Multi-Targeted Neutron Capture Therapy Combined with an 18 kDa Translocator Protein-Targeted Boron Compound Is an Effective Strategy in a Rat Brain Tumor Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Boron Compounds
2.2. Cell Culture
2.3. The F98 Rat Glioma Bearing Brain Tumor Model and the Implantation of the Alzet Osmotic Pump
2.4. Cellular Uptake of Boron in Rat Glioma Cell Lines
2.5. Estimating the Compound Biological Effectiveness on an In Vitro Neutron Irradiation Experiment
2.6. Evaluating the TSPO Expression in F98 Rat Glioma Model
2.7. Biodistribution of Boron Compounds in F98 Rat Glioma Models
2.8. Survival Analysis of F98 Rat Glioma Models on an In Vivo Neutron Irradiation Experiment
2.9. Doses Calculation
2.10. Statistical Analysis
3. Results
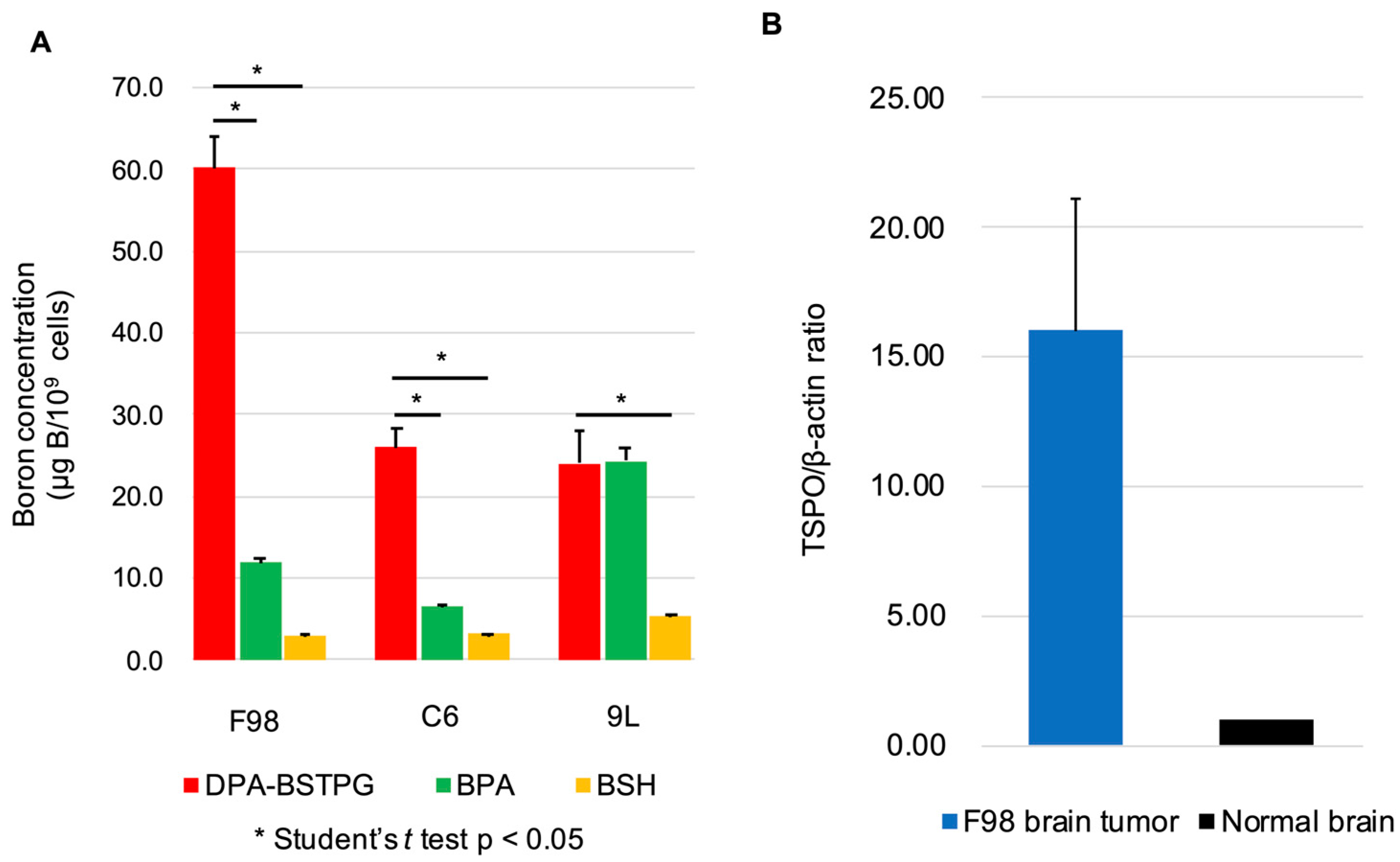
3.1. Cellular Uptake of Boron in Rat Glioma Cell Lines
3.2. The TSPO Expressions in the F98 Rat Glioma Model
3.3. Estimating the Compound Biological Effectiveness on an In Vitro Neutron Irradiation Experiment
3.4. Biodistribution of Boron Compounds in F98 Rat Glioma Models
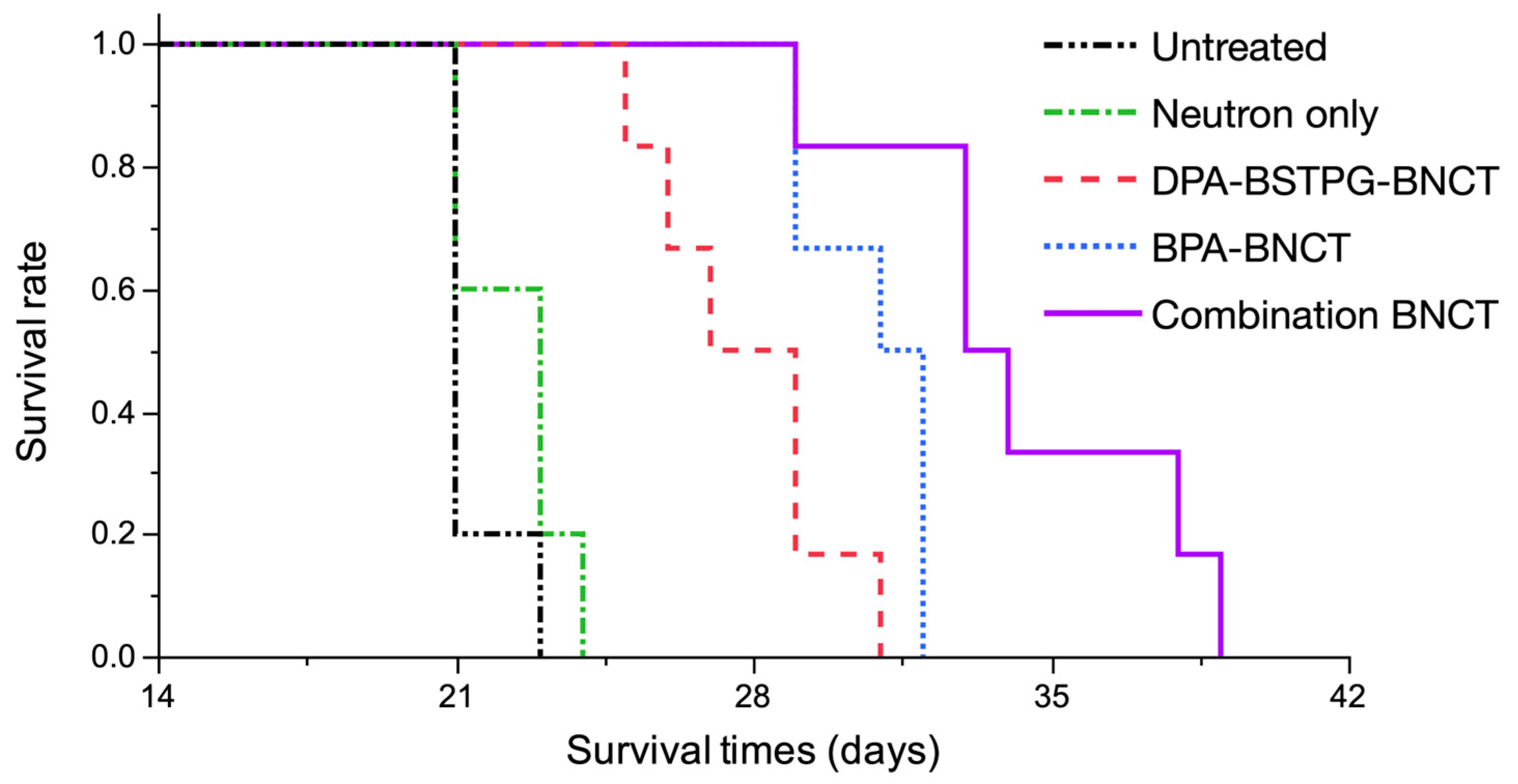
3.5. Survival Analysis of the F98 Rat Glioma Model on an In Vivo Neutron Irradiation Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Boron Compound a | Time b | n c | Boron Concentrations ± SD (μg B/g) d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain | Blood | Heart | Lung | Liver | Kidney | Spleen | Skin | Muscle | |||
| DPA-BSTPG | 2.5 | 6 | 0.8 ± 0.8 | 0.4 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 12 | 4 | 0.8 ± 0.8 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.2 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| 24 | 4 | 1.0 ± 1.4 | 0.4 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | |
References
- Kawabata, S.; Miyatake, S.-I.; Kuroiwa, T.; Yokoyama, K.; Doi, A.; Iida, K.; Miyata, S.; Nonoguchi, N.; Michiue, H.; Takahashi, M.; et al. Boron Neutron Capture Therapy for Newly Diagnosed Glioblastoma. J. Radiat. Res. 2009, 50, 51–60. [Google Scholar] [CrossRef]
- Henriksson, R.; Capala, J.; Michanek, A.; Lindahl, S.A.; Salford, L.G.; Franzén, L.; Blomquist, E.; Westlin, J.E.; Bergenheim, A.T.; Group, S.B.T.S. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: A phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA). Radiother. Oncol. 2008, 88, 183–191. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nakai, K.; Kageji, T.; Kumada, H.; Endo, K.; Matsuda, M.; Shibata, Y.; Matsumura, A. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother. Oncol. 2009, 91, 80–84. [Google Scholar] [CrossRef]
- Sköld, K.; Gorlia, T.; Pellettieri, L.; Giusti, V.; H-Stenstam, B.; Hopewell, J.W. Boron neutron capture therapy for newly diagnosed glioblastoma multiforme: An assessment of clinical potential. Br. J. Radiol. 2010, 83, 596–603. [Google Scholar] [CrossRef]
- Miyatake, S.-I.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J. Neuro-Oncol. 2009, 91, 199–206. [Google Scholar] [CrossRef]
- Pellettieri, L.; H-Stenstam, B.; Rezaei, A.; Giusti, V.; Sköld, K. An investigation of boron neutron capture therapy for recurrent glioblastoma multiforme. Acta Neurol. Scand. 2008, 117, 191–197. [Google Scholar] [CrossRef]
- Hirose, K.; Konno, A.; Hiratsuka, J.; Yoshimoto, S.; Kato, T.; Ono, K.; Otsuki, N.; Hatazawa, J.; Tanaka, H.; Takayama, K.; et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan. Radiother. Oncol. 2021, 155, 182–187. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, Y.Y.; Lin, C.F.; Pan, P.S.; Chen, J.K.; Wang, C.W.; Hsu, S.M.; Kuo, Y.C.; Lan, T.L.; Hsu, S.P.C.; et al. Salvage Boron Neutron Capture Therapy for Malignant Brain Tumor Patients in Compliance with Emergency and Compassionate Use: Evaluation of 34 Cases in Taiwan. Biology 2021, 10, 334. [Google Scholar] [CrossRef]
- Barth, R.F.; H Vicente, M.; Harling, O.K.; Kiger, W.; Riley, K.J.; Binns, P.J.; Wagner, F.M.; Suzuki, M.; Aihara, T.; Kato, I.; et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat. Oncol. 2012, 7, 146. [Google Scholar] [CrossRef]
- Barth, R.F.; Soloway, A.H.; Goodman, J.H.; Gahbauer, R.A.; Gupta, N.; Blue, T.E.; Yang, W.; Tjarks, W. Boron neutron capture therapy of brain tumors: An emerging therapeutic modality. Neurosurgery 1999, 44, 433–450; discussion 450–451. [Google Scholar] [CrossRef]
- Kawabata, S.; Suzuki, M.; Hirose, K.; Tanaka, H.; Kato, T.; Goto, H.; Narita, Y.; Miyatake, S.-I. Accelerator-based BNCT for patients with recurrent glioblastoma: A multicenter phase II study. Neuro-Oncol. Adv. 2021, 3, vdab067. [Google Scholar] [CrossRef]
- Wittig, A.; Sauerwein, W.A.; Coderre, J.A. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat. Res. 2000, 153, 173–180. [Google Scholar] [CrossRef]
- Smith, D.R.; Chandra, S.; Coderre, J.A.; Morrison, G.H. Ion microscopy imaging of 10B from p-boronophenylalanine in a brain tumor model for boron neutron capture therapy. Cancer Res. 1996, 56, 4302–4306. [Google Scholar]
- Ono, K.; Masunaga, S.I.; Kinashi, Y.; Takagaki, M.; Akaboshi, M.; Kobayashi, T.; Akuta, K. Radiobiological evidence suggesting heterogeneous microdistribution of boron compounds in tumors: Its relation to quiescent cell population and tumor cure in neutron capture therapy. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 1081–1086. [Google Scholar] [CrossRef]
- Yokoyama, K.; Miyatake, S.; Kajimoto, Y.; Kawabata, S.; Doi, A.; Yoshida, T.; Asano, T.; Kirihata, M.; Ono, K.; Kuroiwa, T. Pharmacokinetic study of BSH and BPA in simultaneous use for BNCT. J. Neurooncol. 2006, 78, 227–232. [Google Scholar] [CrossRef]
- Elowitz, E.H.; Bergland, R.M.; Coderre, J.A.; Joel, D.D.; Chadha, M.; Chanana, A.D. Biodistribution of p-boronophenylalanine in patients with glioblastoma multiforme for use in boron neutron capture therapy. Neurosurgery 1998, 42, 463–468; discussion 468–469. [Google Scholar] [CrossRef]
- Finkel, G.C.; Poletti, C.E.; Fairchild, R.G.; Slatkin, D.N.; Sweet, W.H. Distribution of 10B after infusion of Na210B12H11SH into a patient with malignant astrocytoma: Implications for boron neutron capture therapy. Neurosurgery 1989, 24, 6–11. [Google Scholar] [CrossRef]
- Ammer, L.M.; Vollmann-Zwerenz, A.; Ruf, V.; Wetzel, C.H.; Riemenschneider, M.J.; Albert, N.L.; Beckhove, P.; Hau, P. The Role of Translocator Protein TSPO in Hallmarks of Glioblastoma. Cancers 2020, 12, 2973. [Google Scholar] [CrossRef]
- Riond, J.; Mattei, M.G.; Kaghad, M.; Dumont, X.; Guillemot, J.C.; Le Fur, G.; Caput, D.; Ferrara, P. Molecular cloning and chromosomal localization of a human peripheral-type benzodiazepine receptor. Eur. J. Biochem. 1991, 195, 305–311. [Google Scholar] [CrossRef]
- Chang, Y.J.; McCabe, R.T.; Rennert, H.; Budarf, M.L.; Sayegh, R.; Emanuel, B.S.; Skolnick, P.; Strauss, J.F. The human “peripheral-type” benzodiazepine receptor: Regional mapping of the gene and characterization of the receptor expressed from cDNA. DNA Cell Biol. 1992, 11, 471–480. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharm. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Jensen, P.; Feng, L.; Law, I.; Svarer, C.; Knudsen, G.M.; Mikkelsen, J.D.; De Nijs, R.; Larsen, V.A.; Dyssegaard, A.; Thomsen, G.; et al. TSPO Imaging in Glioblastoma Multiforme: A Direct Comparison Between 123I-CLINDE SPECT, 18F-FET PET, and Gadolinium-Enhanced MR Imaging. J. Nucl. Med. 2015, 56, 1386–1390. [Google Scholar] [CrossRef]
- Su, Z.; Roncaroli, F.; Durrenberger, P.F.; Coope, D.J.; Karabatsou, K.; Hinz, R.; Thompson, G.; Turkheimer, F.E.; Janczar, K.; Du Plessis, D.; et al. The 18-kDa Mitochondrial Translocator Protein in Human Gliomas: An 11C-(R)PK11195 PET Imaging and Neuropathology Study. J. Nucl. Med. 2015, 56, 512–517. [Google Scholar] [CrossRef]
- Roncaroli, F.; Su, Z.; Herholz, K.; Gerhard, A.; Turkheimer, F.E. TSPO expression in brain tumours: Is TSPO a target for brain tumour imaging? Clin. Transl. Imaging 2016, 4, 145–156. [Google Scholar] [CrossRef]
- Leroy, C.; Saba, W. Contribution of TSPO imaging in the understanding of the state of gliosis in substance use disorders. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 186–200. [Google Scholar] [CrossRef]
- Vlodavsky, E.; Soustiel, J.F. Immunohistochemical expression of peripheral benzodiazepine receptors in human astrocytomas and its correlation with grade of malignancy, proliferation, apoptosis and survival. J. Neuro-Oncol. 2006, 81, 1–7. [Google Scholar] [CrossRef]
- Quach, S.; Holzgreve, A.; Kaiser, L.; Unterrainer, M.; Dekorsy, F.J.; Nelwan, D.V.; Bartos, L.M.; Kirchleitner, S.V.; Weller, J.; Weidner, L.; et al. TSPO PET signal using [18F]GE180 is associated with survival in recurrent gliomas. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 859–869. [Google Scholar] [CrossRef]
- Hattori, Y.; Ishimura, M.; Ohta, Y.; Takenaka, H.; Kawabata, S.; Kirihata, M. Dodecaborate Conjugates Targeting Tumor Cell Overexpressing Translocator Protein for Boron Neutron Capture Therapy. ACS Med. Chem. Lett. 2022, 13, 50–54. [Google Scholar] [CrossRef]
- Coderre, J.A.; Button, T.M.; Micca, P.L.; Fisher, C.D.; Nawrocky, M.M.; Liu, H.B. Neutron capture therapy of the 9L rat gliosarcoma using the p-boronophenylalanine-fructose complex. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 643–652. [Google Scholar] [CrossRef]
- Hiramatsu, R.; Kawabata, S.; Tanaka, H.; Sakurai, Y.; Suzuki, M.; Ono, K.; Miyatake, S.I.; Kuroiwa, T.; Hao, E.; Vicente, M.G.H. Tetrakis(p-Carboranylthio-Tetrafluorophenyl)Chlorin (TPFC): Application for Photodynamic Therapy and Boron Neutron Capture Therapy. J. Pharm. Sci. 2015, 104, 962–970. [Google Scholar] [CrossRef]
- Futamura, G.; Kawabata, S.; Nonoguchi, N.; Hiramatsu, R.; Toho, T.; Tanaka, H.; Masunaga, S.-I.; Hattori, Y.; Kirihata, M.; Ono, K.; et al. Evaluation of a novel sodium borocaptate-containing unnatural amino acid as a boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat. Oncol. 2017, 12, 26. [Google Scholar] [CrossRef]
- Kanemitsu, T.; Kawabata, S.; Fukumura, M.; Futamura, G.; Hiramatsu, R.; Nonoguchi, N.; Nakagawa, F.; Takata, T.; Tanaka, H.; Suzuki, M.; et al. Folate receptor-targeted novel boron compound for boron neutron capture therapy on F98 glioma-bearing rats. Radiat. Environ. Biophys. 2019, 58, 59–67. [Google Scholar] [CrossRef]
- Fukuo, Y.; Hattori, Y.; Kawabata, S.; Kashiwagi, H.; Kanemitsu, T.; Takeuchi, K.; Futamura, G.; Hiramatsu, R.; Watanabe, T.; Hu, N.; et al. The Therapeutic Effects of Dodecaborate Containing Boronophenylalanine for Boron Neutron Capture Therapy in a Rat Brain Tumor Model. Biology 2020, 9, 437. [Google Scholar] [CrossRef]
- Kashiwagi, H.; Kawabata, S.; Yoshimura, K.; Fukuo, Y.; Kanemitsu, T.; Takeuchi, K.; Hiramatsu, R.; Nishimura, K.; Kawai, K.; Takata, T.; et al. Boron neutron capture therapy using dodecaborated albumin conjugates with maleimide is effective in a rat glioma model. Investig. New Drugs 2021, 40, 255–264. [Google Scholar] [CrossRef]
- Hopewell, J.W.; Morris, G.M.; Schwint, A.; Coderre, J.A. The radiobiological principles of boron neutron capture therapy: A critical review. Appl. Radiat. Isot. 2011, 69, 1756–1759. [Google Scholar] [CrossRef]
- Tsuji, Y.; Nonoguchi, N.; Okuzaki, D.; Wada, Y.; Motooka, D.; Hirota, Y.; Toho, T.; Yoshikawa, N.; Furuse, M.; Kawabata, S.; et al. Chronic pathophysiological changes in the normal brain parenchyma caused by radiotherapy accelerate glioma progression. Sci. Rep. 2021, 11, 22110. [Google Scholar] [CrossRef]
- Kawabata, S.; Yang, W.; Barth, R.F.; Wu, G.; Huo, T.; Binns, P.J.; Riley, K.J.; Ongayi, O.; Gottumukkala, V.; Vicente, M.G.H. Convection enhanced delivery of carboranylporphyrins for neutron capture therapy of brain tumors. J. Neuro-Oncol. 2011, 103, 175–185. [Google Scholar] [CrossRef]
- Barca, C.; Foray, C.; Zinnhardt, B.; Winkeler, A.; Herrlinger, U.; Grauer, O.M.; Jacobs, A.H. In Vivo Quantitative Imaging of Glioma Heterogeneity Employing Positron Emission Tomography. Cancers 2022, 14, 3139. [Google Scholar] [CrossRef]
- Unterrainer, M.; Fleischmann, D.F.; Vettermann, F.; Ruf, V.; Kaiser, L.; Nelwan, D.; Lindner, S.; Brendel, M.; Wenter, V.; Stöcklein, S.; et al. TSPO PET, tumour grading and molecular genetics in histologically verified glioma: A correlative. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1368–1380. [Google Scholar] [CrossRef]
- Cai, L.; Kirchleitner, S.V.; Zhao, D.; Li, M.; Tonn, J.C.; Glass, R.; Kälin, R.E. Glioblastoma Exhibits Inter-Individual Heterogeneity of TSPO and LAT1 Expression in Neoplastic and Parenchymal Cells. Int. J. Mol. Sci. 2020, 21, 612. [Google Scholar] [CrossRef]
- Goodman, J.H.; McGregor, J.M.; Clendenon, N.R.; Gahbauer, R.A.; Barth, R.F.; Soloway, A.H.; Fairchild, R.G. Ultrastructural microvascular response to boron neutron capture therapy in an experimental model. Neurosurgery 1989, 24, 701–708. [Google Scholar] [CrossRef]
- Clendenon, N.R.; Barth, R.F.; Gordon, W.A.; Goodman, J.H.; Alam, F.; Staubus, A.E.; Boesel, C.P.; Yates, A.J.; Moeschberger, M.L.; Fairchild, R.G. Boron neutron capture therapy of a rat glioma. Neurosurgery 1990, 26, 47–55. [Google Scholar] [CrossRef]
- James, M.L.; Fulton, R.R.; Vercoullie, J.; Henderson, D.J.; Garreau, L.; Chalon, S.; Dolle, F.; Costa, B.; Guilloteau, D.; Kassiou, M. DPA-714, a new translocator protein-specific ligand: Synthesis, radiofluorination, and pharmacologic characterization. J. Nucl. Med. 2008, 49, 814–822. [Google Scholar] [CrossRef]
- Tang, D.; Li, J.; Buck, J.R.; Tantawy, M.N.; Xia, Y.; Harp, J.M.; Nickels, M.L.; Meiler, J.; Manning, H.C. Evaluation of TSPO PET Ligands [18F] VUIIS1009A and [18F] VUIIS1009B: Tracers for cancer imaging. Mol. Imaging Biol. 2017, 19, 578–588. [Google Scholar] [CrossRef]
- Winkeler, A.; Boisgard, R.; Awde, A.R.; Dubois, A.; Thézé, B.; Zheng, J.; Ciobanu, L.; Dollé, F.; Viel, T.; Jacobs, A.H.; et al. The translocator protein ligand [¹⁸F]DPA-714 images glioma and activated microglia in vivo. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 811–823. [Google Scholar] [CrossRef]
- Awde, A.R.; Boisgard, R.; Thézé, B.; Dubois, A.; Zheng, J.; Dollé, F.; Jacobs, A.H.; Tavitian, B.; Winkeler, A. The translocator protein radioligand 18F-DPA-714 monitors antitumor effect of erufosine in a rat 9L intracranial glioma model. J. Nucl. Med. 2013, 54, 2125–2131. [Google Scholar] [CrossRef]
- Zinnhardt, B.; Roncaroli, F.; Foray, C.; Agushi, E.; Osrah, B.; Hugon, G.; Jacobs, A.H.; Winkeler, A. Imaging of the glioma microenvironment by TSPO PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 174–185. [Google Scholar] [CrossRef]
- Zinnhardt, B.; Müther, M.; Roll, W.; Backhaus, P.; Jeibmann, A.; Foray, C.; Barca, C.; Döring, C.; Tavitian, B.; Dollé, F.; et al. TSPO imaging-guided characterization of the immunosuppressive myeloid tumor microenvironment in patients with malignant glioma. Neuro-Oncol. 2020, 22, 1030–1043. [Google Scholar] [CrossRef]
- Miyata, S.; Kawabata, S.; Hiramatsu, R.; Doi, A.; Ikeda, N.; Yamashita, T.; Kuroiwa, T.; Kasaoka, S.; Maruyama, K.; Miyatake, S. Computed tomography imaging of transferrin targeting liposomes encapsulating both boron and iodine contrast agents by convection-enhanced delivery to F98 rat glioma for boron neutron capture therapy. Neurosurgery 2011, 68, 1380–1387; discussion 1387. [Google Scholar] [CrossRef]

| Boron Compound a | Time b | n c | Boron Concentrations ± SD (μg B/g) d | Ratio | |||
|---|---|---|---|---|---|---|---|
| Tumor | Brain | Blood | T/Br e | T/Bl f | |||
| DPA-BSTPG | 2.5 | 6 | 45.0 ± 18.8 | 0.8 ± 0.8 | 0.4 ± 0.2 | 55.0 | 115.1 |
| 12 | 4 | 32.5 ± 13.1 | 0.8 ± 0.8 | 0.3 ± 0.1 | 40.7 | 106.8 | |
| 24 | 4 | 23.7 ± 13.5 | 1.0 ± 1.4 | 0.4 ± 0.1 | 23.5 | 61.5 | |
| BPA | 2.5 | 4 | 20.6 ± 2.2 | 5.5 ± 0.6 | 7.7 ± 0.5 | 3.8 | 2.7 |
| 12 | 4 | 9.1 ± 3.3 | 2.5 ± 0.6 | 2.9 ± 0.4 | 3.7 | 3.2 | |
| 24 | 4 | 8.2 ± 0.8 | 2.3 ± 0.3 | 2.9 ± 0.4 | 3.6 | 2.8 | |
| Comnination g | 2.5 | 4 | 61.8 ± 20.4 | 5.1 ± 0.7 | 7.3 ± 0.5 | 12.1 | 8.5 |
| Group | Absorbed Dose a (Gy) | CBE b | Photon-Equivalent Dose c (Gy-Eq) | %ILS d | ||
|---|---|---|---|---|---|---|
| Brain | Tumor | Brain | Tumor | |||
| Untreated | 0.0 | 0.0 | - | 0.0 | 0.0 | - |
| Neutron only | 0.9 | 0.9 | - | 1.5 | 1.5 | 9.5 |
| DPA-BSTPG-BNCT | 1.0 | 2.2 | 8.43 | - | 11.8 | 33.3 |
| BPA-BNCT | 1.5 | 3.2 | 3.80 | 2.3 | 10.0 | 50.0 |
| Combination BNCT | - | - | - | - | - | 59.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashiwagi, H.; Hattori, Y.; Kawabata, S.; Kayama, R.; Yoshimura, K.; Fukuo, Y.; Kanemitsu, T.; Shiba, H.; Hiramatsu, R.; Takami, T.; et al. Multi-Targeted Neutron Capture Therapy Combined with an 18 kDa Translocator Protein-Targeted Boron Compound Is an Effective Strategy in a Rat Brain Tumor Model. Cancers 2023, 15, 1034. https://doi.org/10.3390/cancers15041034
Kashiwagi H, Hattori Y, Kawabata S, Kayama R, Yoshimura K, Fukuo Y, Kanemitsu T, Shiba H, Hiramatsu R, Takami T, et al. Multi-Targeted Neutron Capture Therapy Combined with an 18 kDa Translocator Protein-Targeted Boron Compound Is an Effective Strategy in a Rat Brain Tumor Model. Cancers. 2023; 15(4):1034. https://doi.org/10.3390/cancers15041034
Chicago/Turabian StyleKashiwagi, Hideki, Yoshihide Hattori, Shinji Kawabata, Ryo Kayama, Kohei Yoshimura, Yusuke Fukuo, Takuya Kanemitsu, Hiroyuki Shiba, Ryo Hiramatsu, Toshihiro Takami, and et al. 2023. "Multi-Targeted Neutron Capture Therapy Combined with an 18 kDa Translocator Protein-Targeted Boron Compound Is an Effective Strategy in a Rat Brain Tumor Model" Cancers 15, no. 4: 1034. https://doi.org/10.3390/cancers15041034
APA StyleKashiwagi, H., Hattori, Y., Kawabata, S., Kayama, R., Yoshimura, K., Fukuo, Y., Kanemitsu, T., Shiba, H., Hiramatsu, R., Takami, T., Takata, T., Tanaka, H., Watanabe, T., Suzuki, M., Hu, N., Miyatake, S.-I., Kirihata, M., & Wanibuchi, M. (2023). Multi-Targeted Neutron Capture Therapy Combined with an 18 kDa Translocator Protein-Targeted Boron Compound Is an Effective Strategy in a Rat Brain Tumor Model. Cancers, 15(4), 1034. https://doi.org/10.3390/cancers15041034







