Real-World Data Validation of NAPOLI-1 Nomogram for the Prediction of Overall Survival in Metastatic Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
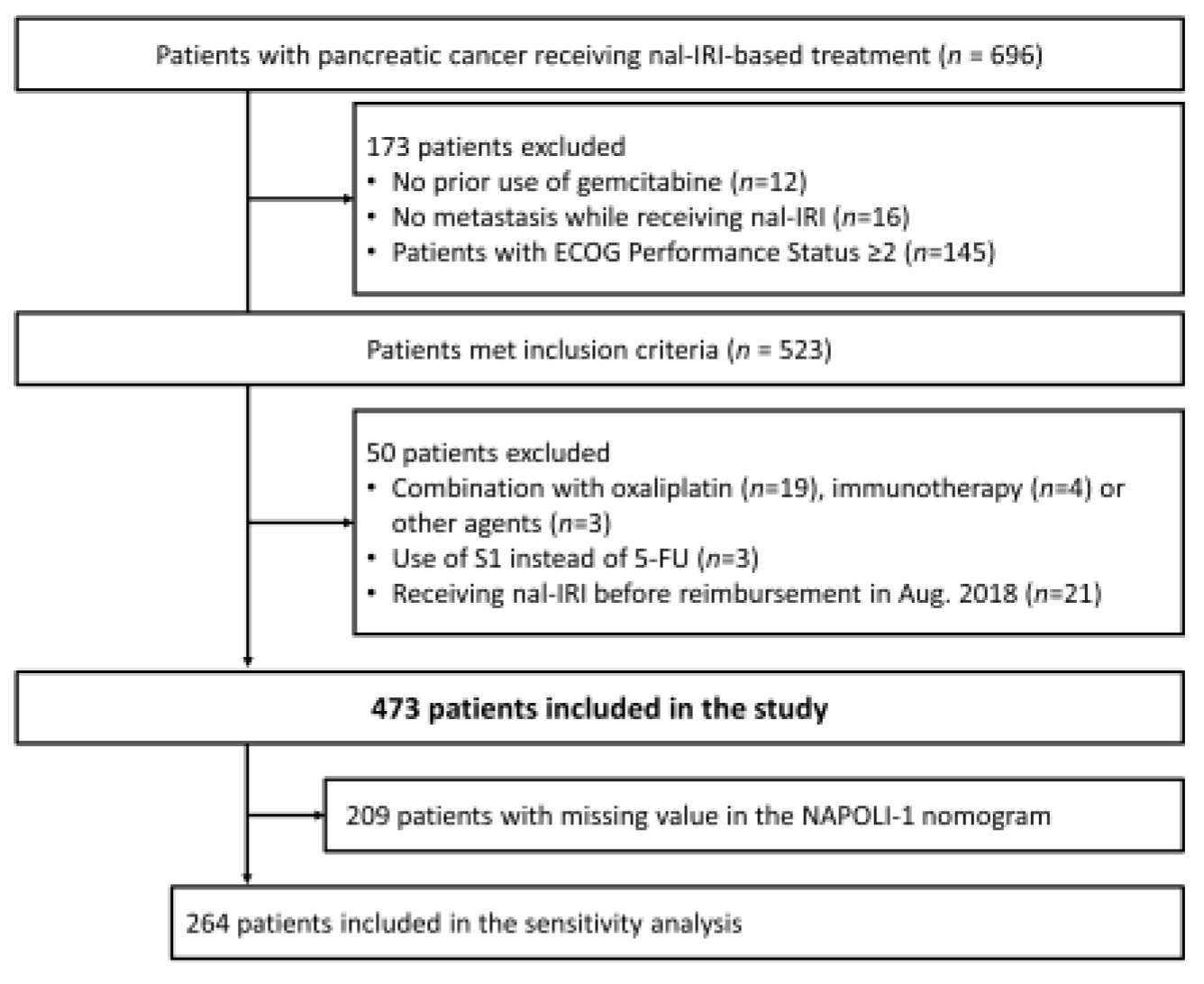
2. Materials and Methods
3. Results
3.1. Patient Demographics
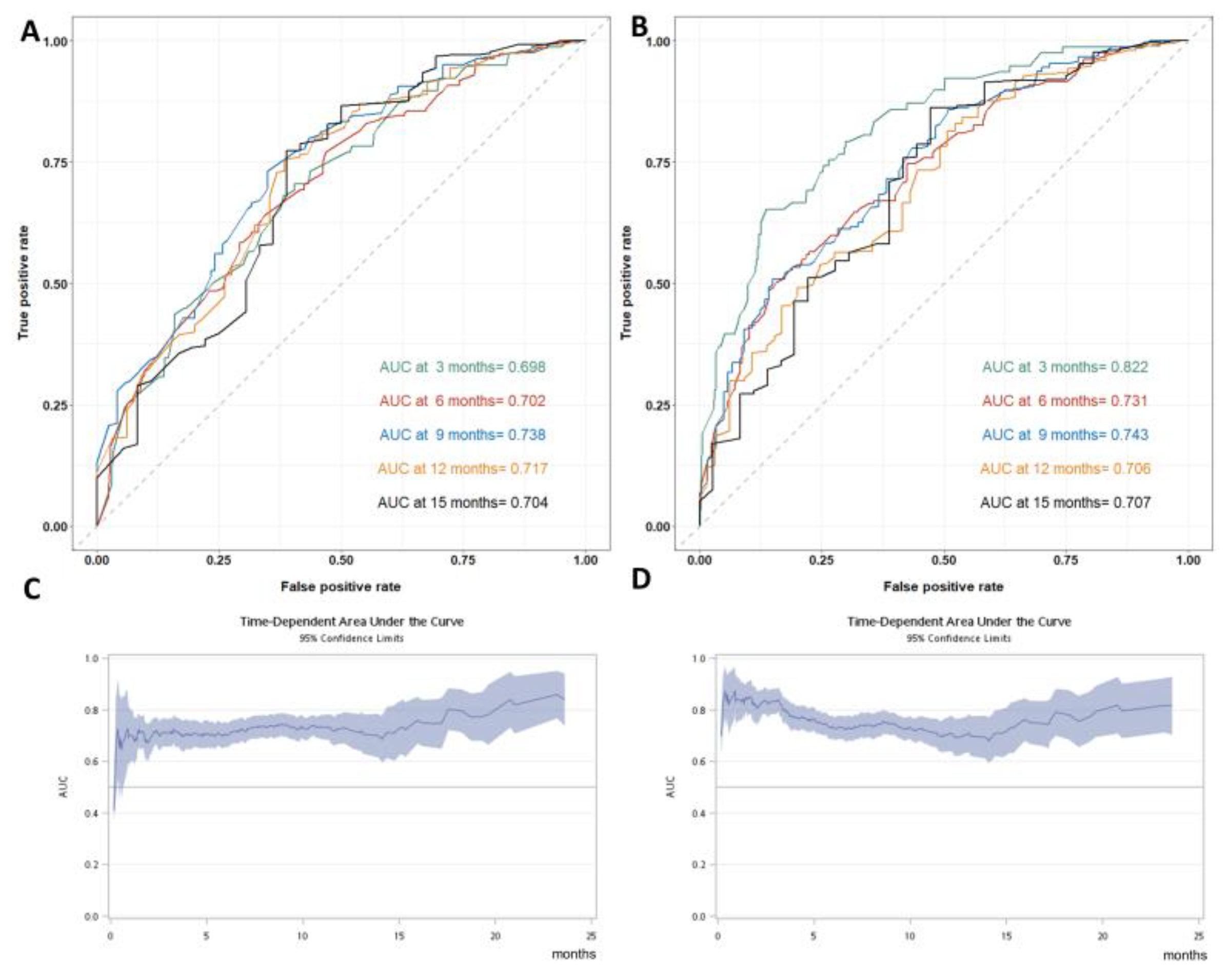
3.2. Model Performance
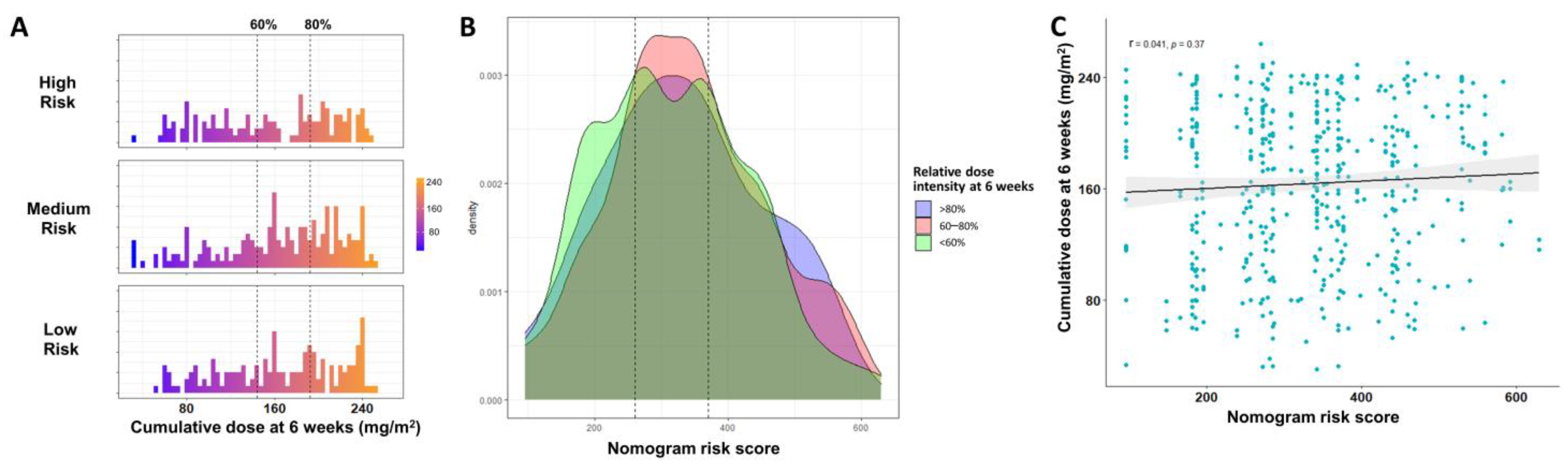
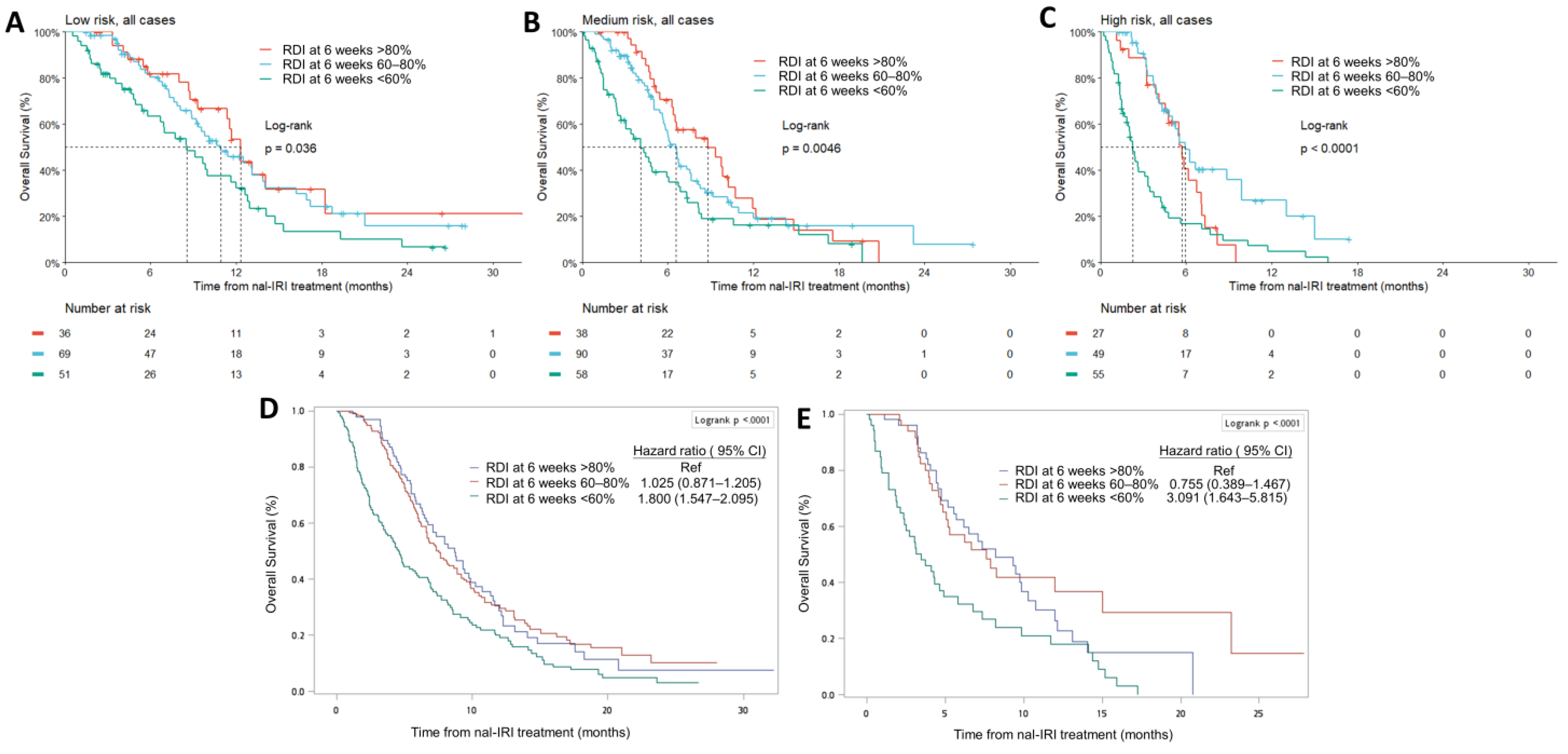
3.3. Relative Dose Intensity at 6 Weeks Is an Independent Prognostic Factor
3.4. Real-World Safety Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Miki, M.; Fujimori, N.; Ueda, K.; Lee, L.; Murakami, M.; Takamatsu, Y.; Shimokawa, Y.; Niina, Y.; Oono, T.; Hisano, T.; et al. Treatment Effect and Safety of Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid after Gemcitabine-Based Therapy in Patients with Advanced Pancreatic Cancer: A Multicenter, Prospective Observational Study. J. Clin. Med. 2022, 11, 5084. [Google Scholar] [CrossRef] [PubMed]
- Glassman, D.C.; Palmaira, R.L.; Covington, C.M.; Desai, A.M.; Ku, G.Y.; Li, J.; Harding, J.J.; Varghese, A.M.; O’Reilly, E.M.; Yu, K.H. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018, 18, 693. [Google Scholar] [CrossRef] [PubMed]
- Kieler, M.; Unseld, M.; Bianconi, D.; Scheithauer, W.; Prager, G.W. A real-world analysis of second-line treatment options in pancreatic cancer: Liposomal-irinotecan plus 5-fluorouracil and folinic acid. Ther. Adv. Med. Oncol. 2019, 11, 1758835919853196. [Google Scholar] [CrossRef]
- Yoo, C.; Im, H.-S.; Kim, K.-P.; Oh, D.-Y.; Lee, K.-H.; Chon, H.J.; Kim, J.H.; Kang, M.; Kim, I.; Lee, G.J.; et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: A study by the Korean Cancer Study Group. Ther. Adv. Med. Oncol. 2019, 11, 1758835919871126. [Google Scholar] [CrossRef]
- Tossey, J.C.; Reardon, J.; VanDeusen, J.B.; Noonan, A.M.; Porter, K.; Arango, M.J. Comparison of conventional versus liposomal irinotecan in combination with fluorouracil for advanced pancreatic cancer: A single-institution experience. Med. Oncol. 2019, 36, 87. [Google Scholar] [CrossRef]
- Barzi, A.; Miksad, R.; Surinach, A.; Corvino, F.A.; Wang, S.; Torres, A.Z.; Mamlouk, K.; Pulgar, S.; Valderrama, A.; Bekaii-Saab, T.; et al. Real-World Dosing Patterns and Outcomes of Patients With Metastatic Pancreatic Cancer Treated With a Liposomal Irinotecan Regimen in the United States. Pancreas 2020, 49, 193–200. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Chiang, N.-J.; Tsai, H.-J.; Yen, C.-J.; Shan, Y.-S.; Chen, L.-T. The Impact of Liposomal Irinotecan on the Treatment of Advanced Pancreatic Adenocarcinoma: Real-World Experience in a Taiwanese Cohort. Sci. Rep. 2020, 10, 7420. [Google Scholar] [CrossRef]
- Kim, G.P.; Surinach, A.; Corvino, F.A.; Cockrum, P.; Belanger, B.; Abushahin, L. Real-world outcomes associated with liposomal irinotecan dose reductions in metastatic pancreatic ductal adenocarcinoma. Futur. Oncol. 2021, 17, 675–688. [Google Scholar] [CrossRef]
- Kasi, A.; McGinnis, T.; Naik, G.; Handa, S.; Williams, G.; Paluri, R. Efficacy and tolerability of the combination of nano-liposomal irinotecan and 5-fluorouracil/leucovorin in advanced pancreatic adenocarcinoma: Post-approval clinic experience. J. Gastrointest. Oncol. 2021, 12, 464–473. [Google Scholar] [CrossRef]
- Park, H.; Kang, B.; Chon, H.; Im, H.-S.; Lee, C.-K.; Kim, I.; Kang, M.; Hwang, J.; Bae, W.; Cheon, J.; et al. Liposomal irinotecan plus fluorouracil/leucovorin versus FOLFIRINOX as the second-line chemotherapy for patients with metastatic pancreatic cancer: A multicenter retrospective study of the Korean Cancer Study Group (KCSG). ESMO Open 2021, 6, 100049. [Google Scholar] [CrossRef] [PubMed]
- Bang, K.; Cheon, J.; Jeong, J.H.; Im, H.-S.; Kim, K.-P.; Ryoo, B.-Y.; Yoo, C. Clinical outcomes of liposomal irinotecan plus fluorouracil/leucovorin for metastatic pancreatic adenocarcinoma in patients previously treated with conventional irinotecan-containing chemotherapy. Ther. Adv. Med. Oncol. 2021, 13, 17588359211003053. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.H.; Hendifar, A.E.; Alese, O.B.; Draper, A.; Abdelrahim, M.; Burns, E.; Khan, G.; Cockrum, P.; Bhak, R.H.; Nguyen, C.; et al. Clinical Outcomes Among Patients With Metastatic Pancreatic Ductal Adenocarcinoma Treated With Liposomal Irinotecan. Front. Oncol. 2021, 11, 678070. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Chiang, N.-J.; Chiu, S.-C.; Chou, W.-C.; Bai, L.-Y.; Li, C.-P.; Su, Y.-Y.; Chiu, T.-J.; Chuang, S.-C.; Peng, C.-M.; et al. The impact of spleen volume on the survival of metastatic pancreatic adenocarcinoma patients receiving nanoliposomal irinotecan. Am. J. Cancer Res. 2022, 12, 1884–1898. [Google Scholar]
- Yu, H.-Y.; Lee, C.-Y.; Lin, L.-G.; Chao, Y.; Li, C.-P. Nanoliposomal irinotecan with 5-fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy: A real-world experience. J. Chin. Med. Assoc. 2022, 85, 42–50. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Hsu, C.; Liu, K.; Chang, P.; Chen, P.; Hung, C.; Hsueh, S.; Yeh, K.; Chen, Y.; Lu, C.; Hung, Y.; et al. Development and validation of a prognostic nomogram to predict survival in patients with advanced pancreatic cancer receiving second-line palliative chemotherapy. J. Gastroenterol. Hepatol. 2020, 35, 1694–1703. [Google Scholar] [CrossRef]
- Chen, L.-T.; Macarulla, T.; Blanc, J.-F.; Mirakhur, B.; de Jong, F.A.; Belanger, B.; Bekaii-Saab, T.; Siveke, J.T. Nomogram for Predicting Survival in Patients Treated with Liposomal Irinotecan Plus Fluorouracil and Leucovorin in Metastatic Pancreatic Cancer. Cancers 2019, 11, 1068. [Google Scholar] [CrossRef]
- Chen, L.-T.; Siveke, J.T.; Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.P.; Shan, Y.-S.; Jameson, G.S.; Macarulla, T.; Lee, K.-H.; et al. Survival with nal-IRI (liposomal irinotecan) plus 5-fluorouracil and leucovorin versus 5-fluorouracil and leucovorin in per-protocol and non-per-protocol populations of NAPOLI-1: Expanded analysis of a global phase 3 trial. Eur. J. Cancer 2018, 105, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Y.; Chiang, N.-J.; Li, C.-P.; Yen, C.-J.; Yang, S.-H.; Chou, W.-C.; Chen, J.-S.; Chiu, T.-J.; Chen, Y.-Y.; Chuang, S.-C.; et al. Dosing Pattern and Early Cumulative Dose of Liposomal Irinotecan in Metastatic Pancreatic Cancer: A Real-World Multicenter Study. Front. Oncol. 2022, 12, 800842. [Google Scholar] [CrossRef] [PubMed]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Bang, Y.; Li, C.; Lee, K.; Chiu, C.; Park, J.O.; Shan, Y.; Kim, J.S.; Chen, J.; Shim, H.; Rau, K.; et al. Liposomal irinotecan in metastatic pancreatic adenocarcinoma in Asian patients: Subgroup analysis of the NAPOLI-1 study. Cancer Sci. 2020, 111, 513–527. [Google Scholar] [CrossRef]
- Adiwijaya, B.; Kim, J.; Lang, I.; Csõszi, T.; Cubillo, A.; Chen, J.-S.; Wong, M.; Park, J.; Rau, K.; Melichar, B.; et al. Population Pharmacokinetics of Liposomal Irinotecan in Patients With Cancer. Clin. Pharmacol. Ther. 2017, 102, 997–1005. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Chiang, N.-J.; Chang, J.; Wang, Y.-W.; Shen, B.-N.; Li, Y.-J.; Hwang, D.-Y.; Shan, Y.-S.; Chen, L.-T. The association between UGT1A1 polymorphisms and treatment toxicities of liposomal irinotecan. ESMO Open 2022, 8, 100746. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Multiple Imputation After 18+ Years. J. Am. Stat. Assoc. 1996, 91, 473–489. [Google Scholar] [CrossRef]
- Rezvan, P.H.; Lee, K.J.; Simpson, J.A. The rise of multiple imputation: A review of the reporting and implementation of the method in medical research. BMC Med. Res. Methodol. 2015, 15, 30. [Google Scholar] [CrossRef]
- Tan, P.-T.; Cro, S.; Van Vogt, E.; Szigeti, M.; Cornelius, V.R. A review of the use of controlled multiple imputation in randomised controlled trials with missing outcome data. BMC Med. Res. Methodol. 2021, 21, 72. [Google Scholar] [CrossRef]
- Ch’Ang, H.-J.; Wang, C.-C.; Cheng, A.-L.; Hsu, C.; Lu, Y.-S.; Chang, M.-C.; Lin, J.-T.; Wang, H.-P.; Shiah, H.-S.; Liu, T.-W.; et al. Phase I study of biweekly gemcitabine followed by oxaliplatin and simplified 48-h infusion of fluorouracil/leucovorin for advanced pancreatic cancer. J. Gastroenterol. Hepatol. 2006, 21, 874–879. [Google Scholar] [CrossRef]
- Ch’Ang, H.-J.; Huang, C.-L.; Wang, H.-P.; Shiah, H.-S.; Chang, M.-C.; Jan, C.-M.; Chen, J.-S.; Tien, Y.-W.; Lin, J.-T.; Cheng, A.-L.; et al. Phase II study of biweekly gemcitabine followed by oxaliplatin and simplified 48-h infusion of 5-fluorouracil/leucovorin (GOFL) in advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2009, 64, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.-J.; Tsai, K.K.; Hsiao, C.-F.; Yang, S.-H.; Hsiao, H.-H.; Shen, W.-C.; Hsu, C.; Lin, Y.-L.; Chen, J.-S.; Shan, Y.-S.; et al. A multicenter, phase I/II trial of biweekly S-1, leucovorin, oxaliplatin and gemcitabine in metastatic pancreatic adenocarcinoma-TCOG T1211 study. Eur. J. Cancer 2020, 124, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-Y.; Chiu, Y.-F.; Li, C.-P.; Yang, S.-H.; Lin, J.; Lin, S.-J.; Chang, P.-Y.; Chiang, N.-J.; Shan, Y.-S.; Ch’Ang, H.-J.; et al. A phase II randomised trial of induction chemotherapy followed by concurrent chemoradiotherapy in locally advanced pancreatic cancer: The Taiwan Cooperative Oncology Group T2212 study. Br. J. Cancer 2022, 126, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.-J.; Shan, Y.-S.; Bai, L.-Y.; Li, C.-P.; Chen, J.-S.; Yang, S.-H.; Kuo, Y.-C.; Chao, Y.; Hsieh, Y.-Y.; Kao, H.-F.; et al. TCOG T5217 trial: A phase II randomized study of SLOG versus modified FOLFIRINOX as the first-line treatment in locally advanced or metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2021, 39, 4143. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Ting, Y.-L.; Wang, C.-J.; Chao, Y.-J.; Liao, T.-K.; Su, P.-J.; Chiang, N.-J.; Liao, I.-C.; Yu, Y.-T.; Liu, Y.-S.; et al. Improved survival with induction chemotherapy and conversion surgery in locally advanced unresectable pancreatic cancer: A single institution experience. Am. J. Cancer Res. 2022, 12, 2189–2202. [Google Scholar]

| Risk Group | Low | Intermediate | High | Overall |
|---|---|---|---|---|
| (n = 156) | (n = 186) | (n = 131) | (n = 473) | |
| Gender | ||||
| Female | 67 (42.9%) | 83 (44.6%) | 48 (36.6%) | 198 (41.9%) |
| Male | 89 (57.1%) | 103 (55.4%) | 83 (63.4%) | 275 (58.1%) |
| Age, median (range) | 62.5 (27–82) | 63.0 (34–86) | 63 (33–86) | 63 (27–86) |
| Disease stage at diagnosis | ||||
| Stage I-III | 108 (69.2%) | 40 (21.5%) | 6 (4.6%) | 154 (32.6%) |
| Stage IV | 48 (30.8%) | 146 (78.5%) | 125 (95.4%) | 319 (67.4%) |
| Primary tumor location | ||||
| Head | 90 (57.7%) | 95 (51.1%) | 64 (48.9%) | 249 (52.6%) |
| Body | 34 (21.8%) | 52 (28.0%) | 22 (16.8%) | 108 (22.8%) |
| Tail | 24 (15.4%) | 32 (17.2%) | 37 (28.2%) | 93 (19.7%) |
| Body + Tail | 7 (4.5%) | 6 (3.2%) | 7 (5.3%) | 20 (4.2%) |
| Head + Body or Tail | 1 (0.6%) | 1 (0.5%) | 1 (0.8%) | 3 (0.6%) |
| Albumin | ||||
| <4 | 45 (28.8%) | 69 (37.1%) | 65 (49.6%) | 179 (37.8%) |
| ≥4 | 55 (35.3%) | 37 (19.9%) | 22 (16.8%) | 114 (24.1%) |
| Not checked | 56 (35.9%) | 80 (43.0%) | 44 (33.6%) | 180 (38.1%) |
| Number of metastatic sites | ||||
| 1 | 117 (75.0%) | 90 (48.4%) | 47 (35.9%) | 254 (53.7%) |
| 2 | 34 (21.8%) | 66 (35.5%) | 41 (31.3%) | 141 (29.8%) |
| 3 | 5 (3.2%) | 27 (14.5%) | 33 (25.2%) | 65 (13.7%) |
| ≥4 | 0 (0%) | 3 (1.6%) | 10 (7.6%) | 13 (2.7%) |
| Site of metastasis | ||||
| Liver | 69 (44.2%) | 125 (67.2%) | 125 (95.4%) | 319 (67.4%) |
| Lung | 35 (22.4%) | 47 (25.3%) | 40 (30.5%) | 122 (25.8%) |
| Peritoneum | 38 (24.4%) | 55 (29.6%) | 37 (28.2%) | 130 (27.5%) |
| CA-19.9 | ||||
| <40 U/mL | 37 (23.7%) | 26 (14.0%) | 6 (4.6%) | 69 (14.6%) |
| ≥40 U/mL | 109 (69.9%) | 138 (74.2%) | 99 (75.6%) | 346 (73.2%) |
| Not checked | 10 (6.4%) | 22 (11.8%) | 26 (19.8%) | 58 (12.3%) |
| Prior treatment | ||||
| Gemcitabine-containing | 156 (100%) | 186 (100%) | 131 (100%) | 473 (100%) |
| Fluorouracil-containing | 112 (71.8%) | 150 (80.6%) | 104 (79.4%) | 366 (77.4%) |
| Irinotecan-containing | 16 (10.3%) | 28 (15.1%) | 20 (15.3%) | 64 (13.5%) |
| Platinum-containing | 59 (37.8%) | 86 (46.2%) | 73 (55.7%) | 218 (46.1%) |
| Taxane-containing | 47 (30.1%) | 56 (30.1%) | 41 (31.3%) | 144 (30.4%) |
| Prior lines of systemic treatment † | ||||
| 0 | 2 (1.3%) | 5 (2.7%) | 0 (0%) | 7 (1.5%) |
| 1 | 101 (64.7%) | 110 (59.1%) | 84 (64.1%) | 295 (62.4%) |
| ≥2 | 53 (34.0%) | 71 (38.2%) | 47 (35.9%) | 171 (36.2%) |
| Operation history | ||||
| No surgery | 74 (47.4%) | 119 (64.0%) | 88 (67.2%) | 281 (59.4%) |
| Whipple operation | 26 (16.7%) | 34 (18.3%) | 17 (13.0%) | 77 (16.3%) |
| Distal pancreatectomy | 20 (12.8%) | 13 (7.0%) | 9 (6.9%) | 42 (8.9%) |
| Total pancreatectomy | 3 (1.9%) | 4 (2.2%) | 3 (2.3%) | 10 (2.1%) |
| Other procedures | 33 (21.2%) | 16 (8.6%) | 14 (10.7%) | 63 (13.3%) |
| Interval between the last therapy and nal-IRI+5-FU/LV | ||||
| Median (IQR) | 0.754 (0.475–1.28) | 0.672 (0.459–1.08) | 0.689 (0.459–1.11) | 0.689 (0.459–1.15) |
| Not recorded | 21 (13.5%) | 28 (15.1%) | 16 (12.2%) | 65 (13.7%) |
| Model 1 NAPOLI-1 Nomogram | Model 2 NAPOLI-1 Nomogram and Cumulative Dose | ||||
|---|---|---|---|---|---|
| Parameter | HR (95%CI) | p-Value | Parameter | HR (95%CI) | p-Value |
| Nomogram risk score | 0.996 (0.995–0.997) | <0.0001 | Nomogram risk score | 0.996 (0.995–0.997) | <0.0001 |
| Age | 1.009 (0.996–1.023) | 0.1607 | RDI6-week > 80% | Reference | - |
| Gender: male | 1.406 (1.118–1.766) | 0.0034 | 60–80% | 1.050 (0.777–1.419) | 0.9148 |
| <60% | 1.975 (1.461–2.670) | <0.0001 | |||
| Age | 1.008 (0.994–1.021) | 0.2786 | |||
| Gender: male | 1.287 (1.022–1.620) | 0.0317 | |||
| Risk Group | Low | Intermediate (n = 186) | High | Overall |
|---|---|---|---|---|
| (n = 156) | (n = 131) | (n = 473) | ||
| Neutropenia | ||||
| All-grade | 69 (44.2%) | 76 (40.9%) | 52 (39.7%) | 197 (41.6%) |
| ≥grade 3 | 36 (23.1%) | 44 (23.7%) | 30 (22.9%) | 110 (23.3%) |
| Febrile neutropenia | 4 (2.6%) | 7 (3.8%) | 5 (3.8%) | 16 (3.4%) |
| Not recorded | 4 (2.1%) | 2 (1.2%) | 3 (2.4%) | 9 (1.9%) |
| Anemia | ||||
| All-grade | 83 (53.2%) | 121 (65.1%) | 97 (74.0%) | 301(63.6%) |
| ≥grade 3 | 23 (14.7%) | 37 (19.9%) | 32 (24.4%) | 92 (19.5%) |
| Not recorded | 2 (1.1%) | 2 (1.2%) | 2 (1.6%) | 6 (1.3%) |
| Thrombocytopenia | ||||
| All-grade | 26 (16.7%) | 47 (25.3%) | 42 (32.1%) | 115 (24.3%) |
| ≥grade 3 | 5 (3.2%) | 10 (5.4%) | 9 (6.9%) | 24 (5.1%) |
| Not recorded | 3 (1.6%) | 1 (0.6%) | 2 (1.6%) | 6 (1.3%) |
| AST or ALT increased | ||||
| All-grade | 43 (27.6%) | 61 (32.8%) | 40 (30.5%) | 144 (30.4%) |
| ≥grade 3 | 6 (3.8%) | 7 (3.8%) | 0 (0%) | 13 (2.7%) |
| Not recorded | 40 (21.4%) | 44 (27.2%) | 33 (26.6%) | 117 (24.7%) |
| Blood bilirubin increased | ||||
| All-grade | 20 (12.8%) | 47 (25.3%) | 35 (26.7%) | 102 (21.6%) |
| ≥grade 3 | 10 (6.4%) | 15 (8.1%) | 10 (7.6%) | 35 (7.4%) |
| Not recorded | 23 (12.3%) | 8 (4.9%) | 9 (7.3%) | 40 (8.5%) |
| Creatinine increased | ||||
| All-grade | 28 (17.9%) | 30 (16.1%) | 22 (16.8%) | 80 (16.9%) |
| ≥grade 3 | 0 (0%) | 2 (1.1%) | 1 (0.8%) | 3 (0.6%) |
| Not recorded | 16 (8.6%) | 3 (1.9%) | 6 (4.8%) | 25 (5.3%) |
| Hypokalemia | ||||
| All-grade | 44 (28.2%) | 61 (32.8%) | 47 (35.9%) | 152 (32.1%) |
| ≥grade 3 | 21 (13.5%) | 21 (11.3%) | 18 (13.7%) | 60 (12.7%) |
| Not recorded | 42 (22.5%) | 34 (21.0%) | 29 (23.4%) | 105 (22.2%) |
| Fatigue | ||||
| All-grade | 64 (41.0%) | 84 (45.2%) | 63 (48.1%) | 211 (44.6%) |
| ≥ grade 3 | 1 (0.6%) | 5 (2.7%) | 2 (1.5%) | 8 (1.7%) |
| Not recorded | 12 (6.4%) | 13 (8.0%) | 10 (8.1%) | 35 (7.4%) |
| Vomiting | ||||
| All grade | 62 (39.7%) | 78 (41.9%) | 46 (35.1%) | 186 (39.3%) |
| ≥grade 3 | 5 (3.2%) | 8 (4.3%) | 2 (1.5%) | 15 (3.2%) |
| Not recorded | 3 (1.6%) | 2 (1.2%) | 4 (3.2%) | 9 (1.9%) |
| Diarrhea | ||||
| All-grade | 53 (34.0%) | 55 (29.6%) | 34 (26.0%) | 142 (30.0%) |
| ≥grade 3 | 3 (1.9%) | 5 (2.7%) | 5 (3.8%) | 13 (2.7%) |
| Not recorded | 5 (2.7%) | 3 (1.9%) | 5 (4.0%) | 13 (2.7%) |
| Hypoalbuminemia | ||||
| All-grade | 29 (18.6%) | 50 (26.9%) | 44 (33.6%) | 123 (26.0%) |
| ≥grade 3 | 1 (0.6%) | 5 (2.7%) | 0 (0%) | 6 (1.3%) |
| Not recorded | 28 (15.0%) | 22 (13.6%) | 15 (12.1%) | 65 (13.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-Y.; Chiang, N.-J.; Yang, Y.-H.; Yen, C.-J.; Bai, L.-Y.; Chiu, C.-F.; Chuang, S.-C.; Yang, S.-H.; Chou, W.-C.; Chen, J.-S.; et al. Real-World Data Validation of NAPOLI-1 Nomogram for the Prediction of Overall Survival in Metastatic Pancreatic Cancer. Cancers 2023, 15, 1008. https://doi.org/10.3390/cancers15041008
Su Y-Y, Chiang N-J, Yang Y-H, Yen C-J, Bai L-Y, Chiu C-F, Chuang S-C, Yang S-H, Chou W-C, Chen J-S, et al. Real-World Data Validation of NAPOLI-1 Nomogram for the Prediction of Overall Survival in Metastatic Pancreatic Cancer. Cancers. 2023; 15(4):1008. https://doi.org/10.3390/cancers15041008
Chicago/Turabian StyleSu, Yung-Yeh, Nai-Jung Chiang, Yi-Hsin Yang, Chia-Jui Yen, Li-Yuan Bai, Chang-Fang Chiu, Shih-Chang Chuang, Shih-Hung Yang, Wen-Chi Chou, Jen-Shi Chen, and et al. 2023. "Real-World Data Validation of NAPOLI-1 Nomogram for the Prediction of Overall Survival in Metastatic Pancreatic Cancer" Cancers 15, no. 4: 1008. https://doi.org/10.3390/cancers15041008
APA StyleSu, Y.-Y., Chiang, N.-J., Yang, Y.-H., Yen, C.-J., Bai, L.-Y., Chiu, C.-F., Chuang, S.-C., Yang, S.-H., Chou, W.-C., Chen, J.-S., Chiu, T.-J., Chen, Y.-Y., Chan, D.-C., Peng, C.-M., Chiu, S.-C., Li, C.-P., Shan, Y.-S., & Chen, L.-T. (2023). Real-World Data Validation of NAPOLI-1 Nomogram for the Prediction of Overall Survival in Metastatic Pancreatic Cancer. Cancers, 15(4), 1008. https://doi.org/10.3390/cancers15041008







