Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
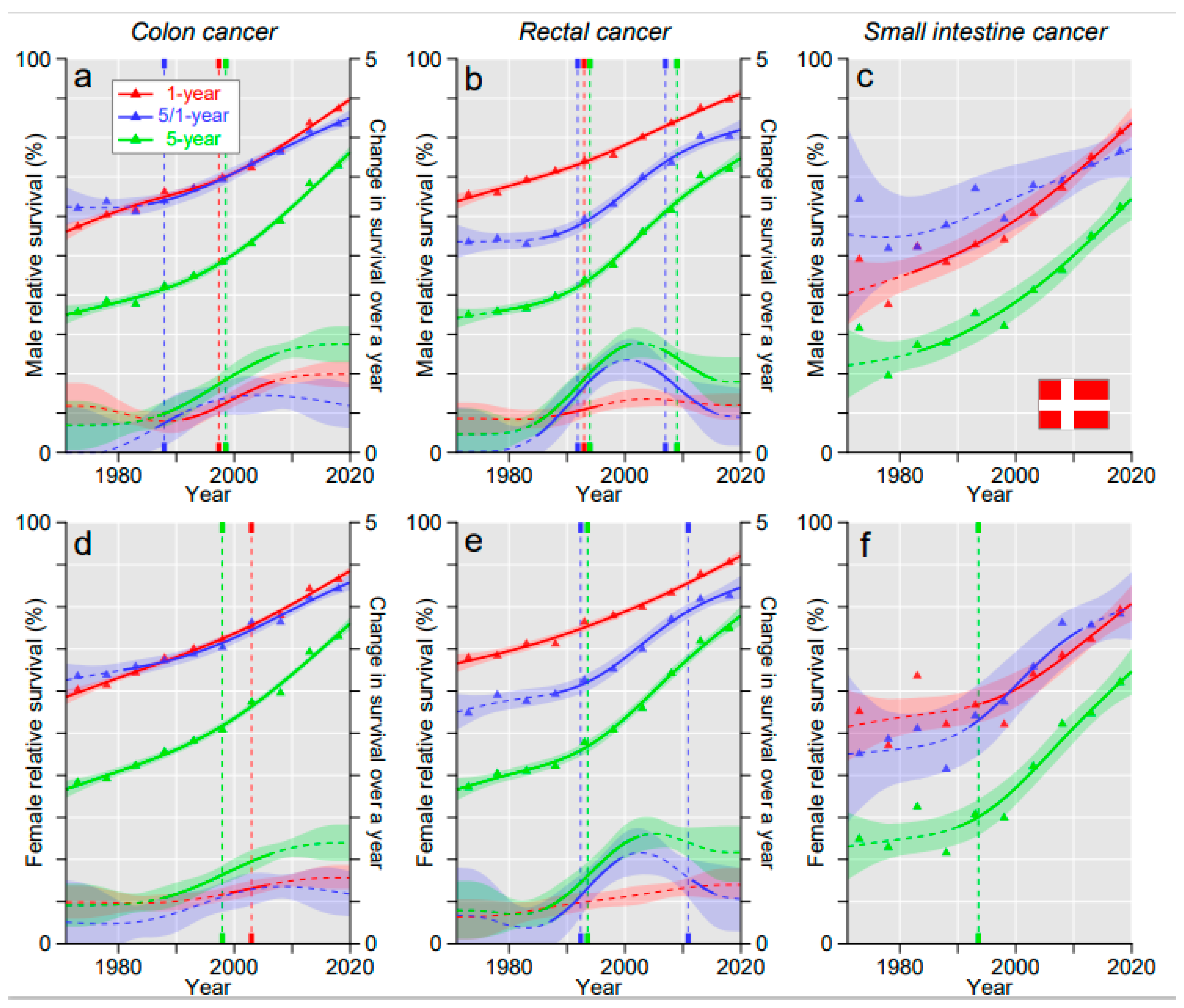
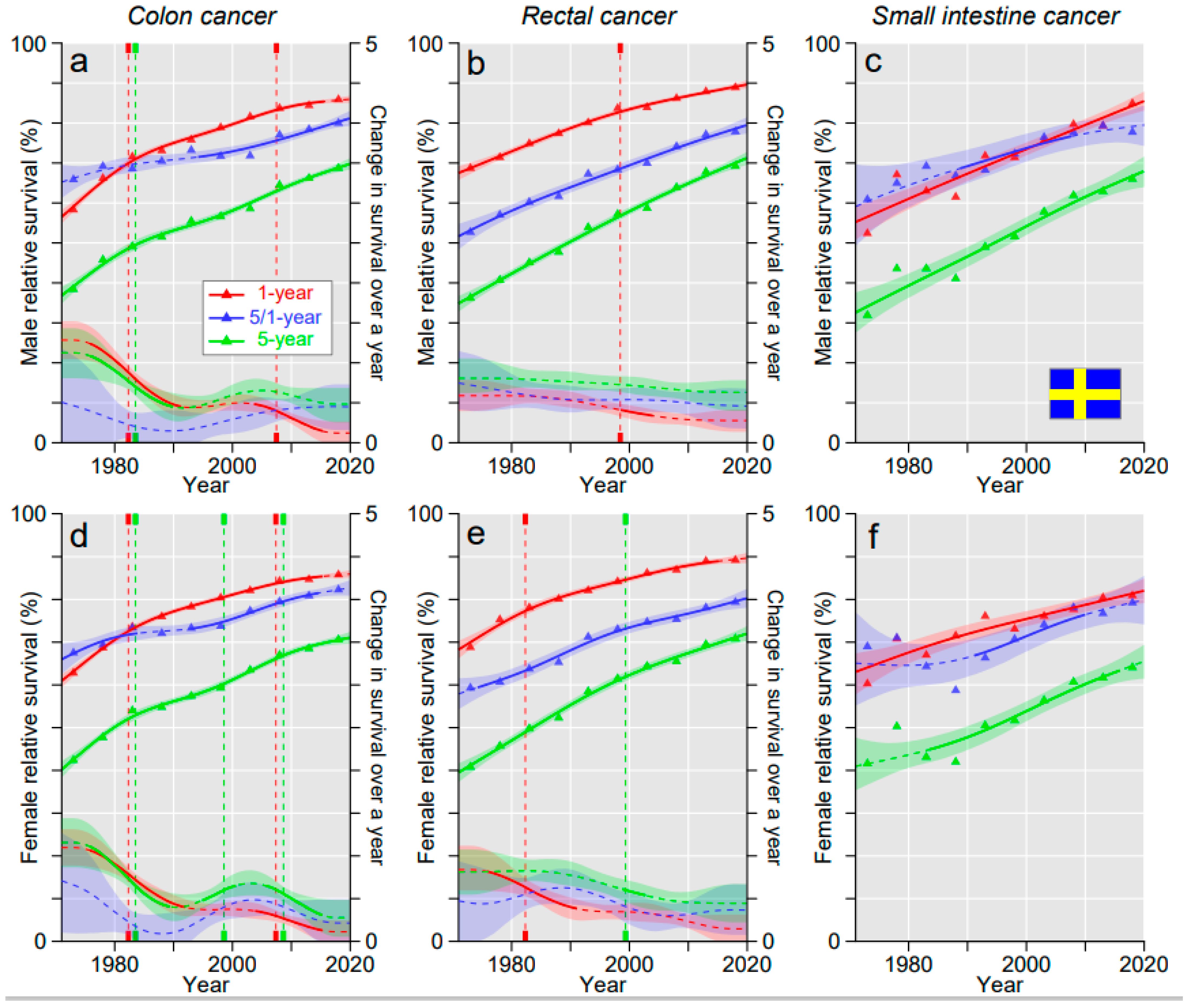
3. Results
3.1. Incidence and Mortality in the Nordic Countries
3.2. Relative Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klint, A.; Engholm, G.; Storm, H.; Tryggvadottir, L.; Gislum, M.; Hakulinen, T.; Bray, F. Trends in survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 578–607. [Google Scholar] [CrossRef] [PubMed]
- Ocasio Quinones, G.A.; Woolf, A. Small Bowel Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kumar, V.; Cotran, R.; Robbins, S. Basic Pathology; W.B. Saunders: Philadelphia, PA, USA, 1997; pp. 1–775. [Google Scholar]
- Hiripi, E.; Bermejo, J.L.; Sundquist, J.; Hemminki, K. Familial gastrointestinal carcinoid tumours and associated cancers. Ann. Oncol. 2009, 20, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Landerholm, K. Time trends in incidence and survival of small intestinal cancer in Sweden. BJS Open 2021, 5, zraa044. [Google Scholar] [CrossRef] [PubMed]
- Bojesen, R.D.; Andersson, M.; Riis, L.B.; Nielsen, O.H.; Jess, T. Incidence of, phenotypes of and survival from small bowel cancer in Denmark, 1994–2010: A population-based study. J. Gastroenterol. 2016, 51, 891–899. [Google Scholar] [CrossRef]
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Dyson, J.K.; Rutter, M.D. Colorectal cancer in inflammatory bowel disease: What is the real magnitude of the risk? World J. Gastroenterol. 2012, 18, 3839–3848. [Google Scholar] [CrossRef]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef]
- Barsouk, A.; Rawla, P.; Barsouk, A.; Thandra, K.C. Epidemiology of Cancers of the Small Intestine: Trends, Risk Factors, and Prevention. Med. Sci. 2019, 7, 46. [Google Scholar] [CrossRef]
- Liu, X.; Hemminki, K.; Försti, A.; Sundquist, K.; Sundquist, J.; Ji, J. Cancer risk in patients with type 2 diabetes mellitus and their relatives. Int. J. Cancer 2015, 137, 903–910. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Cancer risks in ulcerative colitis patients. Int. J. Cancer 2008, 123, 1417–1421. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X.; Sundquist, J.; Sundquist, K. Cancer risks in Crohn disease patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 574–580. [Google Scholar] [CrossRef]
- IARC. Personal Habits and Indoor Combustions; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100E, p. 575. [Google Scholar]
- Hemminki, K.; Sundquist, K.; Sundquist, J.; Försti, A.; Hemminki, A.; Li, X. Familial Risks and Proportions Describing Population Landscape of Familial Cancer. Cancers 2021, 13, 4385. [Google Scholar] [CrossRef]
- Kharazmi, E.; Pukkala, E.; Sundquist, K.; Hemminki, K. Familial Risk of Small Intestinal Carcinoid and Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 944–949. [Google Scholar] [CrossRef]
- Hemminki, K.; Li, X. Familial carcinoid tumors and subsequent cancers: A nation-wide epidemiologic study from Sweden. Int. J. Cancer 2001, 94, 444–448. [Google Scholar] [CrossRef]
- Yu, H.; Hemminki, A.; Sundquist, K.; Hemminki, K. Familial Associations of Colon and Rectal Cancers With Other Cancers. Dis. Colon Rectum 2019, 62, 189–195. [Google Scholar] [CrossRef]
- Yu, H.; Hemminki, K. Genetic epidemiology of colorectal cancer and associated cancers. Mutagenesis 2019, 35, 207–219. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair vari-ants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef]
- Hemminki, K.; Försti, A.; Hemminki, A. Survival in colon and rectal cancers in Finland and Sweden through 50 years. BMJ Open Gastroenterol. 2021, 8, e000644. [Google Scholar] [CrossRef]
- Mazlom, H.; Teuwen, L.-A.; Peeters, M. Management of small bowel adenocarcinoma: Making the most of the available evidence to inform routine practice. Curr. Opin. Oncol. 2021, 33, 368–371. [Google Scholar] [CrossRef]
- Legué, L.M.; Bernards, N.; Gerritse, S.L.; van Oudheusden, T.R.; de Hingh, I.H.J.T.; Creemers, G.-J.M.; Tije, A.J.T.; Lemmens, V.E.P.P. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: A population-based study in The Netherlands. Acta Oncol. 2016, 55, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Tuma, F. Intestinal Carcinoid Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.; Woods, L.M.; Estève, J.; Eloranta, S.; Coleman, M.P.; Rachet, B. Cancer incidence, survival and mortality: Explaining the concepts. Int. J. Cancer 2014, 135, 1774–1782. [Google Scholar] [CrossRef]
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Storm, H.H. NORDCAN-a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef]
- Pukkala, E.; Engholm, G.; Schmidt, L.K.H.; Storm, H.; Khan, S.; Lambe, M.; Pettersson, D.; Ólafsdóttir, E.; Tryggvadóttir, L.; Hakanen, T.; et al. Nordic Cancer Registries—An overview of their procedures and data comparability. Acta Oncol. 2017, 57, 440–455. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, K.; Sundquist, J.; Hemminki, K. Comparability of cancer identification among Death Registry, Cancer Registry and Hospital Discharge Registry. Int. J. Cancer 2012, 131, 2085–2093. [Google Scholar] [CrossRef]
- Centre for Epidemiology. Cancer Incidence in Sweden 2012; The National Board of Health and Welfare: Stockholm, Sweden, 2013.
- Storm, H.H.; Klint, A.; Tryggvadóttir, L.; Gislum, M.; Engholm, G.; Bray, F. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 694–712. [Google Scholar] [CrossRef]
- Tryggvadóttir, L.; Gislum, M.; Bray, F.; Klint, Å.; Hakulinen, T.; Storm, H.H.; Engholm, G. Trends in the survival of patients diagnosed with breast cancer in the Nordic countries 1964–2003 followed up to the end of 2006. Mater. Methods Acta Oncol. 2010, 49, 624–631. [Google Scholar] [CrossRef]
- Lundberg, F.E.; Andersson, T.M.-L.; Lambe, M.; Engholm, G.; Mørch, L.S.; Johannesen, T.B.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Birgisson, H.; et al. Trends in cancer survival in the Nordic countries 1990–2016: The NORDCAN survival studies. Acta Oncol. 2020, 59, 1266–1274. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.; Myklebust, T. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef]
- Lundberg, F.E.; Birgisson, H.; Johannesen, T.B.; Engholm, G.; Virtanen, A.; Pettersson, D.; Ólafsdóttir, E.J.; Lambe, M.; Lambert, P.C.; Mørch, L.S.; et al. Survival trends in patients diagnosed with colon and rectal cancer in the nordic countries 1990–2016: The NORDCAN survival studies. Eur. J. Cancer 2022, 172, 76–84. [Google Scholar] [CrossRef]
- Probst, H.B.; Hussain, Z.B.; Andersen, O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—A national Danish project. Health Policy 2012, 105, 65–70. [Google Scholar] [CrossRef]
- Moberger, P.; Sköldberg, F.; Birgisson, H. Evaluation of the Swedish Colorectal Cancer Registry: An overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018, 57, 1611–1621. [Google Scholar] [CrossRef]
- Stormark, K.; Søreide, K.; Søreide, J.A.; Kvaløy, J.T.; Pfeffer, F.; Eriksen, M.T. Nationwide implementation of laparoscopic surgery for colon cancer: Short-term outcomes and long-term sur-vival in a population-based cohort. Surg. Endosc. 2016, 30, 4853–4864. [Google Scholar] [CrossRef]
- Araghi, M.; Arnold, M.; Rutherford, M.J.; Guren, M.G.; Cabasag, C.J.; Bardot, A.; Ferlay, J.; Tervonen, H.; Shack, L.; Woods, R.; et al. Colon and rectal cancer survival in seven high-income countries 2010–2014: Variation by age and stage at diagnosis (the ICBP SURVMARK-2 project). Gut 2021, 70, 114–126. [Google Scholar] [CrossRef]
- Pilleron, S.; Charvat, H.; Araghi, M.; Arnold, M.; Fidler-Benaoudia, M.M.; Bardot, A.; Guren, M.G.; Tervonen, H.; Little, A.; O’Connell, D.L.; et al. Age disparities in stage-specific colon cancer survival across seven countries: An International Cancer Benchmarking Partnership SURVMARK -2 population-based study. Int. J. Cancer 2020, 148, 1575–1585. [Google Scholar] [CrossRef]
- Engholm, G.; Kejs, A.M.T.; Brewster, D.H.; Gaard, M.; Holmberg, L.; Hartley, R.; Iddenden, R.; Møller, H.; Sankila, R.; Thomson, C.S.; et al. Colorectal cancer survival in the Nordic countries and the United Kingdom: Excess mortality risk analysis of 5 year relative period survival in the period 1999 to 2000. Int. J. Cancer 2007, 121, 1115–1122. [Google Scholar] [CrossRef]


| (A) Case Numbers, Incidence (ASR) and Cumulative Incidence | |||||
|---|---|---|---|---|---|
| Males | ASR (World) | Cum. Risk % (0–74) | Females | ASR (World) | Cum. Risk % (0–74) |
| Colon | |||||
| Denmark, 16,752 | 27.2 | 3.2 | Denmark, 16,388 | 23.3 | 2.7 |
| Finland, 10,311 | 17.2 | 1.9 | Finland, 10,565 | 14.3 | 1.6 |
| Norway, 14041 | 27.2 | 3 | Norway, 15,330 | 25.5 | 2.8 |
| Sweden, 21,576 | 19.3 | 2.2 | Sweden, 22,122 | 17.4 | 1.9 |
| Rectum | |||||
| Denmark, 9585 | 16.4 | 2 | Denmark, 6047 | 9.5 | 1.1 |
| Finland, 7078 | 12.2 | 1.5 | Finland, 4851 | 7.2 | 0.87 |
| Norway, 7932 | 16.4 | 2 | Norway, 5440 | 10.4 | 1.2 |
| Sweden, 12,548 | 12.1 | 1.5 | Sweden, 8221 | 7.3 | 0.87 |
| Small intestine | |||||
| Denmark, 1070 | 1.9 | 0.24 | Denmark, 852 | 1.4 | 0.18 |
| Finland, 911 | 1.7 | 0.2 | Finland, 756 | 1.2 | 0.15 |
| Norway, 1106 | 2.4 | 0.28 | Norway, 780 | 1.5 | 0.18 |
| Sweden, 1620 | 1.6 | 0.2 | Sweden, 1305 | 1.2 | 0.15 |
| (B) Death numbers, mortality (ASR) and cumulative mortality | |||||
| Colon | |||||
| Denmark, 6639 | 9.9 | 0.99 | Denmark, 6685 | 7.8 | 0.77 |
| Finland, 3936 | 6.0 | 0.61 | Finland, 4145 | 4.5 | 0.47 |
| Norway, 5578 | 9.9 | 0.97 | Norway, 6099 | 8.3 | 0.82 |
| Sweden, 9007 | 7.2 | 0.71 | Sweden, 9562 | 6.1 | 0.61 |
| Rectum | |||||
| Denmark, 2783 | 4.3 | 0.46 | Denmark, 1839 | 2.3 | 0.24 |
| Finland, 2675 | 4.2 | 0.46 | Finland, 1821 | 2.1 | 0.23 |
| Norway, 2294 | 4.3 | 0.47 | Norway, 1588 | 2.4 | 0.25 |
| Sweden, 4926 | 4.1 | 0.45 | Sweden, 3457 | 2.4 | 0.26 |
| Small intestine | |||||
| Denmark, 371 | 0.57 | 0.07 | Denmark, 349 | 0.47 | 0.06 |
| Finland, 359 | 0.57 | 0.07 | Finland, 290 | 0.34 | 0.04 |
| Norway, 333 | 0.61 | 0.07 | Norway, 281 | 0.46 | 0.05 |
| Sweden, 646 | 0.55 | 0.06 | Sweden, 579 | 0.43 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichanek, F.; Försti, A.; Liska, V.; Hemminki, A.; Hemminki, K. Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century. Cancers 2023, 15, 991. https://doi.org/10.3390/cancers15030991
Tichanek F, Försti A, Liska V, Hemminki A, Hemminki K. Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century. Cancers. 2023; 15(3):991. https://doi.org/10.3390/cancers15030991
Chicago/Turabian StyleTichanek, Filip, Asta Försti, Vaclav Liska, Akseli Hemminki, and Kari Hemminki. 2023. "Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century" Cancers 15, no. 3: 991. https://doi.org/10.3390/cancers15030991
APA StyleTichanek, F., Försti, A., Liska, V., Hemminki, A., & Hemminki, K. (2023). Survival in Colon, Rectal and Small Intestinal Cancers in the Nordic Countries through a Half Century. Cancers, 15(3), 991. https://doi.org/10.3390/cancers15030991








