Surgery-Related Muscle Loss after Pancreatic Resection and Its Association with Postoperative Nutritional Intake
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. Baseline and Perioperative Characteristics
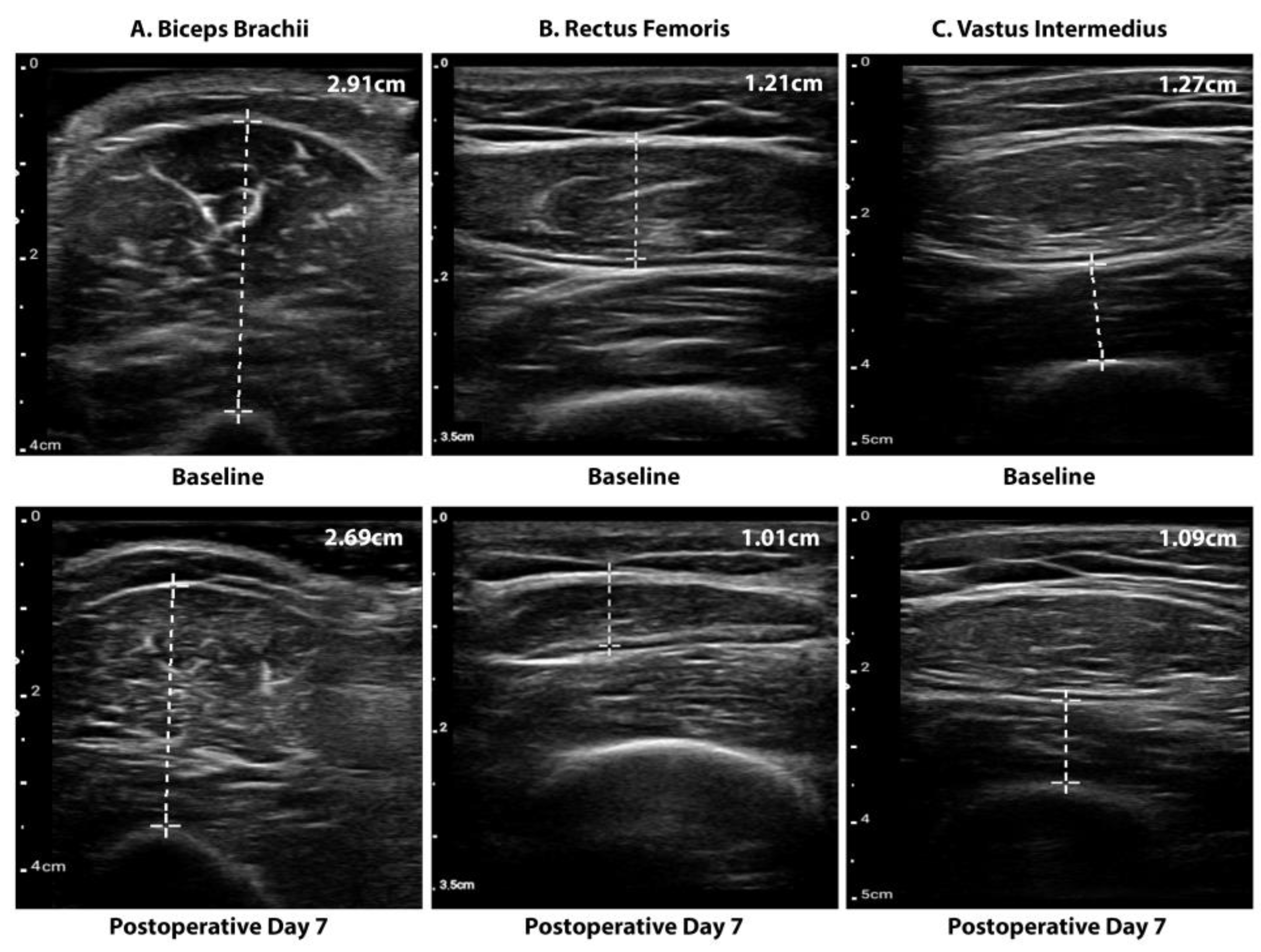
2.2.2. Muscle Mass
2.2.3. Nutritional Intake
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patients
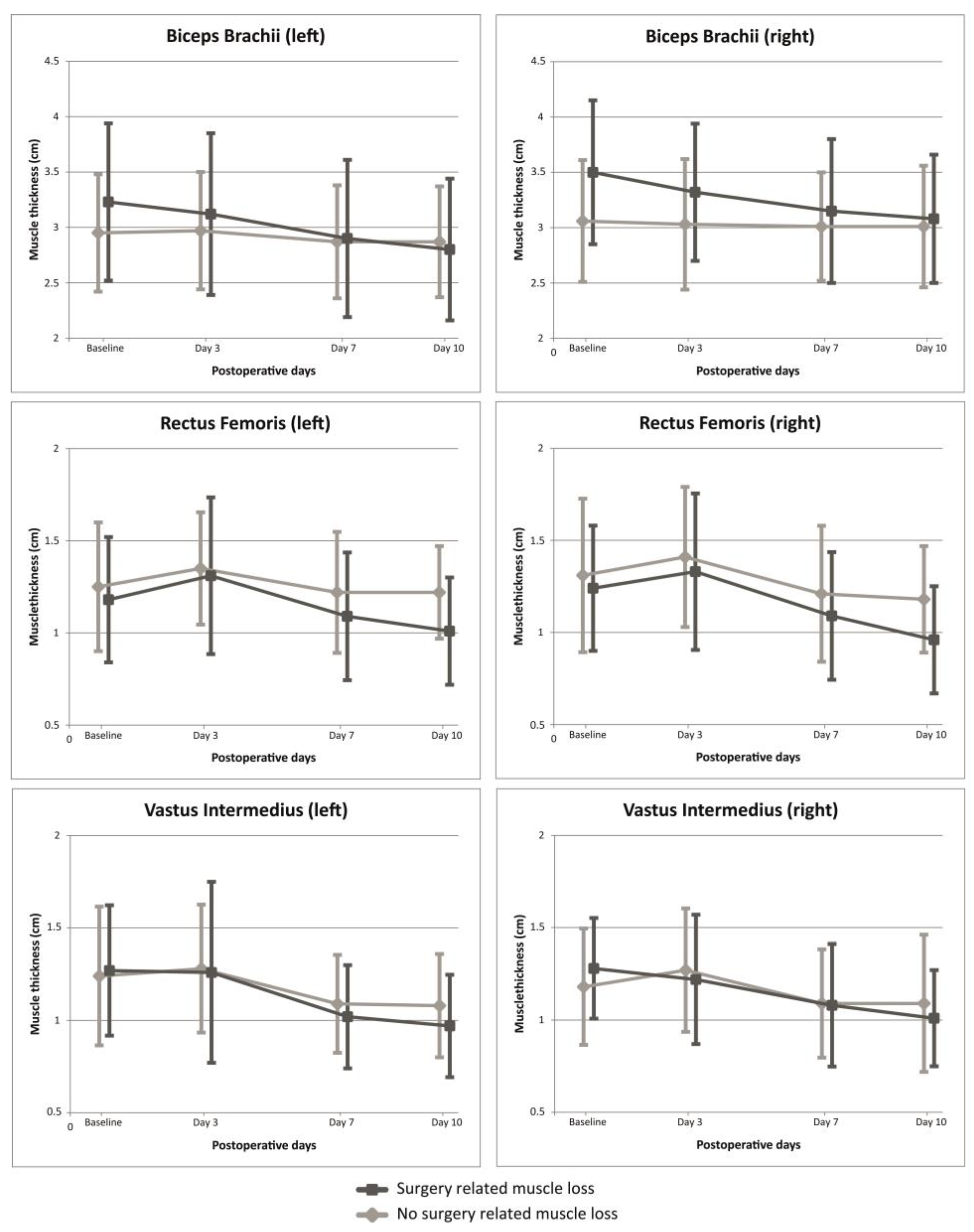
3.2. Presence of Clinically Relevant SRML
3.3. Postoperative Course
3.4. Nutritional INTAKE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puthucheary, Z.A.; Phadke, R.; Rawal, J.; McPhail, M.J.W.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit. Care Med. 2015, 43, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.M.; Looijaard, W.G.P.M.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Oudemans-van Straaten, H.M.; Beishuizen, A. Low Skeletal Muscle Area Is a Risk Factor for Mortality in Mechanically Ventilated Critically Ill Patients. Crit. Care 2014, 18, R12. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, C.E.; Bersten, A.D. Alterations in Respiratory and Limb Muscle Strength and Size in Patients with Sepsis Who Are Mechanically Ventilated. Phys. Ther. 2014, 94, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.; Hussain, S.N.A.; Mathur, S.; Picard, M.; Herridge, M.; Correa, J.; Bain, A.; Guo, Y.; Advani, A.; Advani, S.L.; et al. Mechanisms of Chronic Muscle Wasting and Dysfunction after an Intensive Care Unit Stay: A Pilot Study. Am. J. Respir. Crit. Care Med. 2016, 194, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Herbert, G.; Perry, R.; Andersen, H.K.; Atkinson, C.; Penfold, C.; Lewis, S.J.; Ness, A.R.; Thomas, S. Early Enteral Nutrition within 24 Hours of Lower Gastrointestinal Surgery versus Later Commencement for Length of Hospital Stay and Postoperative Complications. Cochrane Database Syst. Rev. 2019, 7, CD004080. [Google Scholar] [CrossRef]
- Chambers, M.A.; Moylan, J.S.; Reid, M.B. Physical Inactivity and Muscle Weakness in the Critically Ill. Crit. Care Med. 2009, 37, S337–S346. [Google Scholar] [CrossRef]
- Gillis, C.; Carli, F. Promoting Perioperative Metabolic and Nutritional Care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef]
- Huang, D.-D.; Ji, Y.-B.; Zhou, D.-L.; Li, B.; Wang, S.-L.; Chen, X.-L.; Yu, Z.; Zhuang, C.-L. Le Effect of Surgery-Induced Acute Muscle Wasting on Postoperative Outcomes and Quality of Life. J. Surg. Res. 2017, 218, 58. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.B.; Lee, K.; Song, M.; Lee, I.S.; Lee, M.A.; Hong, T.H.; Choi, M.-G. Preoperative Sarcopenia and Post-Operative Accelerated Muscle Loss Negatively Impact Survival after Resection of Pancreatic Cancer. J. Cachexia. Sarcopenia Muscle 2018, 9, 326–334. [Google Scholar] [CrossRef]
- van Wijk, L.; van Duinhoven, S.; Liem, M.S.L.; Bouman, D.E.; Viddeleer, A.R.; Klaase, J.M. Risk Factors for Surgery-Related Muscle Quantity and Muscle Quality Loss and Their Impact on Outcome. Eur. J. Med. Res. 2021, 26, 36. [Google Scholar] [CrossRef]
- Scott, M.J.; Miller, T.E. Pathophysiology of Major Surgery and the Role of Enhanced Recovery Pathways and the Anesthesiologist to Improve Outcomes. Anesthesiol. Clin. 2015, 33, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, C.C.; Mabvuure, N.T.; Kozar, R.A.; Herndon, D.N. The Surgically Induced Stress Response. J. Parenter. Enter. Nutr. 2013, 37, 21S–29S. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.A.A.; Lee, J.Y.; Wort, S.J.; Polkey, M.I.; Kemp, P.R.; Griffiths, M.J.D. Sustained Elevation of Circulating Growth and Differentiation Factor-15 and a Dynamic Imbalance in Mediators of Muscle Homeostasis Are Associated with the Development of Acute Muscle Wasting Following Cardiac Surgery. Crit. Care Med. 2013, 41, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Hadley, J.S.; Hinds, C.J. Anabolic Strategies in Critical Illness. Curr. Opin. Pharmacol. 2002, 2, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Gore, D.C.; Jahoor, F.; Wolfe, R.R.; Herndon, D.N. Acute Response of Human Muscle Protein to Catabolic Hormones. Ann. Surg. 1993, 218, 679–684. [Google Scholar] [CrossRef]
- Matthews, D.E.; Battezzati, A. Regulation of Protein Metabolism during Stress. Curr. Opin. Gen. Surg. 1993, 72–77. [Google Scholar]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Poulia, K.A.; Sarantis, P.; Antoniadou, D.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic Cancer and Cachexia -Metabolic Mechanisms and Novel Insights. Nutrients 2020, 12, 1543. [Google Scholar] [CrossRef]
- Hentzen, J.E.K.R.; van Wijk, L.; Buis, C.I.; Viddeleer, A.R.; de Bock, G.H.; van der Schans, C.P.; van Dam, G.M.; Kruijff, S.; Klaase, J.M. Impact and Risk Factors for Clinically Relevant Surgery-Related Muscle Loss in Patients after Major Abdominal Cancer Surgery: Study Protocol for a Prospective Observational Cohort Study (MUSCLE POWER). Int. J. Clin. Trials 2019, 6, 138–146. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.J.; Goyal, A.; Bansal, P.; Garmon, E.H. American Society of Anesthesiologists Classification; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- De Groot, L.M.; Lee, G.; Ackerie, A.; van der Meij, B.S. Malnutrition Screening and Assessment in the Cancer Care Ambulatory Setting: Mortality Predictability and Calidity of the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) and the GLIM Criteria. Nutrients 2020, 12, 2287. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.A. The Comprehensive Complication Index: A Novel Continuous Scale to Measure Surgical Morbidity. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed Gastric Emptying (DGE) after Pancreatic Surgery: A Suggested Definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 Update of the International Study Group (ISGPS) Definition and Grading of Postoperative Pancreatic Fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy Hemorrhage (PPH): An International Study Group of Pancreatic Surgery (ISGPS) Definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Paiella, S.; De Pastena, M.; Casciani, F.; Pan, T.L.; Bogoni, S.; Andrianello, S.; Marchegiani, G.; Malleo, G.; Bassi, C.; Salvia, R. Chyle Leak after Pancreatic Surgery: Validation of the International Study Group of Pancreatic Surgery Classification. Surgery 2018, 164, 450–454. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.M.; Gallagher, D.; St.-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total Body Skeletal Muscle and Adipose Tissue Volumes: Estimation from a Single Abdominal Cross-Sectional Image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef]
- Boer, B.C.; de Graaff, F.; Brusse-Keizer, M.; Bouman, D.E.; Slump, C.H.; Slee-Valentijn, M.; Klaase, J.M. Skeletal Muscle Mass and Quality as Risk Factors for Postoperative Outcome after Open Colon Resection for Cancer. Int. J. Color. Dis. 2016, 31, 1117–1124. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Gitto, S.; Ruffo, G.; Guarino, S.; Del Grande, F.; Sconfienza, L.M. Sarcopenia: Imaging Assessment and Clinical Application. Abdom. Radiol. 2021, 47, 3205–3216. [Google Scholar] [CrossRef]
- Bahat, G.; Turkmen, B.O.; Aliyev, S.; Catikkas, N.M.; Bakir, B.; Karan, M.A. Cut-off Values of Skeletal Muscle Index and Psoas Muscle Index at L3 Vertebra Level by Computerized Tomography to Assess Low Muscle Mass. Clin. Nutr. 2021, 40, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The Reliability and Validity of Ultrasound to Quantify Muscles in Older Adults: A Systematic Review. J. Cachexia. Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Jager-Wittenaar, H.; Raj, I.S.; van der Schans, C.P.; Hobbelen, H. Reliability and Validity of Ultrasound to Estimate Muscles: A Comparison between Different Transducers and Parameters. Clin. Nutr. ESPEN 2020, 35, 146–152. [Google Scholar] [CrossRef]
- Nascimento, T.S.; de Queiroz, R.S.; Ramos, A.C.C.; Martinez, B.P.; Da Silva e Silva, C.M.; Gomes-Neto, M. Ultrasound Protocols to Assess Skeletal and Diaphragmatic Muscle in People Who Are Critically Ill: A Systematic Review. Ultrasound Med. Biol. 2021, 47, 3041–3067. [Google Scholar] [CrossRef] [PubMed]
- Hogenbirk, R.N.M.; Viddeleer, A.R.; Hentzen, J.E.K.R.; van der Plas, W.Y.; van der Schans, C.P.; de Bock, G.H.; Kruijff, S.; Klaase, J.M. Thickness of Biceps and Quadriceps Femoris Muscle Measured Using Point-of-Care Ultrasound as a Representation of Total Skeletal Muscle Mass. J. Clin. Med. 2022, 11, 6606. [Google Scholar] [CrossRef]
- Turton, P.; Hay, R.; Taylor, J.; McPhee, J.; Welters, I. Human Limb Skeletal Muscle Wasting and Architectural Remodeling during Five to Ten Days Intubation and Ventilation in Critical Care—An Observational Study Using Ultrasound. BMC Anesthesiol. 2016, 16, 119. [Google Scholar] [CrossRef]
- Haaf, D.T.; Hemmen, B.; van de Meent, H.; Bovend’Eerdt, T.J. The Magnitude and Time Course of Muscle Cross-Section Decrease in Intensive Care Unit Patients. Am. J. Phys. Med. Rehabil. 2017, 96, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.M.; El-Ansary, D.; Cartwright, M.S.; Sarwal, A.; Berney, S.; Koopman, R.; Annoni, R.; Puthucheary, Z.; Gordon, I.R.; Morris, P.E.; et al. Ultrasonography in the Intensive Care Setting Can Be Used to Detect Changes in the Quality and Quantity of Muscle and Is Related to Muscle Strength and Function. J. Crit. Care 2015, 30, 1151.e9–1151.e14. [Google Scholar] [CrossRef] [PubMed]
- Annetta, M.G.; Pittiruti, M.; Silvestri, D.; Grieco, D.L.; Maccaglia, A.; La Torre, M.F.; Magarelli, N.; Mercurio, G.; Caricato, A.; Antonelli, M. Ultrasound Assessment of Rectus Femoris and Anterior Tibialis Muscles in Young Trauma Patients. Ann. Intensive Care 2017, 7, 104. [Google Scholar] [CrossRef]
- Gerovasili, V.; Stefanidis, K.; Vitzilaios, K.; Karatzanos, E.; Politis, P.; Koroneos, A.; Chatzimichail, A.; Routsi, C.; Roussos, C.; Nanas, S. Electrical Muscle Stimulation Preserves the Muscle Mass of Critically Ill Patients: A Randomized Study. Crit. Care 2009, 13, R161. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.A.; Oliveira, D.; Cupolilo, E.N.; Miranda, C.S.; Colugnati, F.A.B.; Mansur, H.N.; Fernandes, N.M.D.S.; Bastos, M.G. Rectus Femoris Muscle Mass Evaluation by Ultrasound: Facilitating Sarcopenia Diagnosis in Pre-Dialysis Chronic Kidney Disease Stages. Clinics 2018, 73, e392. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Wu, W.-T.; Huang, K.-C.; Jan, W.H.; Han, D.-S. Limb Muscle Quality and Quantity in Elderly Adults with Dynapenia but Not Sarcopenia: An Ultrasound Imaging Study. Exp. Gerontol. 2018, 108, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Katari, Y.; Srinivasan, R.; Arvind, P.; Hiremathada, S. Point-of-Care Ultrasound to Evaluate Thickness of Rectus Femoris, Vastus Intermedius Muscle, and Fat as an Indicator of Muscle and Fat Wasting in Critically Ill Patients in a Multidisciplinary Intensive Care Unit. Indian J. Crit. Care Med. 2018, 22, 781–788. [Google Scholar] [CrossRef]
- Nozoe, M.; Kubo, H.; Furuichi, A.; Kanai, M.; Takashima, S.; Shimada, S.; Mase, K. Validity of Quadriceps Muscle Thickness Measurement in Patients with Subacute Sroke during Hospitalization for Assessment of Muscle Wasting and Physical Function. J. Stroke Cerebrovasc. Dis. 2017, 26, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, M.; Kanai, M.; Kubo, H.; Kitamura, Y.; Yamamoto, M.; Furuichi, A.; Takashima, S.; Mase, K.; Shimada, S. Changes in Quadriceps Muscle Thickness, Disease Severity, Nutritional Status, and C-Reactive Protein after Acute Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2470–2474. [Google Scholar] [CrossRef] [PubMed]
- Palakshappa, J.A.; Reilly, J.P.; Schweickert, W.D.; Anderson, B.J.; Khoury, V.; Shashaty, M.G.; Fitzgerald, D.; Forker, C.; Butler, K.; Ittner, C.A.; et al. Quantitative Peripheral Muscle Ultrasound in Sepsis: Muscle Area Superior to Thickness. J. Crit. Care 2018, 47, 324–330. [Google Scholar] [CrossRef]
- Arts, I.M.P.; Pillen, S.; Schelhaas, H.J.; Overeem, S.; Zwarts, M.J. Normal Values for Quantitative Muscle Ultrasonography in Adults. Muscle Nerve 2010, 41, 32–41. [Google Scholar] [CrossRef]
- Bongers, B.C.; Dejong, C.H.C.; den Dulk, M. Enhanced Recovery after Surgery Programmes in Older Patients Undergoing Hepatopancreatobiliary Surgery: What Benefits Might Prehabilitation Have? Eur. J. Surg. Oncol. 2021, 47, 551–559. [Google Scholar] [CrossRef]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Li, J.; Hong, D. Enhanced Recovery after Surgery (ERAS) versus Standard Recovery for Elective Gastric Cancer Surgery: A Meta-Analysis of Randomized Controlled Trials. Surg. Oncol. 2020, 32, 75–87. [Google Scholar] [CrossRef]
- Lassen, K.; Coolsen, M.M.E.; Slim, K.; Carli, F.; de Aguilar-Nascimento, J.E.; Schäfer, M.; Parks, R.W.; Fearon, K.C.H.; Lobo, D.N.; Demartines, N.; et al. Guidelines for Perioperative Care for Pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2013, 37, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Nguyen, T.H.; Liberman, A.S.; Carli, F. Nutrition Adequacy in Enhanced Recovery after Surgery: A Single Academic Center Experience. Nutr. Clin. Pract. 2015, 30, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Allard, J.; Vesnaver, E.; Laporte, M.; Gramlich, L.; Bernier, P.; Davidson, B.; Duerksen, D.; Jeejeebhoy, K.; Payette, H. Barriers to Food Intake in Acute Care Hospitals: A Report of the Canadian Malnutrition Task Force. J. Hum. Nutr. Diet. 2015, 28, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Schager, M.; van Dronkelaar, C.; Naumann, E. Protein Intake during Hospital Admission; Dutch National Data on Protein Intake in 339,720 Malnourished Patients from 2009–2009. Clin. Nutr. Open Sci. 2022, 41, 74–81. [Google Scholar] [CrossRef]
- Constansia, R.D.N.; Hentzen, J.E.K.R.; Hogenbirk, R.N.M.; van der Plas, W.Y.; Campmans-Kuijpers, M.J.E.; Buis, C.I.; Kruijff, S.; Klaase, J.M. Actual Postoperative Protein and Calorie Intake in Patients Undergoing Major Open Abdominal Cancer Surgery: A Prospective, Observational Cohort Study. Nutr. Clin. Pract. 2021, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Garth, A.K.; Newsome, C.M.; Simmance, N.; Crowe, T.C. Nutritional Status, Nutrition Practices and Post-Operative Complications in Patients with Gastrointestinal Cancer. J. Hum. Nutr. Diet. 2010, 23, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef]
- Van Dyck, L.; Casaer, M.P.; Gunst, J. Autophagy and Its Implications against Early Full Nutrition Support in Critical Illness. Nutr. Clin. Pr. 2018, 33, 339–347. [Google Scholar] [CrossRef]
- Williams, J.P.; Phillips, B.E.; Smith, K.; Atherton, P.J.; Rankin, D.; Selby, A.L.; Liptrot, S.; Lund, J.; Larvin, M.; Rennie, M.J. Effect of Tumor Burden and Subsequent Surgical Resection on Skeletal Muscle Mass and Protein Turnover in Colorectal Cancer Patients. Am. J. Clin. Nutr. 2012, 96, 1064–1070. [Google Scholar] [CrossRef]
| Patient Characteristics | Total (n = 63) | SRML (n = 24) | No-SRML (n = 39) | p-Value |
|---|---|---|---|---|
| Sex, male | 38 (60.3%) | 18 (75.0%) | 20 (51.3%) | 0.062 |
| Age, years | 67.1 ± 10.2 | 68.1 ± 8.9 | 66.4 ± 10.9 | 0.536 |
| BMI, kg/m2 | 26.3 ± 4.4 | 25.9 ± 4.2 | 26.6 ± 4.5 | 0.512 |
| ASA score ≥ 3 | 17 (27.0%) | 6 (25.0%) | 11 (28.2%) | 0.781 |
| Baseline muscle cross-section, cm | ||||
| Mm. biceps brachii | 3.12 ± 0.60 | 3.31 ± 0.67 | 3.01 ± 0.53 | 0.060 |
| Mm. rectus femoris | 1.23 ± 0.31 | 1.21 ± 0.34 | 1.28 ± 0.36 | 0.460 |
| Mm. vastus intermedius | 1.26 ± 0.35 | 1.27 ± 0.28 | 1.21 ± 0.33 | 0.431 |
| Charlson Comorbidity Index | 3 (2–4) | 3 (2–4) | 3 (2–4) | 1 |
| Comorbidities | ||||
| Diabetes | 14 (22.2%) | 4 (16.7%) | 10 (25.6%) | 0.405 |
| Cardiopulmonary disease | 25 (39.7%) | 8 (33.3%) | 17 (43.6%) | 0.419 |
| Renal comorbidity | 3 (4.8%) | 2 (8.3%) | 1 (2.6%) | 0.296 |
| No comorbidities | 22 (34.9%) | 9 (37.5%) | 13 (33.3%) | 0.736 |
| Smoking | ||||
| Current smoking | 10 (15.9%) | 4 (16.7%) | 6 (15.4%) | 0.892 |
| Stopped smoking | 35 (55.6%) | 13 (54.2%) | 22 (56.4%) | 0.862 |
| Never smoked | 18 (28.6%) | 7 (29.2%) | 11 (28.2%) | 0.935 |
| Preoperative weight loss in 6 months | ||||
| <5% weight loss | 35 (55.6%) | 14 (58.3%) | 21 (53.8%) | 0.728 |
| 5–10% weight loss | 15 (23.8%) | 7 (29.2%) | 8 (20.5%) | 0.434 |
| ≥10% weight loss | 13 (20.6%) | 3 (12.5%) | 10 (25.6%) | 0.211 |
| PG-SGA SF | 2 (1–4) | 2 (1–3.75) | 3 (1–7) | 0.148 |
| PG-SGA SF ≥ 4 | 14 (22.2%) | 2 (8.3%) | 12 (30.8%) | 0.038 |
| Serum albumin prior to surgery | 42.6 ± 3.8 | 42.9 ± 4.0 | 42.4 ±3.7 | 0.600 |
| Serum albumin at discharge | 32.2 ± 3.5 | 32.7 ± 2.8 | 31.9 ± 3.8 | 0.405 |
| Surgical Details | Total Cohort (n = 63) | SRML (n = 24) | No-SRML (n = 39) | p-Value |
|---|---|---|---|---|
| Histological Diagnosis | ||||
| Adenocarcinoma pancreas | 34 (54.0%) | 11 (45.8%) | 23 (59.0%) | 0.310 |
| Adenocarcinoma bile ducts | 15 (23.8%) | 7 (29.2%) | 8 (20.5%) | 0.434 |
| IPMN | 7 (11.1%) | 5 (20.8%) | 2 (5.1%) | 0.054 |
| Neuroendocrine tumor | 3 (4.8%) | 1 (4.2%) | 2 (5.1%) | 0.862 |
| Other malignancy | 1 (1.6%) | 0 (0%) | 1 (2.6%) | 0.429 |
| No malignancy | 3 (4.8%) | 0 (0%) | 3 (7.7%) | 0.164 |
| Neoadjuvant chemotherapy | 8 (12.7%) | 2 (8.3%) | 6 (15.4%) | 0.414 |
| Adjuvant chemotherapy | 29 (46%) | 10 (41.7%) | 19 (48.7%) | 0.586 |
| Surgical procedure | ||||
| PPPD | 43 (68.3%) | 15 (62.5%) | 28 (71.8%) | 0.441 |
| Whipple | 10 (15.9%) | 4 (16.7%) | 6 (15.4%) | 0.892 |
| Total pancreatectomy | 2 (3.2%) | 1 (4.2%) | 1 (2.6%) | 0.725 |
| Distal pancreatectomy | 8 (12.7%) | 4 (16.7%) | 4 (10.3%) | 0.458 |
| Duration of surgery, min | 469 ± 118 | 492 ± 122 | 454 ± 115 | 0.218 |
| Intraoperative blood loss, mL | 500 (300–750) | 500 (312–819) | 400 (300–750) | 0.509 |
| Surgical outcome | ||||
| Length of hospital stay, days | 12 (10–17) | 14 (10–19) | 12 (9–16) | 0.356 |
| Length of ICU stay, days | 1 (0–1) | 1 (1–2) | 1 (0–1) | 0.140 |
| Complications | ||||
| Comprehensive Complication Index | 21 (9–30) | 21 (9–33) | 21 (9–30) | 0.674 |
| Clavien–Dindo ≥ 3 | 14 (22.2%) | 6 (25.0%) | 8 (20.5%) | 0.677 |
| ISGPS definition ≥ grade B or C | ||||
| Delayed gastric emptying | 11 (17.5%) | 5 (20.9%) | 6 (15.4%) | 0.527 |
| Pancreatic fistula | 14 (22.2%) | 5 (20.9%) | 9 (23.1%) | 0.903 |
| Hemorrhage | 5 (7.9%) | 2 (8.3%) | 3 (7.7%) | 0.889 |
| Chylous leakage | 10 (15.9%) | 4 (16.7%) | 6 (15.4%) | 0.836 |
| Biliary fistula | 1 (1.6%) | 0 (0.0%) | 1 (2.6%) | 0.439 |
| Surgical re-intervention | 5 (7.9%) | 3 (12.5%) | 2 (5.1%) | 0.927 |
| Radiological re-intervention | 9 (14.3%) | 4 (16.7%) | 5 (12.8%) | 0.672 |
| Readmission within 30 days | 9 (14.3%) | 5 (20.8%) | 4 (10.3%) | 0.244 |
| Readmission within 90 days | 15 (23.8%) | 5 (21.7%) | 10 (25.6%) | 0.729 |
| Cholangitis | 4 (6.3%) | 2 (8.3%) | 2 (5.1%) | 0.257 |
| Failure to thrive | 4 (6.3%) | 2 (8.3%) | 2 (5.1%) | 0.257 |
| Other | 7 (11.1%) | 1 (4.2%) | 6 (15.4%) | 0.169 |
| Mortality within 90 days | 1 (1.6%) | 1 (4.2%) | 0 | 0.199 |
| Total Protein Intake, g/kg | Total Energy Intake, kcal/kg | |||||||
|---|---|---|---|---|---|---|---|---|
| Day | N | SRML | No-SRML | p-Value | N | SRML | No-SRML | p-Value |
| 1 | 22/35 | 0 (0–0.18) | 0.08 (0–0.19) | 0.380 | 22/35 | 0 (0–3.54) | 1.77 (0–3.43) | 0.399 |
| 2 | 22/35 | 0.06 (0–0.71) | 0.45 (0.10–0.81) | 0.029 | 22/35 | 2.00 (0–13.58) | 10.91 (2.50–14.37) | 0.042 |
| 3 | 22/35 | 0.25 (0–1.04) | 0.96 (0.28–1.20) | 0.029 | 22/35 | 7.03 (0–19.08) | 15.00 (5.20–21.21) | 0.057 |
| 4 | 22/35 | 0.33 (0–1.25) | 0.82 (0.19–1.59) | 0.115 | 22/35 | 6.46 (0.33–20.94) | 14.87 (3.99–26.14) | 0.142 |
| 5 | 21/34 | 0.32 (0.02–1.16) | 0.99 (0.41–1.55) | 0.019 | 21/34 | 6.52 (1.50–21.29) | 18.47 (5.78–28.25) | 0.030 |
| 6 | 21/33 | 0.40 (0.23–1.54) | 1.16 (0.53–1.85) | 0.087 | 21/33 | 8.99 (4.35–28.19) | 18.20 (10.01–32.00) | 0.072 |
| 7 | 19/31 | 0.50 (0.20–1.29) | 1.09 (0.60–1.65) | 0.137 | 19/31 | 11.55 (3.95–21.07) | 17.62 (9.34–29.13) | 0.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hogenbirk, R.N.M.; Hentzen, J.E.K.R.; van der Plas, W.Y.; Campmans-Kuijpers, M.J.E.; Kruijff, S.; Klaase, J.M. Surgery-Related Muscle Loss after Pancreatic Resection and Its Association with Postoperative Nutritional Intake. Cancers 2023, 15, 969. https://doi.org/10.3390/cancers15030969
Hogenbirk RNM, Hentzen JEKR, van der Plas WY, Campmans-Kuijpers MJE, Kruijff S, Klaase JM. Surgery-Related Muscle Loss after Pancreatic Resection and Its Association with Postoperative Nutritional Intake. Cancers. 2023; 15(3):969. https://doi.org/10.3390/cancers15030969
Chicago/Turabian StyleHogenbirk, Rianne N. M., Judith E. K. R. Hentzen, Willemijn Y. van der Plas, Marjo J. E. Campmans-Kuijpers, Schelto Kruijff, and Joost M. Klaase. 2023. "Surgery-Related Muscle Loss after Pancreatic Resection and Its Association with Postoperative Nutritional Intake" Cancers 15, no. 3: 969. https://doi.org/10.3390/cancers15030969
APA StyleHogenbirk, R. N. M., Hentzen, J. E. K. R., van der Plas, W. Y., Campmans-Kuijpers, M. J. E., Kruijff, S., & Klaase, J. M. (2023). Surgery-Related Muscle Loss after Pancreatic Resection and Its Association with Postoperative Nutritional Intake. Cancers, 15(3), 969. https://doi.org/10.3390/cancers15030969





