Traffic-Related Air Pollution and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analyses
3. Results
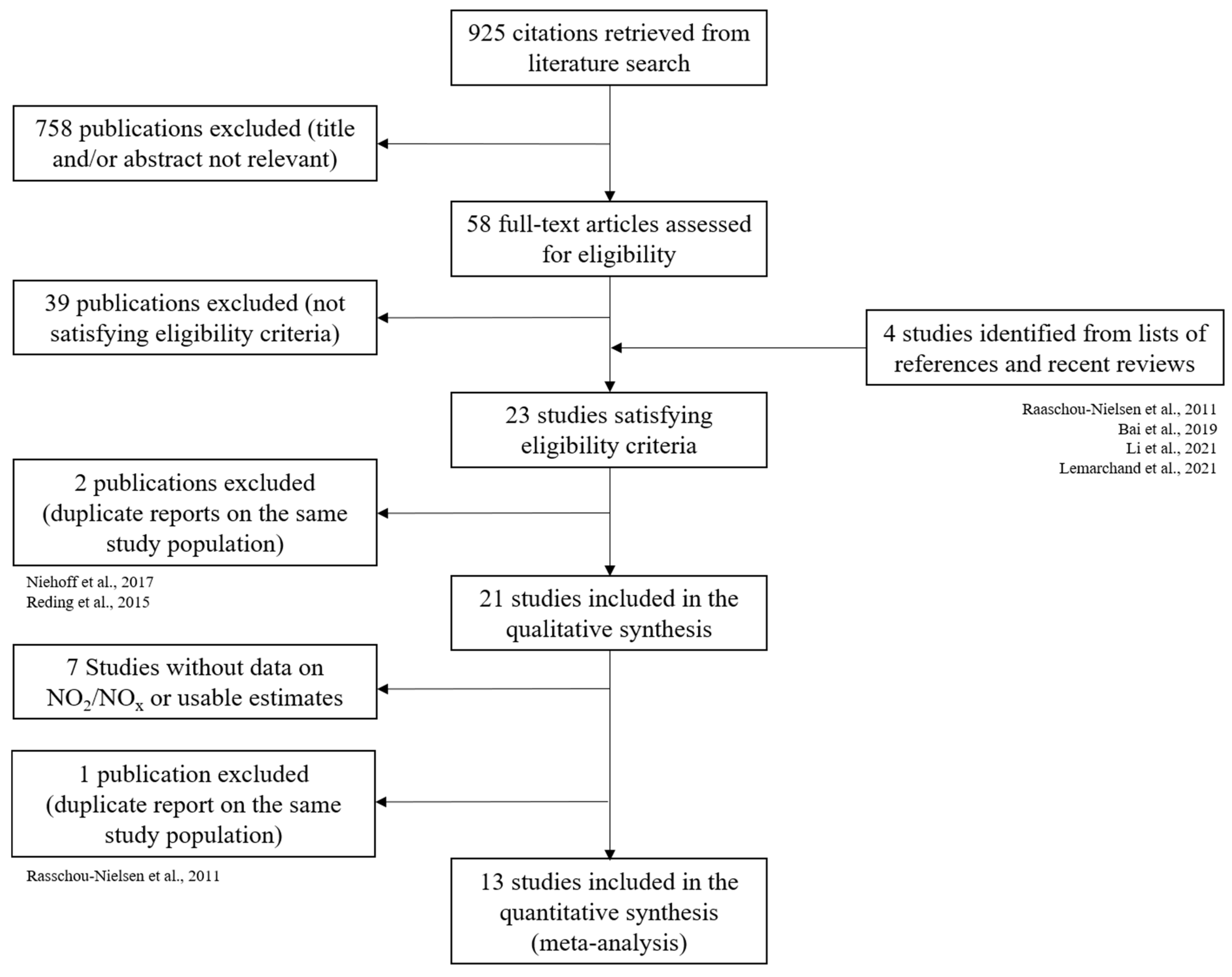
3.1. Studies Selected
3.2. Review of Studies on Traffic-Related Air Pollution Exposure

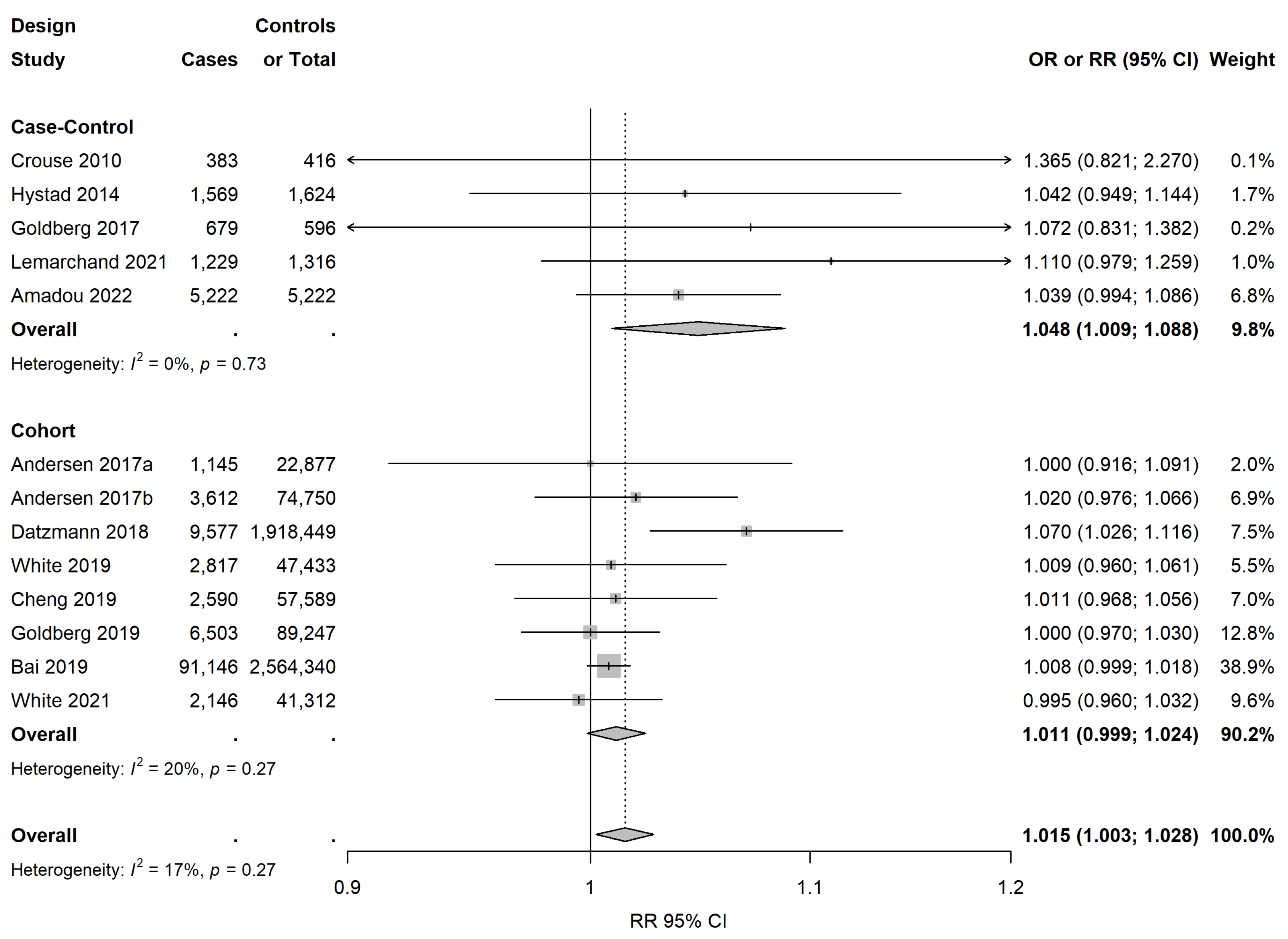
3.3. Meta-Analysis on NO2/NOx Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union Emission Inventory Report 1990–2018—European Environment Agency. Available online: https://www.eea.europa.eu/publications/european-union-emission-inventory-report-1990-2018 (accessed on 10 September 2020).
- Pascal, M.; Corso, M.; Chanel, O.; Declercq, C.; Badaloni, C.; Cesaroni, G.; Henschel, S.; Meister, K.; Haluza, D.; Martin-Olmedo, P.; et al. Assessing the Public Health Impacts of Urban Air Pollution in 25 European Cities: Results of the Aphekom Project. Sci. Total Environ. 2013, 449, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor Air Pollution and Cancer: An Overview of the Current Evidence and Public Health Recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Benbrahim-Tallaa, L.; Baan, R.A.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Loomis, D.; Straif, K. Carcinogenicity of Diesel-Engine and Gasoline-Engine Exhausts and Some Nitroarenes. Lancet Oncol. 2012, 13, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. The Carcinogenicity of Outdoor Air Pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Hamra, G.B.; Guha, N.; Cohen, A.; Laden, F.; Raaschou-Nielsen, O.; Samet, J.M.; Vineis, P.; Forastiere, F.; Saldiva, P.; Yorifuji, T.; et al. Outdoor Particulate Matter Exposure and Lung Cancer: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2014, 122, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Hamra, G.B.; Laden, F.; Cohen, A.J.; Raaschou-Nielsen, O.; Brauer, M.; Loomis, D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 1107–1112. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Butland, B.K.; Anderson, H.R.; Maynard, R.L. Long-Term Concentrations of Nitrogen Dioxide and Mortality. Epidemiol. Camb. Mass 2018, 29, 460–472. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Soulhac, L.; Salizzoni, P. Source Apportionment and Data Assimilation in Urban Air Quality Modelling for NO2: The Lyon Case Study. Atmosphere 2018, 9, 8. [Google Scholar] [CrossRef]
- Baldasano, J.M. COVID-19 Lockdown Effects on Air Quality by NO2 in the Cities of Barcelona and Madrid (Spain). Sci. Total Environ. 2020, 741, 140353. [Google Scholar] [CrossRef]
- Santodonato, J. Review of the Estrogenic and Antiestrogenic Activity of Polycyclic Aromatic Hydrocarbons: Relationship to Carcinogenicity. Chemosphere 1997, 34, 835–848. [Google Scholar] [CrossRef]
- Levy, I.; Mihele, C.; Lu, G.; Narayan, J.; Brook, J.R. Evaluating Multipollutant Exposure and Urban Air Quality: Pollutant Interrelationships, Neighborhood Variability, and Nitrogen Dioxide as a Proxy Pollutant. Environ. Health Perspect. 2014, 122, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mordukhovich, I.; Beyea, J.; Herring, A.H.; Hatch, M.; Stellman, S.D.; Teitelbaum, S.L.; Richardson, D.B.; Millikan, R.C.; Engel, L.S.; Shantakumar, S.; et al. Polymorphisms in DNA Repair Genes, Traffic-Related Polycyclic Aromatic Hydrocarbon Exposure and Breast Cancer Incidence. Int. J. Cancer 2016, 139, 310–321. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Chen, J.; Teitelbaum, S.L.; McCullough, L.E.; Xu, X.; Hee Cho, Y.; Conway, K.; Beyea, J.; Stellman, S.D.; Steck, S.E.; et al. Sources of Polycyclic Aromatic Hydrocarbons Are Associated with Gene-Specific Promoter Methylation in Women with Breast Cancer. Environ. Res. 2016, 145, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.M.; Udesky, J.O.; Rudel, R.A.; Brody, J.G. Environmental Chemicals and Breast Cancer: An Updated Review of Epidemiological Literature Informed by Biological Mechanisms. Environ. Res. 2018, 160, 152–182. [Google Scholar] [CrossRef]
- White, A.J.; Bradshaw, P.T.; Hamra, G.B. Air Pollution and Breast Cancer: A Review. Curr. Epidemiol. Rep. 2018, 5, 92–100. [Google Scholar] [CrossRef]
- Gabet, S.; Lemarchand, C.; Guénel, P.; Slama, R. Breast Cancer Risk in Association with Atmospheric Pollution Exposure: A Meta-Analysis of Effect Estimates Followed by a Health Impact Assessment. Environ. Health Perspect. 2021, 129, 57012. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, W.; Chen, Q.; Zhou, N.; Xu, Y. The Relationship between Exposure to Particulate Matter and Breast Cancer Incidence and Mortality: A Meta-Analysis. Medicine 2019, 98, e18349. [Google Scholar] [CrossRef]
- Hill, P.; Wynder, E.L. Nicotine and Cotinine in Breast Fluid. Cancer Lett. 1979, 6, 251–254. [Google Scholar] [CrossRef]
- Huff, J.E.; Haseman, J.K.; DeMarini, D.M.; Eustis, S.; Maronpot, R.R.; Peters, A.C.; Persing, R.L.; Chrisp, C.E.; Jacobs, A.C. Multiple-Site Carcinogenicity of Benzene in Fischer 344 Rats and B6C3F1 Mice. Environ. Health Perspect. 1989, 82, 125–163. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Sørensen, M.; Hansen, J.; Loft, S.; Overvad, K.; Tjønneland, A. Air Pollution from Traffic and Cancer Incidence: A Danish Cohort Study. Environ. Health Glob. Access Sci. Source 2011, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Goldberg, M.S.; Lavigne, E.; Weichenthal, S.; Martin, R.V.; et al. Exposure to Ambient Air Pollution and the Incidence of Lung Cancer and Breast Cancer in the Ontario Population Health and Environment Cohort. Int. J. Cancer 2019, 146, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Chiou, J.-Y.; Lin, C.-L.; Wei, J.C.-C.; Yeh, M.-H. The Association between Air Pollution Level and Breast Cancer Risk in Taiwan. Medicine 2021, 100, e25637. [Google Scholar] [CrossRef] [PubMed]
- Lemarchand, C.; Gabet, S.; Cénée, S.; Tvardik, N.; Slama, R.; Guénel, P. Breast Cancer Risk in Relation to Ambient Concentrations of Nitrogen Dioxide and Particulate Matter: Results of a Population-Based Case-Control Study Corrected for Potential Selection Bias (the CECILE Study). Environ. Int. 2021, 155, 106604. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Stafoggia, M.; Weinmayr, G.; Pedersen, M.; Galassi, C.; Jørgensen, J.T.; Oudin, A.; Forsberg, B.; Olsson, D.; Oftedal, B.; et al. Long-Term Exposure to Ambient Air Pollution and Incidence of Postmenopausal Breast Cancer in 15 European Cohorts within the ESCAPE Project. Environ. Health Perspect. 2017, 125, 107005. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; Connell, D.O.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: Http://Www.Ohri.ca/Programs/Clinical_epidemiology/Oxford.Asp (accessed on 9 June 2022).
- Vrijheid, M.; Martinez, D.; Manzanares, S.; Dadvand, P.; Schembari, A.; Rankin, J.; Nieuwenhuijsen, M. Ambient Air Pollution and Risk of Congenital Anomalies: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2011, 119, 598–606. [Google Scholar] [CrossRef]
- Crouse, D.L.; Goldberg, M.S.; Ross, N.A.; Chen, H.; Labrèche, F. Postmenopausal Breast Cancer Is Associated with Exposure to Traffic-Related Air Pollution in Montreal, Canada: A Case-Control Study. Environ. Health Perspect. 2010, 118, 1578–1583. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Greenland, S. Quantitative Methods in the Review of Epidemiologic Literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Datzmann, T.; Markevych, I.; Trautmann, F.; Heinrich, J.; Schmitt, J.; Tesch, F. Outdoor Air Pollution, Green Space, and Cancer Incidence in Saxony: A Semi-Individual Cohort Study. BMC Public Health 2018, 18, 715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.; Tseng, C.; Wu, J.; Yang, J.; Conroy, S.M.; Shariff-Marco, S.; Li, L.; Hertz, A.; Gomez, S.L.; Le Marchand, L.; et al. Association between Ambient Air Pollution and Breast Cancer Risk: The Multiethnic Cohort Study. Int. J. Cancer 2020, 146, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Niehoff, N.; White, A.J.; McCullough, L.E.; Steck, S.E.; Beyea, J.; Mordukhovich, I.; Shen, J.; Neugut, A.I.; Conway, K.; Santella, R.M.; et al. Polycyclic Aromatic Hydrocarbons and Postmenopausal Breast Cancer: An Evaluation of Effect Measure Modification by Body Mass Index and Weight Change. Environ. Res. 2017, 152, 17–25. [Google Scholar] [CrossRef]
- Mordukhovich, I.; Beyea, J.; Herring, A.H.; Hatch, M.; Stellman, S.D.; Teitelbaum, S.L.; Richardson, D.B.; Millikan, R.C.; Engel, L.S.; Shantakumar, S.; et al. Vehicular Traffic-Related Polycyclic Aromatic Hydrocarbon Exposure and Breast Cancer Incidence: The Long Island Breast Cancer Study Project (LIBCSP). Environ. Health Perspect. 2016, 124, 30–38. [Google Scholar] [CrossRef]
- Reding, K.W.; Young, M.T.; Szpiro, A.A.; Han, C.J.; DeRoo, L.A.; Weinberg, C.; Kaufman, J.D.; Sandler, D.P. Breast Cancer Risk in Relation to Ambient Air Pollution Exposure at Residences in the Sister Study Cohort. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2015, 24, 1907–1909. [Google Scholar] [CrossRef]
- White, A.J.; Keller, J.P.; Zhao, S.; Carroll, R.; Kaufman, J.D.; Sandler, D.P. Air Pollution, Clustering of Particulate Matter Components, and Breast Cancer in the Sister Study: A U.S.-Wide Cohort. Environ. Health Perspect. 2019, 127, 107002. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Ravnskjær, L.; Andersen, K.K.; Loft, S.; Brandt, J.; Becker, T.; Ketzel, M.; Hertel, O.; Lynge, E.; Bräuner, E.V. Long-Term Exposure to Fine Particulate Matter and Breast Cancer Incidence in the Danish Nurse Cohort Study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017, 26, 428–430. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Villeneuve, P.J.; Crouse, D.; To, T.; Weichenthal, S.A.; Wall, C.; Miller, A.B. Associations between Incident Breast Cancer and Ambient Concentrations of Nitrogen Dioxide from a National Land Use Regression Model in the Canadian National Breast Screening Study. Environ. Int. 2019, 133, 105182. [Google Scholar] [CrossRef]
- Hart, J.E.; Bertrand, K.A.; DuPre, N.; James, P.; Vieira, V.M.; Tamimi, R.M.; Laden, F. Long-Term Particulate Matter Exposures during Adulthood and Risk of Breast Cancer Incidence in the Nurses’ Health Study II Prospective Cohort. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2016, 25, 1274–1276. [Google Scholar] [CrossRef]
- Shmuel, S.; White, A.J.; Sandler, D.P. Residential Exposure to Vehicular Traffic-Related Air Pollution during Childhood and Breast Cancer Risk. Environ. Res. 2017, 159, 257–263. [Google Scholar] [CrossRef]
- Cohen, G.; Levy, I.; Yuval; Kark, J.D.; Levin, N.; Witberg, G.; Iakobishvili, Z.; Bental, T.; Broday, D.M.; Steinberg, D.M.; et al. Chronic Exposure to Traffic-Related Air Pollution and Cancer Incidence among 10,000 Patients Undergoing Percutaneous Coronary Interventions: A Historical Prospective Study. Eur. J. Prev. Cardiol. 2018, 25, 659–670. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Gregoire, A.M.; Niehoff, N.M.; Bertrand, K.A.; Palmer, J.R.; Coogan, P.F.; Bethea, T.N. Air Pollution and Breast Cancer Risk in the Black Women’s Health Study. Environ. Res. 2021, 194, 110651. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Michl, E.L.; Melius, J.M.; Kallenbach, L.R.; Ju, C.L.; Talbot, T.O.; Orr, M.F.; Lauridsen, P.E. Breast Cancer Risk and Residence near Industry or Traffic in Nassau and Suffolk Counties, Long Island, New York. Arch. Environ. Health 1996, 51, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Beyea, J.; Bonner, M.R.; Han, D.; Vena, J.E.; Rogerson, P.; Vito, D.; Muti, P.; Trevisan, M.; Edge, S.B.; et al. Exposure to Traffic Emissions throughout Life and Risk of Breast Cancer: The Western New York Exposures and Breast Cancer (WEB) Study. Cancer Causes Control CCC 2007, 18, 947–955. [Google Scholar] [CrossRef]
- Hystad, P.; Villeneuve, P.J.; Goldberg, M.S.; Crouse, D.L.; Johnson, K. Canadian Cancer Registries Epidemiology Research Group Exposure to Traffic-Related Air Pollution and the Risk of Developing Breast Cancer among Women in Eight Canadian Provinces: A Case-Control Study. Environ. Int. 2015, 74, 240–248. [Google Scholar] [CrossRef]
- Goldberg, M.S.; Labrèche, F.; Weichenthal, S.; Lavigne, E.; Valois, M.-F.; Hatzopoulou, M.; Van Ryswyk, K.; Shekarrizfard, M.; Villeneuve, P.J.; Crouse, D.; et al. The Association between the Incidence of Postmenopausal Breast Cancer and Concentrations at Street-Level of Nitrogen Dioxide and Ultrafine Particles. Environ. Res. 2017, 158, 7–15. [Google Scholar] [CrossRef]
- Amadou, A.; Praud, D.; Coudon, T.; Deygas, F.; Grassot, L.; Dubuis, M.; Faure, E.; Couvidat, F.; Caudeville, J.; Bessagnet, B.; et al. Long-Term Exposure to Nitrogen Dioxide Air Pollution and Breast Cancer Risk: A Nested Case-Control within the French E3N Cohort Study. Environ. Pollut. 2022, 317, 120719. [Google Scholar] [CrossRef]
- Rodríguez, S.; Querol, X.; Alastuey, A.; Viana, M.-M.; Alarcón, M.; Mantilla, E.; Ruiz, C.R. Comparative PM10–PM2.5 Source Contribution Study at Rural, Urban and Industrial Sites during PM Episodes in Eastern Spain. Sci. Total Environ. 2004, 328, 95–113. [Google Scholar] [CrossRef]
- Crouse, D.L.; Peters, P.A.; Hystad, P.; Brook, J.R.; van Donkelaar, A.; Martin, R.V.; Villeneuve, P.J.; Jerrett, M.; Goldberg, M.S.; Pope, C.A.; et al. Ambient PM2.5, O3, and NO2 Exposures and Associations with Mortality over 16 Years of Follow-Up in the Canadian Census Health and Environment Cohort (CanCHEC). Environ. Health Perspect. 2015, 123, 1180–1186. [Google Scholar] [CrossRef]
- Hart, J.E.; Bertrand, K.A.; DuPre, N.; James, P.; Vieira, V.M.; VoPham, T.; Mittleman, M.R.; Tamimi, R.M.; Laden, F. Exposure to Hazardous Air Pollutants and Risk of Incident Breast Cancer in the Nurses’ Health Study II. Environ. Health Glob. Access Sci. Source 2018, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- De Hoogh, K.; Korek, M.; Vienneau, D.; Keuken, M.; Kukkonen, J.; Nieuwenhuijsen, M.J.; Badaloni, C.; Beelen, R.; Bolignano, A.; Cesaroni, G.; et al. Comparing Land Use Regression and Dispersion Modelling to Assess Residential Exposure to Ambient Air Pollution for Epidemiological Studies. Environ. Int. 2014, 73, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Su, J.G.; Apte, J.S.; Lipsitt, J.; Garcia-Gonzales, D.A.; Beckerman, B.S.; de Nazelle, A.; Texcalac-Sangrador, J.L.; Jerrett, M. Populations Potentially Exposed to Traffic-Related Air Pollution in Seven World Cities. Environ. Int. 2015, 78, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Beckerman, B.; Jerrett, M.; Brook, J.R.; Verma, D.K.; Arain, M.A.; Finkelstein, M.M. Correlation of Nitrogen Dioxide with Other Traffic Pollutants near a Major Expressway. Atmos. Environ. 2008, 42, 275–290. [Google Scholar] [CrossRef]
- Park, E.H.; Heo, J.; Kim, H.; Yi, S.-M. Long Term Trends of Chemical Constituents and Source Contributions of PM2.5 in Seoul. Chemosphere 2020, 251, 126371. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.L.; Bonner, M.R.; Nie, J.; Han, D.; Wang, Y.; Tao, M.-H.; Shields, P.G.; Marian, C.; Eng, K.H.; Trevisan, M.; et al. Lifetime Exposure to Ambient Air Pollution and Methylation of Tumor Suppressor Genes in Breast Tumors. Environ. Res. 2018, 161, 418–424. [Google Scholar] [CrossRef]
- Gilbert, N.L.; Woodhouse, S.; Stieb, D.M.; Brook, J.R. Ambient Nitrogen Dioxide and Distance from a Major Highway. Sci. Total Environ. 2003, 312, 43–46. [Google Scholar] [CrossRef]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F.; et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef]
- Choudhary, S.; Sood, S.; Wang, H.-C.R. Dipyridamole Intervention of Breast Cell Carcinogenesis. Mol. Carcinog. 2014, 53, 243–252. [Google Scholar] [CrossRef]
- Hu, Y.; Xiang, J.; Su, L.; Tang, X. The Regulation of Nitric Oxide in Tumor Progression and Therapy. J. Int. Med. Res. 2020, 48, 0300060520905985. [Google Scholar] [CrossRef]
- Mandal, P. Molecular Signature of Nitric Oxide on Major Cancer Hallmarks of Colorectal Carcinoma. Inflammopharmacology 2018, 26, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; von Minckwitz, G.; Weber, S.; Sinn, H.-P.; Schini-Kerth, V.B.; Lobysheva, I.; Nepveu, F.; Wolf, G.; Strebhardt, K.; Kaufmann, M. Expression of Endothelial and Inducible Nitric Oxide Synthase in Benign and Malignant Lesions of the Breast and Measurement of Nitric Oxide Using Electron Paramagnetic Resonance Spectroscopy. Cancer 2002, 95, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.L.; Miles, D.W.; Happerfield, L.; Bobrow, L.G.; Knowles, R.G.; Moncada, S. Nitric Oxide Synthase Activity in Human Breast Cancer. Br. J. Cancer 1995, 72, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Xie, Z.; Zhou, S.; Li, Y.; Zhou, Y.; Sun, M. Nitric Oxide (NO) and NO Synthases (NOS)-Based Targeted Therapy for Colon Cancer. Cancers 2020, 12, 1881. [Google Scholar] [CrossRef]
- Sahay, D.; Terry, M.B.; Miller, R. Is Breast Cancer a Result of Epigenetic Responses to Traffic-Related Air Pollution? A Review of the Latest Evidence. Epigenomics 2019, 11, 701–714. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-Induced Cancers in Animals and in Humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef]
- Yaghjyan, L.; Arao, R.; Brokamp, C.; O’Meara, E.S.; Sprague, B.L.; Ghita, G.; Ryan, P. Association between Air Pollution and Mammographic Breast Density in the Breast Cancer Surveilance Consortium. Breast Cancer Res. BCR 2017, 19, 36. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic Density and the Risk and Detection of Breast Cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef]
- Huynh, S.; von Euler-Chelpin, M.; Raaschou-Nielsen, O.; Hertel, O.; Tjønneland, A.; Lynge, E.; Vejborg, I.; Andersen, Z.J. Long-Term Exposure to Air Pollution and Mammographic Density in the Danish Diet, Cancer and Health Cohort. Environ. Health Glob. Access Sci. Source 2015, 14, 31. [Google Scholar] [CrossRef]
- Zeinomar, N.; Oskar, S.; Kehm, R.D.; Sahebzeda, S.; Terry, M.B. Environmental Exposures and Breast Cancer Risk in the Context of Underlying Susceptibility: A Systematic Review of the Epidemiological Literature. Environ. Res. 2020, 187, 109346. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Delgado-Cruzata, L.; Vin-Raviv, N.; Wu, H.C.; Santella, R.M. DNA Methylation in White Blood Cells: Association with Risk Factors in Epidemiologic Studies. Epigenetics 2011, 6, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Xue, J.; Barzyk, T.M. Evaluating Socioeconomic and Racial Differences in Traffic-Related Metrics in the United States Using a GIS Approach. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Pratt, G.C.; Vadali, M.L.; Kvale, D.L.; Ellickson, K.M. Traffic, Air Pollution, Minority and Socio-Economic Status: Addressing Inequities in Exposure and Risk. Int. J. Environ. Res. Public. Health 2015, 12, 5355–5372. [Google Scholar] [CrossRef]
- Dons, E.; Int Panis, L.; Van Poppel, M.; Theunis, J.; Wets, G. Personal Exposure to Black Carbon in Transport Microenvironments. Atmos. Environ. 2012, 55, 392–398. [Google Scholar] [CrossRef]
- Moreno, T.; Querol, X.; Alastuey, A.; Viana, M.; Gibbons, W. Profiling Transient Daytime Peaks in Urban Air Pollutants: City Centre Traffic Hotspot versus Urban Background Concentrations. J. Environ. Monit. 2009, 11, 1535–1542. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]

| Reference; Region | Study ID a | Exposure | Design | No. of Cases/No. of Total Subjects or No. of Controls b | Exposure Assessment | Variables of Adjustment | Quality Assessment (NOS Score/9) | Important Findings |
|---|---|---|---|---|---|---|---|---|
| Lewis-Michl et al., 1996; USA [46] | - | Traffic density (number of vehicle-miles divided by the number of miles of highway) | CC | 793/966 | Traffic density = vehicle miles/miles of highway (5-km2 grid cells) Weighted annual average of residential address exposures 20 years prior to the index date → based on residential history High density = average ≥ 100,000 vehicle miles/miles of highway | Age, family history, history of benign breast disease, age at first birth, education | 4 | High-density traffic (Nassau): OR = 1.29 (0.77, 2.15) High-density traffic (Suffolk): OR = 0.89 (0.40, 1.99) |
| Nie et al., 2007; USA [47] | - | BaP | CC | 1068/1944 | Exposure to BaP estimated by a geographical model validated and calibrated with measurements at different points in time of life → based on residential history | Age; ethnicity; education; BMI; smoking history; diet; medical history; age at first birth, number of births; family history; age at menarche; age at menopause; history of benign breast disease | 8 | At menarche Premenopausal: Q4/Q1: OR = 2.07 (0.91, 4.72) At first birth Premenopausal: Q4/Q1: OR = 1.22 (0.44, 3.36) Postmenopausal: Q4/Q1: OR = 2.58 (1.15, 5.83) 20 years prior to diagnosis Premenopausal: Q4/Q1: OR = 1.29 (0.59, 2.82) Postmenopausal: Q4/Q1: OR = 0.82 (0.58, 1.18) 10 years prior to diagnosis Premenopausal: Q4/Q1: OR = 1.49 (0.65, 3.43) Postmenopausal: Q4/Q1: OR = 0.80 (0.55, 1.17) |
| Crouse et al., 2010; Canada [29] | 1 | NO2 | CC | 383/416 | Statistical methods (LUR) Annual average in 1985 (10 years before diagnosis), 1996 (at diagnosis), and 2006 at the interview address (1996–1997) c | Age at diagnosis; age at menarche; age at first birth; duration of breastfeeding; age at bilateral oophorectomy; BMI; smoking status; alcohol consumption; education; hospital of diagnosis; family history; ethnicity; oral contraceptive use; duration of HRT; respondent status; history of benign breast disease; occupational exposure to solvents, low magnetic fields, CO, and PAHs; neighborhood income and SES | 6 | Postmenopausal: (per 5 ppb) 2016: OR = 1.52 (0.82, 2.81) 1996: OR = 1.42 (0.81, 2.48) Mean of 1996 and 1985: OR = 1.34 (0.83, 2.16) 1985: OR = 1.28 (0.84, 1.93) |
| Raaschou-Nielsen et al., 2011; Denmark [22] | - | Traffic density (distance from subject’s residence to the closest major road; number of vehicles on the nearest road) | Co | 987/27,735 | -Presence of a street with a traffic density >10,000 vehicles per day within 50 m of the residence -Total number of kilometers travelled by vehicles within 200 m of the residence each day → At baseline address | Age; smoking status; smoking intensity and duration; second-hand smoking; physical activity; BMI; diet; alcohol consumption; breastfeeding; number of births; age at first full-term pregnancy; previous benign breast tumor; previous diagnosis of hypertension; oral contraceptive use; HRT use; skin sensitivity to the sun | 8 | Major street within 50 m (yes/no): IRR = 0.98 (0.78, 1.22) Per 104 vehicle km/day within 200 m: IRR = 0.98 (0.88, 1.10) |
| Hystad et al., 2015; Canada [48] | 2 | NO2/traffic density (distance from subject’s residence to the closest major road) | CC | 1569/1624 | Dispersion model (CTM based on satellite data), statistical methods (interpolation and LUR) Average exposure (1975–1994) from postal codes of residential history Number of years participants resided within 50 m, 100 m, and 300 m of a highway or main road during the 20-year exposure period (1975–1994) → based on residential history postal codes | Age; study province; age at menarche; years of menstruation; parity; age at first birth; breastfeeding; oophorectomy; BMI; smoking status; years since smoking cessation; alcohol consumption; median household income; years of education; second-hand smoking status; meat and vegetable consumption; physical activity; mammography; neighborhood SES; time in urban area | 8 | NO2: OR = 1.04 (0.95; 1.14) Highways (for an increment of 1 additional year of residence) Years ≤ 50 m OR = 0.95 (0.73, 1.23) Years ≤ 100 m OR = 0.95 (0.78, 1.15) Years ≤ 300 m OR = 0.98 (0.86, 1.11) Major roads (for an increment of 1 additional year of residence) Years ≤ 50 m OR = 0.94 (0.82, 1.08) Years ≤ 100 m OR = 1.03 (0.92, 1.15) Years ≤ 300 m OR = 0.98 (0.90, 1.08) |
| Mordukhovich et al., 2016; USA [37] | - | PAH | CC | 1274/1334 | Geographical model validated and calibrated with measurements → based on residential histories in Nassau and Suffolk counties only; addresses at which a woman resided for at least 1 year | Age; education; ethnicity; religion; parity; BMI; age at first full-term pregnancy; oral contraceptive use; alcohol consumption; physical activity; breastfeeding; HRT use; SES | 8 | Traffic PAH exposure: - in 1995: 95th/<50th: OR = 1.06 (0.70, 1.60) - in 1960–1990: 95th/<50th: OR = 1.47 (0.70, 3.08) |
| Hart et al., 2016; USA [42] | - | Traffic density (distance from subject’s residence to the closest major road) | Co | 3072/111,545 | Distance of 3 types of roads to the address (time-dependent variable) using the 2007 roads database → based on residential history (every 2 years) (2007 roads database) | Age; ethnicity; family history; age at menarche; parity; age at first full-term pregnancy; height; BMI; BMI at age 18; history of benign breast disease; alcohol consumption; diet; oral contraceptive use; menopausal status; hormone use; smoking status; physical activity; individual SES; neighborhood SES | 7 | 0–49 m from A1–A3 roads (vs. ≥ 200 m): HR = 1.02 (0.75, 1.37) 0–49 m from A1–A2 roads (vs. ≥ 200 m): HR = 1.44 (0.71, 2.92) 0–199 m from A1 roads (vs. ≥ 200 m): HR = 1.52 (0.89, 2.60) |
| Shmuel et al., 2017; USA [43] | - | Traffic density (characteristics of the main road and of the nearest cross-street) | Co | 2028/42,934 | Distance to the nearest intersection/cross-street Characteristics of the main road (number of lanes, presence of median or barrier) Traffic volume during rush hour → At the longest residence before 14 years old | Age, ethnicity; childhood SES; smoking status; education, family history; menopausal status; childhood residence urban/rural status | 5 | Characteristics of the main road at childhood residence: ≥3 lanes (vs. 1–2): HR = 0.8 (0.6, 1.1) With median or barrier of any kind (vs. without) HR = 1.2 (0.9, 1.7) Heavy traffic (vs. light traffic): HR = 0.9 (0.7, 1.1) Characteristics of the nearest cross-street or intersecting road: Within 100 ft., 3+ lanes and/or median/barrier and heavy traffic (vs.100 ft. + and/or (neither 3+ lanes, nor median/barrier)) HR = 1.4 (1.0, 1.9) |
| Goldberg et al., 2017; Canada [49] | 3 | NO2 | CC | 679/596 | Statistical methods (LUR) Annual average in 2005–2006 at baseline address | Age; age at menarche; parity; age at first birth; breastfeeding; oophorectomy; BMI; smoking status; alcohol consumption; education; family history; ethnicity; oral contraceptive use; HRT use; second-hand smoking; marital status; census variables (immigrants; unemployment; education; income) | 9 | Postmenopausal: per increase of IQR 3.75 ppb: OR: 1.07 (0.83; 1.38) |
| Andersen et al., 2017a; Denmark [40] | 4 | NO2 | Co (Danish Nurse Cohort Study) | 1145/22,877 | Method combining a dispersion model (THOR) and a proxy using GIS (Danish AirGis) 3-year annual running average from 1990 to index date based on residential history | Age; age at menarche; parity; age at first birth; BMI; smoking status; alcohol consumption; physical activity; menopausal status; oral contraceptive use; HRT use; urbanization level | 7 | NO2: HR = 1.00 (0.94–1.07) (per interquartile range increase 7.4 μg/m3) |
| Andersen et al., 2017b; 15 cohorts from nine European countries d [26] | 5 | NO2/NOx/traffic Density (number of vehicles on the nearest road) | Co (ESCAPE) | 3612/74,750 | Statistical methods (LUR) At the address at baseline Number of vehicles per day on the nearest road → At baseline address | Age; parity; age at first birth; breastfeeding; BMI; smoking status; years since smoking cessation; alcohol consumption; education; employment; physical activity; oral contraceptive use; HRT use; neighborhood income | 7 | Postmenopausal NO2: HR = 1.02 (0.98–1.07) p = 0.33 (per increase of 10 μg/m3) NOx: HR = 1.04 (1.00–1.08) p = 0.04 (per increase of 20 μg/m3) |
| Datzmann et al., 2018; Germany [34] | 6 | NO2 | Co | 9577/1,918,449 | Statistical methods (LUR) Annual average in 2007 in the residential district at the baseline address | Age; alcohol-related disorder; unemployment; district number of physician contacts, population density and proportion of unemployment | 6 | NO2: RR = 1.07 (1.03–1.12) (per increase of 10 μg/m3) |
| Cohen et al., 2018; Israël [44] | 7 | NOx | Co | 41/2307 | Statistical methods (LUR) Average of estimated annual concentrations between 2004 and 2012 at baseline address | Age; smoking status; neighborhood SES; ethnicity; hypertension; diabetes; chronic heart failure; renal failure; hemoglobin levels | 5 | NOx: adjusted HR = 1.43 (1.12–1.83) (for a 10-ppb increase) |
| White et al., 2019; USA [39] | 8 | NO2 | Co (The Sister Study) | 2203/47,433 | Statistical methods (kriging) Annual average in 2006 at baseline address | Age; parity; BMI; smoking status; education; ethnicity; HRT use; income; marital status; census tract level income; geographic region | 7 | All: HR = 1.06 (1.01–1.11) Invasive: HR = 1.01 (0.96–1.07) In situ (DCIS): HR = 1.23 (1.12–1.36) (for an increase in the IQR difference 5.8 ppb) |
| Goldberg et al., 2019; Canada [41] | 9 | NO2 | Co (Canadian National Breast Screening Study) | 6503/89,247 | Statistical methods (LUR) Annual average in 2006 at baseline address | Age; age at menarche; pregnancy; BMI; smoking status; education; employment; occupation; family history; oral contraceptive use; HRT use; breast self-examination; contextual measures (education; income; unemployment) | 6 | Premenopausal: Rate ratio: 1.17 (1.00–1.38) for increase of 9.7 ppb Postmenopausal Rate ratio: 1.00 (0.95–1.06) for increase of 9.7 ppb |
| Bai et al., 2019; Canada [23] | 10 | NO2 | Co (Ontario Population Health and Environment Cohort) | 91,146/2,564,340 | Statistical methods (LUR) Time-varying variables using a 3-year running average without taking into account the 4 years before the index date, using residential history | Age; census tract-level recent immigrants, unemployment rate, education and income; urban residency and a north/south indicator | 7 | NO2: HR = 1.02 (0.99–1.04) for 8.2 ppb |
| Cheng et al., 2020; USA [35] | 11 | NO2/NOx | Co (The multi-ethnic cohort study) | ≃2, 500 e/57,589 | Statistical methods (kriging, LUR), dispersion model A set of cumulative average exposures for a series of time intervals during monitoring from residential history | Age; age at menarche; parity; age at first birth; BMI; smoking status; alcohol consumption; family history; ethnicity; HRT use; menopausal status; physical activity; energy intake; neighborhood SES and education | 8 | NOx Kriging: HR = 1.12 (0.96–1.31) (increase of 50.2 ppb) LUR: HR = 1.08 (0.96–1.22) (increase of 45.6 ppb) CALINE4: HR = 0.97 (0.73–1.26) (increase of 8.7 ppb) NO2 Kriging: HR = 1.09 (0.91–1.31) (increase of 16.5 ppb) LUR: HR = 1.04 (0.90–1.20) (increase of 18.6 ppb) |
| White et al., 2021; USA [45] | 12 | NO2 | Co | 2146/41,312 | Statistical methods (LUR) Annual average in 2000–2010 at baseline residence and time-varying air pollution exposure throughout follow-up | Age; education; smoking status; parity; HRT use; BMI; census geographic region; menopausal status and menopausal status*BMI. | 7 | HR = 0.94 (0.87–1.02) for 9.90 ppb increase |
| Lemarchand et al., 2021; France [25] | 13 | NO2 | CC (CECILE study) | 1229/1316 | Chemistry transport model 10-year period prior the reference date using residential history | Age; study area; family history; age at first full-term pregnancy; HRT use; physical activity | 9 | OR = 1.11 (0.96; 1.26) |
| Li et al., 2021; Taiwan [24] | - | NO2 | Co | 1603/98,017 | Monitoring station | Age; monthly income and urbanization level | 6 | Q4/Q1 NO2: HR = 1.79 (1.48, 2.15) |
| Amadou et al., 2022; France [50] | 14 | NO2 | Nested CC (XENAIR) | 5222/5222 | Statistical methods (LUR), chemistry transport model Cumulative exposure from inclusion to index date at each address from inclusion to index date | Age; date; department of residence; menopausal at baseline; physical activity; smoking status; education; rural urban status at inclusion; BMI; family history; history of benign breast disease; age at menarche; parity and age at first full-term pregnancy; breastfeeding; oral contraceptive use and HRT use | 8 | RR = 1.04 (0.99; 1.09) |
| N Studies | RR (95% CI) | I2 (%) | p for Heterogeneity | p for Heterogeneity between Strata | ID of Included Articles a | ||
|---|---|---|---|---|---|---|---|
| Overall meta-estimate | 13 | 1.015 (1.003; 1.028) | 16.9 | 0.27 | 1–6, 8–14 | ||
| Exposure assessment method | 1.00 | ||||||
| LUR | 10 | 1.016 (1.002; 1.030) | 26.5 | 0.20 | 1–3, 5, 6, 9–12, 14 | ||
| Other | 6 | 1.037 (1.006; 1.069) | 9.8 | 0.35 | 2, 4, 8, 11, 13, 14 | ||
| Address used for exposure assessment | 0.24 | ||||||
| At baseline | 7 | 1.018 (0.993; 1.043) | 38.7 | 0.14 | 1, 3, 5, 6, 8, 9, 12 | ||
| Residential history | 6 | 1.010 (1.001; 1.019) | 0.0 | 0.49 | 2, 4, 10, 11, 13, 14 | ||
| Geographic area | 0.10 | ||||||
| North America | 8 | 1.007 (0.999; 1.016) | 0.0 | 0.90 | 1–3, 8–11, 12 | ||
| Europe | 5 | 1.043 (1.017; 1.069) | 6.6 | 0.37 | 4–6, 13, 14 | ||
| Menopausal status | 0.76 | ||||||
| Postmenopausal | 9 | 1.014 (0.995; 1.033) | 0 | 0.51 | 1–3, 5, 8, 9 | ||
| Premenopausal | 6 | 1.022 (0.963; 1.085) | 37.8 | 0.15 | 2, 8, 9 | ||
| ER/PR status of the tumor | 0.32 | ||||||
| ER+PR+ | 5 | 1.034 (0.992; 1.077) | 0.0 | 0.52 | 3, 8, 11, 13, 14 | ||
| ER−PR− | 5 | 0.988 (0.925; 1.055) | 6.6 | 0.37 | 3, 8, 11, 13, 14 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praud, D.; Deygas, F.; Amadou, A.; Bouilly, M.; Turati, F.; Bravi, F.; Xu, T.; Grassot, L.; Coudon, T.; Fervers, B. Traffic-Related Air Pollution and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Cancers 2023, 15, 927. https://doi.org/10.3390/cancers15030927
Praud D, Deygas F, Amadou A, Bouilly M, Turati F, Bravi F, Xu T, Grassot L, Coudon T, Fervers B. Traffic-Related Air Pollution and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Cancers. 2023; 15(3):927. https://doi.org/10.3390/cancers15030927
Chicago/Turabian StylePraud, Delphine, Floriane Deygas, Amina Amadou, Maryline Bouilly, Federica Turati, Francesca Bravi, Tingting Xu, Lény Grassot, Thomas Coudon, and Béatrice Fervers. 2023. "Traffic-Related Air Pollution and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies" Cancers 15, no. 3: 927. https://doi.org/10.3390/cancers15030927
APA StylePraud, D., Deygas, F., Amadou, A., Bouilly, M., Turati, F., Bravi, F., Xu, T., Grassot, L., Coudon, T., & Fervers, B. (2023). Traffic-Related Air Pollution and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Cancers, 15(3), 927. https://doi.org/10.3390/cancers15030927





