E.; Iorfida, M.; Mazza, M.; Mazzarol, G.; Dellapasqua, S.; Munzone, E.; et al. Metronomic Chemotherapy for First-Line Treatment of Metastatic Triple-Negative Breast Cancer: A Phase II Trial. Breast Care 2018, 13, 177–181. [Google Scholar] [CrossRef]
- Samaritani, R.; Corrado, G.; Vizza, E.; Sbiroli, C. Cyclophosphamide “Metronomic” Chemotherapy for Palliative Treatment of a Young Patient with Advanced Epithelial Ovarian Cancer. BMC Cancer 2007, 7, 65. [Google Scholar] [CrossRef]
- Ferrandina, G.; Corrado, G.; Mascilini, F.; Malaguti, P.; Samaritani, R.; Distefano, M.; Masciullo, V.; di Legge, A.; Savarese, A.; Scambia, G. Metronomic Oral Cyclophosphamide (MOC) in the Salvage Therapy of Heavily Treated Recurrent Ovarian Cancer Patients: A Retrospective, Multicenter Study. BMC Cancer 2014, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Pereira, L.; Liu, J.; Lee, H.; Lee, J.; Whalen, C.; Campos, S.; Atkinson, T.; Hill, M.; Berlin, S. Sequential Bevacizumab and Oral Cyclophosphamide for Recurrent Ovarian Cancer. Gynecol. Oncol. 2012, 126, 41–46. [Google Scholar] [CrossRef]
- Gulia, S.; Ghosh, J.; Bajpai, J.; Rath, S.; Maheshwari, A.; Shylasree, T.S.; Deodhar, K.; Thakur, M.; Gupta, S. Pazopanib and Oral Cyclophosphamide in Women With Platinum-Resistant or -Refractory Epithelial Ovarian Cancer. JCO Glob. Oncol. 2020, 6, 542–547. [Google Scholar] [CrossRef]
- ten Bokkel Huinink, W.; Gore, M.; Carmichael, J.; Gordon, A.; Malfetano, J.; Hudson, I.; Broom, C.; Scarabelli, C.; Davidson, N.; Spanczynski, M.; et al. Topotecan versus Paclitaxel for the Treatment of Recurrent Epithelial Ovarian Cancer. J. Clin. Oncol. 1997, 15, 2183–2193. [Google Scholar] [CrossRef]
- Gore, M.; Oza, A.; Rustin, G.; Malfetano, J.; Calvert, H.; Clarke-Pearson, D.; Carmichael, J.; Ross, G.; Beckman, R.A.; Fields, S.Z. A Randomised Trial of Oral versus Intravenous Topotecan in Patients with Relapsed Epithelial Ovarian Cancer. Eur. J. Cancer 2002, 38, 57–63. [Google Scholar] [CrossRef]
- Clarke-Pearson, D.L.; van Le, L.; Iveson, T.; Whitney, C.W.; Hanjani, P.; Kristensen, G.; Malfetano, J.H.; Beckman, R.A.; Ross, G.A.; Lane, S.R.; et al. Oral Topotecan as Single-Agent Second-Line Chemotherapy in Patients with Advanced Ovarian Cancer. J. Clin. Oncol. 2001, 19, 3967–3975. [Google Scholar] [CrossRef]
- Nagle, C.M.; Dixon, S.C.; Jensen, A.; Kjaer, S.K.; Modugno, F.; deFazio, A.; Fereday, S.; Hung, J.; Johnatty, S.E.; Fasching, P.A.; et al. Obesity and Survival among Women with Ovarian Cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.S.; Kim, H.J.; Hong, J.H.; Lee, J.K.; Lee, N.W.; Song, J.Y. Obesity and Epithelial Ovarian Cancer Survival: A Systematic Review and Meta-Analysis. J. Ovarian Res. 2014, 7, 41. [Google Scholar] [CrossRef]
- Bandera, E.V.; Lee, V.S.; Rodriguez-Rodriguez, L.; Bethan Powell, C.; Kushi, L.H. Impact of Chemotherapy Dosing on Ovarian Cancer Survival According to Body Mass Index. JAMA Oncol. 2015, 1, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Scharovsky, O.G.; Mainetti, L.E.; Rozados, V.R. Metronomic Chemotherapy: Changing the Paradigm That More Is Better. Curr. Oncol. 2009, 16, 7. [Google Scholar] [CrossRef]

| N | % | ||
|---|---|---|---|
| Population | Total number of patients | 72 | |
| Age | Median (years) | 60.5 | |
| <60 years of age | 34 | 47.2% | |
| ≥60 years of age | 38 | 52.8% | |
| Histological type | Serous | 48 | 66.7% |
| Other | 24 | 33.3% | |
| Histological grade | Low | 17 | 23.6% |
| High | 35 | 48.6% | |
| Unknown | 20 | 27.8% | |
| Stage at diagnosis (FIGO) | I | 5 | 6.9% |
| II | 4 | 5.6% | |
| III | 26 | 36.1% | |
| IV | 8 | 11.1% | |
| Unknown | 29 | 40.3% | |
| Body-Mass Index (BMI) | <25 | 23 | 31.9% |
| ≥25 | 31 | 43.1% | |
| Unknown | 18 | 25.0% | |
| Duration of palliative systemic treatment before initiation of metronomic chemotherapy | ≤12 months | 24 | 33.3% |
| >12 months | 45 | 62.5% | |
| Unknown | 3 | 4.2% | |
| Previous lines of chemotherapy | 1 | 17 | 24.3% |
| 2 | 19 | 27.1% | |
| 3 | 8 | 11.4% | |
| ≥4 | 14 | 20.0% | |
| Platinum-resistance | Yes | 31 | 43.1% |
| No | 40 | 55.6% | |
| Unknown | 1 | 1.4% | |
| Number of sites with recurrent/metastatic disease | 1 | 4 | 5.6% |
| 2 | 17 | 23.6% | |
| 3–4 | 26 | 36.1% | |
| ≥5 | 16 | 22.2% | |
| Metronomic chemotherapy | Topotecan | 18 | 25.0% |
| Topotecan → CyTo | 9 | 12.50% | |
| CyTo | 45 | 62.50% |
| G 1–4 | G 3–4 | |||
|---|---|---|---|---|
| N | % | N | % | |
| Any AE | 57 | 89.1% | 21 | 32.8% |
| neutropenia | 35 | 64.8% | 10 | 18.5% |
| thrombocytopenia | 18 | 34.6% | 2 | 3.8% |
| anemia | 45 | 83.3% | 14 | 25.9% |
| transaminase elevation | 21 | 42.0% | 2 | 4.0% |
| bilirubine elevation | 3 | 6.0% | 1 | 2.0% |
| creatinine elevation | 16 | 31.4% | 0 | 0.0% |
| nausea | 9 | 14.1% | 0 | 0.0% |
| constipation | 4 | 6.3% | 0 | 0.0% |
| diarrhea | 0 | 0.0% | 0 | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocki, P.J.; Łobacz, M.; Potocki, P.; Kwinta, Ł.; Michałowska-Kaczmarczyk, A.; Słowik, A.; Konopka, K.; Buda-Nowak, A. Metronomic Chemotherapy Based on Topotecan or Topotecan and Cyclophosphamide Combination (CyTo) in Advanced, Pretreated Ovarian Cancer. Cancers 2023, 15, 1067. https://doi.org/10.3390/cancers15041067
Wysocki PJ, Łobacz M, Potocki P, Kwinta Ł, Michałowska-Kaczmarczyk A, Słowik A, Konopka K, Buda-Nowak A. Metronomic Chemotherapy Based on Topotecan or Topotecan and Cyclophosphamide Combination (CyTo) in Advanced, Pretreated Ovarian Cancer. Cancers. 2023; 15(4):1067. https://doi.org/10.3390/cancers15041067
Chicago/Turabian StyleWysocki, Piotr J., Mateusz Łobacz, Paweł Potocki, Łukasz Kwinta, Anna Michałowska-Kaczmarczyk, Agnieszka Słowik, Kamil Konopka, and Anna Buda-Nowak. 2023. "Metronomic Chemotherapy Based on Topotecan or Topotecan and Cyclophosphamide Combination (CyTo) in Advanced, Pretreated Ovarian Cancer" Cancers 15, no. 4: 1067. https://doi.org/10.3390/cancers15041067
APA StyleWysocki, P. J., Łobacz, M., Potocki, P., Kwinta, Ł., Michałowska-Kaczmarczyk, A., Słowik, A., Konopka, K., & Buda-Nowak, A. (2023). Metronomic Chemotherapy Based on Topotecan or Topotecan and Cyclophosphamide Combination (CyTo) in Advanced, Pretreated Ovarian Cancer. Cancers, 15(4), 1067. https://doi.org/10.3390/cancers15041067







