Add-On Effect of Hemagglutinating Virus of Japan Envelope Combined with Chemotherapy or Immune Checkpoint Inhibitor against Malignant Pleural Mesothelioma: An In Vivo Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Mouse
2.2. Preparation of HVJ-E
2.3. Labeling of PKH26 to HVJ-E
2.4. Cytotoxic Assay
2.5. ELISpot Assay
2.6. Subcutaneous Tumor Model
2.7. Orthotopic Pleural Tumor-Bearing Mouse Model
2.7.1. MSTO-H211-Bearing CB-17/Icr-scid/scidJcl or NOG (NOD/Shi-scid, IL2Rγ Null) Model
2.7.2. AB22G2-Bearing BALB/c AJcl Mouse Model
2.7.3. Add-On Effect of HVJ-E for ICIs (@PD-1 mAb., @PD-L1 mAb.) on AB22G2-Bearing Mouse Model
2.7.4. Add-On Effect of the Local Administration of HVJ-E for ICIs (@PD-1 mAb., @PD-L mAb.) on AB22G2-Bearing Mouse Model
2.8. Statistical Analyses
3. Results
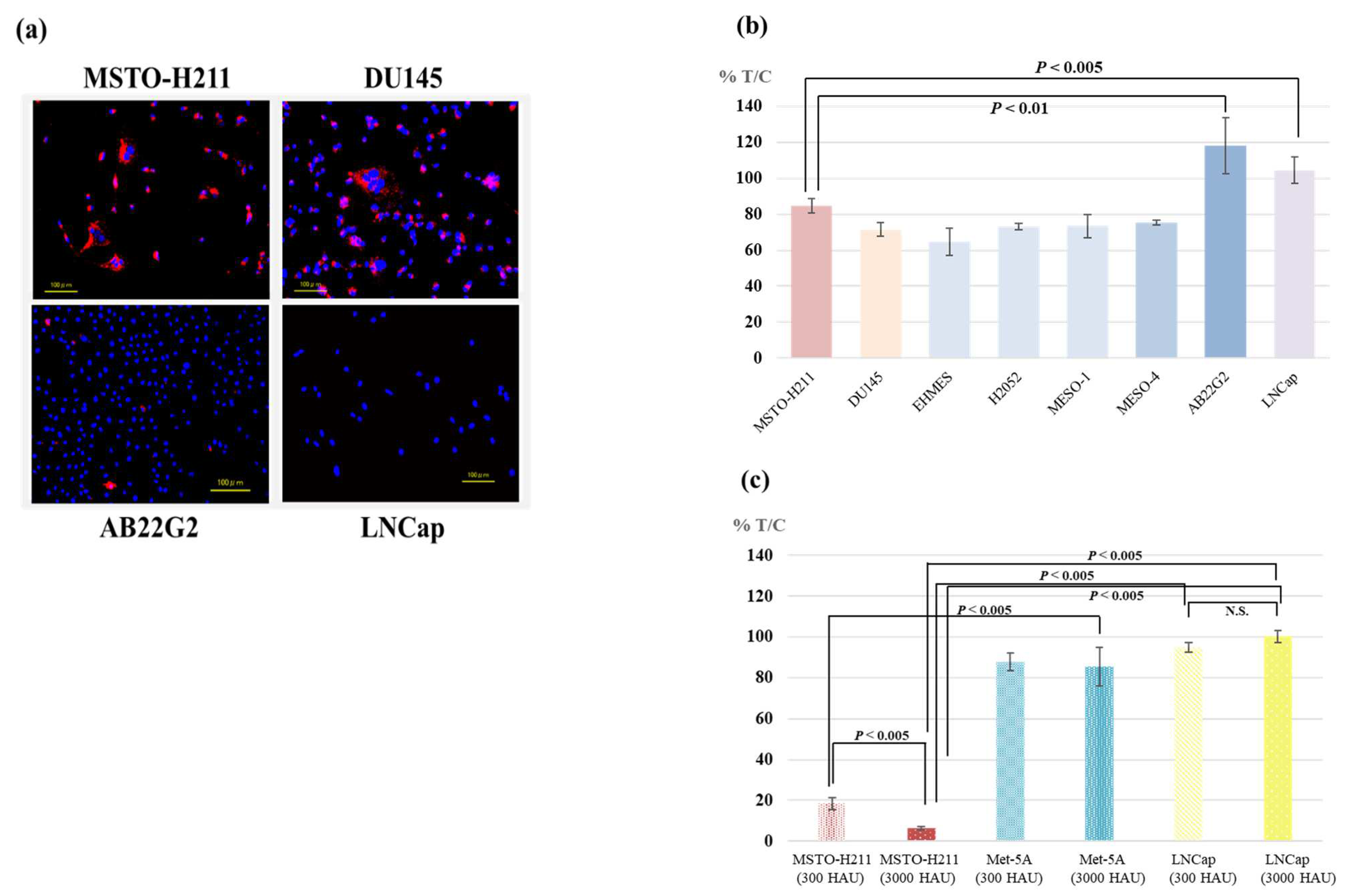
3.1. Affinity of HVJ-E to Mesothelioma and Prostate Cancer Cell Lines
3.2. Cytotoxicity of HVJ-E against Various Tumor Cell Lines
3.3. Survival of Human Malignant Pleural Mesothelioma-Bearing CB-17/SCID Mouse or NOG Mouse
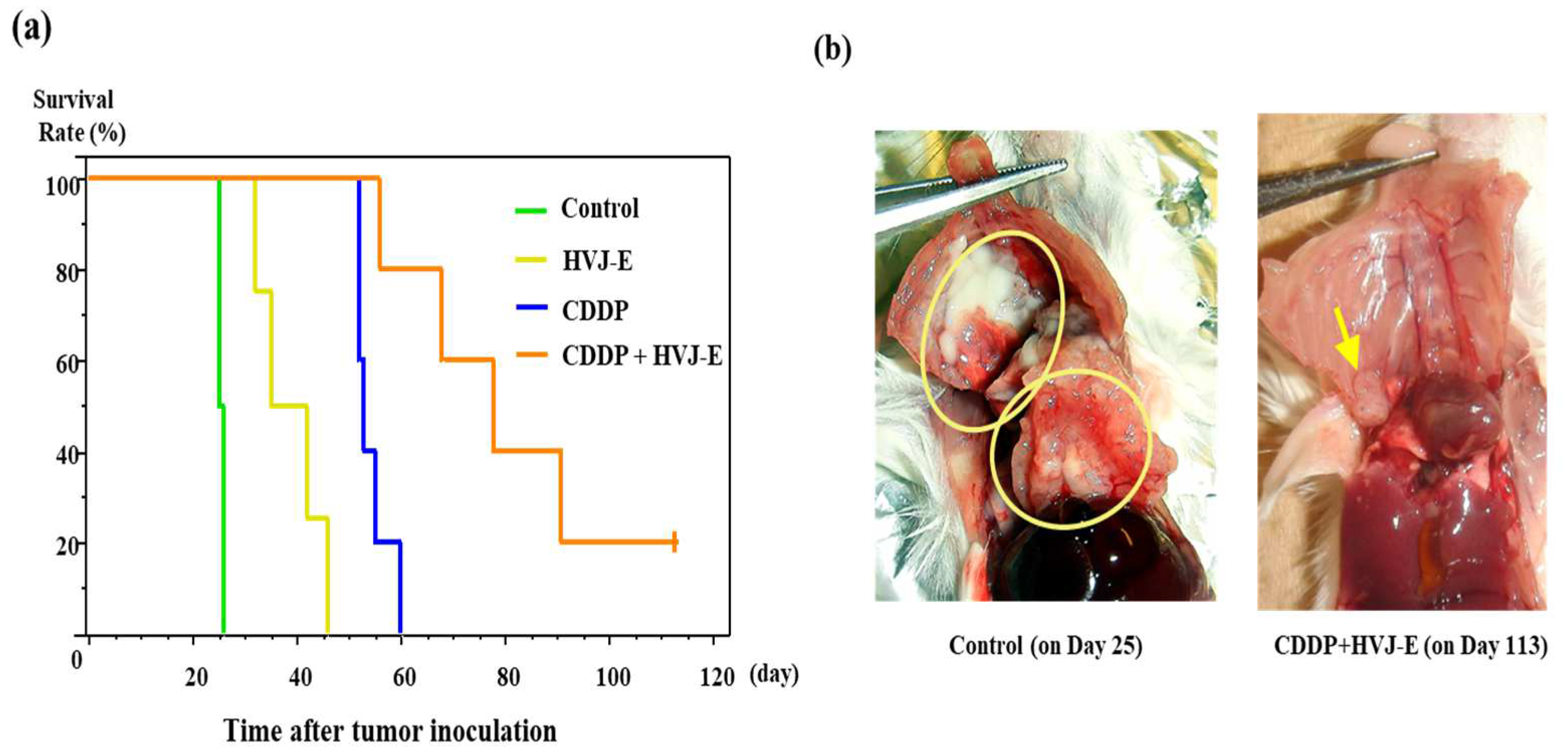
3.4. Survival of Murine Malignant Pleural Mesothelioma-Bearing Mouse
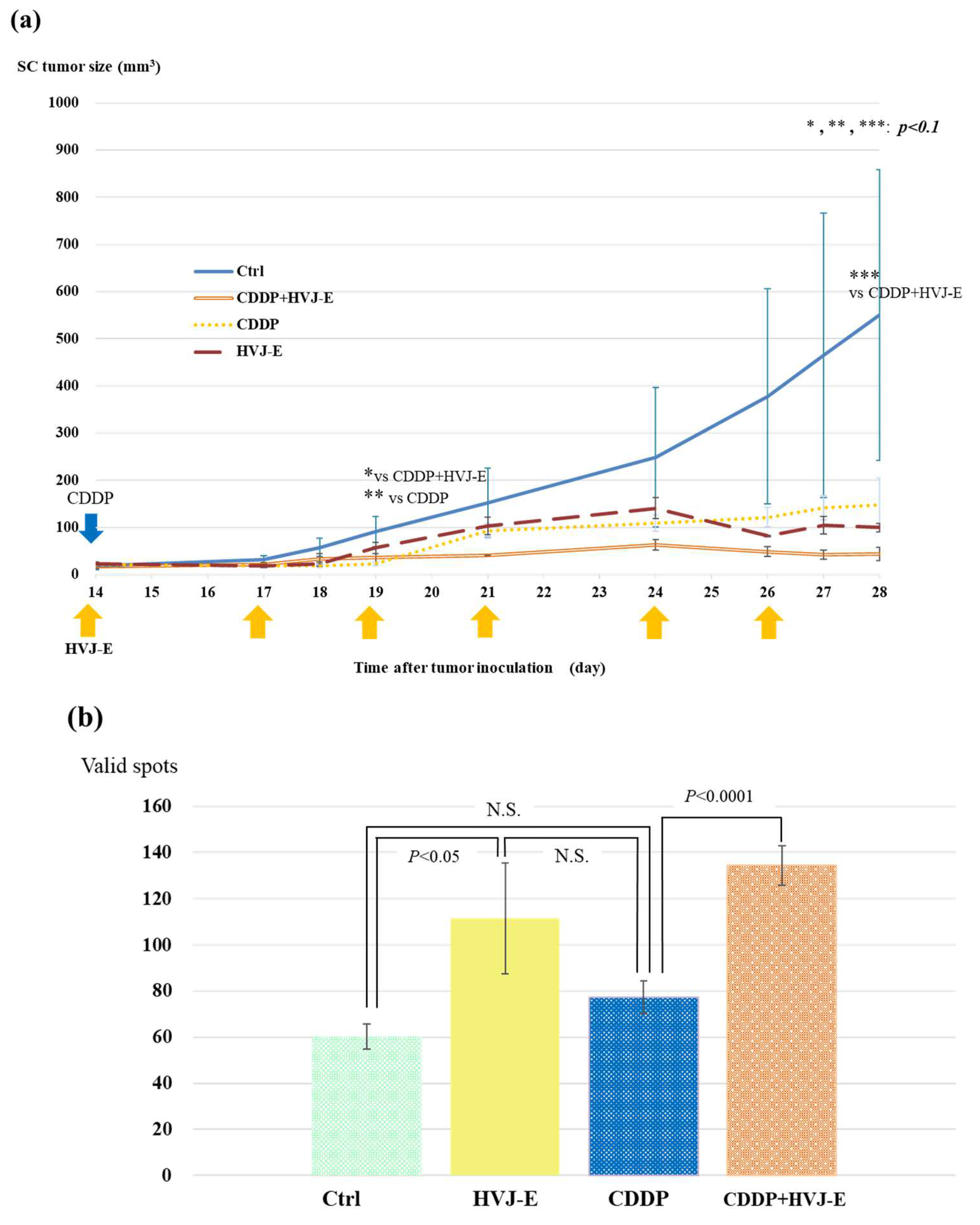
3.5. AB22G2 Specific INF-γ Response on AB22G2 Subcutaneous Tumor Model Treated by HVJ-E and CDDP
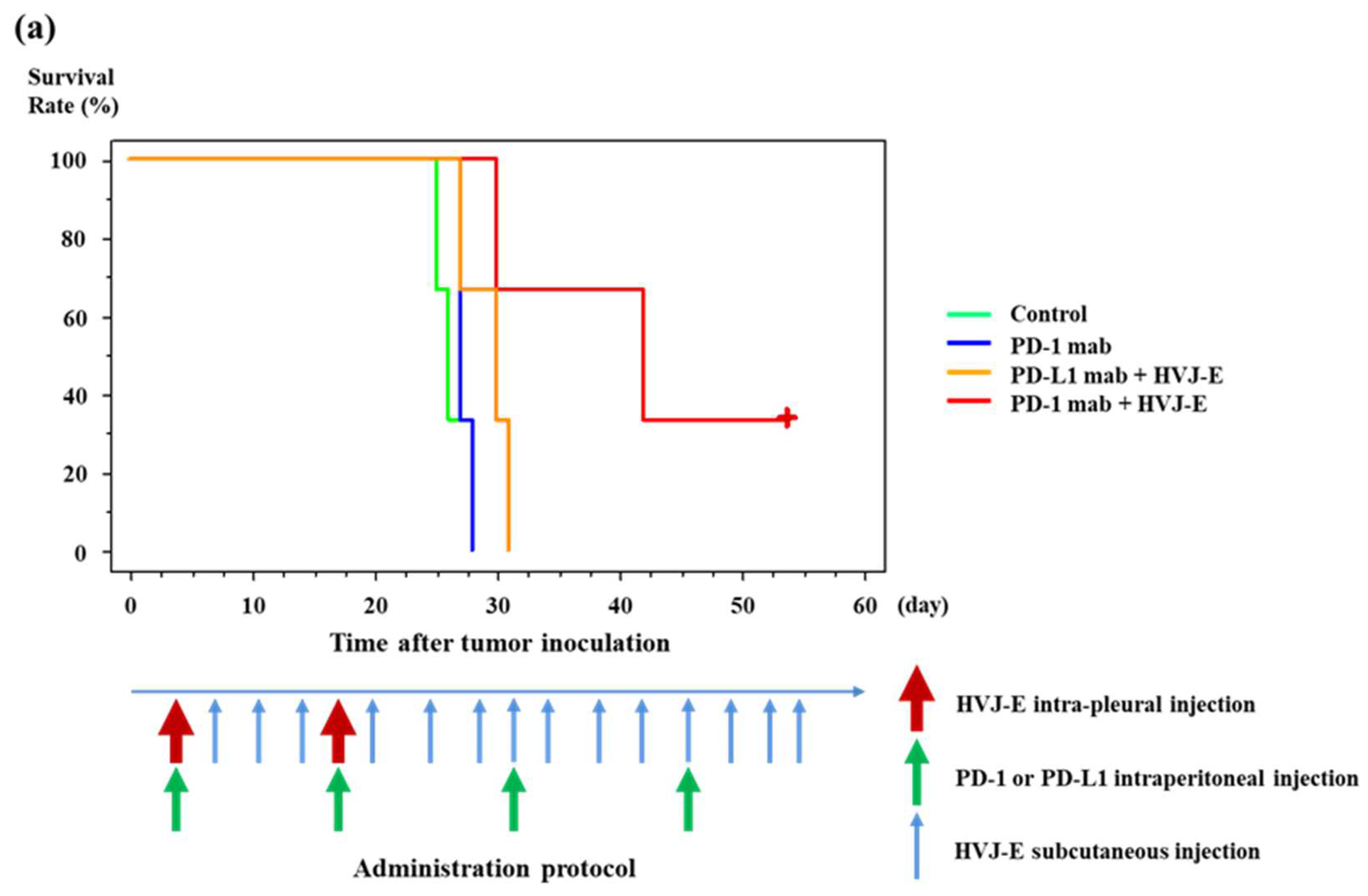
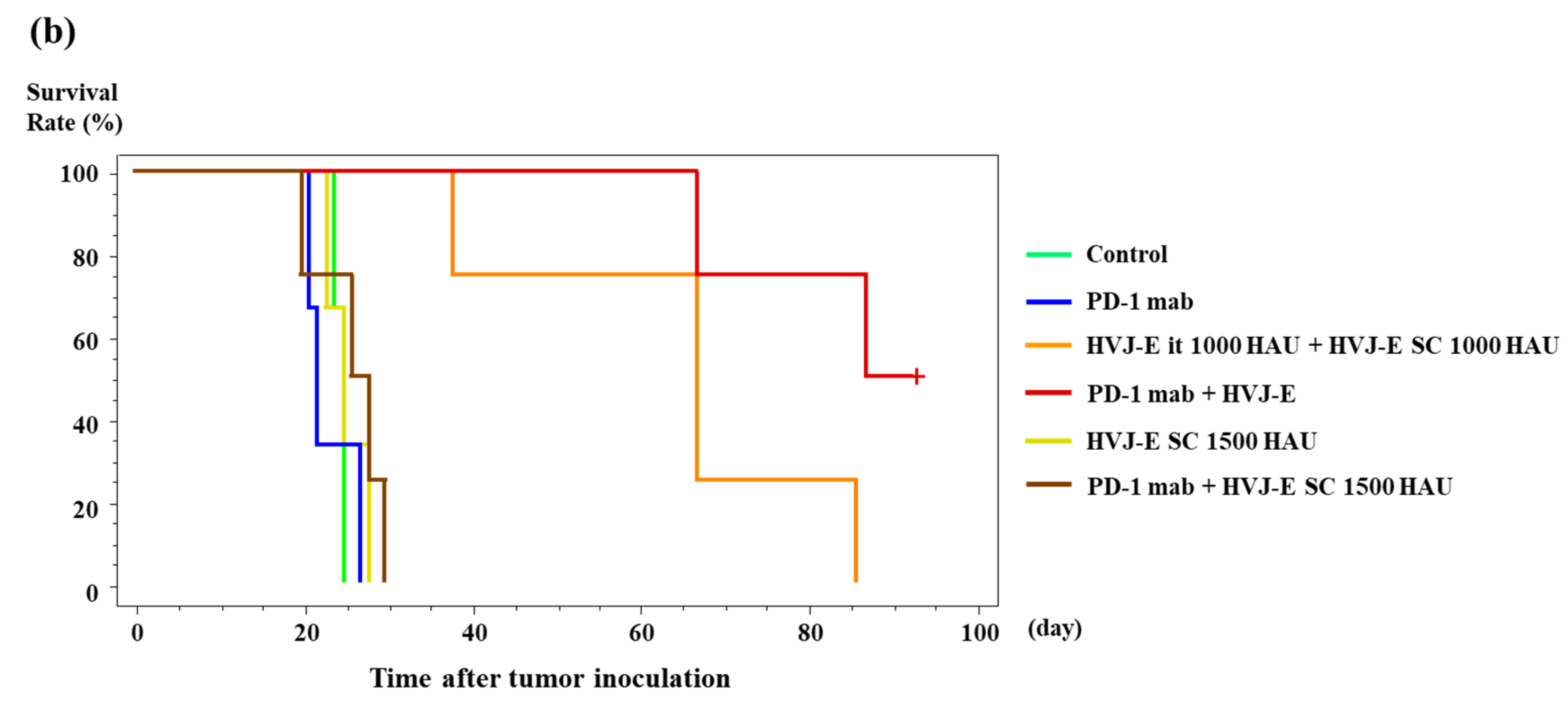
3.6. Survival of Murine Malignant Pleural Mesothelioma-Bearing Mouse Treated with HVJ-E and PD-1 (CD279) Monoclonal Antibody
4. Discussion
4.1. Affinity and Cytotoxicity of HVJ-E against Various Cell Lines
4.2. Antitumor Effects of HVJ-E
4.3. Investigating the Effects on the Acquired Immunity
4.4. Effects of HVJ-E on Cytotoxic T Cells
4.5. Investigate the Effect of Combination with ICIs
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, A.S.; Wistuba, I.; Roth, J.A.; Kindler, H.L. Malignant pleural mesothelioma. J. Clin. Oncol. 2009, 27, 2081–2090. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Reid, G.; Frank, A.L. Asbestos-related cancers: The ‘Hidden Killer’ remains a global threat. Expert Rev. Anticancer Ther. 2020, 20, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sinn, K.; Mosleh, B.; Hoda, M.A. Malignant pleural mesothelioma: Recent developments. Curr. Opin. Oncol. 2021, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Brims, F.J.H.; Gandhi, A.; Olsen, N.; Musk, A.W.; Maskell, N.A.; Lee, Y.C.G. Postmortem findings of malignant pleural mesothelioma: A two-center study of 318 patients. Chest 2012, 142, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Joshi, N.; Raj, K.P.; Wakelee, H.; Neal, J.W. PD-1/PD-L1 Checkpoint Inhibitor Immunotherapy for Malignant Pleural Mesothelioma: Case Series and Literature Review. Clin Lung Cancer 2021, 22, e329–e335. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Brcic, L.; Klikovits, T.; Megyesfalvi, Z.; Mosleh, B.; Sinn, K.; Hritcu, R.; Laszlo, V.; Cufer, T.; Rozman, A.; Kern, I.; et al. Prognostic impact of PD-1 and PD-L1 expression in malignant pleural mesothelioma: An international multicenter study. Transl. Lung Cancer Res. 2021, 10, 1594–1607. [Google Scholar] [CrossRef]
- Shin, J.; Chung, J.H.; Kim, S.H.; Lee, K.S.; Suh, K.J.; Lee, J.Y.; Kim, J.W.; Lee, J.O.; Kim, J.W.; Kim, Y.J.; et al. Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Res. Treat. 2019, 51, 1086–1097. [Google Scholar] [CrossRef]
- Kaneda, Y. A non-replicating oncolytic vector as a novel therapeutic tool against cancer. BMB Rep. 2010, 43, 773–780. [Google Scholar] [CrossRef]
- Kurooka, M.; Kaneda, Y. Inactivated Sendai virus particles eradicate tumors by inducing immune responses through blocking regulatory T cells. Cancer Res. 2007, 67, 227–236. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Miyamoto, Y.; Inoue, T.; Kaneda, Y. Efficient eradication of hormone-resistant human prostate cancers by inactivated Sendai virus particle. Int. J. Cancer 2009, 124, 2478–2487. [Google Scholar] [CrossRef]
- Nakajima, T.; Itai, T.; Wada, H.; Yamauchi, T.; Kiyohara, E.; Kaneda, Y. A Novel Therapy for Melanoma and Prostate Cancer Using a Non-Replicating Sendai Virus Particle (HVJ-E). In Novel Gene Therapy Approaches; Ming, W., David, G., Eds.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Kiyohara, E.; Tanemura, A.; Nishioka, M.; Yamada, M.; Tanaka, A.; Yokomi, A.; Saito, A.; Sakura, K.; Nakajima, T.; Myoui, A.; et al. Intratumoral injection of hemagglutinating virus of Japan-envelope vector yielded an antitumor effect for advanced melanoma: A phase I/IIa clinical study. Cancer Immunol. Immunother. 2020, 69, 1131–1140. [Google Scholar] [CrossRef]
- Kiyohara, E.; Tanemura, A.; Sakura, K.; Nakajima, T.; Myoui, A.; Yamazaki, N.; Kiyohara, Y.; Katayama, I.; Fujimoto, M.; Kaneda, Y. A phase I dose-escalation, safety/tolerability, and preliminary efficacy study of the intratumoral administration of GEN0101 in patients with advanced melanoma. Cancer Immunol. Immunother. 2022, 71, 2041–2049. [Google Scholar] [CrossRef]
- Matsushima-Miyagi, T.; Hatano, K.; Nomura, M.; Li-Wen, L.; Nishikawa, T.; Saga, K.; Shimbo, T.; Kaneda, Y. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles. Clin. Cancer Res. 2012, 18, 6271–6283. [Google Scholar] [CrossRef]
- Matsuda, S.; Nakajima, E.; Nakanishi, T.; Hitsuji, A.; Zhang, H.; Tanaka, A.; Matsuda, H.; Momma, T.; Osaka, T. Effective induction of death in mesothelioma cells with magnetite nanoparticles under an alternating magnetic field. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 90–96. [Google Scholar] [CrossRef]
- Usami, N.; Fukui, T.; Kondo, M.; Taniguchi, T.; Yokoyama, T.; Mori, S.; Yokoi, K.; Horio, Y.; Shimokata, K.; Sekido, Y.; et al. Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients. Cancer Sci. 2006, 97, 387–394. [Google Scholar] [CrossRef]
- Nakataki, E.; Yano, S.; Matsumori, Y.; Goto, H.; Kakiuchi, S.; Muguruma, H.; Bando, Y.; Uehara, H.; Hamada, H.; Kito, K.; et al. Novel orthotopic implantation model of human malignant pleural mesothelioma (EHMES-10 cells) highly expressing vascular endothelial growth factor and its receptor. Cancer Sci. 2006, 97, 183–191. [Google Scholar] [CrossRef]
- Davis, M.R.; Manning, L.S.; Whitaker, D.; Garlepp, M.J.; Robinson, B.W. Establishment of a murine model of malignant mesothelioma. Int. J. Cancer 1992, 52, 881–886. [Google Scholar] [CrossRef]
- Morikawa, K.; Walker, S.M.; Jessup, J.M.; Fidler, I.J. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988, 48, 1943–1948. [Google Scholar]
- Mezzapelle, R.; Rrapaj, E.; Gatti, E.; Ceriotti, C.; Marchis, F.D.; Preti, A.; Spinelli, A.E.; Perani, L.; Venturini, M.; Valtorta, S.; et al. Human malignant mesothelioma is recapitulated in immunocompetent BALB/c mice injected with murine AB cells. Sci. Rep. 2016, 6, 22850. [Google Scholar] [CrossRef]
- Chang, C.Y.; Tai, J.A.; Li, S.; Nishikawa, T.; Kaneda, Y. Virus-stimulated neutrophils in the tumor microenvironment enhance T cell-mediated anti-tumor immunity. Oncotarget 2016, 7, 42195–42207. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.C.; Nishikawa, T.; Chang, C.Y.; Tai, J.A.; Kaneda, Y. CXCL2 combined with HVJ-E suppresses tumor growth and lung metastasis in breast cancer and enhances anti-PD-1 antibody therapy. Mol. Ther. Oncolytics 2020, 20, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Tanaka, T.; Murai, T.; Kondo, M.; Kimura, J.; Su, W.; Kitagawa, T.; Ito, T.; Matsuda, H.; Miyasaka, M. Novel chondroitin sulfate-binding cationic liposomes loaded with cisplatin efficiently suppress the local growth and liver metastasis of tumor cells in vivo. Cancer Res. 2002, 62, 4282–4288. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Gauvrit, A.; Brandler, S.; Sapede-Peroz, C.; Boisgerault, N.; Tangy, F.; Gregoire, M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008, 68, 4882–4892. [Google Scholar] [CrossRef]
- Iwai, H. Susceptibility of Mice to Sendai Virus Infection. Jikken Dobutsu. Exp. Anim. 1984, 33, 275–282. [Google Scholar] [CrossRef]
- Ito, Y.; Yamamoto, F.; Takano, M.; Maeno, K.; Shimokata, K.; Iinuma, M.; Hara, K.; Iijima, S. Detection of cellular receptors for Sendai Virus in mouse tissue sections. Arch. Virol. 1983, 75, 103–113. [Google Scholar] [CrossRef]
- Kurino, T.; Matsuda, R.; Terui, A.; Suzuki, H.; Kokubo, T.; Uehara, T.; Arano, Y.; Hisaka, A.; Hatakeyama, H. Poor outcome with anti-programmed death-ligand 1 (PD-L1) antibody due to poor pharmacokinetic properties in PD-1/PD-L1 blockade-sensitive mouse models. J. Immunother. Cancer 2020, 8, e000400. [Google Scholar] [CrossRef]
- Lee, J.H.; Shklovskaya, E.; Lim, S.Y.; Carlino, M.S.; Menzies, A.M.; Stewart, A.; Pedersen, B.; Irvine, M.; Alavi, S.; Yang, J.Y.H.; et al. Transcriptional downregulation of MHC class I and melanoma de- differentiation in resistance to PD-1 inhibition. Nat. Commun. 2020, 11, 1897. [Google Scholar] [CrossRef]
- Garrido, F.; Ruiz-Cabello, F.; Aptsiauri, N. Rejection versus escape: The tumor MHC dilemma. Cancer Immunol. Immunother. 2017, 66, 259–271. [Google Scholar] [CrossRef]
- Šmahel, M. PD-1/PD-L1 Blockade Therapy for Tumors with Downregulated MHC Class I Expression. Int. J. Mol. Sci. 2017, 18, 1331. [Google Scholar] [CrossRef]
- Fournel, L.; Wu, Z.; Stadler, N.; Damotte, D.; Lococo, F.; Boulle, G.; Ségal-Bendirdjian, E.; Bobbio, A.; Icard, P.; Trédaniel, J.; et al. Cisplatin increases PD-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett. 2019, 464, 5–14. [Google Scholar] [CrossRef]
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.; Tseng, G.; Kim, E.; et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019, 38, 2380–2393. [Google Scholar] [CrossRef]
- Noguchi, T.; Ward, J.P.; Gubin, M.M.; Arthur, C.D.; Lee, S.H.; Hundal, J.; Selby, M.J.; Graziano, R.F.; Mardis, E.R.; Korman, A.J.; et al. Temporally Distinct PD-L1 Expression by Tumor and Host Cells Contributes to Immune Escape. Cancer Immunol. Res. 2017, 5, 106–117. [Google Scholar] [CrossRef]
- Dafni, U.; Tsourti, Z.; Vervita, K.; Peters, S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019, 134, 127–140. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakura, K.; Sasai, M.; Funaki, S.; Shintani, Y.; Okumura, M.; Kaneda, Y. Add-On Effect of Hemagglutinating Virus of Japan Envelope Combined with Chemotherapy or Immune Checkpoint Inhibitor against Malignant Pleural Mesothelioma: An In Vivo Study. Cancers 2023, 15, 929. https://doi.org/10.3390/cancers15030929
Sakura K, Sasai M, Funaki S, Shintani Y, Okumura M, Kaneda Y. Add-On Effect of Hemagglutinating Virus of Japan Envelope Combined with Chemotherapy or Immune Checkpoint Inhibitor against Malignant Pleural Mesothelioma: An In Vivo Study. Cancers. 2023; 15(3):929. https://doi.org/10.3390/cancers15030929
Chicago/Turabian StyleSakura, Kazuma, Masao Sasai, Soichiro Funaki, Yasushi Shintani, Meinoshin Okumura, and Yasufumi Kaneda. 2023. "Add-On Effect of Hemagglutinating Virus of Japan Envelope Combined with Chemotherapy or Immune Checkpoint Inhibitor against Malignant Pleural Mesothelioma: An In Vivo Study" Cancers 15, no. 3: 929. https://doi.org/10.3390/cancers15030929
APA StyleSakura, K., Sasai, M., Funaki, S., Shintani, Y., Okumura, M., & Kaneda, Y. (2023). Add-On Effect of Hemagglutinating Virus of Japan Envelope Combined with Chemotherapy or Immune Checkpoint Inhibitor against Malignant Pleural Mesothelioma: An In Vivo Study. Cancers, 15(3), 929. https://doi.org/10.3390/cancers15030929






