Methanodibenzo[b,f][1,5]dioxocins as Novel Glutaminase Inhibitor with Anti-Glioblastoma Potential
Abstract
Simple Summary
Abstract
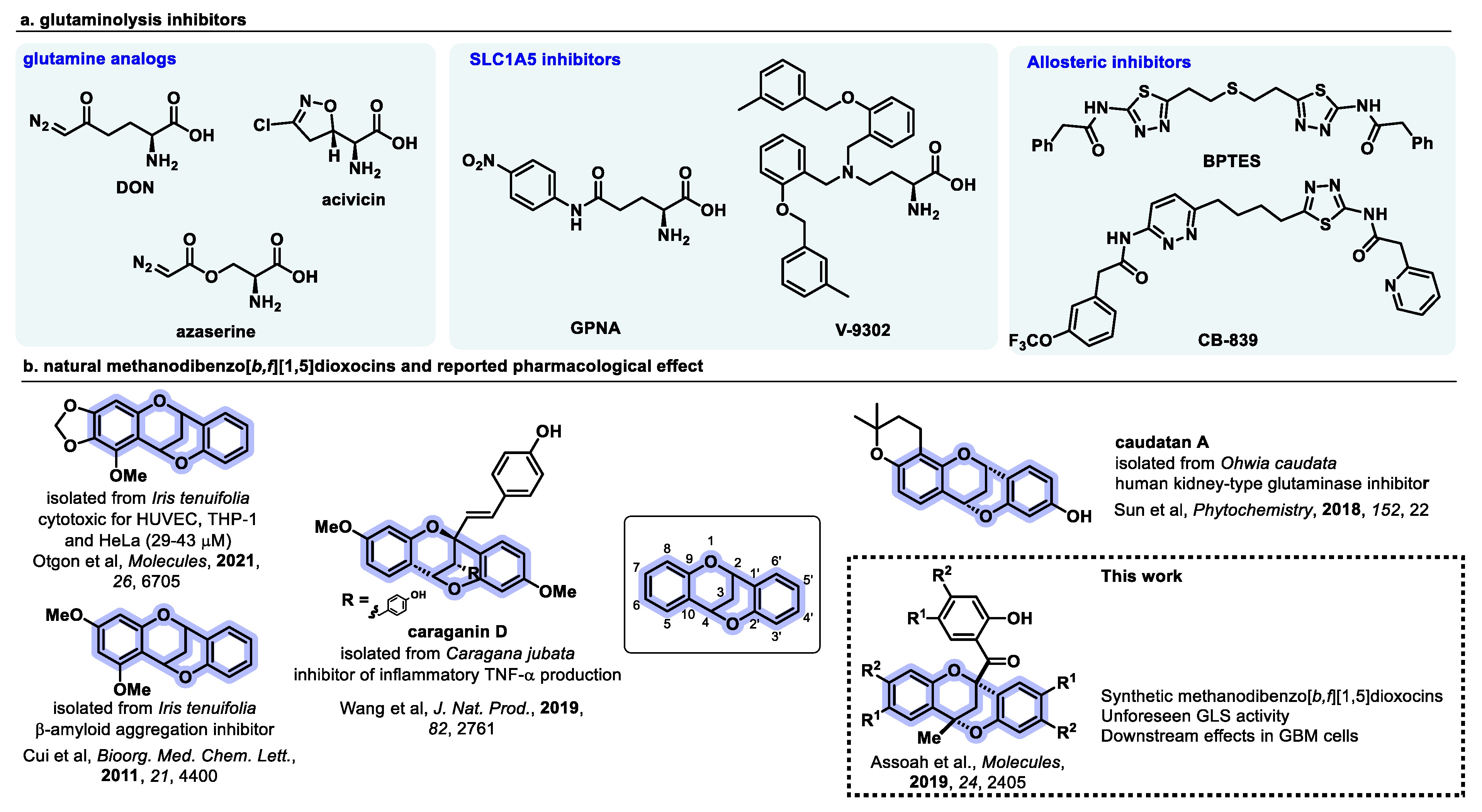
1. Introduction
2. Methodology
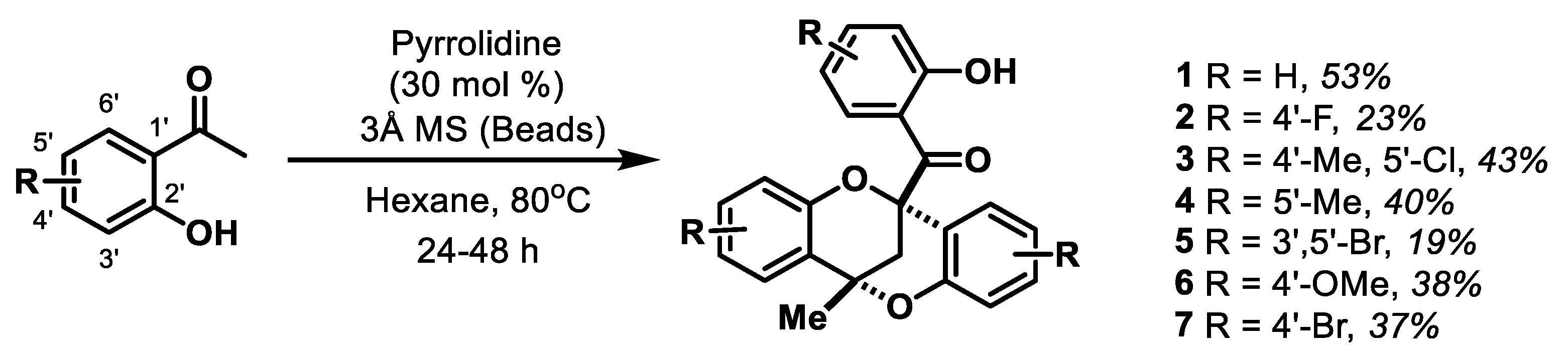
2.1. Synthesis of Novel Dioxocin Derivatives
2.2. Computational Assessment of Ligand–Glutaminase Interaction
2.3. Absorption, Distribution, Metabolism, Elimination, and Toxicity (ADMET) Prediction
2.4. Cell Culture Materials
2.5. Cell Culture and Drug Preparation
2.6. Measuring Plasma Membrane Integrity with Trypan Blue
2.7. In Vitro Measurement of Dose-Dependent Cytotoxicity
2.8. Reactive Oxygen Species Assay (ROS)
2.9. Apoptosis Assay
2.10. Time-Dependent Effect of Inhibition
2.11. Cell Migration Assay
2.12. Statistical Analysis
3. Result
3.1. Dioxocins Interact with Human Glutaminase
3.2. Drug-Likeness Properties of Dioxocin Analogs
3.3. Dioxocin 6 Effectively Reduces Cell Viability of GBM Cells
3.4. Dose-Dependent Effect of Compound 6 in GBM Cells
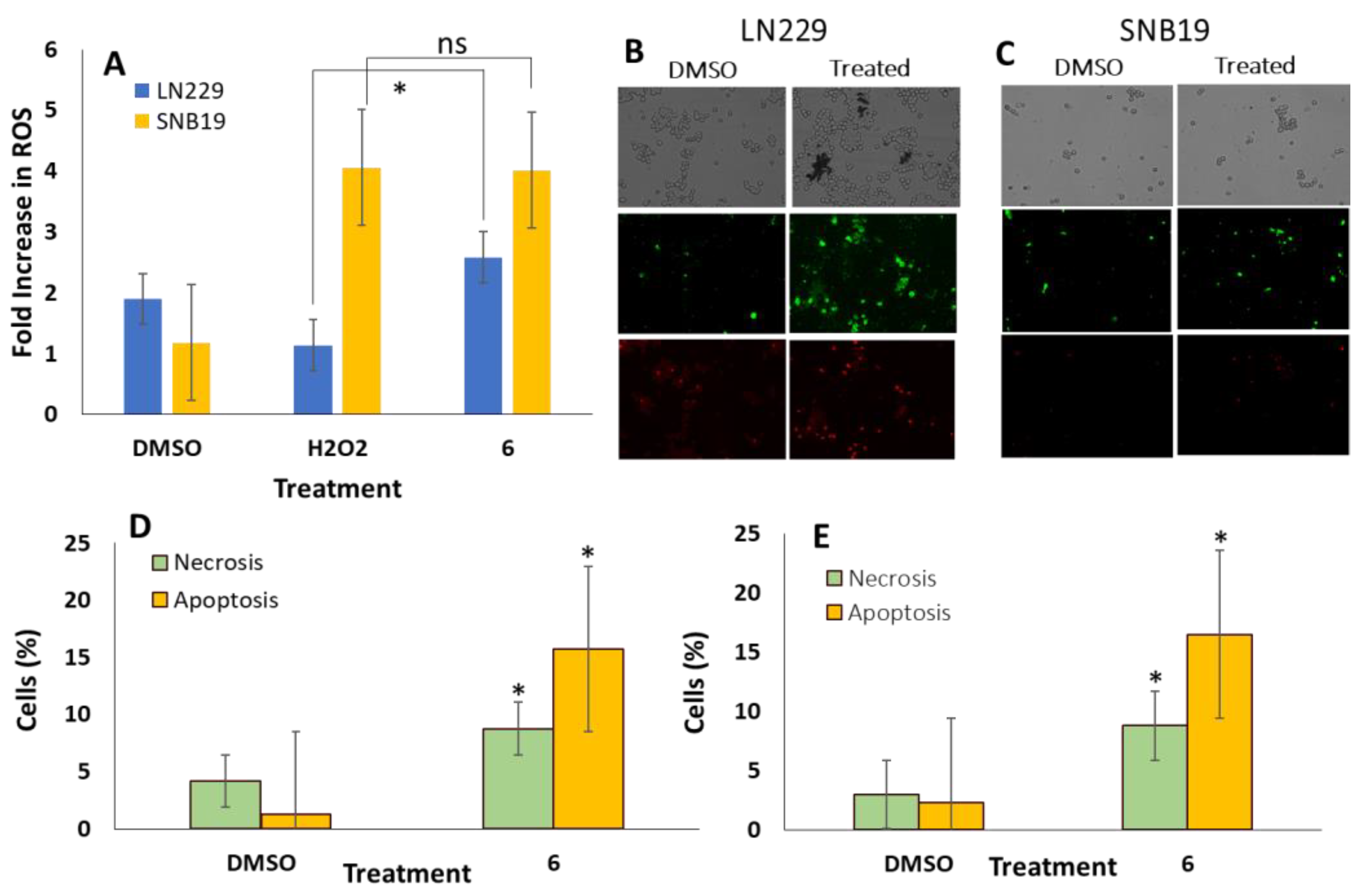
3.5. Interaction of Compound 6 with Glutaminase Elevates Intracellular ROS and Apoptosis in GBM Cells
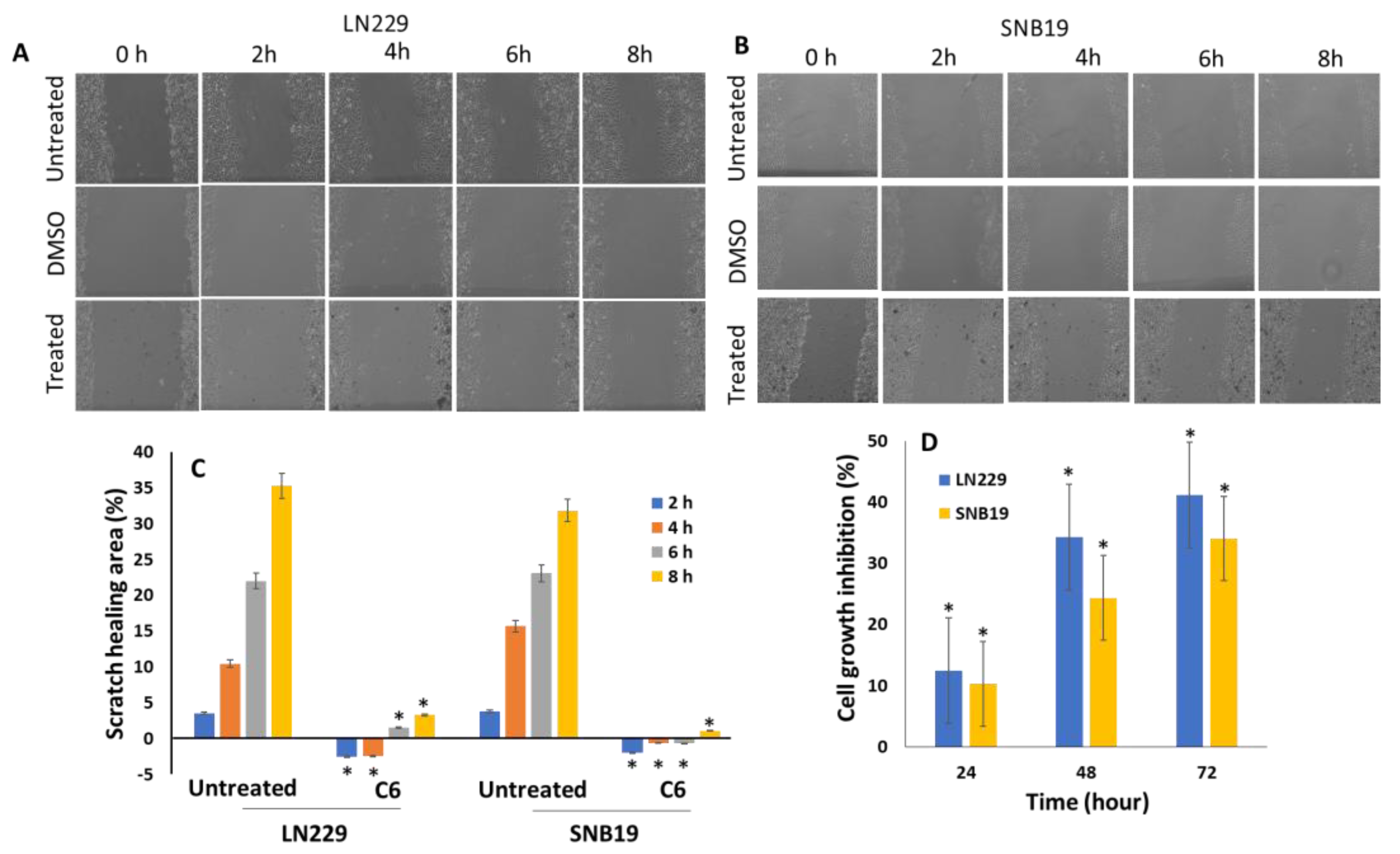
3.6. Compound 6 Inhibits GBM Cell Migration and Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Márquez, J.; Alonso, F.J.; Matés, J.M.; Segura, J.A.; Martín-Rufián, M.; Campos-Sandoval, J.A. Glutamine Addiction In Gliomas. Neurochem. Res. 2017, 42, 1735–1746. [Google Scholar] [CrossRef]
- Doan, P.; Musa, A.; Murugesan, A.; Sipilä, V.; Candeias, N.R.; Emmert-Streib, F.; Ruusuvuori, P.; Granberg, K.; Yli-Harja, O.; Kandhavelu, M. Glioblastoma Multiforme Stem Cell Cycle Arrest by Alkylaminophenol through the Modulation of EGFR and CSC Signaling Pathways. Cells 2020, 9, 681. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Dion, H.W.; Fusari, S.A.; Jakubowski, Z.L.; Zora, J.G.; Bartz, Q.R. 6-Diazo-5-Oxo-L-Norleucine, a New Tumor-Inhibitory Substance. II. Isolation and Characterization. J. Am. Chem. Soc. 1956, 78, 3075–3077. [Google Scholar] [CrossRef]
- Hidalgo, M.; Rodriguez, G.; Kuhn, J.; Brown, T.; Weiss, G.; MacGovren, J.P.; Hoff, D.V.V.; Rowinsky, E. A Phase I and Pharmacological Study of the Glutamine Antagonist Acivicin with the Amino Acid Solution Aminosyn in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 1998, 4, 2763–2770. [Google Scholar]
- Lemberg, K.M.; Vornov, J.J.; Rais, R.; Slusher, B.S. We’re Not “DON” Yet: Optimal Dosing and Prodrug Delivery of 6-Diazo-5-Oxo-L-Norleucine. Mol. Cancer Ther. 2018, 17, 1824. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.D.; Sant, M.E.; Christopherson, R.I. Cytotoxic Mechanisms of Glutamine Antagonists in Mouse L1210 Leukemia. J. Biol. Chem. 1990, 265, 11377–11381. [Google Scholar] [CrossRef]
- Zimmermann, S.C.; Duvall, B.; Tsukamoto, T. Recent Progress in the Discovery of Allosteric Inhibitors of Kidney-Type Glutaminase. J. Med. Chem. 2019, 62, 46–59. [Google Scholar] [CrossRef]
- Ramachandran, S.; Pan, C.Q.; Zimmermann, S.C.; Duvall, B.; Tsukamoto, T.; Low, B.C.; Sivaraman, J. Structural Basis for Exploring the Allosteric Inhibition of Human Kidney Type Glutaminase. Oncotarget 2016, 7, 57943–57954. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, F.; Fan, N.; Zhou, C.; Li, D.; Macvicar, T.; Dong, Q.; Bruns, C.J.; Zhao, Y. Targeting Glutaminolysis: New Perspectives to Understand Cancer Development and Novel Strategies for Potential Target Therapies. Front. Oncol. 2020, 10, 589508. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An Important Scaffold for Medicinal Chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Otgon, O.; Nadmid, S.; Paetz, C.; Dahse, H.-M.; Voigt, K.; Bartram, S.; Boland, W.; Dagvadorj, E. Chromane Derivatives from Underground Parts of Iris Tenuifolia and Their In Vitro Antimicrobial, Cytotoxicity and Antiproliferative Evaluation. Molecules 2021, 26, 6705. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Zhang, Y.; Chen, R.; Cui, Y.; Wang, Q. Anti-Inflammatory Chalcone–Isoflavone Dimers and Chalcone Dimers from Caragana Jubata. J. Nat. Prod. 2019, 82, 2761–2767. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, X.; Liu, X.; Qian, C.; Che, X.; Cao, F.; Jin, S.; Meng, D. Caudatan A, an Undescribed Human Kidney-Type Glutaminase Inhibitor with Tetracyclic Flavan from Ohwia Caudata. Phytochemistry 2018, 152, 22–28. [Google Scholar] [CrossRef]
- Kulkarni, V.S.; Hosangadi, B.D. A Facile Synthesis of Anhydro-Dimers of o-Hydroxybenzaldehydes. Synth. Commun. 1986, 16, 191–193. [Google Scholar] [CrossRef]
- Ragot, J.P.; Prime, M.E.; Archibald, S.J.; Taylor, R.J.K. A Novel Route to Preussomerins via 2-Arylacetal Anions. Org. Lett. 2000, 2, 1613–1616. [Google Scholar] [CrossRef]
- Du, J.-Y.; Ma, Y.-H.; Meng, F.-X.; Chen, B.-L.; Zhang, S.-L.; Li, Q.-L.; Gong, S.-W.; Wang, D.-Q.; Ma, C.-L. Lewis Acid Catalyzed Tandem 1,4-Conjugate Addition/Cyclization of in Situ Generated Alkynyl o-Quinone Methides and Electron-Rich Phenols: Synthesis of Dioxabicyclo[3.3.1]Nonane Skeletons. Org. Lett. 2018, 20, 4371–4374. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Guo, X.; Huo, L.; Xu, Z.; Zhang, W.; Qiu, S.; Yang, B.; Tan, H. A Bioinspired Cascade Sequence Enables Facile Assembly of Methanodibenzo[b,f][1,5]Dioxocin Flavonoid Scaffold. Org. Lett. 2018, 20, 546–549. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, A.; Zhang, W.; Lu, X.; Chen, H.; Tan, H. Concise Total Syntheses of Two Flavans and Structure Revision Assisted by Quantum NMR Calculations. Org. Biomol. Chem. 2022, 20, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, S.J.; Jegadeesan, S.; Vishwakarma, D.S. Total Synthesis of Myristinins A–F and 3′-Hydroxy-5,7-Dimethoxy-4-O-2′-Cycloflavan by Iterative Generation of o-Quinone Methides. New J. Chem. 2022, 46, 5460–5463. [Google Scholar] [CrossRef]
- Assoah, B.; Riihonen, V.; Vale, J.R.; Valkonen, A.; Candeias, N.R. Synthesis of 6,12-Disubstituted Methanodibenzo[b,f][1,5]Dioxocins: Pyrrolidine Catalyzed Self-Condensation of 2′-Hydroxyacetophenones. Molecules 2019, 24, 2405. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, P.S.; Ali, A.A.; Mohammad, A.A.; Kandhavelu, J.; Kandhavelu, M. Time Lapse Microscopy Observation of Cellular Structural Changes and Image Analysis of Drug Treated Cancer Cells to Characterize the Cellular Heterogeneity. Environ. Toxicol. 2015, 30, 724–734. [Google Scholar] [CrossRef]
- Karjalainen, A.; Doan, P.; Chandraseelan, J.G.; Sandberg, O.; Yli-Harja, O.; Candeias, N.R.; Kandhavelu, M. Synthesis of Phenol-Derivatives and Biological Screening for Anticancer Activity. Anti-Cancer Agents Med. Chem. 2017, 17, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Kari, S.; Subramanian, K.; Altomonte, I.A.; Murugesan, A.; Yli-Harja, O.; Kandhavelu, M. Programmed Cell Death Detection Methods: A Systematic Review and a Categorical Comparison. Apoptosis 2022, 1, 482–508. [Google Scholar] [CrossRef]
- Ling, L.-U.; Tan, K.-B.; Lin, H.; Chiu, G.N.C. The Role of Reactive Oxygen Species and Autophagy in Safingol-Induced Cell Death. Cell Death Dis. 2011, 2, e129. [Google Scholar] [CrossRef]
- Doan, P.; Musa, A.; Candeias, N.R.; Emmert-Streib, F.; Yli-Harja, O.; Kandhavelu, M. Alkylaminophenol Induces G1/S Phase Cell Cycle Arrest in Glioblastoma Cells through P53 and Cyclin-Dependent Kinase Signaling Pathway. Front. Pharmacol. 2019, 10, 330. [Google Scholar] [CrossRef]
- Rashid, M. Design, Synthesis and ADMET Prediction of Bis-Benzimidazole as Anticancer Agent. Bioorganic Chem. 2020, 96, 103576. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef]
- Jin, L.; Alesi, G.N.; Kang, S. Glutaminolysis as a Target for Cancer Therapy. Oncogene 2016, 35, 3619–3625. [Google Scholar] [CrossRef]
- Strickland, M.; Stoll, E.A. Metabolic Reprogramming in Glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sasayama, T.; Irino, Y.; Takata, K.; Nagashima, H.; Satoh, N.; Kyotani, K.; Mizowaki, T.; Imahori, T.; Ejima, Y.; et al. Compensatory Glutamine Metabolism Promotes Glioblastoma Resistance to MTOR Inhibitor Treatment. J. Clin. Investig. 2015, 125, 1591–1602. [Google Scholar] [CrossRef]
- Han, K.; Wang, H.; Song, B.; Li, Y.; Ding, W.N. Design, Synthesis and Biological Activity Evaluation of Novel Anticancer Agent 5- (2-Carboxyethenyl) Indole Derivatives. J. Chem. Pharm. Res. 2014, 6, 376–380. [Google Scholar]
- Srivastava, V.; Yadav, A.; Sarkar, P. Molecular Docking and ADMET Study of Bioactive Compounds of Glycyrrhiza Glabra against Main Protease of SARS-CoV2. Mater. Today Proc. 2022, 49, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS Stress in Cancer Cells and Therapeutic Implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Fang, J.; Seki, T.; Maeda, H. Therapeutic Strategies by Modulating Oxygen Stress in Cancer and Inflammation. Adv. Drug Deliv. Rev. 2009, 61, 290–302. [Google Scholar] [CrossRef]
- Kovacic, P.; Jacintho, J. Mechanisms of Carcinogenesis Focus on Oxidative Stress and Electron Transfer. Curr. Med. Chem. 2012, 8, 773–796. [Google Scholar] [CrossRef]
- Shi, J.; Zuo, H.; Ni, L.; Xia, L.; Zhao, L.; Gong, M.; Nie, D.; Gong, P.; Cui, D.; Shi, W.; et al. An IDH1 Mutation Inhibits Growth of Glioma Cells via GSH Depletion and ROS Generation. Neurol. Sci. 2014, 35, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Kodama, S.; Matsuda, N.; Suzuki, K.; Watanabe, M. Involvement of Reactive Oxygen Species (ROS) in the Induction of Genetic Instability by Radiation. J. Radiat. Res. 2004, 45, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Singh, A.; Singh, A.; Pathak, L.P.; Shrivastava, N.; Tripathi, P.K.; Singh, M.P.; Singh, K. Inhibition of P. falciparum pfatp6 by curcumin and its derivatives: A bioinformatic study. Cell. Mol. Biol. 2012, 58, 182–186. [Google Scholar] [CrossRef] [PubMed]
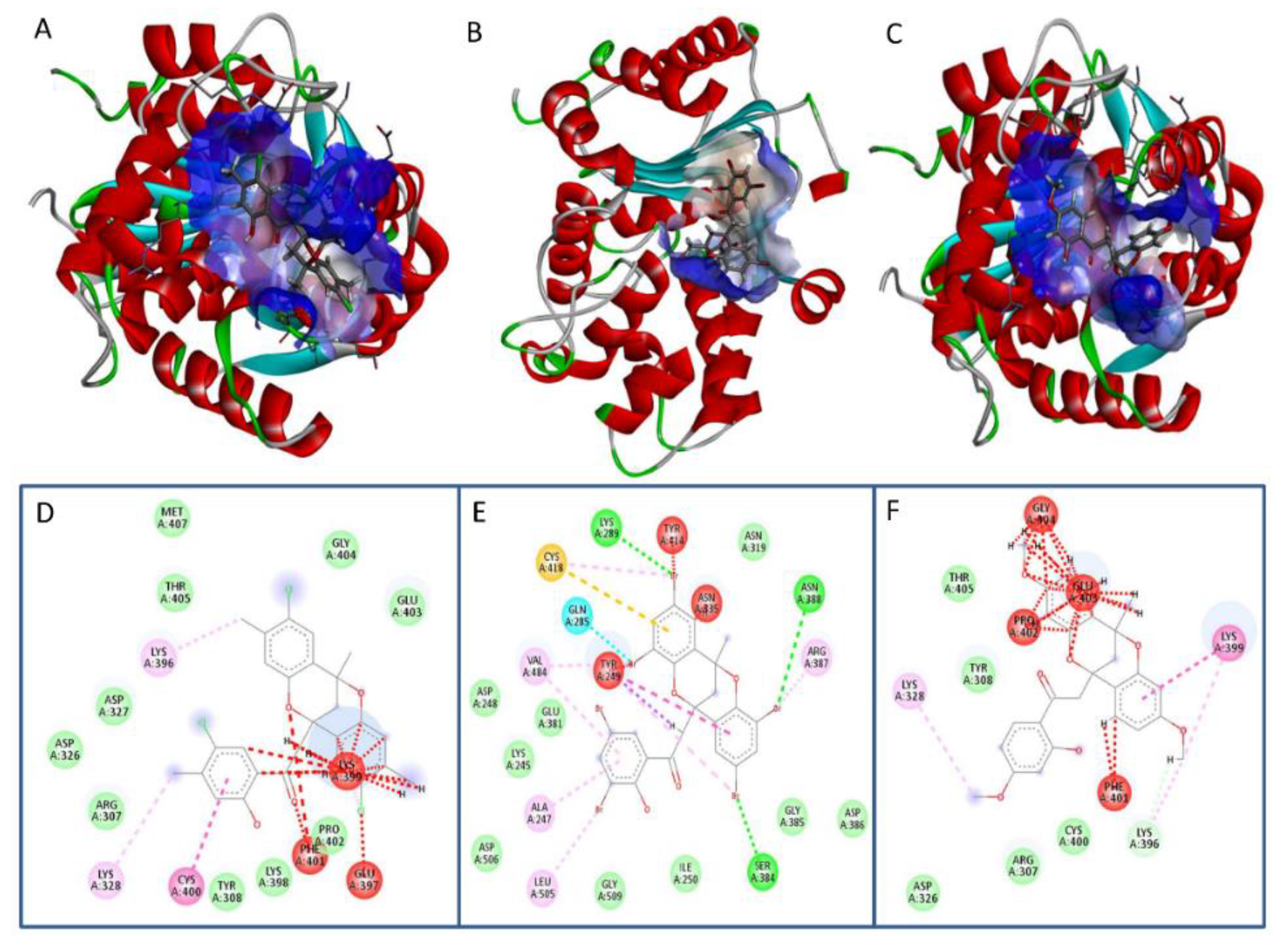

| Compounds | Binding Energy (kcal/mol) |
|---|---|
| 1 | −8.836 |
| 2 | −9.816 |
| 3 | −10.248 |
| 4 | −9.576 |
| 5 | −10.021 |
| 6 | −9.820 |
| 7 | −9.360 |
| Properties | Descriptor | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| Absorption | Water solubility | Moderate | Moderate | Poor | Poor | Insoluble | Moderate | Poor |
| HIA (% Absorbed) | 97.448 | 96.131 | 94.899 | 97.544 | 88.634 | 99.653 | 93.225 | |
| CaCO2 permeability (log Papp in 10 cm/s) | 1.046 | 1.177 | 1.022 | 1.032 | 0.975 | 1.245 | 1.014 | |
| Distribution | P-glycoprotein substrate | Yes | No | Yes | Yes | Yes | No | Yes |
| P-glycoprotein I inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| P-glycoprotein II inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| BBB permeability | Yes | No | No | No | No | No | No | |
| CNS permeability (log PS) | −1.751 | −2.85 | −1.155 | −1.518 | −0.912 | −3.036 | −1.309 | |
| Metabolism | CYP2D6 substrate | No | No | No | No | No | No | No |
| CYP3A4 substrate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| CYP1A2 inhibitor | Yes | No | No | No | No | No | No | |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| CYP2C9 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | |
| CYP3A4 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Excretion | Total Clearance (log mL/min/kg) | 0.185 | 0.266 | −0.393 | 0.113 | −0.545 | 0.576 | −0.197 |
| Renal OCT2 substrate | No | No | No | No | No | No | No | |
| Toxicity | AMES toxicity | No | No | No | No | No | No | No |
| Max. tolerated dose (human)(log mg/kg/day) | 0.115 | 0.457 | 0.116 | 0.022 | 0.103 | 0.175 | 0.09 | |
| Oral Rat Acute Toxicity (LD50, mol/kg) | 2.516 | 2.977 | 2.805 | 2.598 | 2.733 | 2.838 | 2.707 | |
| Hepatotoxicity | Yes | Yes | No | Yes | No | No | Yes | |
| Lipinski violations | 0 | 1 | 2 | 0 | 2 | 0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugesan, A.; Kari, S.; Shrestha, A.; Assoah, B.; Saravanan, K.M.; Murugesan, M.; Thiyagarajan, R.; Candeias, N.R.; Kandhavelu, M. Methanodibenzo[b,f][1,5]dioxocins as Novel Glutaminase Inhibitor with Anti-Glioblastoma Potential. Cancers 2023, 15, 1010. https://doi.org/10.3390/cancers15041010
Murugesan A, Kari S, Shrestha A, Assoah B, Saravanan KM, Murugesan M, Thiyagarajan R, Candeias NR, Kandhavelu M. Methanodibenzo[b,f][1,5]dioxocins as Novel Glutaminase Inhibitor with Anti-Glioblastoma Potential. Cancers. 2023; 15(4):1010. https://doi.org/10.3390/cancers15041010
Chicago/Turabian StyleMurugesan, Akshaya, Sana Kari, Anita Shrestha, Benedicta Assoah, Konda Mani Saravanan, Monica Murugesan, Ramesh Thiyagarajan, Nuno R. Candeias, and Meenakshisundaram Kandhavelu. 2023. "Methanodibenzo[b,f][1,5]dioxocins as Novel Glutaminase Inhibitor with Anti-Glioblastoma Potential" Cancers 15, no. 4: 1010. https://doi.org/10.3390/cancers15041010
APA StyleMurugesan, A., Kari, S., Shrestha, A., Assoah, B., Saravanan, K. M., Murugesan, M., Thiyagarajan, R., Candeias, N. R., & Kandhavelu, M. (2023). Methanodibenzo[b,f][1,5]dioxocins as Novel Glutaminase Inhibitor with Anti-Glioblastoma Potential. Cancers, 15(4), 1010. https://doi.org/10.3390/cancers15041010







