Online Adaptive MRI-Guided Radiotherapy for Primary Tumor and Lymph Node Boosting in Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
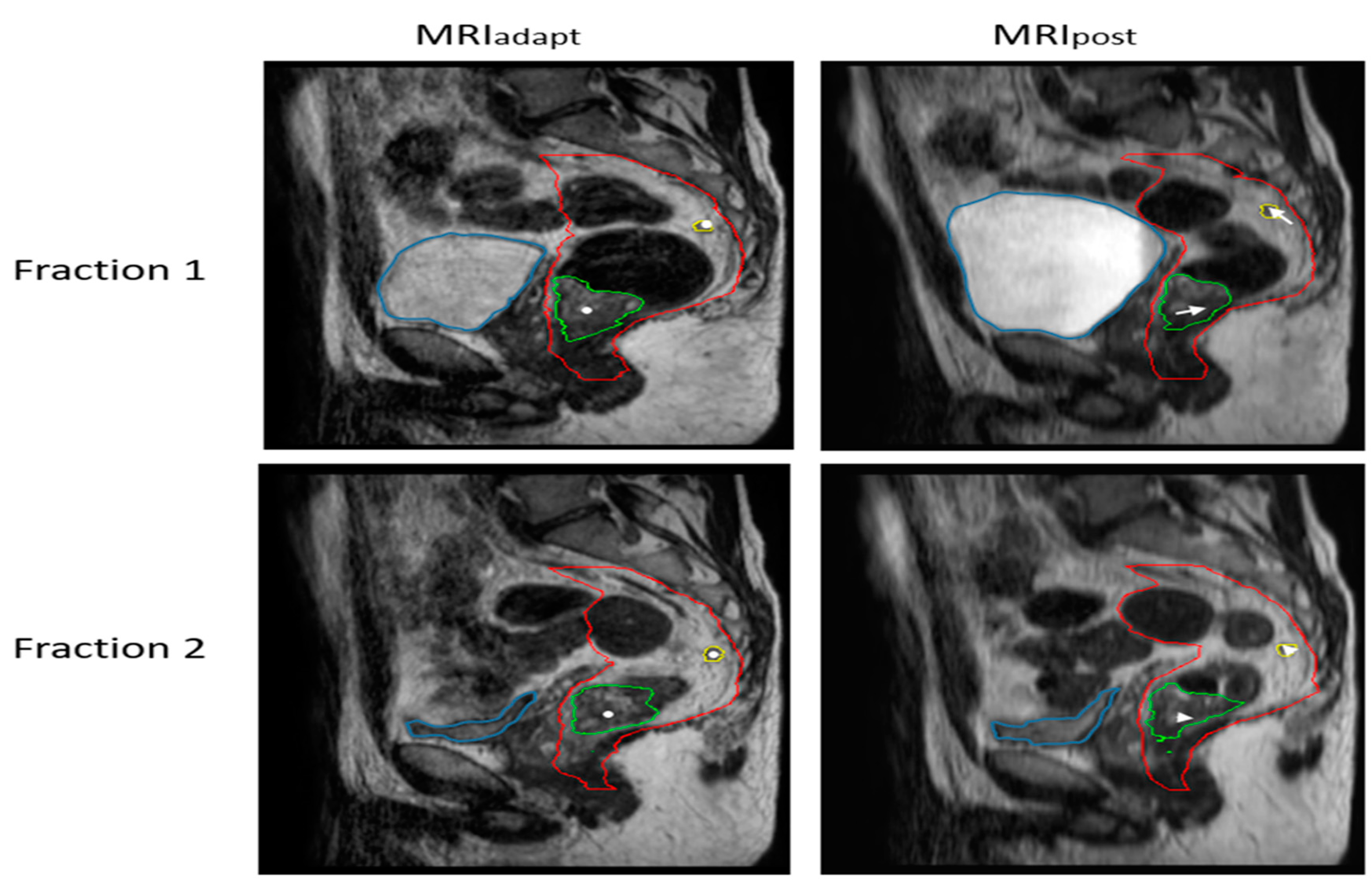
2.1. Patients and MR-Linac Protocol
2.2. Inter- and Intrafraction Displacement
2.2.1. ATS: Intrafraction Displacement of GTVln and GTVprim
2.2.2. ATP: Interfraction Displacement of GTVln with respect to GTVprim
2.3. Margin Calculation
2.4. Relationship between Lymph Node Motion and Other Factors
3. Results
3.1. Patient Characteristics
3.2. Inter- and Intrafraction Displacement
3.2.1. ATS: Intrafraction Displacement of GTVln and GTVprim
3.2.2. ATP: Interfraction Displacement of GTVln with respect to GTVprim
3.3. Relationship between Lymph Node Motion and Other Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Perez, R.O.; Nadalin, W.; Sabbaga, J.; Ribeiro, U., Jr.; e Sousa, A.H.S., Jr.; Campos, F.G.; Kiss, D.R.; Gama-Rodrigues, J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann. Surg. 2004, 240, 711. [Google Scholar] [CrossRef]
- Habr-Gama, A.; Sabbaga, J.; Gama-Rodrigues, J.; São Julião, G.P.; Proscurshim, I.; Aguilar, P.B.; Nadalin, W.; Perez, R.O. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: Are we getting closer to anal cancer management? Dis. Colon Rectum 2013, 56, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Habr-Gama, A.; Gama-Rodrigues, J.; São Julião, G.P.; Proscurshim, I.; Sabbagh, C.; Lynn, P.B.; Perez, R.O. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Malcomson, L.; Emsley, R.; Gollins, S.; Maw, A.; Myint, A.S.; Rooney, P.S.; Susnerwala, S.; Blower, A.; Saunders, M.P. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol. 2016, 17, 174–183. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Harling, H.; Jensen, F.S.; Jensen, L.H.; Jørgensen, J.C.; Lindebjerg, J.; Rafaelsen, S.R.; Jakobsen, A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol. 2015, 16, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Rullier, E.; Rouanet, P.; Tuech, J.-J.; Valverde, A.; Lelong, B.; Rivoire, M.; Faucheron, J.-L.; Jafari, M.; Portier, G.; Meunier, B. Organ preservation for rectal cancer (GRECCAR 2): A prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017, 390, 469–479. [Google Scholar] [CrossRef]
- Bach, S.P.; Gilbert, A.; Brock, K.; Korsgen, S.; Geh, I.; Hill, J.; Gill, T.; Hainsworth, P.; Tutton, M.G.; Khan, J. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): A randomised, open-label feasibility study. Lancet Gastroenterol. Hepatol. 2021, 6, 92–105. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Leijtens, J.; Beets, G.L. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. 2011, 29, 4633–4640. [Google Scholar]
- Bujko, K.; Partycki, M.; Pietrzak, L. Neoadjuvant radiotherapy (5 × 5 Gy): Immediate versus delayed surgery. Early Gastrointest. Cancers II Rectal Cancer 2014, 203, 171–187. [Google Scholar]
- Erlandsson, J.; Lörinc, E.; Ahlberg, M.; Pettersson, D.; Holm, T.; Glimelius, B.; Martling, A. Tumour regression after radiotherapy for rectal cancer–Results from the randomised Stockholm III trial. Radiother. Oncol. 2019, 135, 178–186. [Google Scholar] [CrossRef]
- Hoendervangers, S.; Couwenberg, A.M.; Intven, M.P.; van Grevenstein, W.M.; Verkooijen, H.M. Comparison of pathological complete response rates after neoadjuvant short-course radiotherapy or chemoradiation followed by delayed surgery in locally advanced rectal cancer. Eur. J. Surg. Oncol. 2018, 44, 1013–1017. [Google Scholar] [CrossRef]
- Appelt, A.L.; Pløen, J.; Vogelius, I.R.; Bentzen, S.M.; Jakobsen, A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Burbach, J.P.M.; den Harder, A.M.; Intven, M.; van Vulpen, M.; Verkooijen, H.M.; Reerink, O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: A systematic review and meta-analysis. Radiother. Oncol. 2014, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Couwenberg, A.M.; Burbach, J.P.; Berbee, M.; Lacle, M.M.; Arensman, R.; Raicu, M.G.; Wessels, F.J.; Verdult, J.; Roodhart, J.; Reerink, O. Efficacy of dose-escalated chemoradiation on complete tumor response in patients with locally advanced rectal cancer (RECTAL-BOOST): A phase 2 randomized controlled trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Movsas, B.; Diratzouian, H.; Hanlon, A.; Cooper, H.; Freedman, G.; Konski, A.; Sigurdson, E.; Hoffman, J.; Meropol, N.J.; Weiner, L.M. Phase II trial of preoperative chemoradiation with a hyperfractionated radiation boost in locally advanced rectal cancer. Am. J. Clin. Oncol. 2006, 29, 435–441. [Google Scholar] [CrossRef]
- Meade, P.G.; Blatchford, G.J.; Thorson, A.G.; Christensen, M.A.; Tement, C.A. Preoperative chemoradiation downstages locally advanced ultrasound-staged rectal cancer. Am. J. Surg. 1995, 170, 609–613. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.W.; Eppinga, W.S.C.; Tijssen, R.H.N.; Kerkmeijer, L.G.W.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Kleijnen, J.-P.J.; Van Asselen, B.; Burbach, J.P.; Intven, M.; Philippens, M.E.; Reerink, O.; Lagendijk, J.J.; Raaymakers, B.W. Evolution of motion uncertainty in rectal cancer: Implications for adaptive radiotherapy. Phys. Med. Biol. 2015, 61, 1. [Google Scholar] [CrossRef]
- van den Ende, R.P.; Kerkhof, E.M.; Rigter, L.S.; van Leerdam, M.E.; Peters, F.P.; van Triest, B.; Staring, M.; Marijnen, C.A.; van der Heide, U.A. Feasibility of Gold Fiducial Markers as a Surrogate for Gross Tumor Volume Position in Image-Guided Radiation Therapy of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1151–1159. [Google Scholar] [CrossRef]
- Eijkelenkamp, H.; Boekhoff, M.R.; Verweij, M.E.; Peters, F.P.; Meijer, G.J.; Intven, M.P. Planning target volume margin assessment for online adaptive MR-guided dose-escalation in rectal cancer on a 1.5 T MR-Linac. Radiother. Oncol. 2021, 162, 150–155. [Google Scholar] [CrossRef]
- Kensen, C.M.; Janssen, T.M.; Betgen, A.; Wiersema, L.; Peters, F.P.; Remeijer, P.; Marijnen, C.A.; van der Heide, U.A. Effect of intrafraction adaptation on PTV margins for MRI guided online adaptive radiotherapy for rectal cancer. Radiat. Oncol. 2022, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- de Mol van Otterloo, S.R.; Christodouleas, J.P.; Blezer, E.L.; Akhiat, H.; Brown, K.; Choudhury, A.; Eggert, D.; Erickson, B.A.; Faivre-Finn, C.; Fuller, C.D. The MOMENTUM study: An international registry for the evidence-based introduction of MR-guided adaptive therapy. Front. Oncol. 2020, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Beets, G.L.; Kim, M.-J.; Kessels, A.G.; Beets-Tan, R.G. High-resolution MR imaging for nodal staging in rectal cancer: Are there any criteria in addition to the size? Eur. J. Radiol. 2004, 52, 78–83. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Wang, Z.; Zheng, Y.; Zhao, G.; Yu, Y.; Cheng, Z.; Chen, D.; Liu, W. Patterns of neoplastic foci and lymph node micrometastasis within the mesorectum. Langenbeck’s Arch. Surg. 2005, 390, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.; van der Heide, U.; Remeijer, P.; Sonke, J.; van der Bijl, E. SP-0702 A theoretical framework for treatment margins for online adaptive radiotherapy. Radiother. Oncol. 2022, 170, S618–S619. [Google Scholar] [CrossRef]
- Van Herk, M.; Remeijer, P.; Rasch, C.; Lebesque, J.V. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, J.; de Jong, R.; Sonke, J.-J.; Remeijer, P.; van Vliet, C.; Marijnen, C. Target volume shape variation during hypo-fractionated preoperative irradiation of rectal cancer patients. Radiother. Oncol. 2009, 92, 202–209. [Google Scholar] [CrossRef]
- Boldrini, L.; Chiloiro, G.; Cusumano, D.; Romano, A.; Placidi, L.; Turco, G.; Antonelli, M.V.; Nardini, M.; Galetto, M.; Indovina, L. Mesorectal motion evaluation in rectal cancer MR-guided radiotherapy: An exploratory study to quantify treatment margins. Radiat. Oncol. 2023, 18, 1–8. [Google Scholar] [CrossRef]
- Heijnen, L.A.; Lambregts, D.M.; Lahaye, M.J.; Martens, M.H.; van Nijnatten, T.J.; Rao, S.-X.; Riedl, R.G.; Buijsen, J.; Maas, M.; Beets, G.L. Good and complete responding locally advanced rectal tumors after chemoradiotherapy: Where are the residual positive nodes located on restaging MRI? Abdom. Radiol. 2016, 41, 1245–1252. [Google Scholar] [CrossRef]
- White, I.; Hunt, A.; Bird, T.; Settatree, S.; Soliman, H.; Mcquaid, D.; Dearnaley, D.; Lalondrelle, S.; Bhide, S. Interobserver variability in target volume delineation for CT/MRI simulation and MRI-guided adaptive radiotherapy in rectal cancer. Br. J. Radiol. 2021, 94, 20210350. [Google Scholar] [CrossRef]
- van Kranen, S.; Van Herk, M.; Sonke, J. Margin Design for Deforming and Differential Doving Target Volumes. In Radiotherapy and Oncology; Elsevier House: Clare, Ireland, 2008; Volume 88, p. S154. [Google Scholar]
- Raaymakers, B.W.; Jürgenliemk-Schulz, I.; Bol, G.; Glitzner, M.; Kotte, A.; Van Asselen, B.; De Boer, J.; Bluemink, J.; Hackett, S.; Moerland, M. First patients treated with a 1.5 T MRI-Linac: Clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys. Med. Biol. 2017, 62, L41. [Google Scholar] [CrossRef] [PubMed]
- Tijssen, R.H.; Philippens, M.E.; Paulson, E.S.; Glitzner, M.; Chugh, B.; Wetscherek, A.; Dubec, M.; Wang, J.; van der Heide, U.A. MRI commissioning of 1.5 T MR-linac systems–a multi-institutional study. Radiother. Oncol. 2019, 132, 114–120. [Google Scholar] [CrossRef]
- Nijkamp, J.; Swellengrebel, M.; Hollmann, B.; de Jong, R.; Marijnen, C.; van Vliet-Vroegindeweij, C.; van Triest, B.; van Herk, M.; Sonke, J.-J. Repeat CT assessed CTV variation and PTV margins for short-and long-course pre-operative RT of rectal cancer. Radiother. Oncol. 2012, 102, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Van den Begin, R.; Kleijnen, J.-P.; Engels, B.; Philippens, M.; van Asselen, B.; Raaymakers, B.; Reerink, O.; De Ridder, M.; Intven, M. Tumor volume regression during preoperative chemoradiotherapy for rectal cancer: A prospective observational study with weekly MRI. Acta Oncol. 2018, 57, 723–727. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | n = 28 (%) |
|---|---|
| Age in years, median (range) | 60 (52–67) |
| Sex | |
| Male | 18 (64) |
| Female | 10 (36) |
| Tumor stage | |
| cT2 | 4 (14) |
| cT3 | 21 (75) |
| cT4 | 3 (11) |
| Nodal stage | |
| cN1 | 23 (82) |
| cN2 | 5 (18) |
| Tumor location (distance with respect to anal verge) | |
| Lower rectum (0 to ≤5 cm) | 17 (61) |
| Mid rectum (>5 to ≤10 cm) | 11 (39) |
| Upper rectum (>10 cm) | - |
| Number of lymph nodes | 84 (100) |
| Number of nodes per patient, median (range) | 3 (1–4) |
| Lymph node location with respect to tumor | |
| Proximal | 62 (74) |
| Peritumoral (at the same level of the tumor) | 22 (26) |
| Lymph node location with respect to mesorectum coronal midline | |
| Anterior | 38 (45) |
| Posterior | 46 (55) |
| Lymph node location with respect to anal verge | |
| Lower rectum (0 to ≤5 cm) | 9 (11) |
| Mid rectum (>5 to ≤10 cm) | 52 (62) |
| Upper rectum (>10 cm) | 23 (27) |
| Target Volume | LR (mm) | CC (mm) | AP (mm) | ||
|---|---|---|---|---|---|
| Intrafraction displacement | GTVln (n = 28) ** | GM | −0.2 (−0.3–−0.1) | 0.7 (0.7–0.8) * | −0.3 (−0.4–−0.3) * |
| Σ | 1.2 (1.1–1.3) | 1.2 (1.01–1.3) | 1.9 (1.8–1.9) | ||
| σ | 1.4 (1.34–1.5) | 1.9 (1.8–2.0) | 1.7 (1.6–1.8) | ||
| MPTV (ATS) | 3.5 (3.3–3.7) | 3.1 (2.9–3.3|caudal) 4.5 (4.3–4.7 |cranial) | 5.1 (5.0–5.3 |anterior) 5.7 (5.6–5.9 |posterior) | ||
| GTVprim (n = 28) | GM | 0.0 | 0.2 | −1.2 * | |
| Σ | 0.6 | 1.7 | 1.6 | ||
| σ | 1.0 | 1.4 | 1.3 | ||
| MPTV (ATS) | 1.7 | 4.7 | 3.2. (anterior) 5.6 (posterior) | ||
| Interfraction displacement with respect to GTVprim | GTVln (n = 28) ** | GM | 0.5 (0.4–0.6) | 0.8 (0.6–0.9) | −0.2 (−0.4–0.0) |
| Σ | 3.4 (3.3–3.5) | 6.3 (6.0–6.6) | 3.4 (3.1–3.6) | ||
| σ | 3.3 (3.2–3.4) | 3.5 (3.4–3.6) | 3.9 (3.8–4.0) | ||
| MPTV (ATP) | 10.8 (10.6–11.0) | 18.1 (17.5–18.7) | 11.6 (11.1–12.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kensen, C.M.; Betgen, A.; Wiersema, L.; Peters, F.P.; Kayembe, M.T.; Marijnen, C.A.M.; van der Heide, U.A.; Janssen, T.M. Online Adaptive MRI-Guided Radiotherapy for Primary Tumor and Lymph Node Boosting in Rectal Cancer. Cancers 2023, 15, 1009. https://doi.org/10.3390/cancers15041009
Kensen CM, Betgen A, Wiersema L, Peters FP, Kayembe MT, Marijnen CAM, van der Heide UA, Janssen TM. Online Adaptive MRI-Guided Radiotherapy for Primary Tumor and Lymph Node Boosting in Rectal Cancer. Cancers. 2023; 15(4):1009. https://doi.org/10.3390/cancers15041009
Chicago/Turabian StyleKensen, Chavelli M., Anja Betgen, Lisa Wiersema, Femke P. Peters, Mutamba T. Kayembe, Corrie A. M. Marijnen, Uulke A. van der Heide, and Tomas M. Janssen. 2023. "Online Adaptive MRI-Guided Radiotherapy for Primary Tumor and Lymph Node Boosting in Rectal Cancer" Cancers 15, no. 4: 1009. https://doi.org/10.3390/cancers15041009
APA StyleKensen, C. M., Betgen, A., Wiersema, L., Peters, F. P., Kayembe, M. T., Marijnen, C. A. M., van der Heide, U. A., & Janssen, T. M. (2023). Online Adaptive MRI-Guided Radiotherapy for Primary Tumor and Lymph Node Boosting in Rectal Cancer. Cancers, 15(4), 1009. https://doi.org/10.3390/cancers15041009






