Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Patients, Data Collection, and Study Design
2.2. Surgical Procedure
2.3. Statistical Analysis
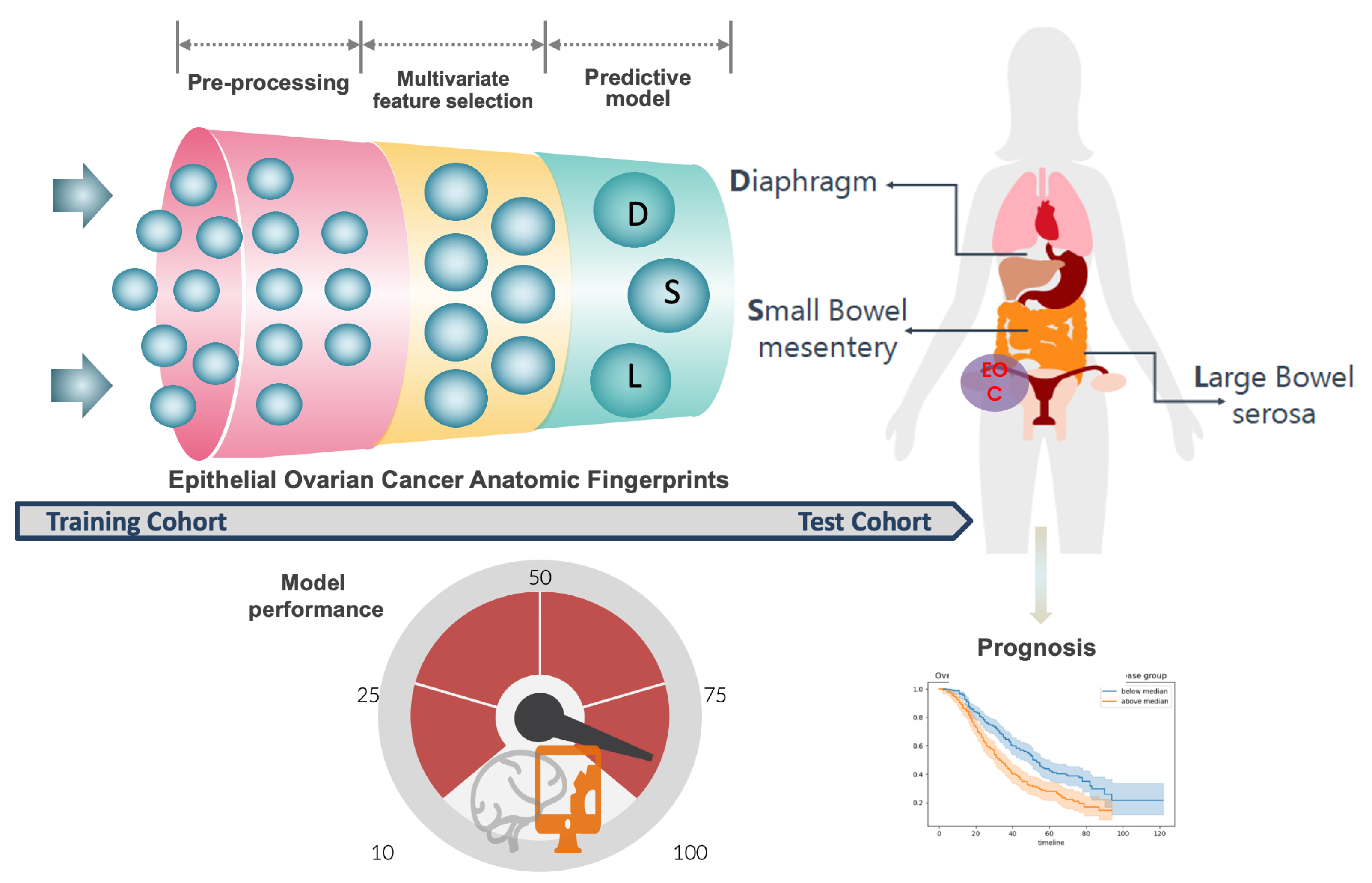
2.4. Predictive Model Development and Performance
2.5. Model Explainability
3. Results
3.1. ANAFI Score Development
3.2. ANAFI Score Evaluation
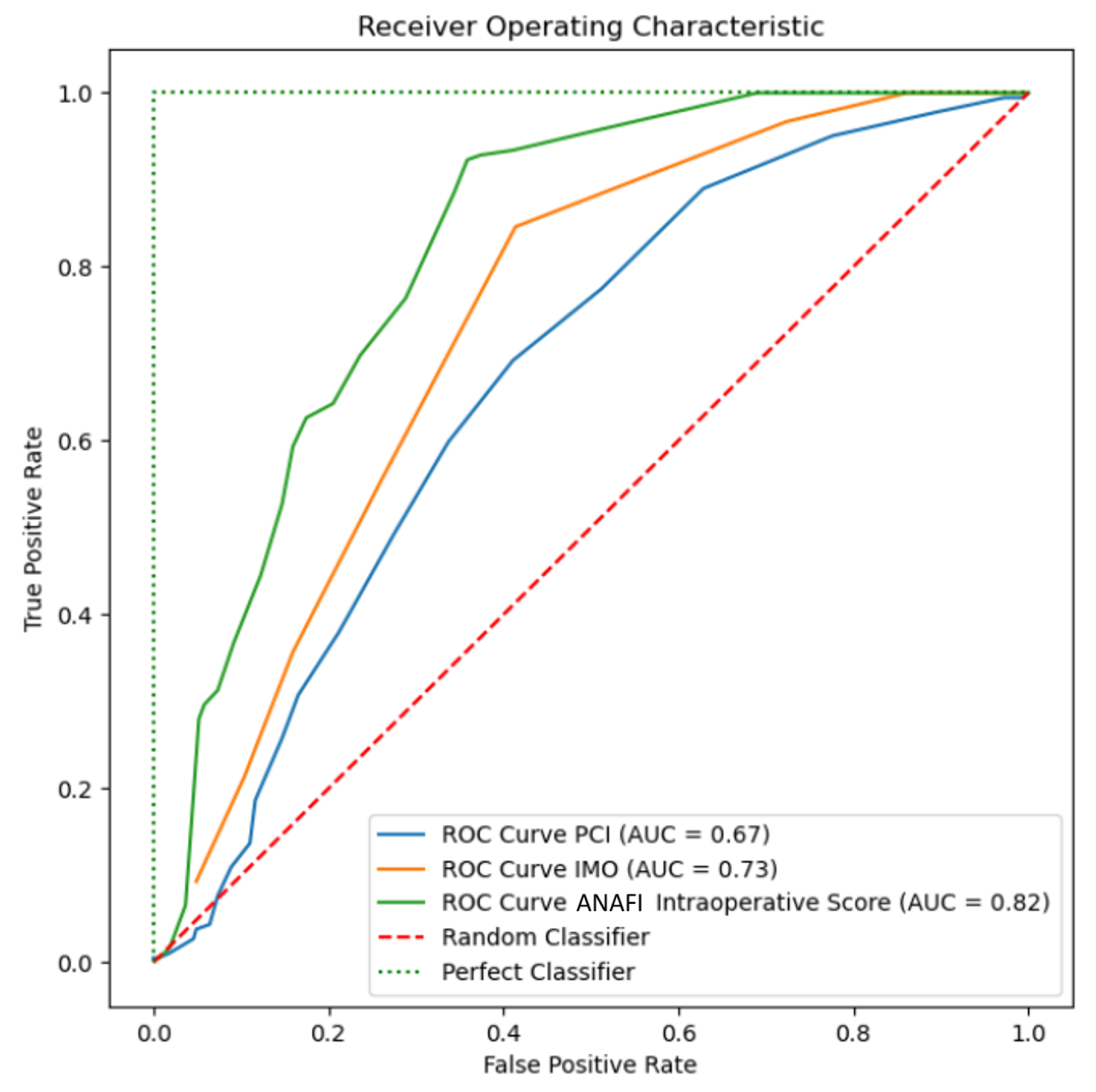
3.2.1. Receiver Operator Curves
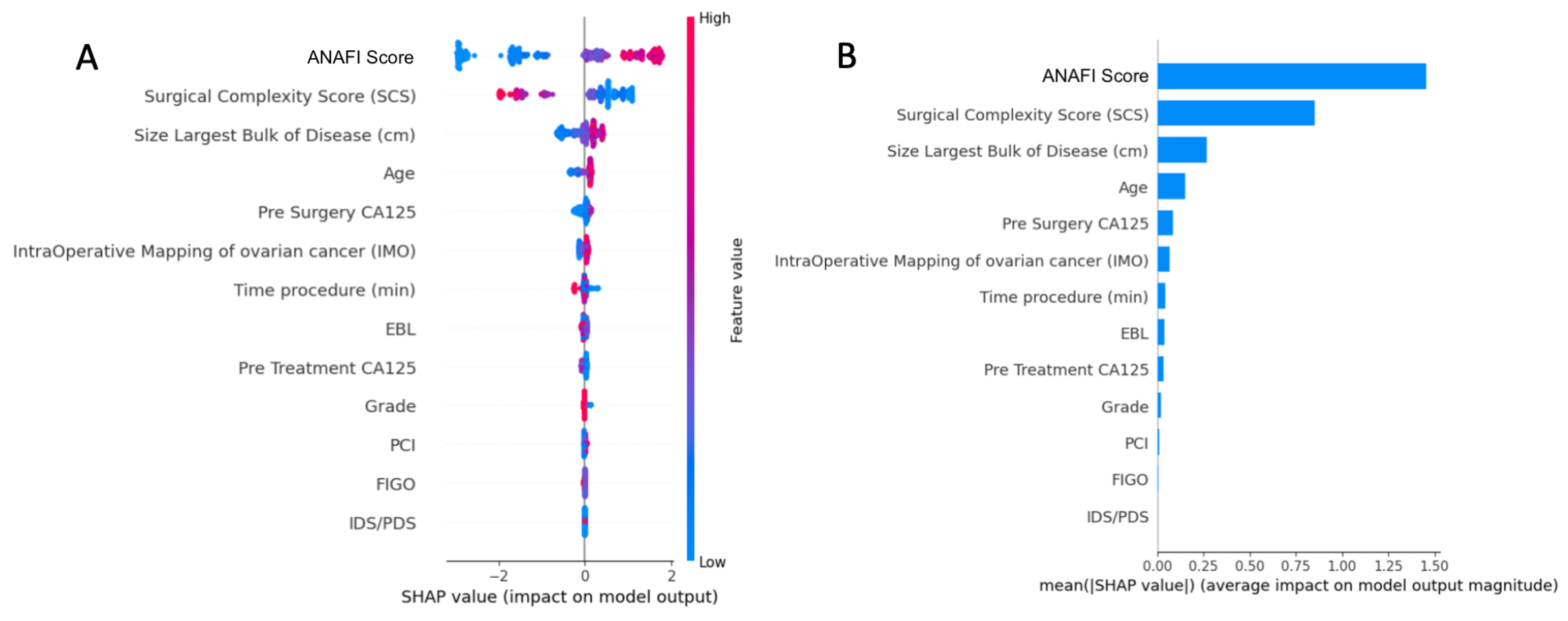
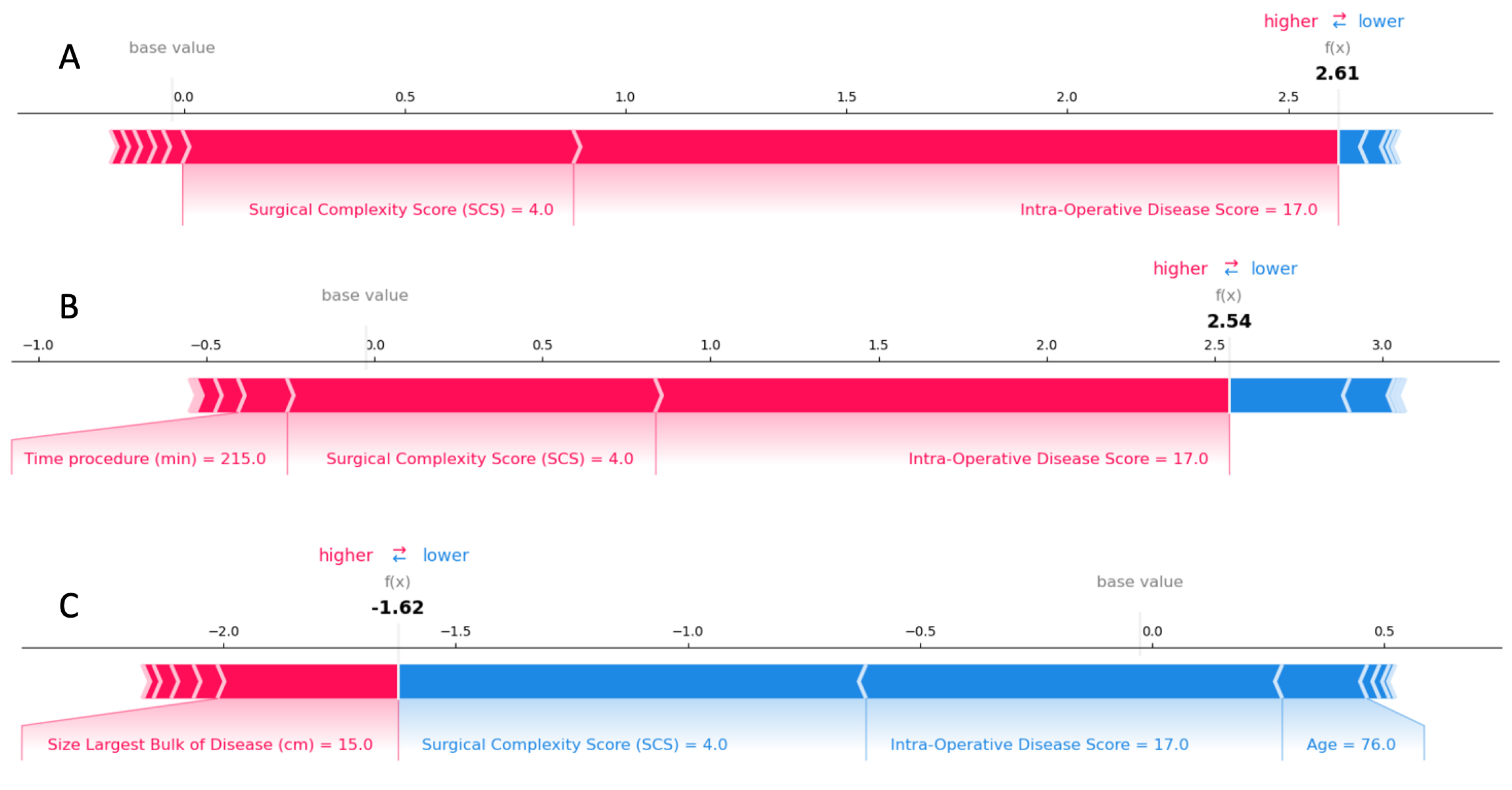
3.2.2. Predictive Model and Explainability
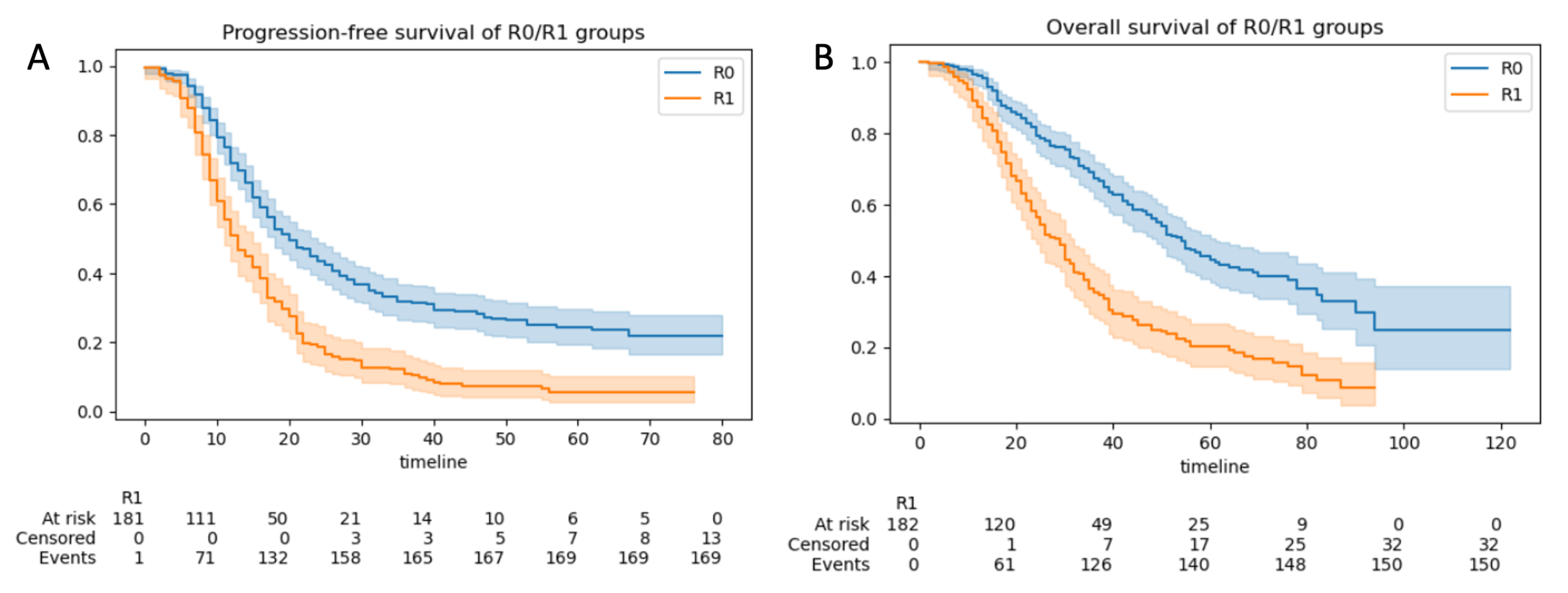
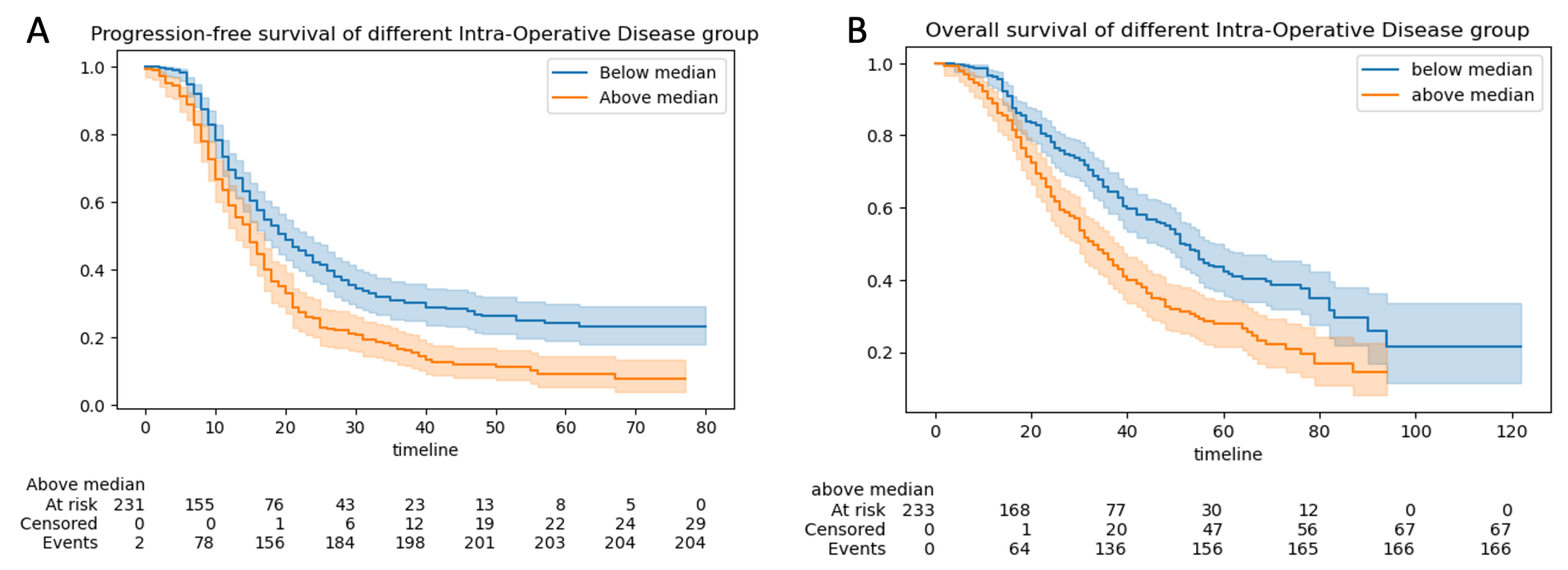
3.2.3. Progression-Free and Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| XAI | Explainable Artificial Intelligence |
| XGBoost | eXreme Gradient Boosting |
| SHAP | Shapley Additive Explanations |
| AUC-ROC | Area under Curve-Receiver Operator Curve |
| CT | Computer Tomography |
| DS | Disease Score |
| ECOG | Eastern Cooperative Oncology Group |
| EOC | Epithelial Ovarian Cancer |
| FIGO | Federation International of Obstetrics and Gynaecology |
| IDS | Interval Debulking Surgery |
| PDS | Primary Debulking Surgery |
| CEA | Carcinoembryonic antigen |
| HE4 | Human Epididymis 4 |
| NHS | National Health System |
| ML | Machine Learning |
| NACT | Neoadjuvant Chemotherapy |
| ACT | Adjuvant Chemotherapy |
| PPM | Patient Pathway Manager |
| MDT | Multidisciplinary team |
| BGCS | British Gynaecologic Cancer Society |
| CPEX | Cardiopulmonary exercise |
| ESGO | European Society Gynaecological Oncology |
| CCU | Critical Care Admission |
| SD | Standard Deviation |
| CV | Cross Validation |
| IMO | Intra-operative Mapping for Ovarian Cancer |
| PCI | Peritoneal Cancer Index |
| NSQIP | National Surgical Quality Improvement Program |
| PS | Performance Status |
| RD | Residual Disease |
| R0 | No Residual-Complete Cytoreduction |
| SCS | Surgical Complexity Score |
| SD | Standard Deviation |
| SJUH | St James’s University Hospital |
Appendix A. ANAFI Score Development
| EOC Anatomic Fingerprints | Overall (n = 508) | Non-CC0 (n = 182) | CC0 (n = 326) | p-Value |
|---|---|---|---|---|
| Disease Liver | 53 (0.1) | 28 (0.15) | 25 (0.08) | 0.01 |
| Disease Diaphragm | 186 (0.37) | 117 (0.64) | 69 (0.21) | <0.001 |
| Disaease Spleen | 30 (0.06) | 18 (0.1) | 12 (0.04) | 0.008 |
| Disease Pancreas | 4 (0.008) | 4 (0.02) | 0 (0) | 0.03 |
| Disease Head/Body Pancreas | 2 (0.004) | 2 (0.02) | 0 (0) | 0.25 |
| Disease Coeliac Trunk/Porta Hepatis | 13 (0.03) | 6 (0.03) | 7 (0.02) | 0.62 |
| Disease Galbladder | 18 (0.04) | 13 (0.07) | 5 (0.02) | 0.002 |
| Disease Lesser Omentum | 52 (0.1) | 39 (0.21) | 13 (0.04) | <0.001 |
| Disease Stomach | 38 (0.07) | 28 (0.15) | 10 (0.03) | <0.001 |
| Disease Greater Omentum | 456 (0.9) | 173 (0.95) | 283 (0.87) | 0.005 |
| Disease Large Bowel | 159 (0.31) | 104 (0.57) | 55 (0.17) | <0.001 |
| Disease Mesentery Large Bowel | 197 (0.39) | 111 (0.61) | 86 (0.26) | <0.001 |
| Disease Appendix | 62 (0.12) | 19 (0.1) | 43 (0.13) | 0.44 |
| Disease Small Bowel | 95 (0.19) | 66 (0.36) | 29 (0.09) | <0.001 |
| Disease Mesentery Small Bowel | 173 (0.34) | 114 (0.63) | 59 (0.18) | <0.001 |
| Abdominal Wall/SMJ nodule | 23 (0.05) | 4 (0.02) | 19 (0.06) | 0.1 |
| Disease Upper abdominal peritoneum | 158 (0.31) | 86 (0.47) | 72 (0.22) | <0.001 |
| Disease Pelvic Peritoneum | 335 (0.66) | 151 (0.83) | 184 (0.56) | <0.001 |
| Disease Bladder peritoneum | 267 (0.53) | 129 (0.71) | 138 (0.42) | <0.001 |
| Disease Inguinal LN | 10 (0.02) | 4 (0.02) | 6 (0.02) | 1 |
| Disease Para-Aortal LN | 114 (0.22) | 30 (0.16) | 84 (0.26) | 0.02 |
| Dissease Pelvic LN | 83 (0.16) | 13 (0.07) | 70 (0.21) | <0.001 |
| Disease Ovaries/Fallopian Tube | 498 (0.98) | 180 (0.99) | 318 (0.98) | 0.47 |
| Disease Uterus/Cervix | 359 (0.71) | 147 (0.81) | 212 (0.65) | <0.001 |
| Disease Pouch of Douglas | 311 (0.61) | 150 (0.82) | 161 (0.49) | <0.001 |
| Algorithm | Hyperparameters |
|---|---|
| XGBoost | ’colsample_bylevel’: 1, ’gamma’: 0.7, ’learning_rate’: 0.01, ’max_delta_step’: 1, ’max_depth’: 5, ’min_child_weight’: 2, ’n_estimators’: 250, ’scale_pos_weight’: 1.79, ’subsample’: 0.75 |
| Precision | Recall | F1-Score | |
|---|---|---|---|
| CC0 | 0.90 | 0.77 | 0.83 |
| Non-CC0 | 0.70 | 0.86 | 0.77 |
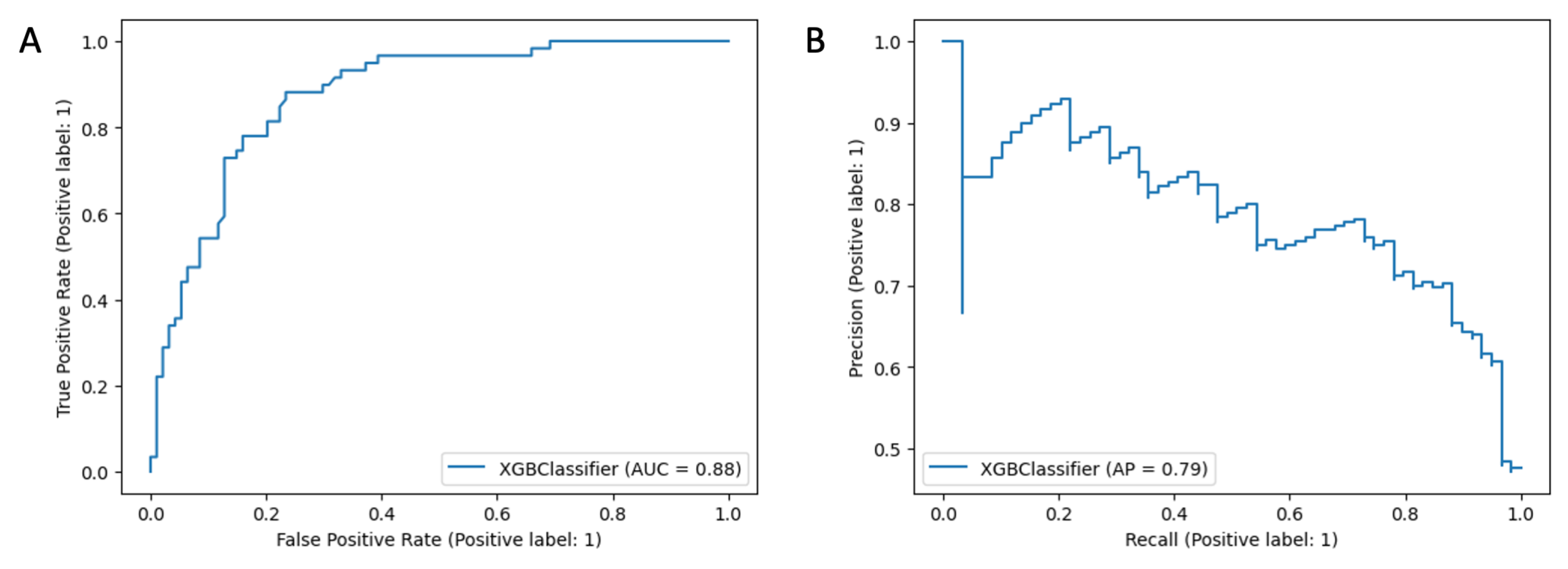
Appendix B. ANAFI Score Evaluation
Appendix B.1. Predictive Model Development
| Demographic Characteristics | Overall (n = 508) | Train Set (n = 355) | Test Set (n = 153) | p-Value | Non-CC0 (n = 182) | CC0 (n = 326) | p-Value |
|---|---|---|---|---|---|---|---|
| PCI | 7.64 ± 4.51 | 7.41 ± 4.4 | 8.18 ± 4.73 | 0.09 | 8.97 ± 4.11 | 6.9 ± 4.56 | <0.001 |
| IMO | 5.08 ± 1.95 | 4.98 ± 1.92 | 5.31 ± 2.01 | 0.08 | 6.03 ± 1.6 | 4.55 ± 1.93 | <0.001 |
| ANAFI Score | 6.95 ± 6.45 | 6.65 ± 6.26 | 7.64 ± 6.84 | 0.12 | 11.51 ± 5.37 | 4.4 ± 5.54 | <0.001 |
| SCS | 3.77 ± 2.18 | 3.69 ± 2.15 | 3.96 ± 2.24 | 0.2 | 3.04 ± 1.4 | 4.18 ± 2.42 | <0.001 |
| Age | 63.74 ± 10.9 | 63.74 ± 11.01 | 63.73 ± 10.67 | 0.99 | 65.98 ± 9.8 | 62.49 ± 11.29 | <0.001 |
| Grade (Low = 0/High = 1) | 459 (0.9) | 323 (0.9) | 136 (0.89) | 0.92 | 160 (0.88) | 299 (0.92) | 0.21 |
| FIGO | 322 (0.63) | 231 (0.65) | 91 (0.59) | 0.11 | 120 (0.66) | 202 (0.62) | 0.005 |
| PDS = 0/IDS = 1 | 129 (0.25) | 85 (0.24) | 91 (0.29) | 0.12 | 47 (0.26) | 82 (0.25) | 0.95 |
| EBL | 523.7 ± 377.6 | 518.9 ± 392.4 | 534.6 ± 341.9 | 0.65 | 531.2 ± 289.9 | 519.4 ± 419.1 | 0.71 |
| Pre Treatment CA125 | 1560 ± 2696 | 1521 ± 2422 | 1650 ± 3252 | 0.66 | 1595 ± 2366 | 1540 ± 2867 | 0.82 |
| Pre Surgery CA125 | 365 ± 863 | 374 ± 934 | 344 ± 672 | 0.18 | 414 ± 895 | 338 ± 844 | 0.02 |
| Size Largest Bulk of Disease (cm) | 8.9 ± 5.6 | 8.7 ± 5.3 | 9.4 ± 6.2 | 0.25 | 9.9 ± 5.2 | 8.4 ± 5.7 | 0.003 |
| Time procedure (min) | 172 ± 79 | 171 ± 80 | 173 ± 78 | 0.77 | 160.44 ± 63.7 | 177.67 ± 85.97 | 0.01 |
| Precision | Recall | F1-Score | |
|---|---|---|---|
| CC0 | 0.89 | 0.88 | 0.89 |
| Non-CC0 | 0.82 | 0.83 | 0.82 |
| Algorithm | Hyperparameters |
|---|---|
| XGBoost | ’colsample_bylevel’: 1, ’gamma’: 0.7, ’learning_rate’: 0.01, ’max_delta_step’: 0, ’max_depth’: 2, ’min_child_weight’: 2, ’n_estimators’: 400, ’scale_pos_weight’: 1.79, ’subsample’: 0.75 |
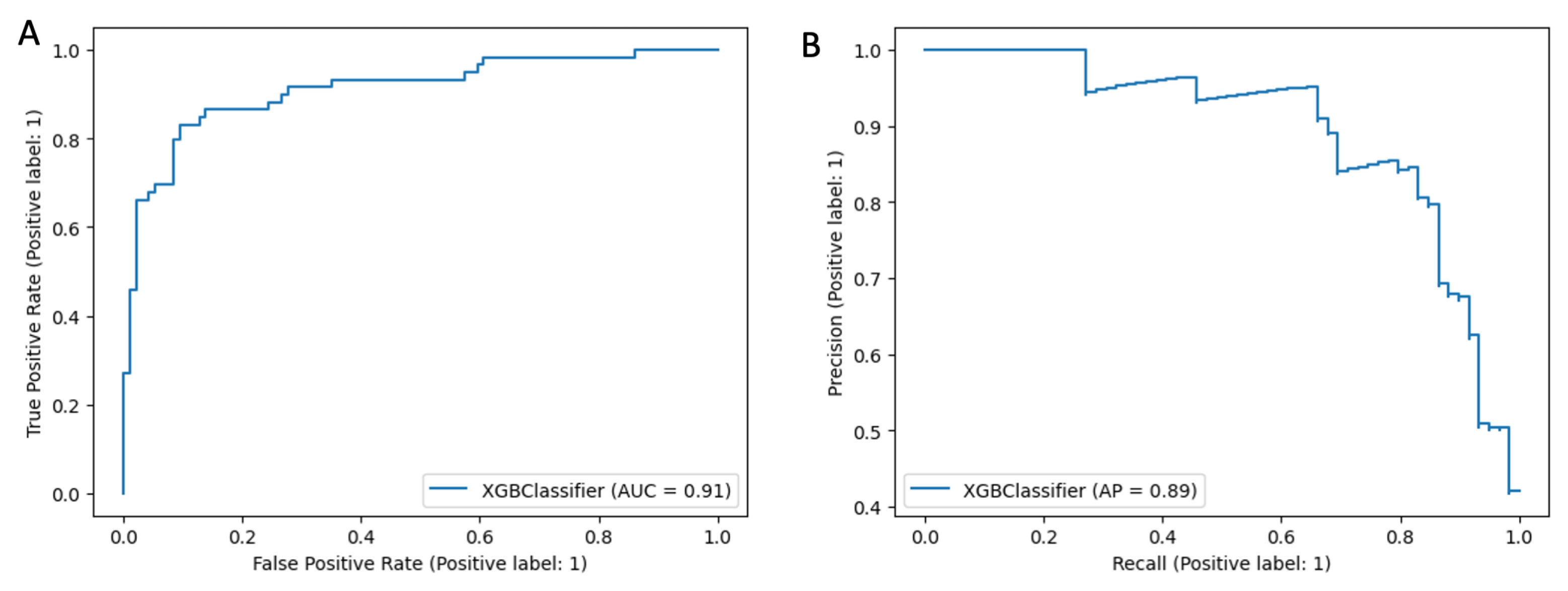
Appendix B.2. Survival Analysis

References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.C.; Vasey, P.A.; Paul, J.; Hay, A.; Davis, J.A.; Kaye, S.B. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J. Clin. Oncol. 2005, 23, 8802–8811. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Eisenhauer, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Levine, D.A.; Guile, M.W.; Bristow, R.E.; Aghajanian, C.; Barakat, R.R. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol. Oncol. 2009, 114, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Ercoli, A.; Lorusso, D.; Rossi, M.; Scambia, G. A Laparoscopy-Based Score To Predict Surgical Outcome in Patients With Advanced Ovarian Carcinoma: A Pilot Study. Ann. Surg. Oncol. 2006, 13, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Sehouli, J.; Könsgen, D.; Mustea, A.; Oskay-Özcelik, G.; Katsares, I.; Weidemann, H.; Lichtenegger, W. “IMO”—Intraoperatives Mapping des Ovarialkarzinoms. Zentralbl Gynakol 2003, 125, 129–135. [Google Scholar] [PubMed]
- Eisenhauer, E.L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Poynor, E.A.; Aghajanian, C.; Jarnagin, W.R.; DeMatteo, R.P.; D’Angelica, M.I.; Barakat, R.R.; et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC–IV epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 1083–1090. [Google Scholar] [CrossRef]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef]
- Shen, P.; Ng, J.L.; Ong, W.S.; Chia, C.S.; Tan, G.H.C.; Soo, K.C.; Teo, M.C.C. Prognostic Relevance of the Peritoneal Surface Disease Severity Score Compared to the Peritoneal Cancer Index for Colorectal Peritoneal Carcinomatosis. Int. J. Surg. Oncol. 2016, 2016, 2495131. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Springer: Boston, MA, USA, 1996; pp. 359–374. [Google Scholar] [CrossRef]
- Tentes, A.A.; Tripsiannis, G.; Markakidis, S.; Karanikiotis, C.; Tzegas, G.; Georgiadis, G.; Avgidou, K. Peritoneal cancer index: A prognostic indicator of survival in advanced ovarian cancer. Eur. J. Surg. Oncol. (EJSO) 2003, 29, 69–73. [Google Scholar] [CrossRef]
- Gouy, S.; Belghiti, J.; Uzan, C.; Canlorbe, G.; Gauthier, T.; Morice, P. Accuracy and Reproducibility of the Peritoneal Cancer Index in Advanced Ovarian Cancer During Laparoscopy and Laparotomy. Int. J. Gynecol. Cancer 2013, 23, 1699–1703. [Google Scholar] [CrossRef]
- Jónsdóttir, B.; Lomnytska, M.; Poromaa, I.S.; Silins, I.; Stålberg, K. The Peritoneal Cancer Index is a Strong Predictor of Incomplete Cytoreductive Surgery in Ovarian Cancer. Ann. Surg. Oncol. 2021, 28, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Lomnytska, M.; Karlsson, E.; Jonsdottir, B.; Lejon, A.M.; Stålberg, K.; Poromaa, I.S.; Silins, I.; Graf, W. Peritoneal cancer index predicts severe complications after ovarian cancer surgery. Eur. J. Surg. Oncol. 2021, 47, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, P.; Biacchi, D.; Cornali, T.; Accarpio, F.; Sibio, S.; Luraschi, B.; Impagnatiello, A.; Di Giorgio, A. Computerized System for Staging Peritoneal Surface Malignancies. Ann. Surg. Oncol. 2016, 23, 1454–1460. [Google Scholar] [CrossRef]
- Lampe, B.; Kroll, N.; Piso, P.; Forner, D.M.; Mallmann, P. Prognostic Significance of Sugarbaker’s Peritoneal Cancer Index for the Operability of Ovarian Carcinoma. Int. J. Gynecol. Cancer 2015, 25, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.; Harter, P.; Alesina, P.F.; Walz, M.K.; Lorenz, D.; Groeben, H.; Heikaus, S.; Fisseler-Eckhoff, A.; Schneider, S.; Ataseven, B.; et al. Pattern of and reason for postoperative residual disease in patients with advanced ovarian cancer following upfront radical debulking surgery. Gynecol. Oncol. 2016, 141, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Thangavelu, A.; Tarabanis, C.; Nugent, D.; De Jong, D. Explainable Artificial Intelligence for Prediction of Complete Surgical Cytoreduction in Advanced-Stage Epithelial Ovarian Cancer. J. Pers. Med. 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016. KDD ’16. pp. 785–794. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. In Proceedings of the Advances in Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Guyon, I., Luxburg, U.V., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; Volume 30. [Google Scholar]
- Newsham, A.C.; Johnston, C.; Hall, G.; Leahy, M.G.; Smith, A.B.; Vikram, A.; Donnelly, A.M.; Velikova, G.; Selby, P.J.; Fisher, S.E. Development of an advanced database for clinical trials integrated with an electronic patient record system. Comput. Biol. Med. 2011, 41, 575–586. [Google Scholar] [CrossRef]
- Johnson, R.L.; Laios, A.; Jackson, D.; Nugent, D.; Orsi, N.M.; Theophilou, G.; Thangavelu, A.; de Jong, D. The Uncertain Benefit of Adjuvant Chemotherapy in Advanced Low-Grade Serous Ovarian Cancer and the Pivotal Role of Surgical Cytoreduction. J. Clin. Med. 2021, 10, 5927. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Hall, M.; Cruickshank, D.; Gabra, H.; Ganesan, R.; Hughes, C.; Kehoe, S.; Ledermann, J.; Morrison, J.; Naik, R.; et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 123–139. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Haidopoulos, D.; Hasenburg, A.; Hughes, C.; et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer 2021, 31, 1199–1206. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Podratz, K.C.; Cliby, W.A. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am. J. Obstet. Gynecol. 2007, 197, 676.e1–676.e7. [Google Scholar] [CrossRef] [PubMed]
- Freund, Y.; Schapire, R.E. A Decision-Theoretic Generalization of On-Line Learning and an Application to Boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.I. From local explanations to global understanding with explainable AI for trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Nair, B.; Vavilala, M.S.; Horibe, M.; Eisses, M.J.; Adams, T.; Liston, D.E.; Low, D.K.W.; Newman, S.F.; Kim, J.; et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat. Biomed. Eng. 2018, 2, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Laios, A.; Kalampokis, E.; Johnson, R.; Munot, S.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Theophilou, G.; Leach, C.; Nugent, D.; et al. Factors Predicting Surgical Effort Using Explainable Artificial Intelligence in Advanced Stage Epithelial Ovarian Cancer. Cancers 2022, 14, 3447. [Google Scholar] [CrossRef]
- Karamanou, A.; Kalampokis, E.; Tarabanis, K. Linked Open Government Data to Predict and Explain House Prices: The Case of Scottish Statistics Portal. Big Data Res. 2022, 30, 100355. [Google Scholar] [CrossRef]
- Parsa, A.B.; Movahedi, A.; Taghipour, H.; Derrible, S.; Mohammadian, A.K. Toward safer highways, application of XGBoost and SHAP for real-time accident detection and feature analysis. Accid. Anal. Prev. 2020, 136, 105405. [Google Scholar] [CrossRef]
- Hosoya, S.; Ueda, K.; Odajima, S.; Ogawa, K.; Komazaki, H.; Seki, T.; Takenaka, M.; Saito, M.; Tanabe, H.; Yamada, K.; et al. Scoring Systems of Peritoneal Dissemination for the Prediction of Operative Completeness in Advanced Ovarian Cancer. Anticancer Res. 2022, 42, 115–124. [Google Scholar] [CrossRef]
- Maubert, A.; Birtwisle, L.; Bernard, J.; Benizri, E.; Bereder, J. Can machine learning predict resecability of a peritoneal carcinomatosis? Surg. Oncol. 2019, 29, 120–125. [Google Scholar] [CrossRef]
- Llueca, A.; Escrig, J. MUAPOS working group (multidisciplinary unit of abdominal pelvic oncology surgery). Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur. J. Surg. Oncol. 2018, 44, 163–169. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Jones, B.P.; Savvatis, K.; Campbell, J.; Kyrgiou, M.; Farthing, A.; Brett, S.; Roux, R.; Hall, M.; Rustin, G.; et al. Maximal effort cytoreductive surgery for disseminated ovarian cancer in a UK setting: Challenges and possibilities. Arch. Gynecol. Obstet. 2016, 294, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma During the Platinum Era: A Meta-Analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Vidal, F.; Al Thani, H.; Haddad, P.; Luyckx, M.; Stoeckle, E.; Morice, P.; Leblanc, E.; Lecuru, F.; Daraï, E.; Classe, J.M.; et al. Which Surgical Attitude to Choose in the Context of Non-Resectability of Ovarian Carcinomatosis: Beyond Gross Residual Disease Considerations. Ann. Surg. Oncol. 2016, 23, 434–442. [Google Scholar] [CrossRef]
- Collins, A.; Spooner, S.; Horne, J.; Chainrai, M.; Runau, F.; Bourne, T.; Moss, E.L.; Davies, Q.; Chattopadhyay, S.; Bharathan, R. Peri-operative Variables Associated with Prolonged Intensive Care Stay Following Cytoreductive Surgery for Ovarian Cancer. Anticancer Res. 2021, 41, 3059–3065. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, M.; Harter, P.; Bjørn, S.F.; Høgdall, C. Specific Regions, Rather than the Entire Peritoneal Carcinosis Index, are Predictive of Complete Resection and Survival in Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2018, 28, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Soleymani majd, H.; Ferrari, F.; Manek, S.; Gubbala, K.; Campanile, R.G.; Hardern, K.; Tozzi, R. Diaphragmatic peritonectomy vs. full thickness resection with pleurectomy during Visceral-Peritoneal Debulking (VPD) in 100 consecutive patients with stage IIIC–IV ovarian cancer: A surgical-histological analysis. Gynecol. Oncol. 2016, 140, 430–435. [Google Scholar] [CrossRef]
- Tozzi, R.; Ferrari, F.; Nieuwstad, J.; Campanile, R.G.; Soleymani Majd, H. Tozzi classification of diaphragmatic surgery in patients with stage IIIC–IV ovarian cancer based on surgical findings and complexity. J. Gynecol. Oncol. 2020, 31. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Spirtos, N.M. What Are the Current Surgical Objectives, Strategies, and Technical Capabilities of Gynecologic Oncologists Treating Advanced Epithelial Ovarian Cancer? Gynecol. Oncol. 2001, 82, 489–497. [Google Scholar] [CrossRef]
- Tozzi, R.; Traill, Z.; Campanile, R.G.; Kilic, Y.; Baysal, A.; Giannice, R.; Morotti, M.; Soleymani majd, H.; Valenti, G. Diagnostic flow-chart to identify bowel involvement in patients with stage IIIC-IV ovarian cancer: Can laparoscopy improve the accuracy of CT scan? Gynecol. Oncol. 2019, 155, 207–212. [Google Scholar] [CrossRef]
- Cerci, Z.C.; Sakarya, D.K.; Yetimalar, M.H.; Bezircioglu, I.; Kasap, B.; Baser, E.; Yucel, K. Computed tomography as a predictor of the extent of the disease and surgical outcomes in ovarian cancer. Ginekol. Pol. 2016, 87, 326–332. [Google Scholar] [CrossRef] [PubMed]
- van Stein, R.; Engbersen, M.; Stolk, T.; Lopez-Yurda, M.; Lahaye, M.; Beets-Tan, R.; Lok, C.; Sonke, G.; Van Driel, W. Peroperative extent of peritoneal metastases affects the surgical outcome and survival in advanced ovarian cancer. Gynecol. Oncol. 2022, 167, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Santillan, A.; Eisenhauer, E.L.; Hu, J.; Aletti, G.; Podratz, K.C.; Bristow, R.E.; Chi, D.S.; Cliby, W.A. A new frontier for quality of care in gynecologic oncology surgery: Multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol. Oncol. 2007, 107, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Uzan, J.; Bonsang-Kitzis, H.; Rossi, L.; Rance, B.; Bats, A.S.; Gosset, M.; Deloménie, M.; Pujade-Lauraine, E.; Lécuru, F.; Ngô, C. Prognostic impact of initial tumor load and intraperitoneal disease dissemination patterns in patients with advanced ovarian cancer undergoing complete cytoreductive surgery. Eur. J. Surg. Oncol. 2019, 45, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, C.; Morotti, M.; Casarin, J.; Tozzi, R.; Ghezzi, F.; Mavroeidis, V.K.; Alazzam, M.; majd, H.S. Interval Debulking Surgery for Advanced Ovarian Cancer in Elderly Patients (≥70 y): Does the Age Matter? J. Investig. Surg. 2021, 34, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Ekmann-Gade, A.W.; Schnack, T.H.; Seibæk, L.; Noer, M.C.; Høgdall, C. Impact of surgery and chemotherapy timing on outcomes in older versus younger epithelial ovarian cancer patients: A nationwide Danish cohort study. J. Geriatr. Oncol. 2022, in press. [CrossRef]
- Narasimhulu, D.M.; Kumar, A.; Weaver, A.L.; McGree, M.E.; Langstraat, C.L.; Cliby, W.A. Using an evidence-based triage algorithm to reduce 90-day mortality after primary debulking surgery for advanced epithelial ovarian cancer. Gynecol. Oncol. 2019, 155, 58–62. [Google Scholar] [CrossRef]
- Winter, W.E.; Maxwell, G.L.; Tian, C.; Carlson, J.W.; Ozols, R.F.; Rose, P.G.; Markman, M.; Armstrong, D.K.; Muggia, F.; McGuire, W.P. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2007, 25, 3621–3627. [Google Scholar] [CrossRef]
- Lyons, Y.A.; Reyes, H.D.; McDonald, M.E.; Newtson, A.; Devor, E.; Bender, D.P.; Goodheart, M.J.; Gonzalez Bosquet, J. Interval debulking surgery is not worth the wait: A National Cancer Database study comparing primary cytoreductive surgery versus neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2020, 30, 845–852. [Google Scholar] [CrossRef]
- Bristow, R.E.; Eisenhauer, E.L.; Santillan, A.; Chi, D.S. Delaying the primary surgical effort for advanced ovarian cancer: A systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecol. Oncol. 2007, 104, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Leiserowitz, G.S.; Lin, J.F.; Tergas, A.I.; Cliby, W.A.; Bristow, R.E. Factors Predicting Use of Neoadjuvant Chemotherapy Compared With Primary Debulking Surgery in Advanced Stage Ovarian Cancer—A National Cancer Database Study. Int. J. Gynecol. Cancer 2017, 27, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Avesani, G.; Arshad, M.; Lu, H.; Fotopoulou, C.; Cannone, F.; Melotti, R.; Aboagye, E.; Rockall, A. Radiological assessment of Peritoneal Cancer Index on preoperative CT in ovarian cancer is related to surgical outcome and survival. La Radiol. Medica 2020, 125, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Barriga, K.; Medina, C.; Gomez-Quiles, L.; Marco-Domenech, S.F.; Escrig, J.; Llueca, A. CT Enterography for Preoperative Evaluation of Peritoneal Carcinomatosis Index in Advanced Ovarian Cancer. J. Clin. Med. 2022, 11, 476. [Google Scholar] [CrossRef] [PubMed]


| Multivariate Analysis PFS | Multivariate Analysis OS | |||||
|---|---|---|---|---|---|---|
| Covariates | HR | p | 95% CI | HR | p | 95% CI |
| Age | 1.000 | 0.53 | 0.01–0.99 | 1.000 | 0.67 | 0.99–1.01 |
| Grade | 1.53 | 0.06 | 0.87–0.98 | 1.32 | <0.14 | 0.92–1.91 |
| PDS/IDS | 0.53 | <0.005 | 0.39–0.71 | 0.61 | <0.005 | 0.48–0.79 |
| Intra Operative Mapping (IMO) | 1.04 | 0.49 | 0.92–1.18 | 1.05 | 0.36 | 0.94–1.17 |
| Peritoneal Carcinomatosis Index (PCI) | 1.03 | 0.23 | 0.98–1.08 | 1.03 | 0.16 | 0.99–1.08 |
| Intra-operative Disease score | 1.06 | <0.005 | 1.03–1.09 | 1.04 | <0.005 | 1.01–1.07 |
| Surgical Complexity Score (SCS) | 0.88 | <0.005 | 0.83–0.94 | 0.91 | <0.005 | 0.87–0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laios, A.; Kalampokis, E.; Johnson, R.; Munot, S.; Thangavelu, A.; Hutson, R.; Broadhead, T.; Theophilou, G.; Nugent, D.; De Jong, D. Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers 2023, 15, 966. https://doi.org/10.3390/cancers15030966
Laios A, Kalampokis E, Johnson R, Munot S, Thangavelu A, Hutson R, Broadhead T, Theophilou G, Nugent D, De Jong D. Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers. 2023; 15(3):966. https://doi.org/10.3390/cancers15030966
Chicago/Turabian StyleLaios, Alexandros, Evangelos Kalampokis, Racheal Johnson, Sarika Munot, Amudha Thangavelu, Richard Hutson, Tim Broadhead, Georgios Theophilou, David Nugent, and Diederick De Jong. 2023. "Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence" Cancers 15, no. 3: 966. https://doi.org/10.3390/cancers15030966
APA StyleLaios, A., Kalampokis, E., Johnson, R., Munot, S., Thangavelu, A., Hutson, R., Broadhead, T., Theophilou, G., Nugent, D., & De Jong, D. (2023). Development of a Novel Intra-Operative Score to Record Diseases’ Anatomic Fingerprints (ANAFI Score) for the Prediction of Complete Cytoreduction in Advanced-Stage Ovarian Cancer by Using Machine Learning and Explainable Artificial Intelligence. Cancers, 15(3), 966. https://doi.org/10.3390/cancers15030966









