The Role of P16, P53, KI-67 and PD-L1 Immunostaining in Primary Vaginal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Patients
2.3. Tissue Microarray (TMA) Creation and Immunhistochemistry
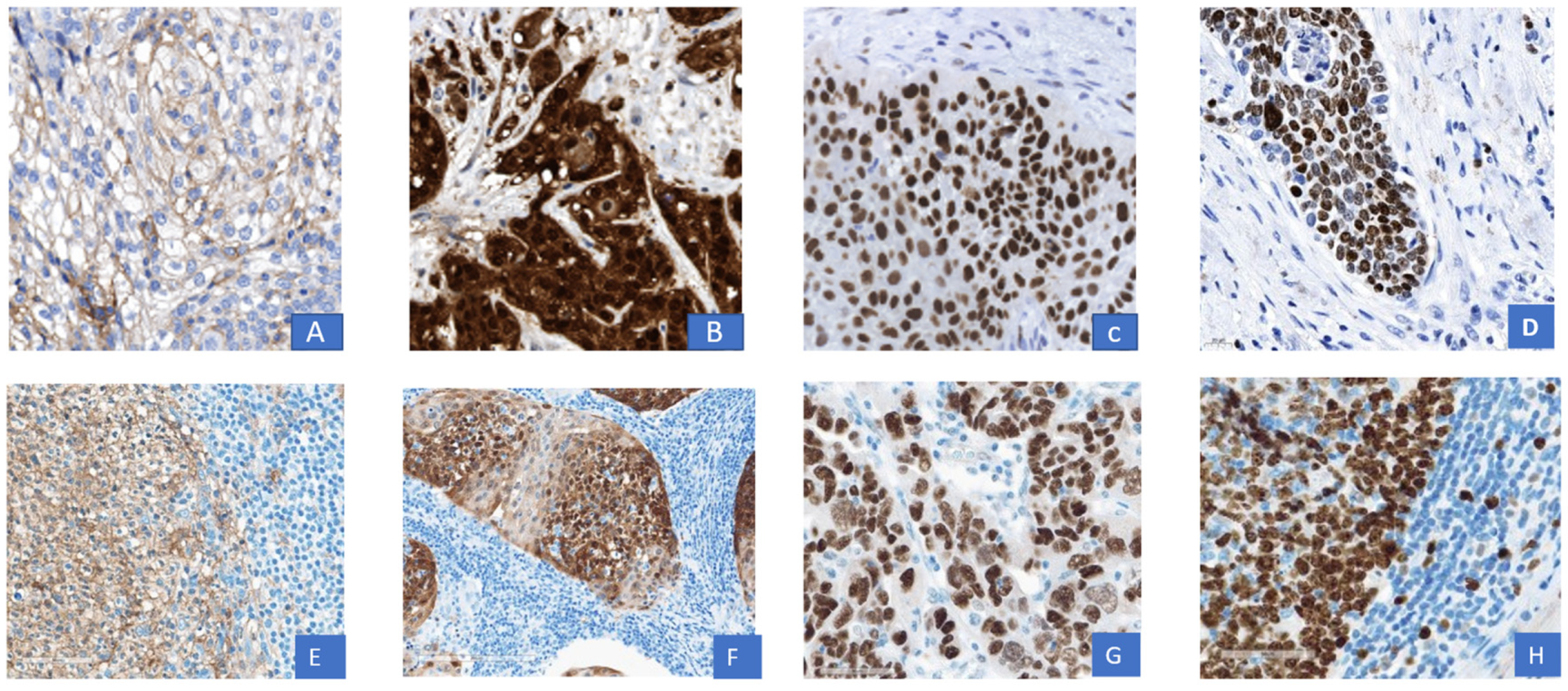
2.4. Evaluation of Immunohistochemistry (IHC)
2.5. Statistical Analysis
3. Results
3.1. General Patient Characteristics
3.2. 5-Year-Survival Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.C.; Höhn, A.K.; Hampl, M.; Mehlhorn, G.; Follmann, M.; Schnürch, H.G. Interdisciplinary S2k guidelines on the diagnosis and treatment of vaginal carcinoma and its precursors-recommendations on surgical pathology for histopathological workup, diagnostics, and reporting. Pathologe 2021, 42, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.J.; Deavers, M.T.; Jhingran, A.; Bodurka, D.C.; Eifel, P.J. Primary adenocarcinoma of the vagina not associated with diethylstilbestrol (DES) exposure. Gynecol. Oncol. 2007, 105, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the Vagina. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Laliscia, C.; Gadducci, A.; Fabrini, M.G.; Barcellini, A.; Guerrieri, M.E.; Parietti, E.; Ursino, S.; Morganti, R.; Cafaro, I.; Paiar, F.; et al. Definitive Radiotherapy for Primary Squamous Cell Carcinoma of the Vagina: Are High-Dose External Beam Radiotherapy and High-Dose-Rate Brachytherapy Boost the Best Treatment? Experience of Two Italian Institutes. Oncol. Res. Treat. 2017, 40, 697–701. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Usta, E.H.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef]
- Kunos, C.A.; Andrews, S.J.; Moore, K.N.; Chon, H.S.; Ivy, S.P. Randomized Phase II Trial of Triapine-Cisplatin-Radiotherapy for Locally Advanced Stage Uterine Cervix or Vaginal Cancers. Front. Oncol. 2019, 15, 1067. [Google Scholar] [CrossRef]
- Naumann, R.W.; Hollebecque, A.; Meyer, T.; Devlin, M.J.; Oaknin, A.; Kerger, J.; López-Picazo, J.M.; Machiels, J.P.; Delord, J.P.; Evans, T.F.J.; et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: Results from the Phase I/II CheckMate 358 Trial. J. Clin. Oncol. 2019, 37, 2825–2834. [Google Scholar] [CrossRef]
- How, J.A.; Jazaeri, A.A.; Soliman, P.T.; Fleming, N.D.; Gong, J.; Piha-Paul, S.A.; Janku, F.; Stephen, B.; Naing, A. Pembrolizumab in vaginal and vulvar squamous cell carcinoma: A case series from a phase II basket trial. Sci. Rep. 2021, 11, 3667. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Cassier, P.; Rolland, F.; Makhloufi, H.; Bendjama, K.; Delord, J.P. 63MO TG4001 therapeutic vaccination combined with PD-L1 blocker avelumab remodels the tumor microenvironement (TME) and drives antitumor responses in human papillomavirus (HPV)+ malignancies. Ann. Oncol. 2020, 7, 1442. [Google Scholar] [CrossRef]
- Ferenzcy, A.S.; Colgan, T.J.; Herrington, C.S.; Hirschowitz, L.; Löning, T.; Park, K.J.; Stoler, M.; Wells, M.; Wilbur, D.C.; Wright, T. Epithelial Tumors of the Vagina; Kurman, R., Ed.; IARC Press: Lyon, France, 2014; pp. 210–217. ISBN 9789283224358. [Google Scholar]
- Wittekind, C. TNM: Klassifikation maligner Tumoren, 8th ed.; Wittekind, C., Ed.; John Wiley & Sons: Leipzig, Germany, 2016; pp. 213–215. ISBN 978-3-527-34280-8. [Google Scholar]
- Adams, T.S.; Cuello, M.A. Cancer of the vagina. Int. J. Gynecol. Obstet. 2018, 143, 14–21. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Kortekaas, K.E.; Thompson, E.; Pors, J.; Chen, J.; Ho, J.; Prentice, L.M.; McConechy, M.K.; Chow, C.; Proctor, L.; et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correlation with TP53 mutation status. Mod. Pathol. 2020, 33, 1595–1605. [Google Scholar] [CrossRef]
- Pirog, E.C. Immunohistochemistry and in situ hybridization for the diagnosis and classification of squamous lesions of the anogenital region. Semin. Diagn. Pathol. 2015, 32, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Egger, E.K.; Ralser, D.J.; Lindner, K.; Recker, F.; Marinova, M.; Savchenko, O.; Lau, J.F.; Mustea, A. Diagnostic and Therapeutic Approach in a Metastatic Vaginal Adenocarcinoma: A Case Report. Front. Immunol. 2021, 12, 686879. [Google Scholar] [CrossRef] [PubMed]
- Schnürch, H.G.; Ackermann, S.; Alt-Radtke, C.D.; Angleitner, L.; Barinoff, J.; Beckmann, M.W.; Böing, C.; Dannecker, C.; Fehm, T.; Gaase, R.; et al. Diagnosis, Therapy and Follow-up of Vaginal Cancer and Its Precursors. Guideline of the DGGG and the DKG (S2k-Level, AWMF Registry No. 032/042). Geburtshilfe Frauenheilkd. 2019, 79, 1060–1078. [Google Scholar] [CrossRef]
- Jhingran, A. Updates in the treatment of vaginal cancer. Int. J. Gynecol. Cancer 2022, 32, 344–351. [Google Scholar] [CrossRef]
- Yang, J.; Delara, R.; Magrina, J.; Magtibay, P.; Langstraat, C.; Dinh, T.; Karlin, N.; Vora, S.A.; Butler, K. Management and outcomes of primary vaginal Cancer. Gynecol. Oncol. 2020, 159, 456–463. [Google Scholar] [CrossRef]
- Hellman, K.; Lindquist, D.; Ranhem, C.; Wilander, E.; Andersson, S. Human papillomavirus, p16(INK4A), and Ki-67 in relation to clinicopathological variables and survival in primary carcinoma of the vagina. Br. J. Cancer 2014, 110, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Larsson, G.L.; Helenius, G.; Andersson, S.; Sorbe, B.; Karlsson, M.G. Prognostic impact of human papilloma virus (HPV) genotyping and HPV-16 subtyping in vaginal carcinoma. Gynecol. Oncol. 2013, 129, 406–411. [Google Scholar] [CrossRef]
- Alonso, I.; Felix, A.; Torné, A.; Fusté, V.; Del Pino, M.; Castillo, P.; Balasch, J.; Pahisa, J.; Rios, J.; Ordiet, J. Human papillomavirus as a favorable prognostic biomarker in squamous cell carcinomas of the vagina. Gynecol. Oncol. 2012, 125, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.H.; Grimm, C.; Polterauer, S.; Hefler, L.; Stani, J.; Heinze, G.; Horvat, R. The prognostic role of human papillomavirus in patients with vaginal cancer. Int. J. Gynecol. Cancer 2011, 21, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, C.R.; Harris, J.P.; Chin, A.; Von Eyben, R.; Giaretta, S.; Shaffer, J.L.; Hiniker, S.M.; Kapp, D.S.; Folkins, A.K.; Kidd, E.A. Prognostic Significance of P16 Expression and P53 Expression in Primary Vaginal Cancer. Int. J. Gynecol. Pathol. 2019, 38, 588–596. [Google Scholar] [CrossRef]
- Fuste, V.; Del Pino, M.; Perez, A.; Garcia, A.; Torne, A.; Pahisa, J.; Ordi, J. Primary squamous cell carcinoma of the vagina: Human papillomavirus detection, p16(INK4A) overexpression and clinicopathological correlations. Histopathology 2010, 57, 907–916. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, J.P.; Bryant, C.S.; Imudia, A.N.; Ali-Fehmi, R.; Malone, J.M.; Morris, R.T. Prognostic significance of keratinization in squamous cell cancer of uterine cervix: A population based study. Arch. Gynecol. Obstet. 2009, 280, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, W.K.; Kim, Y.J.; Kim, Y.S.; Park, W. Significance of the number of high-risk factors in patients with cervical cancer treated with radical hysterectomy and concurrent chemoradiotherapy. Gynecol. Oncol. 2020, 157, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Welters, M.J.; Van Der Sluis, T.C.; Van Meir, H.; Loof, N.M.; Van Ham, V.J.; Van Duikeren, S.; Saskia, J.; Santegoets, R.A.; de Kam, M.L.; Cohen, A.F.; et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 2016, 8, 334ra52. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Zeng, Q.; Guo, X.J.; Wang, H.; Liu, H.H.; Dong, Z.Y. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Reports 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Tian, H.; Du, W.; Zhao, L.; Feng, J.; Yuan, D.; Li, Z. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 2017, 15, 1063–1070. [Google Scholar] [CrossRef]
- Rice, A.E.; Latchman, Y.E.; Balint, J.P.; Lee, J.H.; Gabitzsch, E.S.; Jones, F.R. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther. 2015, 22, 454–462. [Google Scholar] [CrossRef]
- Rotman, J.; de Otter, L.A.S.; Bleeker, M.C.G.; Samuels, S.S.; Heeren, A.M.; Roemer, M.G.M.; Kenter, G.G.; Zijlmans, H.J.M.A.A.; van Trommel, N.E.; de Gruijl, T.D.; et al. PD-L1 and PD-L2 Expression in Cervical Cancer: Regulation and Biomarker Potential. Front. Immunol. 2020, 11, 3281. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Limet, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Ros, W.; Delord, J.P.; Perets, R.; Italiano, A.; Shapira-Frommer, R.; Manzuk, L.; Piha-Paul, S.A.; Xu, L.; Zeigenfuss, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 37, 1470–1478. [Google Scholar] [CrossRef]
- Shapira-Frommer, R.; Mileshkin, L.; Manzyuk, L.; Penel, L.; Burge, M.; Piha-Paul, S.A.; Girda, E.; Lopez Martin, J.A.; van Dongen, M.G.J.; Italiano, A.; et al. Efficacy and safety of pembrolizumab for patients with previously treated advanced vulvar squamous cell carcinoma: Results from the phase 2 KEYNOTE-158 study. Gynecol. Oncol. 2022, 166, 211–218. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Monk, B.J.; Penson, R.T.; Long, H.J.; Poveda, A.; Landrum, L.M.; Leitao, M.M.; Brown, J.; Reid, T.J.A.; et al. Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG Oncology/GOG study. Clin. Cancer Res. 2015, 21, 5480–5487. [Google Scholar] [CrossRef]
- Smith, J.S.; Backes, D.M.; Hoots, B.E.; Kurman, R.J.; Pimenta, J.M. Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet. Gynecol. 2009, 113, 917–924. [Google Scholar] [CrossRef]
- Haręża, D.A.; Wilczyński, J.R.; Paradowska, E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022, 23, 1818. [Google Scholar] [CrossRef] [PubMed]
- Choschzick, M.; Hantaredja, W.; Tennstedt, P.; Gieseking, F.; Wölber, L.; Simon, R. Role of TP53 mutations in vulvar carcinomas. Int. J. Gynecol. Pathol. 2011, 30, 497–504. [Google Scholar] [CrossRef]
- Xing, D.; Fadare, O. Molecular events in the pathogenesis of vulvar squamous cell carcinoma. Semin. Diagn. Pathol. 2021, 38, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.L.; Bertoli, H.K.; Sand, F.L.; Kjær, A.K.; Thomsen, L.T.; Kjær, S.K. The prognostic significance of HPV, p16, and p53 protein expression in vaginal cancer: A systematic review. Acta Obstet. Gynecol. Scand. 2021, 100, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Koyamatsu, Y.; Yokoyama, M.; Nakao, Y.; Fukuda, K.; Saito, T.; Matsukuma, K.; Iwasaka, T. A comparative analysis of human papillomavirus types 16 and 18 and expression of p53 gene and Ki-67 in cervical, vaginal, and vulvar carcinomas. Gynecol. Oncol. 2003, 90, 547–551. [Google Scholar] [CrossRef] [PubMed]
| Pt | Age | 1st Line | 2nd Line | 3rd Line | 4th Line | 5th Line | Rec 1. No 2. Yes | OS (mo) | Survival 1. Dead 2. Alive | FIGO | KI-67 1. >60% 2. ≤60% | PDL1 +≥1 −<1 | p53 1. wt 2. mut | P16 +/− | Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | CC/PL | 1 | 13 | 2 | II | 1 | + | 1 | + | KSCC | ||||
| 2 | 61 | CC/PL | 1 | 25 | 2 | I | 1 | + | 1 | + | NKSCC | ||||
| 3 | 62 | PC/PL | 1 | 26 | 2 | I | 2 | + | 2 | + | KSCC | ||||
| 4 | 87 | PC/SEN | 1 | 24 | 2 | I | 1 | + | 1 | + | NKSCC | ||||
| 5 | 64 | RHPC/PL | 1 | 48 | 2 | I | 2 | + | 1 | + | NKSCC | ||||
| 6 | 81 | PS | 1 | 3 | 1 | IV | 2 | + | 1 | + | KSCC | ||||
| 7 | 47 | PE | 1 | 156 | 2 | II | 1 | − | 2 | + | NKSCC | ||||
| 8 | 53 | PE | 1 | 108 | 2 | IV | 2 | + | 1 | + | ASCC | ||||
| 9 | 72 | PE | 1 | 4 | 2 | IV | 2 | + | 1 | − | KSCC | ||||
| 10 | 66 | PE | 1 | 12 | 2 | II | 2 | + | 1 | − | CCAC | ||||
| 11 | 87 | PE | 1 | 17 | 2 | II | 2 | − | 1 | + | KSCC | ||||
| 12 | 78 | PE | 1 | 4 | 1 | II | 2 | + | 2 | − | KSCC | ||||
| 13 | PE | IL->RT | HC-> GOG240 | 2 | 68 | 2 | II | 2 | + | 1 | + | KSCC | |||
| 14 | 71 | PE | CARB | 2 | 14 | 1 | IV | 2 | + | 1 | + | NKSCC | |||
| 15 | 68 | PE | IL | 2 | 12 | 1 | III | 2 | − | 2 | − | NKSCC | |||
| 16 | 65 | PE | 2 | 9 | 1 | IV | 2 | + | 2 | + | NKSCC | ||||
| 17 | 72 | CC/PL | 2 | 96 | I | 2 | + | 1 | + | NKSCC | |||||
| 18 | 32 | RHPC/PL ->CCRT | CIS/PAC | PE | 2 | 17 | 1 | II | 1 | + | 1 | + | KSCC | ||
| 19 | 51 | GOG240 | CIS | PAC/TRA | TD-M1 | PEM/RT | 2 | 48 | 2 | IV | 2 | + | 2 | + | CCAC |
| 20 | 75 | CCRT | 2 | 7 | 1 | II | 1 | + | 2 | − | NKSCC | ||||
| 21 | 52 | CCRT | TACP | 2 | 18 | 1 | III | 2 | + | 2 | + | KSCC | |||
| 22 | 49 | CCRT | PE | RT | PS | 2 | 38 | 1 | III | 2 | + | 2 | + | KSCC |
| Parameter | N/% | 5-Year DFS | 5-Year OS |
|---|---|---|---|
| FIGO | N = 22 | ||
| I | 5/22.7% | 100% | 100.00% |
| II | 8/36.4% | 50% | 56.25% |
| III | 3/13.6% | 0% | 0% |
| IV | 6/27.3% | 20.83% | 41.67% |
| Log-rank: | p-value: 0.223 | p-value: 0.147 | |
| N | N = 21 + | ||
| N0 | 11/50% | 81.22% | 90.91% |
| N1 | 10/45.5% | 11.43% | 22.86% |
| Log-Rank | p-value: 0.004 | p-value: 0.004 | |
| L | N = 21 * | ||
| L0 | 14/63.6% | 71.43% | 68.57% |
| L1 | 7/31.8% | 0% | 17.14% |
| Log-Rank | p-value: <0.001 | p-value: 0.009 | |
| H | N = 21 * | ||
| H0 | 17/77.3% | 63.103% | 67.97% |
| H1 | 4/18.2% | 0% | 0% |
| Log-rank | p-value: 0.003 | p-value: 0.002 | |
| Tumor location | N = 22 | ||
| Upper third | 6/27.3% | 50.00% | 66.67% |
| Other Location | 16/72.7% | 50.91% | 47.40% |
| Log rank | p-value: 0.846 | p-value: 0.482 | |
| Tumor size | N = 22 | ||
| </=4 cm | 11/50% | 60% | 68.57% |
| >4 cm | 11/50% | 36.36% | 33.94% |
| Log-Rank | p-value: 0.249 | p-value: 0.141 | |
| </=2 cm | 4/18.2% | 75% | 75% |
| >2 cm | 18/81.8% | 39.68% | 36.77% |
| Log-Rank | p-value: 0.406 | p-value: 0.322 | |
| T | N = 22 | ||
| T1 | 7/31.8% | 71.43% | 71.43% |
| T2 | 10/45.5% | 33.33% | 35.56% |
| T3/T4 | 5/22.7% | 26.67% | 40.00% |
| Log-Rank | p-value: 0.627 | p-value:0.624 | |
| R | N = 19 # | ||
| R0 | 13/59.1% | 75.52% | 73.43% |
| R1 | 6/27.3% | 25.00% | 50.00% |
| Log-Rank | p-value:0.116 | p-value: 0.466 | |
| Age | N = 22 | ||
| </=64 years | 11/50% | 53.03% | 64% |
| >64 years | 11/50% | 40.91% | 32.91% |
| Log-Rank | p-value: 0.221 | p-value: 0.029 | |
| Depth of infiltration in mm | N = 20 − | ||
| </=5 | 8/36.5% | 75% | 58.33% |
| >5 | 12/54.5% | 37.04% | 51.33% |
| Log-rank | p-value: 0.043 | p-value: 0.248 | |
| P16 | N = 22 | ||
| negative | 5/22.7% | 26.67% | 26.67% |
| Positive | 17/77.3% | 52.29% | 57.30% |
| Log-rank | p-value:0.230 | p-value: 0.010 | |
| P53 | N = 22 | ||
| Wild type | 13/59.1% | 67.69% | 71.80% |
| mutated | 9/40.9% | 22.22% | 22.22% |
| Log rank | p-value: 0.164 | p-value: 0.091 | |
| KI-67 | N = 22 | ||
| <60% | 15/68.2% | 42.42% | 50.05% |
| >/=60% | 7/31.8% | 57.14% | 53.57% |
| Log rank | p-value: 0.541 | p-value: 0.814 |
| Factor | p-Value: |
|---|---|
| Age | 0.365 |
| Lymph node metastasis | 0.555 |
| Hemangiosis | 0.953 |
| Lymphangiosis | 0.947 |
| P 16 | 0.920 |
| Factor | PD-L1-CPS > 1 | Ki67 | P53 | P16 |
|---|---|---|---|---|
| FIGO I versus FIGO II-IV | p-value: 1.000 | p-value: 0.2743 | p-value: 1.0000 | p-value: 0.2899 |
| Tumor location in the cranial Vagina versus other locations | p-value: 1.000 | p-value: 0.1207 | p-value: 0.3330 | p-value: 1.0000 |
| Tumor size ≤4/>4 cm | p-value: 0.0152 | p-value: 0.1984 | p-value: 0.0805 | p-value: 1.0000 |
| Depth of infiltration ≤5/>5 mm | p-value: 1.000 | p-value: 0.6126 | p-value:1.000 | p-value: 1.0000 |
| Lymph node negative versus lymph node metastasis | p-value: 0.5765 | p-value: 1.0000 | p-value: 0.0805 | p-value: 1.0000 |
| L0 versus L1 | p-value: 1.000 | p-value: 0.6384 | p-value: 0.0260 | p-value: 1.0000 |
| V0 versus V1 | p-value: 0.465 | p-value: 0.5743 | p-value: 0.0096 | p-value: 0.2098 |
| PD-L1-CPS | N/% | DFS | OS |
|---|---|---|---|
| </=5 | 7 | 71.43% | 85, 71% |
| >5 | 15 | 34.91% | 36.11% |
| Log-rank | p-value: 0.110 | p-value: 0.116 | |
| PD-L1-TPS | |||
| <10 | 11 | 60% | 60% |
| >/=10 | 11 | 30.49% | 41.56% |
| Log-rank | p-value: 0.110 | p-value:0.205 | |
| PD-L1-IC | |||
| </=1 | 12 | 48.61% | 54.69% |
| >1 | 10 | 45.71% | 49.22% |
| Log-rank | p-value: 0.650 | p-value: 0.923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egger, E.K.; Condic, M.; Ralser, D.J.; Marinova, M.; Mustea, A.; Recker, F.; Kristiansen, G.; Thiesler, T. The Role of P16, P53, KI-67 and PD-L1 Immunostaining in Primary Vaginal Cancer. Cancers 2023, 15, 1046. https://doi.org/10.3390/cancers15041046
Egger EK, Condic M, Ralser DJ, Marinova M, Mustea A, Recker F, Kristiansen G, Thiesler T. The Role of P16, P53, KI-67 and PD-L1 Immunostaining in Primary Vaginal Cancer. Cancers. 2023; 15(4):1046. https://doi.org/10.3390/cancers15041046
Chicago/Turabian StyleEgger, Eva K., Mateja Condic, Damian J. Ralser, Milka Marinova, Alexander Mustea, Florian Recker, Glen Kristiansen, and Thore Thiesler. 2023. "The Role of P16, P53, KI-67 and PD-L1 Immunostaining in Primary Vaginal Cancer" Cancers 15, no. 4: 1046. https://doi.org/10.3390/cancers15041046
APA StyleEgger, E. K., Condic, M., Ralser, D. J., Marinova, M., Mustea, A., Recker, F., Kristiansen, G., & Thiesler, T. (2023). The Role of P16, P53, KI-67 and PD-L1 Immunostaining in Primary Vaginal Cancer. Cancers, 15(4), 1046. https://doi.org/10.3390/cancers15041046









