Is There a Role of Warburg Effect in Prostate Cancer Aggressiveness? Analysis of Expression of Enzymes of Lipidic Metabolism by Immunohistochemistry in Prostate Cancer Patients (DIAMOND Study)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry (IHC)
2.2. Statistical Analysis
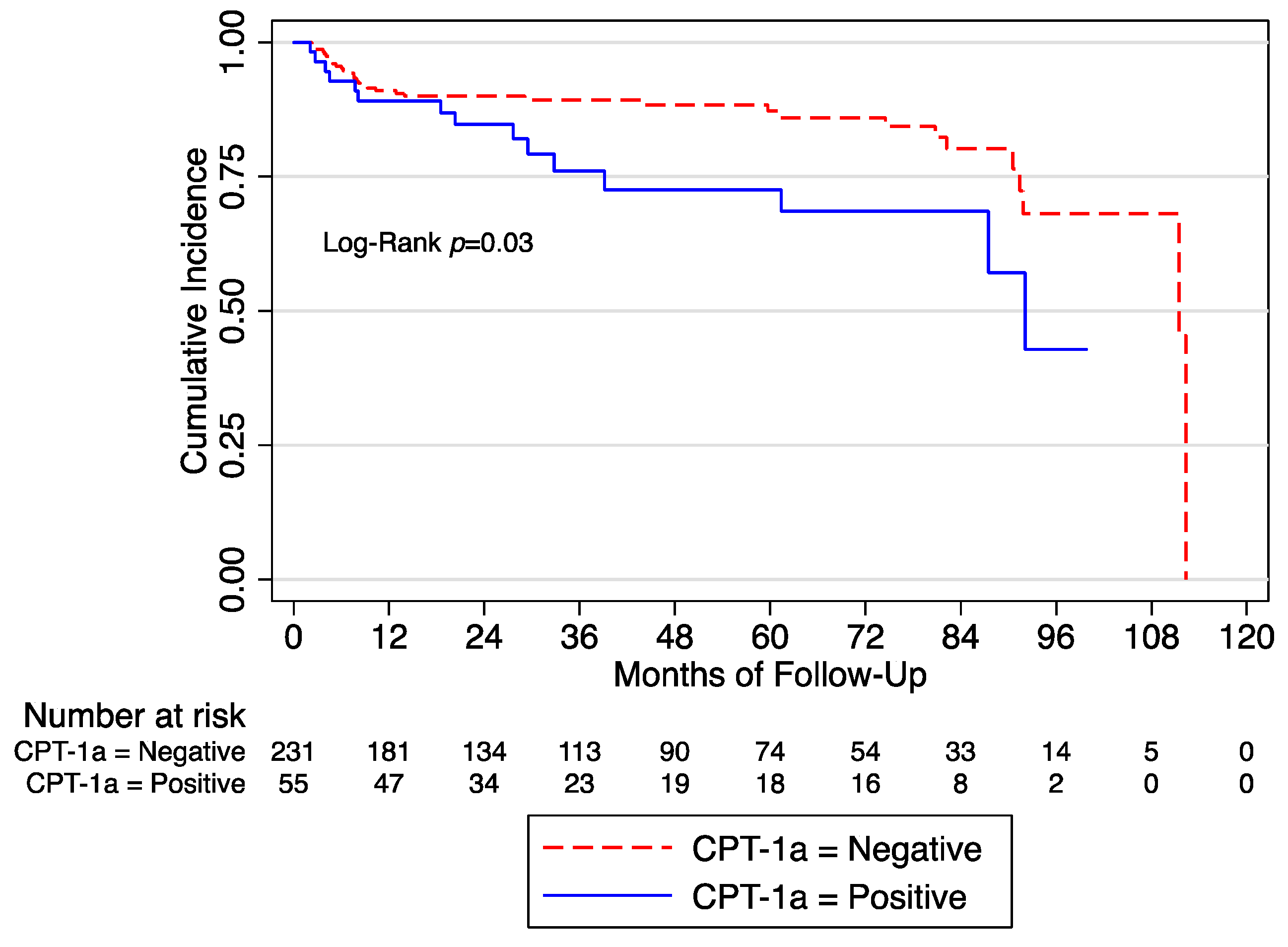
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef]
- Russo, G.I.; Bonacci, P.; Bivona, D.; Privitera, G.F.; Broggi, G.; Caltabiano, R.; Vella, J.; lo Giudice, A.; Asmundo, M.G.; Cimino, S.; et al. Genomic Landscape Alterations in Primary Tumor and Matched Lymph Node Metastasis in Hormone-Naïve Prostate Cancer Patients. Cancers 2022, 14, 4212. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Russo, G.I.; De Nunzio, C.; Sebastianelli, A.; Salvi, M.; Vignozzi, L.; Tubaro, A.; Morgia, G.; Serni, S. Meta-Analysis of Metabolic Syndrome and Prostate Cancer. Prostate Cancer Prostatic Dis. 2017, 20, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Aronson, W.; Freedland, S.J.; Giovannucci, E.; Parsons, J.K. The Correlation Between Metabolic Syndrome and Prostatic Diseases. Eur. Urol. 2012, 61, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Liu, Y. Fatty Acid Oxidation Is a Dominant Bioenergetic Pathway in Prostate Cancer. Prostate Cancer Prostatic Dis. 2006, 9, 230–234. [Google Scholar] [CrossRef]
- Wu, X.; Daniels, G.; Lee, P.; Monaco, M.E. Lipid Metabolism in Prostate Cancer. Am. J. Clin. Exp. Urol. 2014, 2, 111–120. [Google Scholar]
- Carrer, A.; Wellen, K.E. Metabolism and Epigenetics: A Link Cancer Cells Exploit. Curr. Opin. Biotechnol. 2015, 34, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Flavin, R.; Zadra, G.; Loda, M. Metabolic Alterations and Targeted Therapies in Prostate Cancer. J. Pathol. 2011, 223, 284–295. [Google Scholar] [CrossRef]
- Shah, S.; Carriveau, W.J.; Li, J.; Campbell, S.L.; Kopinski, P.K.; Lim, H.-W.; Daurio, N.; Trefely, S.; Won, K.-J.; Wallace, D.C.; et al. Targeting ACLY Sensitizes Castration-Resistant Prostate Cancer Cells to AR Antagonism by Impinging on an ACLY-AMPK-AR Feedback Mechanism. Oncotarget 2016, 7, 43713–43730. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Kim, S.-H.; Hahm, E.-R.; Pore, S.K.; Jacobs, B.L.; Singh, S.V. Prostate Cancer Chemoprevention by Sulforaphane in a Preclinical Mouse Model Is Associated with Inhibition of Fatty Acid Metabolism. Carcinogenesis 2018, 39, 826–837. [Google Scholar] [CrossRef]
- Olsson, M.; Gustafsson, O.; Skogastierna, C.; Tolf, A.; Rietz, B.D.; Morfin, R.; Rane, A.; Ekström, L. Regulation and Expression of Human CYP7B1 in Prostate: Overexpression of CYP7B1 during Progression of Prostatic Adenocarcinoma. Prostate 2007, 67, 1439–1446. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Hao, S.; Qin, Y.; Wu, Y. Knockdown of Sterol O-Acyltransferase 1 (SOAT1) Suppresses SCD1-Mediated Lipogenesis and Cancer Procession in Prostate Cancer. Prostaglandins Other Lipid Mediat. 2021, 153, 106537. [Google Scholar] [CrossRef]
- Broggi, G.; lo Giudice, A.; di Mauro, M.; Pricoco, E.; Piombino, E.; Ferro, M.; Caltabiano, R.; Morgia, G.; Russo, G.I. Insulin Signaling, Androgen Receptor and PSMA Immunohistochemical Analysis by Semi-Automated Tissue Microarray in Prostate Cancer with Diabetes (DIAMOND Study). Transl. Res. 2021, 238, 25–35. [Google Scholar] [CrossRef]
- Broggi, G.; lo Giudice, A.; di Mauro, M.; Asmundo, M.G.; Pricoco, E.; Piombino, E.; Caltabiano, R.; Morgia, G.; Russo, G.I. SRSF-1 and Microvessel Density Immunohistochemical Analysis by Semi-automated Tissue Microarray in Prostate Cancer Patients with Diabetes (DIAMOND Study). Prostate 2021, 81, 882–892. [Google Scholar] [CrossRef]
- Shah, U.; Dhir, R.; Gollin, S.; Chandran, U.; Lewis, D.; Acquafondata, M.; Pflug, B. Fatty Acid Synthase Gene Overexpression and Copy Number Gain in Prostate Adenocarcinoma. Hum. Pathol. 2006, 37, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fu, Y.; Hu, F.; Luo, X.; Hu, J.; Wang, G. PIK3R3 Regulates PPARα Expression to Stimulate Fatty Acid β-Oxidation and Decrease Hepatosteatosis. Exp. Mol. Med. 2018, 50, e431. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Hahm, E.-R.; Pore, S.K.; Singh, S.V. Leelamine Is a Novel Lipogenesis Inhibitor in Prostate Cancer Cells In Vitro and In Vivo. Mol. Cancer Ther. 2019, 18, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Igal, R.A. Stearoyl-CoA Desaturase-1: A Novel Key Player in the Mechanisms of Cell Proliferation, Programmed Cell Death and Transformation to Cancer. Carcinogenesis 2010, 31, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, S.; Schoelch, C.; Thomas, L.; Rist, W.; Rippmann, J.F.; Neubauer, H. Acetyl-CoA Carboxylases 1 and 2 Show Distinct Expression Patterns in Rats and Humans and Alterations in Obesity and Diabetes. Diabetes Metab. Res. Rev. 2009, 25, 577–586. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Asmundo, M.G.; Broggi, G.; Cimino, S.; Morgia, G.; di Trapani, E.; Luzzago, S.; Musi, G.; Ferro, M.; de Cobelli, O.; et al. The Clinical Role of SRSF1 Expression in Cancer: A Review of the Current Literature. Appl. Sci. 2022, 12, 2268. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; de Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Zaidi, N.; Swinnen, J.V.; Smans, K. ATP-Citrate Lyase: A Key Player in Cancer Metabolism. Cancer Res. 2012, 72, 3709–3714. [Google Scholar] [CrossRef]
- Watson, J.A.; Fang, M.; Lowenstein, J.M. Tricarballylate and Hydroxycitrate: Substrate and Inhibitor of ATP: Citrate Oxaloacetate Lyase. Arch. Biochem. Biophys. 1969, 135, 209–217. [Google Scholar] [CrossRef]
- Bauer, D.E.; Hatzivassiliou, G.; Zhao, F.; Andreadis, C.; Thompson, C.B. ATP Citrate Lyase Is an Important Component of Cell Growth and Transformation. Oncogene 2005, 24, 6314–6322. [Google Scholar] [CrossRef]
- Migita, T.; Narita, T.; Nomura, K.; Miyagi, E.; Inazuka, F.; Matsuura, M.; Ushijima, M.; Mashima, T.; Seimiya, H.; Satoh, Y.; et al. ATP Citrate Lyase: Activation and Therapeutic Implications in Non–Small Cell Lung Cancer. Cancer Res. 2008, 68, 8547–8554. [Google Scholar] [CrossRef]
- Hatzivassiliou, G.; Zhao, F.; Bauer, D.E.; Andreadis, C.; Shaw, A.N.; Dhanak, D.; Hingorani, S.R.; Tuveson, D.A.; Thompson, C.B. ATP Citrate Lyase Inhibition Can Suppress Tumor Cell Growth. Cancer Cell 2005, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Eidelman, E.; Twum-Ampofo, J.; Ansari, J.; Siddiqui, M.M. The Metabolic Phenotype of Prostate Cancer. Front. Oncol. 2017, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Deep, G.; Schlaepfer, I. Aberrant Lipid Metabolism Promotes Prostate Cancer: Role in Cell Survival under Hypoxia and Extracellular Vesicles Biogenesis. Int. J. Mol. Sci. 2016, 17, 1061. [Google Scholar] [CrossRef] [PubMed]
- Dłubek, J.; Rysz, J.; Jabłonowski, Z.; Gluba-Brzózka, A.; Franczyk, B. The Correlation between Lipid Metabolism Disorders and Prostate Cancer. Curr. Med. Chem. 2021, 28, 2048–2061. [Google Scholar] [CrossRef] [PubMed]
- Lounis, M.A.; Péant, B.; Leclerc-Desaulniers, K.; Ganguli, D.; Daneault, C.; Ruiz, M.; Zoubeidi, A.; Mes-Masson, A.-M.; Saad, F. Modulation of de Novo Lipogenesis Improves Response to Enzalutamide Treatment in Prostate Cancer. Cancers 2020, 12, 3339. [Google Scholar] [CrossRef]
- Abudurexiti, M.; Zhu, W.; Wang, Y.; Wang, J.; Xu, W.; Huang, Y.; Zhu, Y.; Shi, G.; Zhang, H.; Zhu, Y.; et al. Targeting CPT1B as a Potential Therapeutic Strategy in Castration-resistant and Enzalutamide-resistant Prostate Cancer. Prostate 2020, 80, 950–961. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Rider, L.; Rodrigues, L.U.; Gijón, M.A.; Pac, C.T.; Romero, L.; Cimic, A.; Sirintrapun, S.J.; Glodé, L.M.; Eckel, R.H.; et al. Lipid Catabolism via CPT1 as a Therapeutic Target for Prostate Cancer. Mol. Cancer Ther. 2014, 13, 2361–2371. [Google Scholar] [CrossRef]
- Russo, G.I.; Musso, N.; Romano, A.; Caruso, G.; Petralia, S.; Lanzanò, L.; Broggi, G.; Camarda, M. The Role of Dielectrophoresis for Cancer Diagnosis and Prognosis. Cancers 2021, 14, 198. [Google Scholar] [CrossRef]
- Russo, G.I.; Soeterik, T.; Puche-Sanz, I.; Broggi, G.; lo Giudice, A.; de Nunzio, C.; Lombardo, R.; Marra, G.; Gandaglia, G. Oncological Outcomes of Cribriform Histology Pattern in Prostate Cancer Patients: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2022, 1–9. [Google Scholar] [CrossRef]
| ATP Citrate Lyase | p-Value | ||
|---|---|---|---|
| Negative (n = 203) | Positive (n = 187) | ||
| Age (years), median (IQR) | 71.0 (65.0–77.0) | 68.0 (63.0–72.0) | <0.01 |
| PSA (ng/mL), median IQR) | 5.7 (2.11–8.9) | 7.57 (5.6–11.5) | <0.01 |
| Fasting glucose (mg/dL), median (IQR) | 98.0 (88.0–111.0) | 95.0 (87.0–108.5) | 0.23 |
| Total cholesterol (mg/dL), median (IQR) | 183.0 (157.0–210.0 | 190.5 (159.0–216.0) | 0.38 |
| Triglycerides (mg/dL), median (IQR) | 100.0 (65.0–150.0) | 101.5 (73.0–136.0) | 0.84 |
| Diabetes, n (%) | 28 (26.92) | 44 (15.38) | <0.01 |
| Group, n (%) | <0.01 | ||
| BPH | 86 (42.36) | 18 (9.63) | |
| PC | 117 (57.64) | 169 (90.37) | |
| ISUP Gleason score, n (%) | 0.21 | ||
| 1 | 35 (29.91) | 47 (27.81) | |
| 2 | 52 (44.44) | 58 (34.32) | |
| 3 | 22 (18.80) | 44 (26.04) | |
| 4 | 3 (2.56) | 11 (6.51) | |
| 5 | 5 (4.27) | 9 (5.33) | |
| Pathological stage, n (%) | 0.55 | ||
| T2 | 84 (71.79) | 113 (66.86) | |
| T3 | 21 (17.95) | 32 (19.05) | |
| T4 | 12 (10.26) | 24 (14.29) | |
| Classification risk of PC, n (%) | 0.57 | ||
| Low risk | 42 (35.90) | 58 (34.32) | |
| Intermediate risk | 54 (46.15) | 72 (42.60) | |
| High risk | 21 (17.95) | 39 (23.08) | |
| Ki-67 positive score, n (%) | 20 (9.85) | 33 (17.65) | 0.02 |
| AR positive score, n (%) | 84 (41.38) | 98 (52.41) | 0.03 |
| PSMA positive score, n (%) | 58 (28.57) | 90 (48.13) | <0.01 |
| IR-α positive score, n (%) | 105 (51.72) | 154 (82.35) | <0.01 |
| IR-β positive score, n (%) | 9 (4.43) | 14 (7.49) | 0.20 |
| IGF-1R positive score, n (%) | 23 (11.33) | 41 (21.93) | <0.01 |
| SRSF-1 positive score, n (%) | 80 (39.41) | 108 (57.75) | <0.01 |
| CPT1-a positive score, n (%) | 30 (14.78) | 35 (18.72) | 0.30 |
| SCD-1 positive score, n (%) | 24 (11.82) | 41 (21.93) | <0.01 |
| SREBP1 positive score, n (%) | 39 (19.21) | 57 (30.48) | 0.01 |
| FAS positive score, n (%) | 68 (33.50) | 144 (77.01) | <0.01 |
| ACC-1 positive score, n (%) | 31 (15.27) | 113 (60.43) | <0.01 |
| Carnitine Palmitoyltransferase-1a | p-Value | ||
|---|---|---|---|
| Low-IRS (n = 325) | High-IRS (n = 65) | ||
| Age (years), median (IQR) | 70.0 (64.0–74.0) | 68.0 (64.0–74.0) | <0.01 |
| PSA (ng/mL), median IQR) | 6.43 (4.05–10.0) | 7.0 (4.9–10.01) | <0.01 |
| Fasting glucose (mg/dL), median (IQR) | 96.0 (88.0–109) | 99.0 (87.0–111.0) | 0.71 |
| Total cholesterol (mg/dL), median (IQR) | 187.0 (158.0–214.0) | 179.0 (152.0–200.0) | <0.01 |
| Triglycerides (mg/dL), median (IQR) | 99.0 (68.0–137.0) | 121.0 (73.0–170.0) | <0.01 |
| Diabetes, n (%) | 61 (18.77) | 11 (16.92) | 0.73 |
| Group, n (%) | 0.02 | ||
| BPH | 94 (28.92) | 10 (15.38) | |
| PC | 231 (71.08) | 55 (84.62) | |
| ISUP Gleason score, n (%) | 0.84 | ||
| 1 | 68 (29.44) | 14 (25.45) | |
| 2 | 89 (38.53) | 21 (38.18) | |
| 3 | 52 (22.51) | 14 (25.45) | |
| 4 | 12 (5.19) | 2 (3.64) | |
| 5 | 10 (4.33) | 4 (7.27) | |
| Pathological stage, n (%) | 0.95 | ||
| T2 | 159 (69.13) | 37 (67.27) | |
| T3 | 42 (18.26) | 11 (20.0) | |
| T4 | 29 (12.61 | 7 (12.73) | |
| Classification risk of PC, n (%) | 0.14 | ||
| Low risk | 87 (37.6) | 13 (23.64) | |
| Intermediate risk | 98 (42.42) | 28 (50.91) | |
| High risk | 46 (19.91) | 14 (25.45) | |
| Ki-67 positive score, n (%) | 53 (15.73) | 12 (22.64) | 0.21 |
| AR positive score, n (%) | 25 (12.02) | 40 (21.98) | <0.01 |
| PSMA positive score, n (%) | 36 (14.88) | 29 (19.59) | 0.22 |
| IR-α positive score, n (%) | 10 (7.63) | 55 (21.24) | <0.01 |
| IR-β positive score, n (%) | 57 (15.53) | 8 (34.78) | 0.02 |
| IGF-1R positive score, n (%) | 53 (16.26) | 12 (18.75) | 0.62 |
| SRSF-1 positive score, n (%) | 23 (11.39) | 42 (22.34) | <0.01 |
| ATP-citrate lyase positive score, n (%) | 30 (14.78) | 35 (18.72) | 0.29 |
| SCD-1 positive score, n (%) | 49 (15.08) | 16 (24.62) | 0.06 |
| SREBP1 positive score, n (%) | 41 (13.95) | 24 (25.00) | 0.01 |
| FAS positive score, n (%) | 15 (8.43) | 50 (23.58) | <0.01 |
| ACC-1 positive score, n (%) | 29 (11.79) | 36 (25.00) | <0.01 |
| ATPLy + vs. − (OR 95% CI) | CPT1a, + vs. − (OR 95% CI) | SCD + vs. − (OR 95% CI) | SREBP + vs. − (OR 95% CI) | FAS + vs. − (OR 95% CI) | AC-1 + vs. − (OR 95% CI) | |
|---|---|---|---|---|---|---|
| PSA, continuous | 1.01 (0.98–1.03) | 1.00 (0.97–1.02) | 0.98 (0.95–1.01) | 0.96 (0.92–1.00) | 1.00 (0.98–1.03) | 0.99 (0.97–1.01) |
| Fasting blood glucose, continuous | 0.99 (0.98–1.01) | 1.00 (0.99–1.02) | 0.99 (0.98–1.01) | 1.00 (0.99–1.01) | 0.99 (0.98–1.00) | 0.99 (0.98–1.01) |
| Total cholesterol, continuous | 0.99 (0.98–1.00) | 0.99 (0.98–1.01) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.99–1.00) | 0.99 (0.98–1.00) |
| Triglycerides, continuous | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.99.1.00) | 0.99 (0.98–1.00) |
| Diabetes, yes vs. no | 1.11 (0.58–2.16) | 0.82 (0.49–2.43) | 1.58 (0.74–3.39) | 0.51 (0.22–1.21) | 0.50 (0.26–0.97) | 1.60 (0.83–3.06) |
| Pathological stage, pT3/4 vs. pT2 | 1.27 (0.76–2.12) | 1.08 (0.79–2.04) | 0.94 (0.49–1.80) | 1.04 (0.60–1.85) | 0.71 (0.42–1.20) | 1.30 (0.93–1.80) |
| ISUP Gleason, ≥4 vs. <4 | 1.82 (0.77–4.30) | 0.75 (0.44–3.02) | 1.53 (0.61–3.82) | 0.79 (0.30–2.04) | 1.47 (0.60–3.60) | 1.21 (0.98–1.52) |
| AR, + vs. − | 1.71 (1.06–2.77) † | 2.27 (1.24–4.16) † | 2.87 (1.53–5.39) † | 2.16 (1.25–3.73) † | 2.19 (1.30–3.69) † | 3.65 (2.22–5.93) † |
| PSMA, + vs. − | 1.12 (0.70–1.80) | 0.97 (0.54–1.75) | 1.16 (0.64–2.12) | 0.94 (0.55–1.61) | 1.64 (1.00–2.71) † | 1.80 (1.13–2.89) † |
| Ki-67, + vs. − | 1.33 (0.71–2.50) | 1.37 (0.66–2.84) | 2.16 (1.07–4.32) † | 1.02 (0.51–2.04) | 1.67 (0.83–3.38) | 1.11 (0.60–2.03) |
| IR-α, + vs. − | 2.56 (1.43–4.56) † | 2.55 (1.03–6.27) † | 1.20 (0.56–2.56) | 1.93 (0.92–4.05) | 3.31 (1.84–5.95) † | 9.99 (4.35–22.93) † |
| IR-β, + vs. − | 1.08 (0.45–2.59) | 2.45 (1.01–6.11) † | 1.24 (0.44–3.51) | 1.33 (0.52–3.38) | 1.77 (0.63–4.95) | 1.85 (0.77–4.43) |
| IGF-1R, + vs. − | 1.30 (0.73–2.32) | 0.96 (0.47–1.95) | 1.01 (0.49–2.07) | 0.35 (0.16–0.78) † | 0.80 (0.44–1.44) | 1.18 (0.67–2.05) |
| ATPLy + vs. − | - | 1.26 (0.69–2.32) | 1.43 (0.76–2.68) | 1.41 (0.81–2.47) | 4.84 (2.84–8.25) † | 4.97 (2.95–8.39) † |
| CPT1a, + vs. − | 1.26 (0.69–2.32) | - | 2.15 (1.08–4.24) † | 2.95 (1.58–5.49) † | 2.16 (1.05–4.41) † | 2.12 (1.16–3.87) † |
| SCD + vs. − | 1.43 (0.76–2.68) | 2.15 (1.08–4.24) † | - | 2.87 (1.53–5.39) † | 3.17 (1.42–7.04) † | 2.63 (1.40–4.91) † |
| SREBP + vs. − | 1.41 (0.81–2.47) | 2.95 (1.57–5.48) † | 2.87 (1.53–5.39) † | - | 1.74 (0.94–3.21) | 2.53 (1.45–4.40) † |
| FAS + vs. − | 4.84 (2.84–8.25) † | 2.16 (1.05–4.41) † | 3.17 (1.42–7.04) † | 1.74 (0.94–3.21) | - | 11.29 (5.76–22.14) † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, G.I.; Asmundo, M.G.; Lo Giudice, A.; Trefiletti, G.; Cimino, S.; Ferro, M.; Lombardo, R.; De Nunzio, C.; Morgia, G.; Piombino, E.; et al. Is There a Role of Warburg Effect in Prostate Cancer Aggressiveness? Analysis of Expression of Enzymes of Lipidic Metabolism by Immunohistochemistry in Prostate Cancer Patients (DIAMOND Study). Cancers 2023, 15, 948. https://doi.org/10.3390/cancers15030948
Russo GI, Asmundo MG, Lo Giudice A, Trefiletti G, Cimino S, Ferro M, Lombardo R, De Nunzio C, Morgia G, Piombino E, et al. Is There a Role of Warburg Effect in Prostate Cancer Aggressiveness? Analysis of Expression of Enzymes of Lipidic Metabolism by Immunohistochemistry in Prostate Cancer Patients (DIAMOND Study). Cancers. 2023; 15(3):948. https://doi.org/10.3390/cancers15030948
Chicago/Turabian StyleRusso, Giorgio Ivan, Maria Giovanna Asmundo, Arturo Lo Giudice, Giuseppe Trefiletti, Sebastiano Cimino, Matteo Ferro, Riccardo Lombardo, Cosimo De Nunzio, Giuseppe Morgia, Eliana Piombino, and et al. 2023. "Is There a Role of Warburg Effect in Prostate Cancer Aggressiveness? Analysis of Expression of Enzymes of Lipidic Metabolism by Immunohistochemistry in Prostate Cancer Patients (DIAMOND Study)" Cancers 15, no. 3: 948. https://doi.org/10.3390/cancers15030948
APA StyleRusso, G. I., Asmundo, M. G., Lo Giudice, A., Trefiletti, G., Cimino, S., Ferro, M., Lombardo, R., De Nunzio, C., Morgia, G., Piombino, E., Failla, M., Caltabiano, R., & Broggi, G. (2023). Is There a Role of Warburg Effect in Prostate Cancer Aggressiveness? Analysis of Expression of Enzymes of Lipidic Metabolism by Immunohistochemistry in Prostate Cancer Patients (DIAMOND Study). Cancers, 15(3), 948. https://doi.org/10.3390/cancers15030948












